Abstract
Background
Endocrine therapy (ET) is the mainstay of treatment for hormone receptor-positive human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer; however, adaptive mechanisms emerge in about 25–30% of cases through alterations in the estrogen receptor ligand-binding domain, with a consequent ligand-independent estrogen receptor activity. Epigenetic-mediated events are less known and potentially involved in alternative mechanisms of resistance. The aim of this study was to test the feasibility of estrogen receptor 1 (ESR1) epigenetic characterization through liquid biopsy and to show its potential longitudinal application for an early ET sensitivity assessment.
Methods
A cohort of 49 women with hormone receptor-positive HER2-negative MBC was prospectively enrolled and characterized through circulating tumor DNA using methylation-specific droplet digital PCR (MS-ddPCR) before treatment start (BL) and after 3 months concomitantly with computed tomography (CT) scan restaging (EV1). ESR1 epigenetic status was defined by assessing the methylation of its main promoters (promA and promB). The most established cell-free tumor DNA (ctDNA) factors associated with ET resistance [ESR1 and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutations] were assessed through next-generation sequencing. Associations were tested through Mann–Whitney U test, matched pairs variations through Wilcoxon signed rank test, and survival was analyzed by log-rank test.
Results
The ET backbone was mainly based on aromatase inhibitors (AIs) (70.83%) in association with CDK4/6 inhibitors (93.75%). Significantly lower promA levels at baseline were observed in patients with liver metastases (P = 0.0212) and in patients with ESR1 mutations (P = 0.0091). No significant impact on PFS was observed for promA (P = 0.3777) and promB (P = 0.7455) dichotomized at the median while a ≥2-fold increase in promB or in either promA or promB at EV1 resulted in a significantly worse prognosis (respectively P = 0.0189, P = 0.0294). A significant increase at EV1 was observed for promB among patients with PIK3CA mutation (P = 0.0173). A trend was observed for promB in ESR1 wild-type patients and for promA in the ESR1 mutant subgroup.
Conclusion
The study proofed the concept of an epigenetic characterization strategy based on ctDNA and is capable of being integrated in the current clinical workflow to give useful insights on treatment sensitivity.
Keywords: circulating tumor DNA, DNA methylation, endocrine treatment, ESR1, liquid biopsy
Background
Breast cancer (BC) is a complex disease encompassing clinically and molecularly heterogeneous tumors. Approximately 70% of BC express hormone receptors and are, therefore, eligible for endocrine treatment (ET). In the last decades, several ETs have been introduced in the management of hormone receptor-positive human epidermal growth factor receptor 2 (HER2)-negative (luminal-like) BC, including aromatase inhibitors (AIs), selective estrogen receptor modulators (SERM), and degraders (SERD), that dramatically decreased mortality (1–3). Despite that, about 25–30% of metastatic BC (MBC) previously treated with ET develop resistance due to alterations in the estrogen receptor (ER) ligand-binding domain, showing a ligand-independent ER activity and in particular, up to 20% develop mutations in the ER gene (ESR1) (4–7). Although the main known ET resistance mechanisms rely on the mutations of few genes, they account only for 40% of cases, as genetic-mediated events are not the only mechanisms capable to perturbate ESR1 activity and expression (8, 9). Despite being initially neglected, ESR1 mutations are currently one of the main known ET resistance factors in luminal-like MBC. As a matter of fact, ESR1 mutations are not often present in primary tumors but are rather selected during AI-based therapies and eventually characterize the dominant clone when disease progression occurs (10). Moreover, their onset is associated with a lower treatment benefit in subsequent lines when an AI-based backbone is selected, while discordant data are available with respect to SERDS (5, 11). DNA methylation, an epigenetic phenomenon, leads to gene silencing through the addition of a methyl group to the fifth carbon of the cytosine residue in the context of GcP islands (CGIs) and cis-regulatory elements (CREs), as promoter and enhancer regions (12–15). In luminal-like MBC, the ESR1 promoter methylation drives the silencing of ESR1, with a loss of ER tissue expression and consequent ET resistance (16). ESR1 is located in an extremely complex locus of 450 kb in chromosome 6q25.1 and its expression is regulated by several promoters. The different transcripts generated by each promoter show a unique 5’-untranslated region, and they are subject to splicing to form a single mRNA (17). Several promoters are involved in ESR1’s tissue-specific regulation and in particular, two proximal promoters (promoter A and B) are located within ∼2 kb of the transcription start site and are transcriptionally active in BC (18–20).
Liquid biopsy, based on the analysis of circulating cell-free tumor DNA (ctDNA) and circulating tumor cells (CTCs), is gaining momentum as a noninvasive real-time tumor-monitoring tool that may reflect tumor biology and evolution. This concept was explored by analyzing the most established ET resistance factors, ESR1 and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutations, in the PALOMA-3 study. While it has been highlighted that a relative change in PIK3CA allele frequency after 15 days strongly predicted PFS on palbociclib and fulvestrant, on the other hand, ESR1 dynamics offered limited information on the long-term clinical outcome, probably due to early divergent response of tumor subclones to treatment and the more gradual onset of new ESR1 mutations (21). On the other hand, few studies have proved the association between ET sensitivity and ESR1 promoter methylation (22).
The aim of this study was to test the feasibility of ESR1 epigenetic characterization through liquid biopsy and to show its potential longitudinal application for an early ET sensitivity assessment.
Materials and Methods
Study Population and Ethics Statement
A cohort of 49 women with luminal-like MBC was prospectively enrolled in the CRO-2018-56 multicenter pragmatic study, between 2018 and 2019. All patients were diagnosed with luminal-like MBC and received either fulvestrant or AIs with or without CDK4/6 inhibitors as first-line ET according to the investigator’s choice. Diagnosis of any secondary malignancy within the last 3 years and prior ET for MBC were the two main exclusion criteria. Patients could have received both ET and chemotherapy in the adjuvant and neoadjuvant setting. Samples were collected before treatment start [baseline (BL)] and after 3 months concomitantly with computed tomography (CT) scan restaging [first evaluation (EV1)]. The study was approved by the ethics committee under the CEUR-2018-Sper-056-CRO protocol.
Extraction of Circulating Tumor DNA From Plasma Samples
Blood samples were collected using the PAXgene Blood ccfDNA Tubes (Qiagen) or the Cell-Free DNA BCT tubes (Streck). Plasma was then recovered and stored at −80°C. ctDNA was isolated from 4.8 ml aliquots of plasma with the QIAsymphony PAXgene Blood ccfDNA Kit (Qiagen) through the QIAsymphony SP instrument (Qiagen) using the recommended Standard Protocol Line (STA) for small fragment enrichment and eluted in 60 μl of elution buffer (Qiagen). ctDNA concentration was estimated using the Qubit 1X dsDNA HS Assay Kit (Qiagen).
Next-Generation Sequencing
Primers (Sigma) were designed to amplify the regions of the genes that contained hotspot mutation and were built with different 5′-adapter region. To prepare the next-generation sequencing (NGS) library, we carried out two consecutive rounds of PCR, both of which used Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Fisher). In the first round, primers recognized and amplified the region(s) of interest, and in the second one, PCR products from first round were diluted 1:100 and then amplified with Index primers (IDT) that barcoded each sample for subsequent univocal identification. After amplification, Wizard® SV Gel and PCR Clean-Up System (Promega) were used to purify the fragments, following the manufacturer’s instructions. Finally, all samples were diluted to a final concentration of 2 nM, to create the library. Phix sequencing Control (Illumina) was added and the library was finally denatured and loaded into the cartridge (Illumina).
Sequencing data were exported as BAM and VCF files and analyzed using the IGV software and the VariantStudio software (Illumina), respectively (Supplementary Data Sheet 1). After quality check controls and filtering, reads and variants distributed in target regions were analyzed and annotated.
Methylation-Specific Droplet Digital PCR
CpG island prediction and primer/probe design were assessed on the Methprimer 2.0 online platform according to standard recommendations (23). Probes’ sequences included at least three potential methylation sites, and probe-specific binding sites included several cytosines, ensuring specificity for converted DNA. No CpG were included in primer pairs to enforce methylation-independent primer efficiencies. The amplicon size was kept on 100 bp or less, to efficiently anneal on ctDNA fragments. Two probes were designed: one was labeled in 6-FAM and was specific for methylated DNA and the other was labeled in HEX specific for un-methylated DNA.
The EZ DNA Methylation-GoldTM Kit (Zymo Research) was used for bisulfite conversion of 10 ng ctDNA, according to the manufacturer’s standard protocol. Bisulfite-converted ctDNA was eluted in 20 μl elution buffer for downstream analysis. Ten nanograms of Human WGA Methylated and Non-methylated DNA Sets (Zymo Research) were bisulfite converted and processed with each droplet digital PCR (ddPCR) experiment as positive and negative controls.
Five microliters of bisulfite-converted ctDNA were then used for absolute quantification of ESR1 promoter methylation levels. Samples were considered positive for methylation when at least three positive droplets were evaluated. The total number of copies/reactions was assessed, and methylation levels were expressed as fractional abundance of methylated DNA alleles [beta value (β)]. Samples were run in duplicated and then merged for further analysis. Droplets were generated on the automated droplet generator QX200 AutoDGTM (Bio-Rad), and after PCR amplification, droplets were read on the QX200TM Droplet Reader (Bio-Rad). Data were analyzed using the QuantaSoftTM 1.7.4 Software (Bio-Rad).
Statistical Analysis
Clinico-pathological characteristics were summarized through descriptive analysis. Categorical variables were described through frequency distribution, whereas continuous variables were reported through median and interquartile range (IQR). The BL staging was performed concomitantly to liquid biopsy. Distant localizations were categorized based on the presence of specific organ involvement (e.g., liver involvement, yes vs. no) independently from other metastatic sites.
Associations between promoter methylation, clinico-pathological characteristics, and detectable mutations were explored through Mann–Whitney U test. The association between metastatic sites and median level of promA/B was investigated through uni- and multivariate logistic regression. Matched pairs variations across BL and EV1 were tested through Wilcoxon signed rank test.
Progression-free survival (PFS) was defined as the time from study enrollment to disease progression (according to RECIST criteria) or death from any cause or date of last follow-up, while overall survival (OS) was defined as the time from study enrollment to death from any cause. Censoring was applied to patients without an endpoint event at the last follow-up visit. Survival was represented by Kaplan-Meier estimator plot and analyzed by log-rank test. The differential prognostic impact of promA/B across clinically relevant subgroups was tested by Cox regression and represented though forest plot.
All P-values were two sided, with P < 0.05 considered the threshold for statistical significance.
Statistical analysis was conducted using the StataCorp 2016 Stata Statistical Software: Release 15.1 (College Station, TX, United States), R (The R foundation for Statistical Computing. version 3.3.1) (2016-06-21), and JMP (SAS Institute, version 14).
Results
A cohort of 49 patients with luminal-like MBC treated with first-line ET was prospectively enrolled between May 2018 and November 2019. One patient rapidly progressed during the induction of ovarian suppression and was therefore not eligible for the analysis at EV1. The first-line ET backbone was mainly based on AIs (70.83%) in association with CDK4/6 inhibitors (93.75%). Of the 11 patients treated with a fulvestrant-based ET, 9 patients relapsed during adjuvant ET. Among them, five relapsed during the extended adjuvant phased (i.e., after more than 5 years of adjuvant treatment).
Consistently with literature, bone metastases were the most represented (Table 1) (24, 25). Median follow-up was 11.9 months (IQR, 10.1–19.3).
TABLE 1.
Clinico-pathological characteristics of the study cohort.
| Median | IQR | |
| Age (years) | 63 | 52–71 |
| ER (%) | 95 | 90–95 |
| PR (%) | 50 | 20–75 |
| N | % | |
| Age | ||
| <50 | 10 | 20 |
| ≥50 | 39 | 80 |
| ESR1 status | ||
| Wild type | 40 | 82 |
| Mutated | 7 | 14 |
| Not evaluable | 2 | 4 |
| PIK3CA status | ||
| Wild type | 36 | 72 |
| Mutated | 11 | 22 |
| Not evaluable | 2 | 4 |
| Liver involvement | ||
| No | 35 | 73 |
| Yes | 14 | 27 |
| Lung involvement | ||
| No | 38 | 78 |
| Yes | 11 | 22 |
| CNS involvement | ||
| No | 49 | 100 |
| Yes | 0 | 0 |
| Bone involvement | ||
| No | 17 | 35 |
| Yes | 32 | 65 |
| Lymph nodes involvement | ||
| No | 32 | 65 |
| Yes | 17 | 35 |
| Soft tissue involvement | ||
| No | 45 | 92 |
| Yes | 4 | 8 |
| First-line treatment agents | ||
| AI single agent | 1 | 2 |
| Fulvestrant single agent | 2 | 4 |
| AI and CDK4/6i | 34 | 71 |
| Fulvestrant and CDK4/6i | 11 | 23 |
IQR, interquartile range; ER, estrogen receptor; PR, progesterone receptor; ESR1, estrogen receptor 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha.
ESR1 and PIK3CA mutations are currently the most established ctDNA factors associated with ET resistance and were therefore assessed through an amplicon-based NGS panel for a more complete baseline characterization.
ESR1 and PIK3CA were mutated in 7 (14%) and 11 (23%) patients, respectively. ESR1 variants were Y537S (3/7, 44%), Y537N (1/7, 14%), H377R (1/7, 14%), and D538G (2/7, 28%), while PIK3CA variants were H1047R (8/11, 73%), E545K (2/11, 18%), and H1047L (1/11, 9%). Full details are available in Supplementary Table 1.
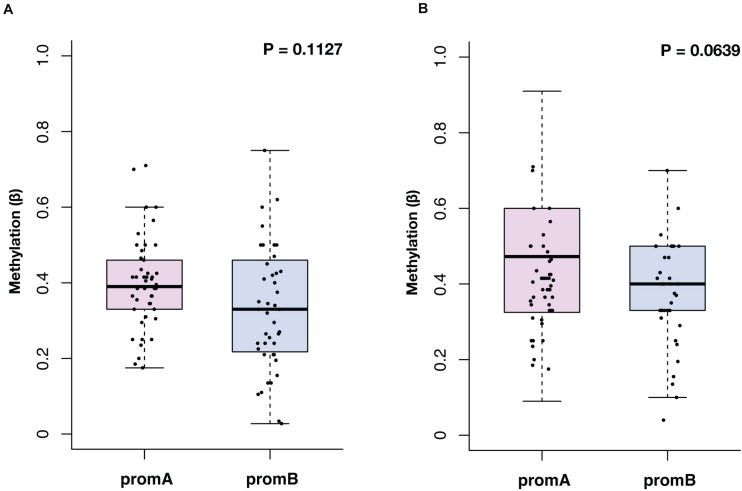
The median methylation at BL for promoter A (promA) was 0.39 (IQR, 0.33–0.43) and 0.33 (IQR, 0.22–0.46) for promB, with no significant differences in the overall population (P = 0.1127) (Figure 1A). At EV1, promA and promB medians were, respectively, 0.47 (IQR, 0.33–0.60) and 0.40 (IQR, 0.33–0.50) at EV1 (P = 0.0639) (Figure 1B). No direct correlation was observed after Shapiro–Wilk normality testing at BL (R2 = 0.0978) and EV1 (R2 = 0.0265).
FIGURE 1.
Overall distribution of ESR1 promoter A (promA, purple) and B (promB, blue) methylation at baseline (A) and at first CT scan evaluation after 3 months of ET (B). Median methylation at baseline was 0.39 for promA and 0.33 for promB (P = 0.1127) (A). At first CT scan evaluation after 3 months of ET median methylation was 0.47 for promA and 0.40 for promB (P = 0.0639) (B).
Baseline promA Varied Across Baseline Characteristics and Was Not Associated With Prognosis
Promoter A and B were investigated at BL to explore potential factors associated with ESR1’s epigenetic status (Table 2). No differences were observed with respect to age, while significantly lower promA levels were observed in patients with liver metastases (P = 0.011). No significant variations were observed across all investigated sites for promB, numerically higher promB levels were detected in patients with soft tissue involvement (Table 2). PromA was significantly lower in patients with ESR1 mutations (median, 0.41; IQR, 0.33–0.46; median, 0.25; IQR, 0.19 0.35, respectively, in ESR1 wild-type and mutated; P = 0.0091) (Figure 2A). No associations were observed with respect to PIK3CA mutational status (Figure 2).
TABLE 2.
Distribution of promoter A and B methylation levels (promA and promB) across age groups and metastatic sites.
| promA | promB | |||||
| Median | IQR | P | Median | IQR | P | |
| Age | ||||||
| <50 | 39 | 28–44.25 | 0.7213 | 32 | 21–50 | 0.8304 |
| ≥50 | 38.5 | 33–46.5 | 33 | 21.8–46 | ||
| Liver involvement | ||||||
| No | 41.5 | 34.5–50 | 0.0196 | 33.75 | 21–48.5 | 0.5009 |
| Yes | 36.5 | 25–38.5 | 28 | 23.3–42 | ||
| Lung involvement | ||||||
| No | 41.3 | 33.8–48.3 | 0.1498 | 32 | 21–45 | 0.2486 |
| Yes | 36.5 | 30.5–40.5 | 40 | 24–50 | ||
| Bone involvement | ||||||
| No | 41.5 | 36.5–46 | 0.1268 | 37.3 | 30.8–50 | 0.1017 |
| Yes | 38.5 | 31–41.5 | 26.8 | 20.3–44 | ||
| Lymph nodes involvement | ||||||
| No | 38.5 | 33–43.5 | 0.9625 | 32 | 21–42.5 | 0.2150 |
| Yes | 39.5 | 31–48.5 | 37.5 | 24–50 | ||
| Soft tissue involvement | ||||||
| No | 40.5 | 33–46.5 | 0.1137 | 32 | 21–42.8 | 0.0688 |
| Yes | 31 | 30.5–35.5 | 50 | 38.3–55 | ||
Methylation levels were significantly lower for promA in patients with liver involvement (P = 0.0196). Significance was tested through the Mann–Whitney U test.
FIGURE 2.
Promoter A and B methylation (promA and promB) according to ESR1 and PIK3CA mutational status. PromA methylation levels were lower in patients with a ctDNA-detectable ESR1 mutation (A). No associations were observed with respect to PIK3CA mutational status or in ESR1 wild type patients (B–D).
The association between metastatic sites and promA/B was further explored through multivariate logistic regression after dichotomization at the median value. The inverse association between promA and liver involvement was confirmed (OR = 0.09; 95% CI = 0.01–0.58; P = 0.0109) (Table 3).
TABLE 3.
A significantly lower promA was confirmed in patients with liver involvement after correction for the most represented metastatic sites (P = 0.0109).
| promA | promA | |||||
| OR | 95% Cl | P | OR | 95% Cl | P | |
| Liver involvement | ||||||
| No | 1 | 0.0109 | 1 | 0.1745 | ||
| Yes | 0.09 | 0.01–0.58 | 0.34 | 0.07–1.61 | ||
| Lung involvement | ||||||
| No | 1 | 0.0635 | 1 | 0.7761 | ||
| Yes | 0.14 | 0.02–1.12 | 1.28 | 0.23–7.06 | ||
| Bone involvement | ||||||
| No | 1 | 0.1118 | 1 | 0.1711 | ||
| Yes | 0.28 | 0.06–1.35 | 0.33 | 0.07–1.61 | ||
| Lymph nodes involvement | ||||||
| No | 1 | 0.0806 | 1 | 0.3078 | ||
| Yes | 5.40 | 0.81–35.80 | 2.43 | 0.44–13.44 | ||
Significance was tested through multivariate logistic regression, promA and B were dichotomized at median.
In this subgroup, median ER expression of the most updated biopsy was 90% (range, 30–100%) while the median ER expression was 95% (range, 80–100%) in patients without liver involvement.
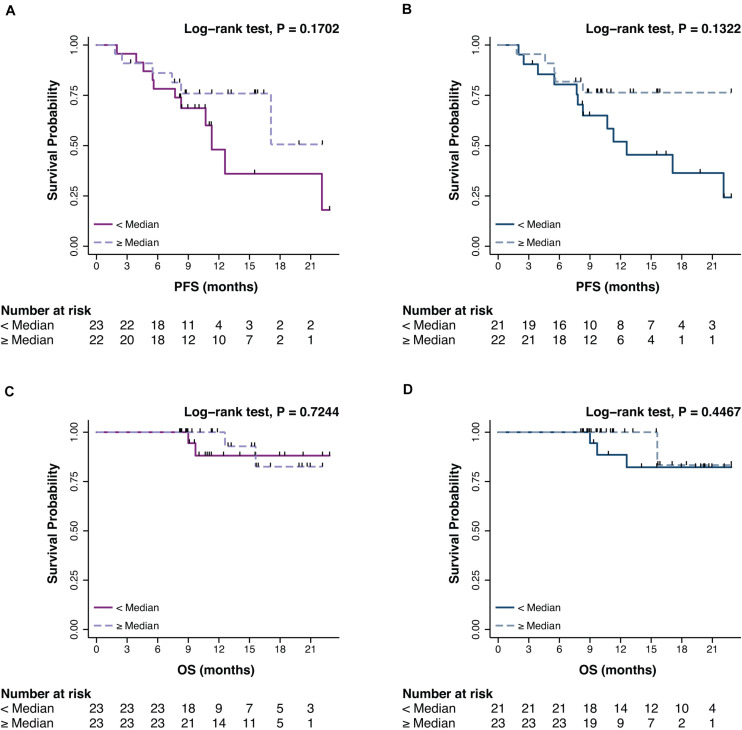
The prognostic impact of promA and promB was then explored in terms of PFS and OS. At BL, no significant impact was observed for promA and promB dichotomized at the median for both PFS (respectively P = 0.1702 and P = 0.1322) (Figures 3A,B) and OS (respectively P = 0.7244 and P = 0.4467) (Figures 3C,D).
FIGURE 3.
Impact on PFS and OS of promA (purple) (A,C) and promB (blue) (B,D). No significant impact was observed for promA and promB dichotomized at the median for both PFS (respectively P = 0.1702 and P = 0.1322) (A,B) and OS (respectively P = 0.7244 and P = 0.4467) (C,D).
The Longitudinal Evaluation of prom A/B Is Feasible and Can Give Insights on Treatment Sensitivity
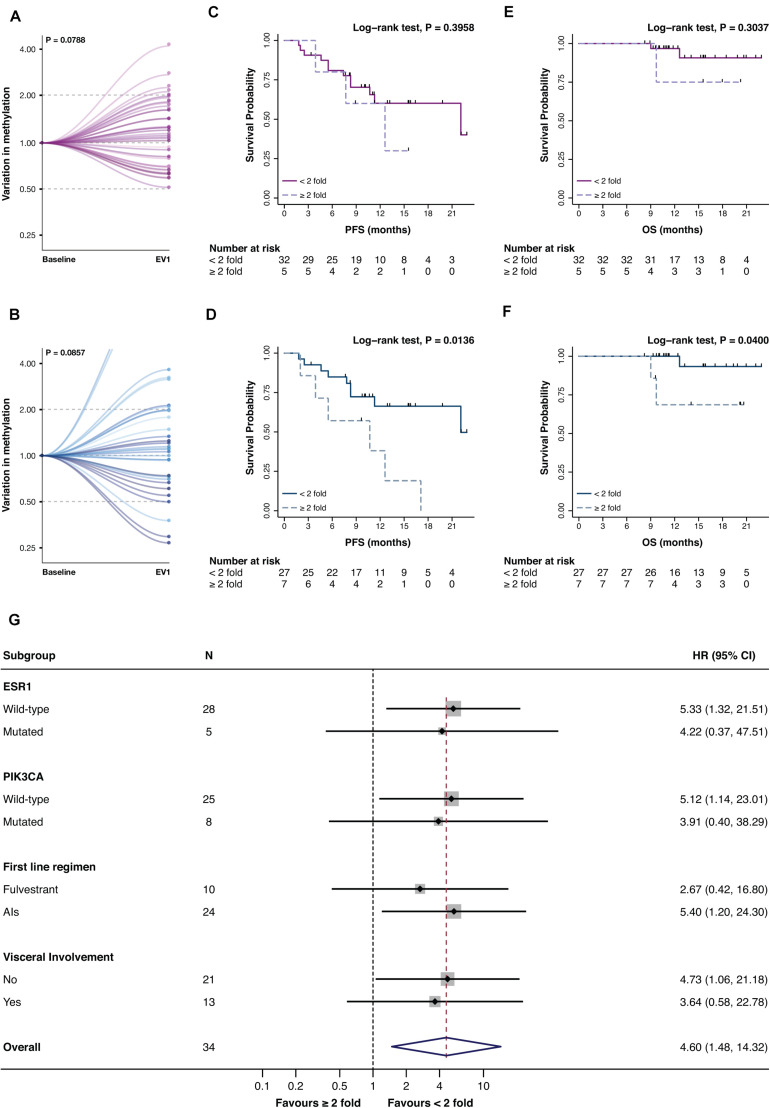
Promoter A and B were then assessed at EV1 (Figure 1B). Only numerical variations were observed from BL to EV1 for promA and promB after normalization on the BL values (respectively P = 0.0788 and P = 0.0857), notably two cases showed a 15- and 18-fold increase in promB levels (Figures 4A,B).
FIGURE 4.
Variation of promA (purple) and promB (blue) between BL and EV1 (A,B) and impact on PFS (C,D) and OS (E,F) of a ≥2-fold increase in promA and promB. Subgroup analysis of the impact on PFS of a ≥2-fold increase in promB (G). A numerical increase in promA was observed after normalization to the baseline levels. For two cases, an 18- and 15-fold increase in promB was observed (B) and were, therefore, out of scale (B). A ≥2-fold increase in promA was not associated with PFS or OS (C,E), while a doubling in promB resulted in a significantly worse prognosis both on PFS and OS (D,F). Notably, subgroup analysis showed a consistent impact on PFS across different ET backbones and according to ESR1 and PIK3CA mutational status.
The impact of a variation in promA and promB between BL and EV1 was investigated both in terms of PFS and OS. To exclude possible biasses derived from the biological meaning of undetectable ctDNA levels, only patents with detectable methylation at BL and EV1 were included in this analysis (26).
While promA doubling alone has no impact on PFS nor OS (Figures 4C,E), a ≥2-fold increase in promB resulted in a significantly worse prognosis both in terms of PFS and OS (respectively P = 0.0136 and P = 0.0400) (Figure 4D,F). The prognostic impact of a 2-fold increase in promB was explored across clinically relevant subgroups. In the total population, hazard ratio (HR) was 3.47 (95% CI, 1.20–10.04; P = 0.0214), consistent results were observed according to visceral involvement, first-line ET backbone, and ESR1 and PIK3CA mutational status (Figure 4G).
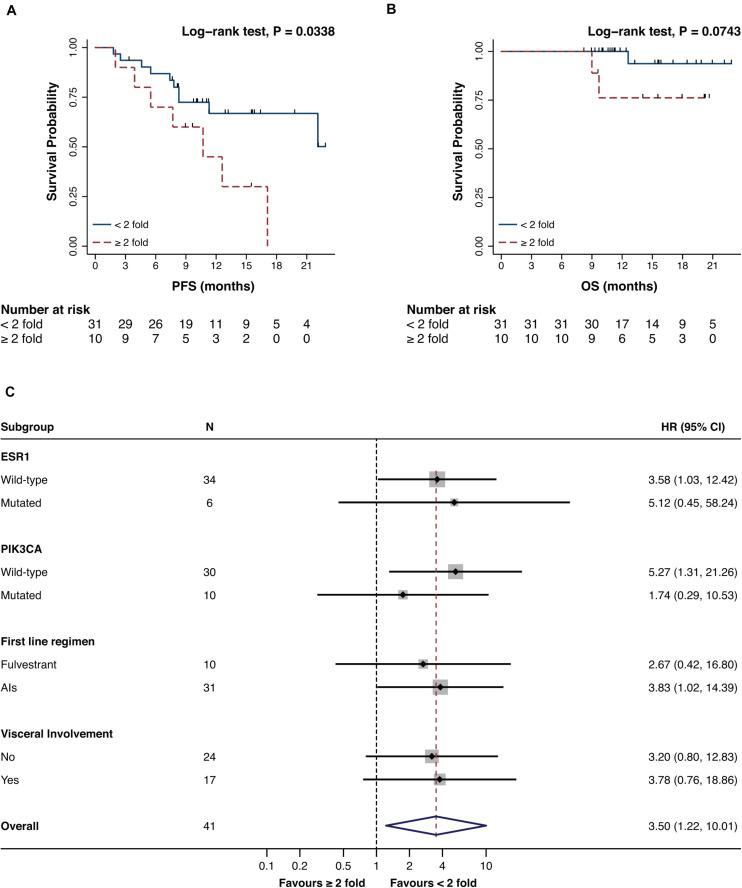
The prognostic impact of a 2-fold increase of either promA or promB was moreover investigated. A ≥2-fold increase in either promA or promB was associated to a significantly worse prognosis in terms of PFS (P = 0.0338) (Figures 5A,B) that was consistent across all considered subgroups (Figure 5C).
FIGURE 5.
Impact on PFS (A) and OS (B) and subgroup analysis in terms of PFS (C) of a ≥2-fold increase in either promA or promB. A ≥2-fold increase in promA or promB was associated with an impact on PFS (A). Subgroup analysis showed a consistent impact on PFS across different ET backbones and according to ESR1 and PIK3CA mutational status (C).
Given the established role of ESR1 and PIK3CA gene mutations in ET resistance, promA and promB variations were investigated according to ESR1 and PIK3CA mutational status (Figure 6). A significant increase was observed for promB among patients with PIK3CA mutation (P = 0.0173) (Figure 6H). A trend was observed for promA in the ESR1 mutant subgroup (Figure 4B) and for promB in ESR1 wild-type patients (Figure 6C).
FIGURE 6.
Promoter A (purple) and B (blue) variations between BL and EV1 according to ESR1 and PIK3CA mutational status. A significant increase was observed for promB in PIK3CA-mutated patients (H). A numerical difference was observed for promA in the ESR1-mutated subgroup (B) and promB in ESR1 wild-type MBC (C). No significant promA variations were observed in ESR1 wild-type patients (A) or with respect to PIK3CA mutational status (E,F). No significant promB variations were observed in ESR1-mutated or in PIK3CA wild-type patients (D,G).
Discussion
De novo and acquired ET resistance result in cancer progression and metastatic spreading, limiting clinical benefit in luminal-like MBC. Mutations of the ESR1 gene have been described in 20% of ET-resistant cases and represents one of the main ET resistance mechanisms, but the relationship between epigenetic events and treatment-induced resistance is still unclear (10, 27–30).
The present study tested the feasibility of evaluating ESR1 CRE hypermethylation in liquid biopsies from luminal-like MBC and explored the potential impact on prognosis of its dynamics.
The most transcriptionally active ESR1 promoters (promoter A and B) were analyzed at baseline and after 3 months of ET. No significant cross-associations were observed between the two, suggesting their independent role and potential for mutual integration. The baseline levels of promA were associated with metastatic liver involvement, which was confirmed also on multivariate analysis. Uncovering the link between metastatic behavior and tumor biology is an unmet clinical need as it can potentially enable a more granular prognostic stratification and guide disease monitoring strategies by suggesting the best imaging technique for each patient (25, 31–33).
The prognostic role of promA and promB was moreover explored. Although no prognostic impact was observed for both BL promA and promB, a 2-fold increase in promB and in either promA and promB after 3 months of ET was associated with a worse PFS and OS. Notably, PFS results were consistent across clinically relevant subgroups, such as ET backbone and ESR1 and PIK3CA mutational status. Although preliminary, these results may suggest the added value of longitudinal genetic and epigenetic monitoring as the prognostic role of ESR1-associated features is likely the result of a complex interplay between inherit biological features and acquired resistance mechanisms induced by ET, rather than a baseline characteristic.
A similar scenario was described by the preliminary results of the PADA-1 study which explored the impact of baseline ESR1 mutation and its dynamics on PFS. Out of the 32 analyzed patients, 25 (78%) had an ESR1 mutation that became undetectable in ctDNA within the first 5 months of ET. Although 14 patients had a recurrence of ESR1 mutation with a consequent progression, 9 patients (36%) were still free from ESR1 mutation and progression at the time of analysis. Intriguingly, 2 patients (8%) experienced a progression without the recurrence of ESR1 mutation. Since the study was based on a genetic-only characterization, these results suggest the need for a wider, genetic, and epigenetic characterization to better characterize the complexity of ET resistance (34).
It has previously been shown that ESR1 promoter methylation was significantly associated with either ER-negative early BC or with a heterogenous ER expression in the luminal-like disease (27). This suggested that silencing by methylation could affect the expression of ER and in turn treatment response, although without evidence of a survival impact (27).
Endocrine-resistant cells are characterized by a distinctive DNA methylome. Moreover, hypermethylation can affect estrogen-regulated enhancers resulting in a reduced ESR1 binding with a consequent decreased gene expression of key effectors of ESR1 activity (12). Furthermore, ESR1-responsive enhancer hypomethylation is critical in the transition from normal epithelial cells to endocrine-responsive luminal-like BC, supporting the concept that dynamic epigenetic variations are pivotal in cancer evolution (12).
Notwithstanding the preliminary follow-up and sample size, our results suggest that the combination of promA and promB dynamics could be more informative than the ESR1 mutational status alone, since epigenetic modifications have a faster onset and could realistically be able to grasp features linked to an inherently lower sensitivity to ET (10, 28–30).
The added value of a longitudinal strategy is also supported by the lack of impact on PFS of baseline promA and promB. If on one hand mutational alterations of ESR1 are late events mainly induced by therapy and often maintained across the subsequent lines, on the other, epigenetic alterations are highly dynamic and reflect the actual real-time changes caused by ET at the moment of the blood draw.
Alternative approaches for a more comprehensive liquid biopsy-based characterization of luminal-like MBC were initially focused on CTCs. A previously reported pilot study based on 17 patients after progression to ET (respectively 7 treated with fulvestrant and 10 with AI) highlighted an association between ET resistance and both enumeration and heterogeneous ER expression of CTCs (35). Notably, all patients treated with AI showed a heterogeneous ER expression in CTCs, while two patients treated with fulvestrant had no detectable CTC expression of ER and three had heterogeneous CTC ER, probably due to an incomplete degradation of the ER target by fulvestrant (35). A larger study characterized 122 MBC patients through a methylation-specific (MS) qPCR assay applied to CTCs and, in a smaller subgroup (58 patients), in matched plasma samples. ESR1 methylation was observed in 10 out of 36 CTC-positive samples (27.8%), was highly concordant with the paired ctDNA samples, and was moreover associated with a lack of response to everolimus and exemestane (22). These results, although supporting the concept of integrated genetic and epigenetic events concurring to ET resistance, are on the other hand limited to patients defined as Stage IVaggressive through the CellSearch platform (i.e., with ≥5 CTCs/7 ml of blood), a population limited to 45% of the overall MBC patients and up to 49% in the luminal-like subgroup (36).
A ctDNA approach, on the other hand, can be applied to a broader cohort of patients also thanks to the higher sensitivity of ddPCR.
In our cohort, ESR1 and PIK3CA mutations were detected, respectively in 14 and 23% of patients and, intriguingly, a significantly lower promA was highlighted in patients with ESR1 mutation.
ESR1 is often affected by activating mutations, such as Y537 and D538 (located in the helix 12 of ESR1 ligand binding domain), which result in an agonist form of ESR1 (28). Given these premises, it is mechanistically tempting to think that promoter methylation could represent a detrimental event to an acquired resistance advantage and that the latter could be hindered by epigenetic silencing.
Although intriguing and prospectively generated, these results may have some limitations. If on one hand the proof of concept study enrolled a homogeneous cohort of luminal MBC patients treated with first-line ET and was therefore an appropriate basis for hypothesis generation and for testing the technological transferability of longitudinal epigenetic characterization, on the other hand, the sample size is limited and hinders a deeper characterization of subgroups of particular interest. Consistently, the median follow-up is limited and may underestimate the real prognostic impact of the analyzed biomarkers. Moreover, additional timepoints could increase promA and promB curve’s resolution and therefore provide precious information for optimal timing definition.
Conclusion
The present study proofed the concept that an epigenetic characterization strategy based on ctDNA can be integrated in the current clinical workflow of patients with metastatic breast cancer to give useful insights on treatment sensitivity. Although exploratory, these results support the emerging new concept of a dynamic epigenetic profiling. Based on these data, a cohort expansion is planned to validate and further develop this approach.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Some datasets presented in this article are not readily available because are part of an ongoing clinical trial. Requests to access the datasets should be directed to corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Comitato Etico Unico Regionale del Friuli Venezia Giulia. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LG, DB, MB, and FP conceived the study, participated in its design and coordination, recruited the patients, created and updated the clinical database, performed the statistical analysis, and wrote the manuscript. AF, LA, and GD optimized the preanalytical and extraction workflow and the MS-ddPCR assay, and helped to draft the manuscript. DV, BB, and GB optimized and performed the ctDNA NGS analysis and helped to draft the manuscript. CC, LB, EB, SB, GT, LD, and SR recruited the patients and updated the clinical database. All authors read and approved the final manuscript.
Conflict of Interest
LG has received reimbursement for travel expenses from Menarini Silicon Biosystems. FP received research funding from AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by AstraZeneca and the Ministry of Health Ricerca Finalizzata grant (Grant Number: RF-2016-02362544) to FP; the CRO Aviano 5x1000 2014, redditi 2013 Cancer Specific Intramural Grant to LG; the Associazione Italiana per la Ricerca sul Cancro (AIRC) grant (IG#20061) to BB; The L.R. 17/2014-Regione FVG (TuMaGiDo) grant to GB. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.550185/full#supplementary-material
ESR1 and PIK3CA mutational status for all patients enrolled in the CRO-2018-56 study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. (2014) 19:608–15. 10.1634/theoncologist.2014-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonotto M, Gerratana L, Di Maio M, De Angelis C, Cinausero M, Moroso S, et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. (2017) 31:114–20. 10.1016/j.breast.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 4.Clatot F, Augusto L, Fiore D. ESR1 mutations in breast cancer. Aging (Albany NY). (2017) 9:3–4. 10.1016/j.breast.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer. JAMA Oncol. (2016) 2:1310. 10.1001/jamaoncol.2016.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fribbens C, O’Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. (2016) 34:2961–8. 10.1200/JCO.2016.67.3061 [DOI] [PubMed] [Google Scholar]
- 7.Davis AA, Zhang Q, Gerratana L, Shah AN, Zhan Y, Qiang W, et al. Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res. (2019) 21:137. 10.1186/s13058-019-1229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. (2019) 125:3714–28. 10.1002/cncr.32345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carausu M, Bidard FC, Callens C, Melaabi S, Jeannot E, Pierga JY, et al. ESR1 mutations: a new biomarker in breast cancer. Expert Rev Mol Diagn. (2019) 19:599–611. 10.1080/14737159.2019.1631799 [DOI] [PubMed] [Google Scholar]
- 10.Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. (2015) 7:313ra182. 10.1126/scitranslmed.aac7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauring J, Wolff AC. Evolving role of the estrogen receptor as a predictive biomarker: ESR1 mutational status and endocrine resistance in breast cancer. J Clin Oncol. (2016) 34:2950–2. 10.1200/JCO.2016.68.4720 [DOI] [PubMed] [Google Scholar]
- 12.Stone A, Zotenko E, Locke WJ, Korbie D, Millar EKA, Pidsley R, et al. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nat Commun. (2015) 6:7758. 10.1038/ncomms8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiefer JC. Epigenetics in development. Dev Dyn. (2007) 236:1144–56. 10.1002/dvdy.21094 [DOI] [PubMed] [Google Scholar]
- 14.Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Mol Biotechnol. (2010) 44:71–81. 10.1007/s12033-009-9216-2 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Spector TD, Deloukas P, Bell JT, Engelhardt BE. Predicting genome-wide DNA methylation using methylation marks, genomic position, and DNA regulatory elements. Genome Biol. (2015) 16:14. 10.1186/s13059-015-0581-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Galán J, Torres-Torres B, Núñez MI, López-Peñalver J, Del Moral R, Ruiz De Almodóvar JM, et al. ESR1 gene promoter region methylation in free circulating DNA and its correlation with estrogen receptor protein expression in tumor tissue in breast cancer patients. BMC Cancer. (2014) 14:59. 10.1186/1471-2407-14-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koš M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERα gene promoter region. Mol Endocrinol. (2001) 15:2057–63. 10.1210/mend.15.12.0731 [DOI] [PubMed] [Google Scholar]
- 18.Grandien K, Bäckdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. (1995) 136:2223–9. 10.1210/endo.136.5.7720671 [DOI] [PubMed] [Google Scholar]
- 19.Grandien KFH, Berkenstam A, Nilsson S, Gustafsson J-A. Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J Mol Endocrinol. (1993) 10:269–77. 10.1677/jme.0.0100269 [DOI] [PubMed] [Google Scholar]
- 20.Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-α gene are generated by alternative splicing and promoter usage. Mol Endocrinol. (1998) 12:1939–54. 10.1210/mend.12.12.0209 [DOI] [PubMed] [Google Scholar]
- 21.O’Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. (2018) 9:896. 10.1038/s41467-018-03215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastoraki S, Strati A, Tzanikou E, Chimonidou M, Politaki E, Voutsina A, et al. ESR1 Methylation: a liquid biopsy–based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res. (2018) 24:1500–10. 10.1158/1078-0432.CCR-17-1181 [DOI] [PubMed] [Google Scholar]
- 23.Barekati Z, Radpour R, Kohler C, Zhong XY. Specificity of methylation assays in cancer research: a guideline for designing primers and probes. Obstet Gynecol Int. (2010) 2010:1–7. 10.1155/2010/870865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. (2015) 32:125–33. 10.1007/s10585-015-9697-2 [DOI] [PubMed] [Google Scholar]
- 25.Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. (2010) 28:3271–7. 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 26.Rossi G, Mu Z, Rademaker A, Austin L, Strickland KS, Lima Barros Costa R, et al. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res. (2017) 24:560–8. 10.1158/1078-0432.CCR-17-2092 [DOI] [PubMed] [Google Scholar]
- 27.Kirn V, Strake L, Thangarajah F, Richters L, Eischeid H, Koitzsch U, et al. ESR1-promoter-methylation status in primary breast cancer and its corresponding metastases. Clin Exp Metastasis. (2018) 35:707–12. 10.1007/s10585-018-9935-5 [DOI] [PubMed] [Google Scholar]
- 28.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. (2013) 45:1439–45. 10.1038/ng.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. (2014) 20:1757–67. 10.1158/1078-0432.CCR-13-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen MPHM, Martens JWM, Helmijr JCA, Beaufort CM, van Marion R, Krol NMG, et al. Cell-free DNA mutations as biomarkers in breast cancer patients receiving tamoxifen. Oncotarget. (2016) 7:43412–8. 10.18632/oncotarget.9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulfoni M, Gerratana L, Del Ben F, Marzinotto S, Sorrentino M, Turetta M, et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. (2016) 18:30. 10.1186/s13058-016-0687-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerratana L, Basile D, Toffoletto B, Bulfoni M, Zago S, Magini A, et al. Biologically driven cut-off definition of lymphocyte ratios in metastatic breast cancer and association with exosomal subpopulations and prognosis. Sci Rep. (2020) 10:7010. 10.1038/s41598-020-63291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis AA, Jacob S, Gerratana L, Shah AN, Wehbe F, Katam N, et al. Landscape of circulating tumour DNA in metastatic breast cancer. EBioMedicine. (2020) 58:102914. 10.1016/j.ebiom.2020.102914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidard FC, Callens C, Dalenc F, Pistilli B, De La Motte Rouge T, Clatot F, et al. Prognostic impact of ESR1 mutations in ER+ HER2- MBC patients prior treated with first line AI and palbociclib: an exploratory analysis of the PADA-1 trial. J Clin Oncol. (2020) 38:1010–1010. 10.1200/JCO.2020.38.15_suppl.1010 32753890 [DOI] [Google Scholar]
- 35.Paoletti C, Larios JM, Muñiz MC, Aung K, Cannell EM, Darga EP, et al. Heterogeneous estrogen receptor expression in circulating tumor cells suggests diverse mechanisms of fulvestrant resistance. Mol Oncol. (2016) 10:1078–85. 10.1016/j.molonc.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cristofanilli M, Pierga J-Y, Reuben J, Rademaker A, Davis AA, Peeters DJ, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol. (2019) 134:39–45. 10.1016/j.critrevonc.2018.12.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ESR1 and PIK3CA mutational status for all patients enrolled in the CRO-2018-56 study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Some datasets presented in this article are not readily available because are part of an ongoing clinical trial. Requests to access the datasets should be directed to corresponding author.