Abstract
Objective:
Tumor-associated macrophages (TAMs) are known to have adverse effects on the survival of women with endometrial cancer. Because monocytes function as progenitors of macrophages, this study examined the association between monocyte count at the first recurrence/progression of endometrial cancer and survival time after recurrence/progression (SAR).
Methods:
This is a retrospective study evaluating 141 consecutive cases of recurrent endometrial cancer after surgical staging (n = 114) and progression after nonsurgical management (n = 27). Complete blood cell counts with cell differentiation at the time of the first recurrence/progression were correlated to SAR.
Results:
Median time of SAR was 7.8 months, and there were 97 (68.8%) patients who died from endometrial cancer with 1-, 2-, and 5-year SAR rates being 51.0%, 32.9%, and 14.2%, respectively. Median monocyte counts at recurrence/progression were 0.5 × 109/L. The strongest correlation to monocyte counts was seen in neutrophil counts (r = 0.57, P < 0.01) followed by platelet counts (r = 0.43, P < 0.01). An elevated monocyte count at recurrence/progression was significantly associated with decreased SAR (hazard ratio per unit, 3.97; 95% confidence interval, 2.00–7.90; P < 0.01). On multivariate analysis controlling for patient demographics, complete blood cell counts, tumor factors, and treatment types for recurrent/progressed disease, higher monocyte counts at recurrence/progression remained an independent predictor for decreased SAR (hazard ratio per unit, 3.12; 95% confidence interval, 1.52–6.67; P < 0.01).
Conclusions:
Our study demonstrated that the increased monocyte counts at recurrence/progression may be a useful biomarker for predicting decreased survival outcome of women with endometrial cancer.
Keywords: Endometrial cancer, Recurrence, Monocyte, Survival, Prognosis
Endometrial cancer is the most common gynecologic malignancy in the United States, with an estimated 60 050 new cases and 10 470 deaths projected in 2016.1 Approximately 70% of patients are given a diagnosis while the disease is confined to the uterus.2 Among these cases, 10% to 15% of patients with early-stage endometrial cancer will experience recurrences.3,4 On the other hand, approximately 50% of patients with advanced-stage endometrial cancer will recur and account for more than 50% of all deaths related to uterine tumors.2 Survival after recurrence/progression (SAR) in advanced-stage endometrial cancer seems to be relatively short.5
Recent studies have shown that the formation of an inflammatory microenvironment plays a pivotal role in endometrial cancer development.6,7 Chronic inflammation and tumor progression are strongly linked.5,7 Key features of cancer-related inflammation are the activation of oncogenes (PTEN, KRAS, and p53), the release of inflammatory cytokines (tumor necrosis factor α, IL-1, IL-6, and COX2), and a prominent leukocyte infiltrate such as marcrophages.8,9
Macrophages play a pivotal role in the immune system in the tumor microenvironment. Tumor-associated macrophages (TAMs) originate from circulating monocytes in the blood. Recruited monocytes differentiate into mature macrophages in the tumor microenvironment.10 Studies have shown that an increased macrophage infiltration in the uterine tumors is correlated with aggressive tumor behaviors, progression, and growth of endometrial cancer.11,12 In addition, an increased number of circulating monocytes have been associated with aggressive tumor behavior and decreased survival in women with endometrial cancer.13 However, the significance of monocyte levels at recurrence/progression remains unclear in the setting of endometrial cancer. The aim of this study was to examine the association between monocyte counts at the first recurrence/progression and SAR in women with endometrial cancer.
MATERIALS AND METHODS
Study Design and Eligibility
After institutional review board approval was obtained at the University of Southern California, the institutional database for endometrial cancer was used to identify cases. Eligibility criteria for this study were (1) patients with endometrial cancer who were found to have the first recurrence after hysterectomy-based surgical staging and (2) patients with endometrial cancer who initially were treated with chemotherapy but progressed at the Los Angeles County Medical Center and Keck Medical Center of University of Southern California between January 1, 2003, and August 31, 2015. Patients were excluded from this study if they were without laboratory results at the time of recurrence/progression or if they had other histologic diagnoses including uterine sarcoma, carcinosarcoma, and endometrial hyperplasia. The Strengthening the Reporting of Observational Studies in Epidemiology guideline was consulted for this retrospective cohort study.14 Some of the patients in this study were within the context of our previous studies.13,15,16
Clinical Information
With the eligible cases, the following information was abstracted from medical records: (1) patient demographics, (2) pathology results for hysterectomy-based surgical staging or endometrial biopsy, (3) laboratory results for complete blood cell counts (CBCs) obtained at the time of initial endometrial cancer diagnosis and the first recurrence/progression, and (4) survival outcomes. The patient demographics included age at recurrence/progression, ethnicity, use of surgical staging at the initial endometrial cancer diagnosis, progression-free survival (PFS), anatomical sites of recurrent/progressed disease, number of recurrence sites, and the first-line treatment for recurrence/progression (chemotherapy, radiation therapy, or surgery). The pathology results included histologic subtype, tumor grade, and cancer stage. The laboratory results for CBC included absolute neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts (×109/L); hemoglobin (Hb; g/dL); platelet counts (×109/L); blood urea nitrogen (BUN) (mg/dL); creatinine (mg/dL); and albumin levels (mg/L). For survival time, PFS and SAR were obtained.
Definition
Cancer stage was reclassified based on the 2009 International Federation of Gynecology and Obstetrics system.17 Histologic subtypes were grouped as endometrioid, serous, clear cell, or other adenocarcinoma. Tumor grade was divided into a low-grade group and a high-grade group. Grade 1 to 2 endometrioid tumors were categorized as low grade. Grade 3 endometrioid, serous, and clear cell tumors were categorized as high grade. The cutoff values for CBC were divided into 3 groups (1–33, 34–66, and 67–100 percentiles) as follows: monocyte count (≤0.4, 0.5–0.6, ≥0.7 × 109/L), lymphocyte count (≤1.3, 1.9–1.4, ≥2.0 × 109/L), and Hb (≤10.0, 10.1–12.9, ≥13.0 g/dL). Chemotherapy response was determined by the RECIST criteria. Progression-free survival was defined as the time interval between the date of hysterectomy and the date of the first recurrence among surgically treated cases or between the date of diagnosis and the date of progression among medically treated cases. Survival after recurrence/progression was defined as the time interval between the date of first recurrence/progression and either the date of death due to endometrial cancer or the last follow-up date if the patient was alive. The coinvestigators (H.M., M.Y.D., and M.S.H.) entered the data into the de-identified data sheet, and the principal investigator (K.M.) of the study examined the accuracy, consistency, and quality of the data.
Statistical Analysis
The primary interest of analysis was to correlate monocyte counts at the time of the first recurrence/progression to clinicopathological factors. The secondary aim of the analysis was to examine the significance of monocyte counts on SAR in patients with endometrial cancer. Continuous variables were examined for normality by the Kolmogorov-Smirnov test and were expressed with mean (SD) or median (range) as appropriate. Statistical significance of the continuous variables was assessed with Student t test or Mann-Whitney U test as appropriate. Categorical or ordinal variables were expressed with number (%), and statistical significance was examined by χ2 test or the Fisher exact test as appropriate. Complete blood cell counts at the time of the initial endometrial cancer diagnosis and the first recurrence/progression were compared using paired t test. Spearman correlation coefficient was used to examine associations between monocyte counts and other laboratory parameters.
Comparison of median values in multiple groups (more than 2 groups) was examined by the Kruskal-Wallis test. For survival analysis, the Kaplan-Meier method was used to construct survival curves, and statistical significance was examined by log-rank test for univariate analysis. A Cox proportional hazard regression model was used for multivariate analysis. Covariates with P < 0.10 in the univariate analysis were initially entered into the multivariate model. With a conditional backward method, the final model in the multivariate analysis only retained the significant factors for SAR. The statistical significance of the survival analysis was expressed with a hazard ratio (HR) and 95% confidence interval (CI). Receiver operating characteristic curve analysis was used to determine the cutoff values for CBC to maximize SAR and to assess the use of our predictive model for SAR expressed with area under curve (AUC). All statistical tests were 2-tailed, and a P value less than 0.05 was considered to be statistically significant. Statistical Package for the Social Sciences (SPSS version 22.0; Chicago, IL) was used for the analyses.
RESULTS
There were 694 cases of endometrial cancer identified during the study period. Of those, 141 patients had recurrence or progression of disease and were examined for the analysis: recurrence of disease after surgical staging (n = 114) and progression of disease after nonsurgical management with chemotherapy (n = 27). Clinicopathological demographics are listed in Table 1. Median age was 58.2 years, and most patients were Hispanic (60.4%). Chemotherapy was the most common modality for treatment of recurrent/progressed disease (71.6%) followed by radiotherapy (24.8%). Among women who developed endometrial cancer recurrence/progression, platinum agent was the most common chemotherapeutic agent (77.8%) followed by platinum (70%; Table S1, http://links.lww.com/IGC/A433). Whole pelvis radiotherapy was the most common type of radiotherapy for the recurrent/progressive disease (48.5%).
TABLE 1.
Patient demographics and tumor characteristics
| No. | n = 141 |
|---|---|
| Age at recurrence, y | 58.2 (8.1) |
| <60 | 77 (54.6%) |
| ≥60 | 64 (45.4%) |
| Ethnicity | |
| White | 14 (9.9%) |
| African American | 15 (10.6%) |
| Hispanic | 85 (60.4%) |
| Asian | 27 (19.1%) |
| Histology | |
| Endometrioid | 69 (48.9%) |
| Serous | 29 (20.6%) |
| Clear cell | 10 (7.1%) |
| Other | 33 (23.4%) |
| Grade | |
| 1 | 23 (16.3%) |
| 2 | 28 (19.9%) |
| 3 | 90 (63.8%) |
| Initial stage | |
| I | 23 (16.3%) |
| II | 12 (8.5%) |
| III | 46 (32.6%) |
| IV | 60 (42.6%) |
| Initial surgical staging | |
| No | 27 (19.1%) |
| Yes | 114 (80.9%) |
| PFS, mo | 11.9 (0.6–88.1) |
| <6 | 35 (24.8%) |
| 6–11.9 | 36 (25.5%) |
| 12–23.9 | 39 (27.7%) |
| ≥24 | 31 (22.0%) |
| Recurrence site | |
| Within the pelvis | 39 (29.5%) |
| Outside the pelvis | 93 (70.5%) |
| No. recurrence site | |
| Single | 49 (37.1%) |
| Multiple | 83 (62.9%) |
| Chemotherapy* | |
| No | 49 (34.8%) |
| Yes | 92 (65.2%) |
| Radiotherapy* | |
| No | 106 (75.2%) |
| Yes | 35 (24.8%) |
| Surgery* | |
| No | 132 (93.6%) |
| Yes | 9 (6.4%) |
Median (range) or number (%) is shown. There were 9 pieces of missing data for recurrence site and the number of recurrence. Histology, grade, and stage were data from the initial diagnosis. Chemotherapy includes hormone therapy.
Administered for the first recurrent/progressive disease.
For tumor characteristics, most of the cases had non-endometrioid histology (51.1%), high-grade tumors (63.8%), and advanced stage at the initial endometrial cancer diagnosis (75.2%). The median PFS was 11.9 months. Approximately one half of the recurrence/progression occurred within 1 year after the date of hysterectomy (50.3%). For the anatomical sites at the recurrence/progression, more than two thirds of the patients had tumors outside the pelvis (70.5%), and most had multiple recurrence sites (62.9%) at the time of recurrence/progression.
Monocyte counts were associated with clinicopathological factors (Tables 2, 3). The median monocyte count at the time of recurrence/progression was 0.5 × 109/L (range, 0.0–2.1). There was no significant difference between the monocyte count at the initial endometrial cancer diagnosis and the monocyte counts at recurrence/progression (P = 0.68). When compared with the time of initial endometrial cancer diagnosis, a significant decrease at the time of recurrence/progression was observed for neutrophil (6.0 vs 4.4 × 109/L, P < 0.01), lymphocyte (1.8 vs 1.3 × 109/L, P < 0.01), and platelet (316 vs 274 × 109/L, P < 0.01) counts. Conversely, BUN at the time of recurrence/progression was significantly higher (13.0 vs 14.0 mg/dL, P < 0.01) compared with BUN at the time of initial cancer diagnosis.
TABLE 2.
Correlation between monocyte and clinicopathological factors (continuous variables)
| Median (Range) |
Comparison at Monocyte Counts |
||||||
|---|---|---|---|---|---|---|---|
| No. | Initial Diagnosis | Recurrence | P | Median (Range) | r | P | |
| Neut, × 109/L | 124 | 6.0 (0.7–17.9) | 4.4 (0.3–21.9) | <0.01 | 4.4 (0.3–21.9) | 0.57 | <0.01 |
| Lymph, × 109/L | 124 | 1.8 (0.6–5.5) | 1.3 (0.2–3.8) | <0.01 | 1.3 (0.2–3.8) | 0.24 | <0.01 |
| N-L ratio | 124 | 3.3 (0.3–21) | 3.45 (0.4–38.4) | 0.06 | 3.45 (0.4–38.4) | 0.25 | <0.01 |
| Mono, × 109/L | 124 | 0.6 (0.1–1.6) | 0.5 (0–2.1) | 0.68 | 0.5 (0–2.1) | ||
| L-M ratio | 124 | 3.2 (0.0–33.0) | 2.7 (0.0–8.0) | <0.01 | 2.7 (0.0–8.0) | ||
| Eos, × 109/L | 124 | 0.1 (0–1.0) | 0.1 (0–0.7) | 0.02 | 0.1 (0–0.7) | 0.07 | 0.43 |
| Baso, × 109/L | 124 | 0 (0–0.2) | 0 (0–0.2) | <0.01 | 0 (0–0.2) | 0.25 | <0.01 |
| Hb, g/dL | 133 | 11.7 (5.3–15.7) | 11.8 (6.8–15.7) | 0.32 | 11.8 (6.8–15.7) | −0.15 | 0.10 |
| Plt, × 109/L | 133 | 316 (65–930) | 274 (87–1030) | <0.01 | 274 (87–1030) | 0.43 | <0.01 |
| BUN, mg/dL | 133 | 13.0 (4–57) | 14.0 (4–110) | <0.01 | 14.0 (4–110) | 0.14 | 0.13 |
| Cr, mg/dL | 133 | 0.69 (0.2–3.7) | 0.66 (0.1–5.4) | 0.07 | 0.66 (0.1–5.4) | 0.12 | 0.20 |
| Alb, mg/L | 130 | 4.0 (2.0–5.9) | 4.0 (1.5–8.3) | 0.42 | 4.0 (1.5–8.3) | −0.22 | 0.02 |
Number listed is the value at the time of diagnosis or recurrence; mean (±SD) or median (range). Paired t test was used for P values. All factors except eosinophil counts have significant correlations (P < 0.05). Spearman coefficient was also used for P values. Significant P values with r ≥ 0.20 or r ≤ −0.20 are in bold.
Alb, albumin; Baso, basophil; Cr, creatinine; Eos, eosinophil; L-M ratio, lymphocyte-monocyte ratio; Lymph, lymphocyte; Mono, monocyte; Neut, neutrophil; N-L ratio, neutrophil-lymphocyte ratio; Plt, platelet.
TABLE 3.
Correlations between monocyte counts and clinicopathological factors (categorical and ordinal variables)
| No. | Monocyte Counts Median (Range) | ≤0.4 × 109/L (n = 52) | 0.5–0.6 × 109/L (n = 39) | ≥0.7 × 109/L (n = 33) | P | |
|---|---|---|---|---|---|---|
| Age, y | 0.67 | |||||
| <60 | 69 | 0.5 (0–2.1) | 29 (42.0%) | 18 (26.1%) | 22 (31.9%) | |
| ≥60 | 55 | 0.5 (0.3–1.4) | 23 (41.8%) | 21 (38.2%) | 11 (20.0%) | |
| Ethnicity | 0.86 | |||||
| White | 13 | 0.4 (0.3–0.9) | 7 (53.8%) | 2 (15.4%) | 4 (33.3%) | |
| African American | 12 | 0.55 (0.3–1.1) | 5 (41.7%) | 3 (25.0%) | 4 (33.3%) | |
| Hispanic | 73 | 0.5 (0–2.1) | 30 (41.1%) | 25 (34.2%) | 18 (24.7%) | |
| Asian | 26 | 0.5 (0.1–1.6) | 10 (38.5%) | 9 (34.6%) | 7 (26.9%) | |
| Histology | 0.75 | |||||
| Endometrioid | 60 | 0.5 (0–2.1) | 27 (45.0%) | 18 (30.0%) | 15 (25.0%) | |
| Serous | 26 | 0.5 (0.3–1.2) | 11 (42.3%) | 9 (34.6%) | 6 (23.1%) | |
| Clear cell | 9 | 0.4 (0.3–1.2) | 6 (66.7%) | 0 (0%) | 3 (33.3%) | |
| Other | 29 | 0.6 (0.2–1.1) | 8 (27.6%) | 12 (41.4%) | 9 (31.0%) | |
| Grade | 0.63 | |||||
| 1 | 20 | 0.45 (0.3–2.1) | 10 (50.0%) | 5 (25.0%) | 5 (25.0%) | |
| 2 | 25 | 0.5 (0.1–1.6) | 10 (40.0%) | 10 (40.0%) | 5 (20.0%) | |
| 3 | 79 | 0.5 (0–2.0) | 32 (40.5%) | 24 (30.4%) | 23 (29.1%) | |
| Stage | 0.25 | |||||
| I | 21 | 0.6 (0.3–1.1) | 6 (28.6%) | 9 (42.8%) | 6 (28.6%) | |
| II | 11 | 0.4 (0.2–1.6) | 8 (72.7%) | 1 (9.1%) | 2 (18.2%) | |
| III | 42 | 0.5 (0.3–1.6) | 20 (47.6%) | 13 (31.0%) | 9 (21.4%) | |
| IV | 50 | 0.5 (0–2.1) | 18 (36.0%) | 16 (32.0%) | 16 (32.0%) | |
| Surgical staging | 0.08 | |||||
| No | 22 | 0.6 (0–2.1) | 6 (27.3%) | 7 (31.8%) | 9 (40.9%) | |
| Yes | 102 | 0.5 (0.1–2.0) | 46 (45.1%) | 32 (31.4%) | 24 (23.5%) | |
| PFS, mo | 0.20 | |||||
| <12 | 65 | 0.5 (0–2.1) | 24 (36.9%) | 21 (32.3%) | 20 (30.8%) | |
| ≥12 | 59 | 0.5 (0.2–1.4) | 28 (47.5%) | 18 (30.5%) | 13 (22.0%) | |
| Recurrence site | 0.23 | |||||
| Within the pelvis | 34 | 0.4 (0.2–1.4) | 18 (52.9%) | 9 (26.5%) | 7 (20.6%) | |
| Outside the pelvis | 84 | 0.5 (0–2.1) | 33 (39.3%) | 27 (32.1%) | 24 (28.6%) | |
| No. recurrence site | 0.15 | |||||
| Single | 41 | 0.4 (0.2–1.1) | 21 (51.2%) | 14 (34.1%) | 6 (14.6%) | |
| Multiple | 77 | 0.5 (0–2.1) | 30 (39.0%) | 22 (28.6%) | 25 (32.5%) | |
| Chemotherapy | 0.08 | |||||
| No | 34 | 0.6 (0–2.1) | 11 (32.4%) | 9 (26.5%) | 14 (42.2%) | |
| Yes | 90 | 0.5 (0.1–1.6) | 41 (45.6%) | 30 (33.3%) | 19 (21.1%) | |
| Radiotherapy | 0.99 | |||||
| No | 91 | 0.5 (0–2.0) | 40 (44.0%) | 26 (28.6%) | 25 (27.5%) | |
| Yes | 33 | 0.5 (0.1–2.1) | 12 (36.4%) | 13 (39.4%) | 8 (24.2%) | |
| Surgery for recurrence | 0.78 | |||||
| No | 115 | 0.5 (0–2.1) | 49 (42.6%) | 35 (30.4%) | 31 (27.0%) | |
| Yes | 9 | 0.5 (0.3–0.9) | 3 (33.3%) | 4 (44.4%) | 2 (22.2%) |
Mann-Whitney U test or Kruskal-Wallis test was used for P values. There were 17 cases of missing data for monocyte counts and 9 cases of missing data for recurrence site and the number of recurrence.
Monocyte counts at the time of recurrence/progression were positively correlated to neutrophil-lymphocyte ratio (r = 0.25, P < 0.01), as well as neutrophil (r = 0.57, P < 0.01), lymphocyte (r = 0.24, P < 0.01), basophil (r = 0.25, P < 0.01), and platelet (r = 0.43, P < 0.01) counts. Monocyte counts at the time of recurrence/progression were inversely correlated to albumin levels (r = −0.22, P = 0.02). Monocyte counts at the time of recurrence/progression were not associated with all the collected variables (Table 3). Response to the first-line chemotherapy was correlated to the monocyte counts at the first recurrence/progression (Table S1, http://links.lww.com/IGC/A433). Median monocyte counts were statistically similar across the chemotherapy treatment patterns: complete response, 0.4 × 109/L; partial response, 0.6 × 109/L; stable disease, 0.4 × 109/L; and progressive disease, 0.5 × 109/L (P = 0.24); however, women with progressed disease to the first-line chemotherapy had a higher proportion of high monocyte counts at recurrence (percent proportion of ≥0.7 × 109/L; 12.5%, 18.2%, 17.6%, and 24.0%, respectively). Response on the radiotherapy for the first recurrence/progression of endometrial cancer was not associated with the monocyte counts at the first recurrence/progression (P = 0.25; Table S1, http://links.lww.com/IGC/A433).
A survival analysis was performed (Table 4). The median time for SAR was 7.8 months. There were 97 (68.8%) patients who died of endometrial cancer. Longer PFS time was significantly associated with higher SAR rates (PFS of <6, 6–11.9, 12–23.9, and ≥24 months; 2-year SAR rates; 10.5%, 43.7%, 71.0%, and 82.1%; P < 0.01). In the univariate analysis, elevated monocyte counts were associated with decreased SAR (HRper unit, 3.97; 95% CI, 2.00–7.90; P < 0.01). The cutoff to maximize the survival difference was 0.4 × 109/L (2-year SAR rates > 0.4 vs ≤0.4 × 109/L, 27.3% vs 39.9%, P = 0.029; Fig. 1). Other clinicopathological factors significantly associated with decreased SAR in the univariate analysis included elevated neutrophil counts, decreased lymphocyte counts, elevated neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, anemia, thrombocytosis, elevated BUN, elevated creatinine, hypoalbuminemia, high-grade tumor, advanced stage, no surgical staging, no chemotherapy and/or radiation therapy, and no surgery (all Ps < 0.05). In the multivariate analysis, monocyte counts remained a statistically independent risk factor for SAR (HR, 2.60; 95% CI, 1.17–5.58; P = 0.02). Other independent risk factors associated with decreased SAR included PFS (HR, 0.96; 95% CI, 0.94–0.99; P < 0.01), lymphocyte count (HR, 0.46; 95% CI, 0.31–0.70; P < 0.01), Hb (HR, 0.63; 95% CI, 0.64–0.90; P < 0.01), use of surgical staging at the initial endometrial cancer diagnosis (HR, 0.21; 95% CI, 0.11–0.39; P < 0.01), and use of radiation therapy after recurrence/progression (HR, 0.58; 95% CI, 0.34–0.99; P = 0.046).
TABLE 4.
Survival after the recurrence of endometrioid adenocarcinoma in the multivariate analysis
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| No. | HR (95% CI) | P | HR (95% CI) | P | |
| Age (continuous) | 141 | 1.00 (0.98–1.03) | 0.96 | ||
| PFS (continuous) | 141 | 0.95 (0.93–0.97) | <0.01 | 0.96 (0.94–0.99) | <0.01 |
| Laboratory values | |||||
| Neut (continuous) | 124 | 1.18 (1.10–1.26) | <0.01 | ||
| Lymph (continuous) | 124 | 0.46 (0.31–0.68) | <0.01 | 0.46 (0.31–0.70) | <0.01 |
| Mono (continuous) | 124 | 3.97 (2.00–7.90) | <0.01 | 3.12 (1.52–6.67) | <0.01 |
| N-L ratio (continuous) | 124 | 1.12 (1.08–1.16) | <0.01 | ||
| L-M ratio (continuous) | 124 | 0.62 (0.52–0.75) | <0.01 | ||
| Hb (continuous) | 133 | 0.63 (0.55–0.73) | <0.01 | 0.76 (0.64–0.90) | <0.01 |
| Plt (continuous) | 133 | 1.01 (1.00–1.01) | <0.01 | ||
| BUN (continuous) | 133 | 1.04 (1.02–1.06) | <0.01 | ||
| Cr (continuous) | 133 | 2.09 (1.49–2.92) | <0.01 | ||
| Alb (continuous) | 130 | 0.33 (0.23–0.46) | <0.01 | ||
| Histopathology type | 0.12 | ||||
| Endometrioid | 59 | 1 | |||
| Nonendometrioid | 55 | 1.50 (0.91–2.32) | |||
| Tumor grade | 0.02 | ||||
| Low | 44 | 1 | |||
| High | 70 | 1.77 (1.08–2.89) | |||
| Stage | 0.03 | ||||
| I-III | 81 | 1 | |||
| IV | 40 | 1.58 (1.05–2.37) | |||
| Surgical staging | <0.01 | <0.01 | |||
| No | 27 | 1 | 1 | ||
| Yes | 114 | 0.11 (0.07–0.20) | 0.21 (0.11–0.39) | ||
| Recurrence site | 0.09 | ||||
| Within the pelvis | 34 | 1 | |||
| Outside the pelvis | 84 | 1.57 (0.93–2.64) | |||
| No. recurrence site | 0.20 | ||||
| Single | 44 | 1 | |||
| Multiple | 67 | 1.38 (0.85–2.25) | |||
| Chemotherapy* | <0.01 | ||||
| No | 49 | 1 | |||
| Yes | 92 | 0.55 (0.36–0.85) | |||
| Radiotherapy* | 0.01 | 0.046 | |||
| No | 91 | 1 | 1 | ||
| Yes | 33 | 0.55 (0.31–0.86) | 0.58 (0.34–0.99) | ||
| Surgery* | 0.01 | ||||
| No | 115 | 1 | |||
| Yes | 9 | 0.26 (0.08–0.84) | |||
Log-rank testwas used for the univariate analysis. Only significant variableswere used in the univariate analysis among all the covariates tested. A Cox proportional hazard regression model was used for the multivariate analysis (conditional backward). Significant P values are in bold.
Administered for the first recurrence/progressive disease.
Alb, albumin; Cr, creatinine; L-M ratio, lymphocyte-to-monocyte ratio; Lymph, lymphocyte; Mono, monocyte; Neut, neutrophil; N-L ratio, neutrophil-to-lymphocyte ratio; Plt, platelet.
FIGURE 1.
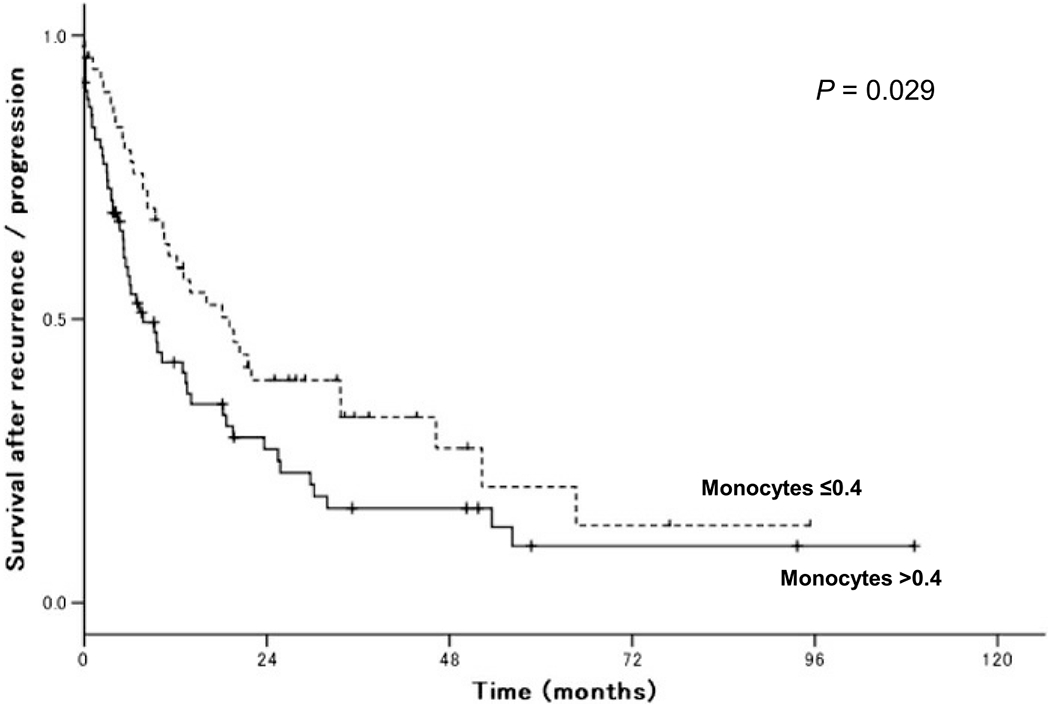
Survival after the recurrence of endometrial cancer. Log-rank test for P values. Kaplan-Meier method for survival curves.
By using the risk factors for SAR at the time of recurrence/progression in endometrial cancer, the predictive model for SAR was determined based on the combinations of the 4 risk factors and the corresponding values of AUC (monocyte counts > 0.4 × 109/L, lymphocyte counts < 2.0 × 109/L, Hb levels < 12.0 g/dL, and PFS < 12 months; Table 5). The number of risk factors was inversely associated with 2-year SAR rates (0, 1, 2, 3, and 4 risk factors: 100%, 67.7%, 37.6%, 22.8%, and 0%, respectively; P < 0.01). The most predictive model was the combination of all 4 risk factors with AUC of 0.76 (95% CI, 0.67–0.85).
TABLE 5.
Predictive model for SAR
| No. | 2-y SAR, % | P | |
|---|---|---|---|
| Any risk factors* | <0.01 | ||
| None | 1 | 100 | |
| 1 | 27 | 67.7 | |
| 2 | 36 | 37.6 | |
| 3 | 34 | 22.8 | |
| 4 | 26 | 0 | |
| Combination | |||
| Lymph alone | 17 | 67.3 | |
| Mono alone | 8 | 100 | |
| Hb alone | 1 | 50.0 | |
| PFS alone | 1 | 0 | |
| Lymph + Mono | 8 | 51.4 | |
| Lymph + Hb | 9 | 33.3 | |
| Lymph + PFS | 10 | 25.9 | |
| Mono + Hb | 4 | 50.0 | |
| Mono + PFS | 4 | 45.0 | |
| Hb + PFS | 1 | n.a. | |
| Lymph + Mono + Hb | 11 | 29.1 | |
| Lymph + Mono + PFS | 7 | 38.5 | |
| Lymph + Hb + PFS | 12 | 16.7 | |
| Mono + Hb + PFS | 4 | 0 | |
| Lymph + Mono + Hb + PFS | 26 | 0 |
Number (%) is shown. χ2 Test was used for P values. Risk factors included lymph < 2.0 × 109/L, mono > 0.4 × 109/L, Hb < 12 g/dL, and PFS < 12 months. P value is significant.
Lymph, lymphocyte; Mono, monocyte; n.a., not available; Neut, neutrophil.
DISCUSSION
The key findings of our study are the following: (1) elevated monocyte counts at the time of recurrence/progression in endometrial cancer are associated with poor survival outcome compared, and (2) 4 perimeters (monocyte counts, lymphocyte counts, Hb levels, and PFS) at recurrence/progression may be useful markers for predicting the survival outcome of women with endometrial cancer. The 4 risk factors identified in this study for decreased overall SAR of endometrial cancer may be partially explained by the theory of cancer-related inflammation and the roles of immune response to cancer, which have been demonstrated in various cancers.6,7
The effect of circulating monocyte counts on survival outcome may be explained by TAMs, which are a key mediator of the immune system. Circulating monocytes are recruited and differentiate into macrophages in the tumor microenvironment (monocyte-macrophage lineage).10 Macrophages can be divided into 2 types: the M1 type acts in immune activation, and the M2 type causes immunosuppression.18,19 Many studies indicate that TAMs express several M2-associated molecular signatures such as CD163 and CD204.18,20 Tumor-associated macrophages are differentiated into polarized M2 macrophages in the tumor microenvironment and are a source and target of cytokines (IL-10, transforming growth factor β), chemokines (CCL5, CCL20, CCL22), growth factors (VEGF), and extracellular matrix proteins that promote tumor angiogenesis, cell proliferation, and invasion of the surrounding tissues.5,21 Furthermore, TAMs express the ligand receptors for programmed death-1 and cytotoxic T-lymphocyte–associated antigen 4. PDL1 triggers apoptosis in target cells, and cytotoxic T-lymphocyte-associated antigen 4 regulates T-cell activation. Through these ligands, TAMs activate the suppression of cytotoxic T cells and result in immune suppression.22 Because circulating monocytes are precursors and the potential origin of TAMs, assessing peripheral monocyte counts may call for a surrogate marker of TAMs.
Decreased lymphocyte counts were found to be associated with poor survival outcomes in this study, and lymphocyte counts at the time of recurrence/progression were significantly lower compared with lymphocyte counts at the time of initial cancer diagnosis. The prognostic significance of peripheral lymphocyte counts in various kinds of cancers has been reported.4,23,24 Lymphocytes play important roles in antitumor immunity, inducing apoptosis, and suppressing tumor proliferation. Cytotoxic T cells and natural killer cells, both derived from T lymphocytes, exhibit antitumor activities of inhibiting the growth and metastasis of tumor.5,25 Several studies have shown that the presence of a lymphocytic infiltration in tumor tissues is associated with improved survival outcome and that the immune system participates in the control and elimination of tumors.6,7,23,26
Hemoglobin levels are also one of the important predictors for SAR in this study. Anemia in endometrial cancer generally can be caused by tumor bleeding or paraneoplastic mechanisms. Tumors can produce or induce cytokines (IL-1, IL-6, interferon γ, tumor necrosis factor α) that can induce cancer-related inflammation, as well as suppress hematopoietic differentiation.8,27 Secondarily, anemia causes hypoxia in tissues, which then induces angiogenesis in the tumor microenvironment and, by this mechanism, can instigate resistance to treatments. In addition, multiple studies have reported that anemia reduces survival times in patients with various malignancies, including endometrial cancer.28,29
Progression-free survival correlates to SAR in this study and can consequently be used as a strong prognostic factor. Patients who had tumor recurrence/progression in a short period have significantly shorter survival than patients with recurrence/progression after a longer period. In part, this result supports previous studies demonstrating short treatment-free interval and decreased overall survival.30,31 Because our study demonstrated the benefit of systemic chemotherapy for a recurrent/progressed disease, this may translate into the choice of chemotherapy based on PFS interval.32
Our estimation model of survival in endometrial cancer used the aforementioned 4 factors. By evaluating these 4 factors at the time of disease recurrence/progression, a risk of survival can be estimated. Risk stratification at the time of disease recurrence/progression may then help decisions regarding further treatment or initiation of adjuvant or salvage therapy. The risk stratification produced by these monograms is consequently valuable for patient counseling. Aggressive treatment may be considered to be withdrawn if survival time is estimated to be limited.
The strength our study is that this study will be the first to examine the significance of monocyte counts at the recurrence/progression correlating to survival outcome in endometrial cancer. A weakness of our study is that this was a retrospective design that may have missed possible confounding factors. The results obtained from our study are hypothesis generating and should be confirmed with larger studies. Another limitation is that our study did not address the direct correlation between circulating monocyte counts and TAMs in the specimen. Our results suggest a potential biological role of monocyte-macrophage lineages in endometrial cancer biology at the time of recurrence/progression.
In conclusion, the monocyte count at recurrence/progression may be a useful biomarker for predicting the survival outcome of women with endometrial cancer. However, the mechanism based on pathogenesis and correlation of TAMs remains unclear in our study and needs further investigation.
Supplementary Material
Acknowledgments
Supported by the Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. [DOI] [PubMed] [Google Scholar]
- 4.Sorbe B, Juresta C, Ahlin C. Natural history of recurrences in endometrial carcinoma. Oncol Lett. 2014;8:1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace AE, Gibson DA, Saunders PT, et al. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010;206:141–157. [DOI] [PubMed] [Google Scholar]
- 7.Modugno F, Ness RB, Chen C, et al. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840–2847. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Vecchi A, Allavena P. Pharmacological modulation of monocytes and macrophages. Curr Opin Pharmacol. 2014;17:38–44. [DOI] [PubMed] [Google Scholar]
- 11.Soeda S, Nakamura N, Ozeki T, et al. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol. 2008;109:122–128. [DOI] [PubMed] [Google Scholar]
- 12.Kubler K, Ayub TH, Weber SK, et al. Prognostic significance of tumor-associated macrophages in endometrial adenocarcinoma. Gynecol Oncol. 2014;135:176–183. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo K, Hom MS, Moeini A, et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015;138:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Ramzan AA, Gualtieri MR, et al. Prediction of concurrent endometrial carcinoma in women with endometrial hyperplasia. Gynecol Oncol. 2015;139:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo K, Garcia-Sayre J, Medeiros F, et al. Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J Surg Oncol. 2015;112:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. [DOI] [PubMed] [Google Scholar]
- 18.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 20.Espinosa I, Jose Carnicer M, Catasus L, et al. Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role. Am J Surg Pathol. 2010;34:1708–1714. [DOI] [PubMed] [Google Scholar]
- 21.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eo WK, Kwon S, Koh SB, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of endometrial cancer. J Cancer. 2016;7:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eo WK, Jeong da W, Chang HJ, et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol. 2015;21:2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl). 2013;91:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–864. [DOI] [PubMed] [Google Scholar]
- 27.Metindir J, Bilir Dilek G. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol. 2009;135:125–129. [DOI] [PubMed] [Google Scholar]
- 28.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 29.Obermair A, Handisurya A, Kaider A, et al. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review. Cancer. 1998;83:726–731. [PubMed] [Google Scholar]
- 30.Ueda Y, Matsumura Y, Egawa-Takata T, et al. Disease-free interval after primary treatment predicts prognosis of recurrent endometrial carcinoma. Anticancer Res. 2010;30:4347–4352. [PubMed] [Google Scholar]
- 31.Miyake T, Ueda Y, Egawa-Takata T, et al. Recurrent endometrial carcinoma: prognosis for patients with recurrence within 6 to 12 months is worse relative to those relapsing at 12 months or later. Am J Obstet Gynecol. 2011;204:535.e1–535.e5. [DOI] [PubMed] [Google Scholar]
- 32.Nagao S, Nishio S, Okada S, et al. What is an appropriate second-line regimen for recurrent endometrial cancer? Ancillary analysis of the SGSG012/GOTIC004/Intergroup study. Cancer Chemother Pharmacol. 2015;76:335–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


