Abstract
Currently, immunotherapy has shown great efficacy in clinical trials, and monoclonal antibodies directed against immune checkpoint PD-1/PD-L1 have shown encouraging results in first-line or second-line treatment of non-small cell lung cancer patients. Meanwhile, anti-PD-1/PD-L1 immune checkpoint drugs combined with other treatments, such as chemotherapy, targeted therapy as well as anti-CTLA-4 checkpoint therapy, are considered an attractive treatment with higher efficacy. However, toxicity associated with PD-1/PD-L1 blockade is worth attention. Understanding the adverse effects caused by anti-PD-1/PD-L1 immunosuppressive agents is vital to guide the clinical rational use of drug. In this review, we summarized the adverse effects that occurred during the clinical use of anti-PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer and discussed how to effectively manage and respond to these adverse reactions.
Keywords: adverse effects, PD-1/PD-L1, immunotherapy, immune checkpoint inhibitor, non-small cell lung cancer
Introduction
Currently, cancer is still a key threat to human health (1). Among them, lung cancer is the leading cause of cancer-related deaths worldwide, and about 80% of lung cancer is non-small cell lung cancer (NSCLC), with poor prognosis (2, 3). Encouragingly, the blockade of immune checkpoints against PD-1/PD-L1 has dramatically changed the treatment prospects for patients with NSCLC (4–6). The traditional treatments of cancer are mainly target at the cancer cells themselves, while the main goal of tumor immunotherapy is to enhance or restore the monitoring and killing effect of the body's immune system on tumors (7–9). There are many immune checkpoint molecules in the body, which are involved in maintaining the body's immune balance and its own immune tolerance (10). Among them, PD-1 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) are classic co-inhibitory molecules that suppress the immune response (11–13). Tumor cells overexpress the immunosuppressive surface ligand PD-L1, which interacts with T cell molecules, leading to T cell failure (14, 15). Knowledge based on the immune escape mechanism of cancer cells has led to the development of immunological checkpoint inhibitors (16, 17).
In recent years, immune checkpoint inhibitors (ICIs) have been widely used in tumor immunotherapy (18, 19). ICIs based on the PD-1/PD-L1 axis have been proved to exhibit promising therapeutic effects in a variety of advanced cancers (20–22). For example, the anti-PD-1 ICIs nivolumab and pembrolizumab have shown exciting results in the treatment of metastatic melanoma and NSCLC (23, 24). Moreover, anti-PD-L1 antibody durvalumab, atezolizumab as well as avelumab have also shown anti-tumor activity in a number of tumor types. However, it is worth noting that as the immune system is reactivated, the body's immune tolerance imbalance occurs (10). Immunotherapy leads to the emergence of novel toxic features, known as immune-related adverse events (irAEs), by reactivating the immune system (14). Although severe irAEs are rare, they can be life-threatening without intervention and proper management (25, 26). In addition, it has also been reported that the combined use of PD-1/PD-L1 ICIs with chemotherapeutics or other targeted therapies leads to the emergence of new toxic reactions (14). Therefore, raising our awareness of these adverse events (AEs) is critical to optimize the clinical efficacy and safety of these new immunotherapeutic.
In this review, we summarized the adverse reactions of the five FDA-approved targeted PD-1/PD-L1 immune checkpoint drugs currently used in the clinic when used alone or in combination with other treatments in NSCLC patients. We aim to raise awareness of the clinical manifestations, diagnosis, and management of these toxic reactions through our summary.
Mechanism Overview of PD-1/PD-L1 Blockade
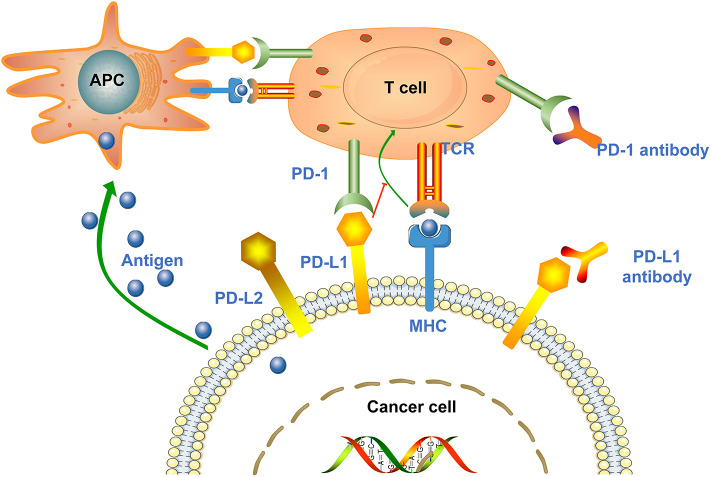
PD-1, also known as CD279, is a type I transmembrane protein of the immunoglobulin superfamily (27). As a transmembrane protein, PD-1 inducibly expressed on the surface of activated T cells, B cells, NKT cells and antigen presenting cells (APC) (15, 28). PD-1 interacts with two major ligands, PD-L1 and PD-L2, resulting in disruption of intracellular signaling and down-regulation of effector T cell function (18, 29). The binding affinity of PD-1 and PD-L1 is three times than of PD-1 and PD-L2 (30). Studies showed that PD-1 is expressed in multiple type of cells, including T cells, B cells, dendritic cells, monocytes as well as tumor-infiltrating lymphocytes (TILs), while PD-L1 is expressed in cancer cells and APC (31, 32). PD-L1 expression is mainly affected by Toll-like receptors (TLRs) (33, 34). TLR-mediated PD-L1 regulation is dependent on activation of MEK/ERK kinase, which enhances PD-L1 messenger RNA (mRNA) transcription by nuclear factor kappa B (35). PD-L1 interacts with PD-1 expressed on T cells, leading to the negative regulation of effector T cell activation, thereby causing cancer cells to secrete the proinflammatory cytokines, such as TNF-α, IL-2, and IFN-γ, and become more aggressive (30). IFN-γ receptors 1 and 2 are also involved in the regulation of PD-L1 expression, primarily through JAK/STAT-mediated IRF-1 activation (35). In addition, other immunosuppressive cells in the tumor microenvironment (TME), such as regulatory T cells, tumor-associated macrophages and myeloid-derived suppressor cells, also express PD-1 to maintain a highly immunosuppressive microenvironment (Figure 1) (36, 37).
Figure 1.
Mechanisms of cancer cell mediated immune escape. Antigen presenting cells (APCs) absorb antigens released by cancer cells and present them to T cells to promote T cells activation and high expression of PD-1. Upon T cell activation, the PD-1 receptor binds to PD-L1/PD-L2 expressed on the surface of cancer cells and suppresses the immune response. In addition, tumor cells can also present antigens directly to activated T cells in the context of MHC. Anti-PD-1/PD-L1 antibodies can block the above process and enhance the body's immune response.
Adverse Effects Based on PD-1/PD-L1 Blockade for NSCLC Therapy
To date, several anti-PD-1/PD-L1 immune checkpoint agents have been approved for the treatment of NSCLC, including two anti-PD-1 drugs pembrolizumab and nivolumab, as well as three anti-PD-L1 drugs atezolizumab, durvalumab and avelumab (38, 39). Blocking of PD-1/PD-L1 immune checkpoint leads to the development of new toxicities by reactivation of the immune system, also known as irAEs (26). These irAEs may affect multiple organ systems and tissues, with clinical manifestations of autoimmune-like/inflammatory side effect that may cause damage to the skin, lungs, gastrointestinal tract, liver, endocrine glands, and skeletal muscle (12). In addition, the most common treatment-related adverse events (TRAEs) include fatigue, fever/chillness and infusion reactions (9). Furthermore, rare and serious TRAEs have been reported, including immune-related encephalitis (40), myasthenia gravis (41), acute renal failure/interstitial nephritis (42), and myocarditis (43). Here, we list the TRAEs caused by PD-1 and PD-L1 inhibitors in the treatment of NSCLC in Tables 1, 2, respectively, both monotherapy and combination therapy are included.
Table 1.
Adverse effects base on anti-PD-1 antibodies.
| Agent | Phase | ClinicalTrials.gov | No. of patients | Therapy schedule | TRAEs (Any grade) | Treatment-related serious AEs (grade 3–5) | References |
|---|---|---|---|---|---|---|---|
| Pembrolizumab | I | NCT01295827 | 495 | 2 or 10 mg/kg, Q3W or 10 mg/kg, Q2W | Total: 70.9%, n = 351 Fatigue (19%, n = 96) Pruritus (11%, n = 53) Decreased appetite (11%, n = 52) Rash (10%, n = 48) Arthralgia (9%, n = 45) Diarrhea (8%, n = 40) Nausea (8%, n = 37) Hypothyroidism (7%, n = 34) |
Total: 9.5%, n = 47 Decreased appetite (1%, n = 5) Asthenia (1%, n = 5) Dyspnea (4%, n = 19) Pneumonitis (2%, n = 9) |
(24) |
| 101 | 2 or 10 mg/kg, Q3W or Q2W | Total: 85%, n = 86 Fatigue (28%, n = 28) Pruritus (15%, n = 15) Hypothyroidism (14%, n = 14) Rash (14%, n = 14) Arthralgia (12%, n = 12) Nausea (12%, n = 12) Dyspnea (9%, n = 9) Diarrhea (8%, n = 8) |
Total: 12%, n = 12 Hypertension (1%, n = 1) Colitis (1%, n = 1) Dehydration (1%, n=1) Dyspnea (1%, n = 1) Pneumonitis (1%, n = 1) |
(44) | |||
| Pembrolizumab | III | NCT02220894 | 636 | 200 mg, Q3W | Total: 63%, n = 399 Hypothyroidism (11%, n = 69) Fatigue (8%, n = 50) Pruritus (7%, n = 46) Rash (7%, n=46) Alanine aminotransferase increased (7%, n = 45) Pneumonitis (7%, n = 43) Decreased appetite (6%, n = 40) Hyperthyroidism (6%, n = 37) |
Total: 18%, n = 113 Pneumonitis (3%, n = 20) Alanine aminotransferase increased (1%, n = 9) Hypothyroidism (<1%, n = 1) Fatigue (<1%, n = 3) |
(45) |
| Pembrolizumab | II/III | NCT01905657 | 691 | 2 or 10 mg/kg, Q3W | Total: 64%, n = 441 Fatigue (28%, n = 95) Decreased appetite (24%, n = 79) Nausea (20%, n = 68) Rash (22%%, n = 73) Diarrhea (13%, n = 46) Asthenia (12%, n = 39) Stomatitis (6%, n = 20) Anemia (7%, n = 24) |
Total: 14%, n = 98 Fatigue (3%, n = 10) Decreased appetite (<2%, n = 4) Nausea (<2%, n = 3) Diarrhea (1%, n = 2) |
(46) |
| Pembrolizumab | III | NCT02142738 | 154 | 200 mg, Q3W | Total: 73%, n = 113 Diarrhea (14%, n = 22) Pyrexia (10%, n=16) Fatigue (10%, n = 16) Nausea (10%, n = 15) Decreased appetite (9%, n = 14) Anemia (5%, n = 8) Constipation (4%, n = 6) Vomiting (3%, n = 4) |
Total: 27%, n = 41 Diarrhea (4%, n = 6) Anemia (2%, n = 3) Fatigue (1%, n = 2) |
(47) |
| Pembrolizumab + pemetrexed + carboplatin | II | NCT02039674 | 59 | Pembrolizumab 200 mg, Q3W plus chemotherapy | Total: 93%, n = 55 Fatigue (61%, n = 36) Nausea (56%, n = 33) Anemia (20%, n = 12) Vomiting (25%, n = 15) Rash (25%, n = 15) Decreased appetite (19%, n = 11) Diarrhea (20%, n = 12) Increased aspartate (17%, n = 10) |
Total: 39%, n = 23 Fatigue (3%, n = 2) Acute kidney injury (3%, n = 1) Anemia (12%, n = 7) Neutropenia (3%, n = 2) Decreased neutrophil count (3%, n = 2) |
(48) |
| Pembrolizumab + pemetrexed + platinum-based drug | III | NCT02578680 | 405 | Pembrolizumab 200 mg, Q3W plus chemotherapy | Total: 99%, n = 404 Nausea (56%, n = 225) Fatigue (46%, n = 187) Anemia (41%, n = 165) Constipation (35%, n = 141) |
Total: 67%, n = 272 Anemia (16%, n = 66) Neutropenia (15.8%, n = 64) Thrombocytopenia (8%, n = 32) Asthenia (6%, n = 25) |
(49) |
| Diarrhea (31%, n = 125) Decreased appetite (28%, n = 114) Neutropenia (27%, n = 110) Vomiting (24%, n = 98) |
Fatigue (6%, n = 23) Diarrhea (5%, n = 21) Nausea (4%, n = 14) |
||||||
| Pembrolizumab + carboplatin + paclitaxel or nab-paclitaxel | III | NCT02775435 | 278 | Pembrolizumab 200 mg, Q3W plus chemotherapy | Total: 98%, n = 273 Anemia (53%, n = 148) Alopecia (46%, n = 128) Neutropenia (38%, n = 105) Nausea (36%, n = 99) Thrombocytopenia (31%, n = 85) Diarrhea (30%, n = 83) Decreased appetite (25%, n = 68) Constipation (23%, n = 64) |
Total: 70%, n = 194 Anemia (16%, n = 43) Neutropenia (23%, n=63) Thrombocytopenia (7%, n = 19) Diarrhea (4%, n = 11) Decreased appetite (2%, n = 6) |
(50) |
| Nivolumab | III | NCT01642004 | 131 | 3 mg/kg, Q2W | Total: 58%, n = 76 Fatigue (16%, n = 21) Decreased appetite (11%, n = 14) Asthenia (10%, n = 13) Nausea (9%, n = 12) Diarrhea (8%, n = 10) Arthralgia (5%, n = 7) Pneumonitis (5%, n = 6) Pyrexia (5%, n = 6) |
Total: 7%, n = 9 Fatigue (1%, n = 1) Decreased appetite (1%, n = 1) Leukopenia (1%, n = 1) |
(51) |
| Nivolumab | III | NCT01673867 | 287 | 3 mg/kg, Q2W | Total: 69%, n = 199 Fatigue (16%, n = 46) Nausea (12%, n = 34) Decreased appetite (10%, n = 30) Asthenia (10%, n = 29) Diarrhea (8%, n = 22) Peripheral edema (3%, n = 8) Myalgia (2%, n = 7) Anemia (2%, n = 6) |
Total: 10%, n = 30 Fatigue (1%, n = 3) Nausea (1%, n = 2) Asthenia (<1%, n = 1) Diarrhea (<1%, n = 2) |
(52) |
| Nivolumab | II | NCT01721759 | 117 | 3 mg/kg, Q2W | Total: 74%, n = 87 Fatigue (33%, n = 38) Asthenia (12%, n = 14) Nausea (15%, n = 18) Diarrhea (10%, n = 12) Decreased appetite (19%, n = 22) Rash (11%, n = 13) Anemia (6%, n = 7) Pneumonitis (5%, n = 6) |
Total: 17%, n = 20 Fatigue (4%, n = 5) Diarrhea (3%, n = 3) Rash (1%, n = 1) Pneumonitis (1%, n = 1) Anemia (1%, n = 1) |
(53) |
| Nivolumab | I | NCT01454102 | 52 | 3 mg/kg, Q2W | Total: 71%, n = 37 Fatigue (29%, n = 15) Rash (19%, n = 10) Nausea (14%, n = 7) Diarrhea (12%, n = 6) Pruritus (12%, n = 6) Arthralgia (6%, n = 3) Constipation (6%, n = 3) Hypothyroidism (6%, n = 3) |
Total: 19%, n = 10 Rash (4%, n = 2) Diarrhea (2%, n = 1) Pneumonitis (2%, n = 1) |
(54) |
| Nivolumab + Ipilimumab | I | NCT01454102 | 38 | Nivolumab 3 mg/kg, Q2W + ipilimumab 1 mg/kg, Q12W | Total: 82%, n = 31 Pruritus (24%, n = 9) Diarrhea (21%, n = 8) Nausea (16%, n = 6) Fatigue (16%, n = 6) Increased amylase (16%, n = 6) Maculopapular rash (13%, n = 5) Pyrexia (13%, n = 5) Rash (16%, n = 6) |
Total: 37%, n = 14 Increased lipase (8%, n = 3) Pneumonitis (5%, n = 2) Diarrhea (3%, n = 1) Fatigue (3%, n = 1) Rash (3%, n = 1) |
(55) |
| 39 | Nivolumab 3 mg/kg Q2W + ipilimumab 1 mg/kg Q6W | Total: 72%, n = 28 Pruritus (13%, n = 5) Diarrhea (21%, n = 8) Nausea (16%, n = 6) Fatigue (23%, n = 9) Maculopapular rash (10%, n = 4) Pyrexia (5%, n = 2) Rash (10%, n = 4) Decreased appetite (13%, n = 5) |
Total: 33%, n = 13 Adrenal insufficiency (5%, n = 2) Colitis (5%, n = 2) Nausea (3%, n = 1) Fatigue (3%, n = 1) Maculopapular rash (3%, n = 1) |
(55) | |||
| Nivolumab +cisplatin + gemcitabine or paclitaxel | I | NCT01454102 | 56 | 5 or 10 mg/kg, Q3W + chemotherapy | Total: 95%, n = 53 Fatigue (71%, n = 40) Nausea (46%, n = 26) Decreased appetite (36%, n = 20) Alopecia (30%, n = 17) Anemia (27%, n = 15) Rash (27%, n = 15) Diarrhea (21%, n = 12) |
Total: 45%, n = 25 Pneumonitis (7%, n = 4) Fatigue (5%, n = 3) Acute renal failure (5%, n = 3) Anemia (4%, n = 2) Neutropenia (4%, n = 2) |
(56) |
| Nivolumab + ALT-803 | Ib | NCT02523469 | 21 | Nivolumab 3 mg / kg, Q2W + ALT-803 6,10,15 or 20μg/ kg, Q1W | Injection-site reaction (90%, n = 19) Flu-like symptoms (71%, n = 15) Fever (67%, n = 10) Chills (29%, n = 6) Nausea (38%, n = 8) Pain (33%, n = 7) Dizziness (24%, n = 5) |
Fatigue (10%, n = 2) Lymphocytopenia (10%, n = 2) Fever (5%, n = 1) Anemia (5%, n = 1) Abdominal pain (5%, n = 1) |
(57) |
Table 2.
Adverse effects base on anti-PD-L1 antibodies.
| Agent | Phase | ClinicalTrials.gov |
No. of patients receiving anti-PD-L1 agent |
Therapy schedule | TRAEs (Any grade) | Treatment-related serious AEs (grade 3–5) | References |
|---|---|---|---|---|---|---|---|
| Atezolizumab | II | NCT02031458 | 659 | 1,200 mg, Q3W | Total: 65%, n = 429 Fatigue (19%, n = 122) Diarrhea (11%, n = 71) Nausea (11%, n = 73) Pruritus (10%, n = 65) Pyrexia (8%, n = 54) Decreased appetite (8%, n = 53) Asthenia (8%, n = 50) Rash (8%, n = 50) |
Total: 12%, n = 82 Fatigue (1%, n = 7) Nausea (1%, n = 4) Asthenia (1%, n = 3) Rash (1%, n = 9) |
(58) |
| Atezolizumab | I | NCT01375842 | 89 | 1-20 mg/kg or 1,200 mg, Q3W | Total: 76%, n = 68 Fatigue (20%, n = 18) Nausea (16%, n = 14) Decreased appetite (14%, n = 12) Asthenia (10%, n = 9) Rash (9%, n = 8) Dyspnea (8%, n = 7) Diarrhea (8%, n = 7) Headache (7%, n = 6) |
Total: 11%, n = 10 Fatigue (2%, n = 2) Dyspnea (2%, n = 2) Nausea (1%, n = 1) Vomiting (1%, n = 1) |
(59) |
| Atezolizumab | III | NCT02008227 | 609 | 1,200 mg, Q3W | Total: 94%, n = 573 Fatigue (27%, n = 163) Decreased appetite (24%, n = 143) Cough (23%, n = 141) Nausea (18%, n = 108) Diarrhea (15%, n = 94) Asthenia (19%, n = 116) Dyspnea (19%, n = 118) Anemia (12%, n = 70) |
Total: 37%, n = 227 Fatigue (3%, n = 17) Dyspnea (3%, n = 15) Anemia (2%, n = 14) Asthenia (1%, n = 8) Back pain (1%, n = 7) |
(60) |
| Atezolizumab | II | NCT01846416 | 137 | 1,200 mg, Q3W | Total: 70%, n = 96 Fatigue (27%, n = 37) Decreased appetite (15%, n = 21) Nausea (15%, n = 20) Diarrhea (10%, n = 13) Pyrexia (8%, n = 11) Pruritus (7%, n = 10) Arthralgia (7%, n = 9) Rash (7%, n = 9) |
Not mentioned | (61) |
| Atezolizumab + carboplatin + paclitaxel or pemetrexed or nab-paclitaxel | I | NCT01633970 | 76 | Atezolizumab 15 mg/kg, Q3W + chemotherapy | Not mentioned | Total: 72%, n = 55 Neutropenia (38%, n = 29) Anemia (21%, n = 16) Fatigue (11%, n = 8) Thrombocytopenia (8%, n = 6) Febrile neutropenia (7%, n = 5) Neutrophil count decreased (7%, n = 5) Platelet count decreased (5%, n = 4) Dehydration (5%, n = 4) |
(62) |
| Atezolizumab + bevacizumab + carboplatin +paclitaxel | III | NCT02366143 | 393 | Atezolizumab 1,200 mg, Q3W + chemotherapy | Total: 94.4%, n = 371 Alopecia (47%, n = 183) Peripheral neuropathy (36%, n = 141) Nausea (30%, n = 119) Fatigue (22%, n = 88) Decreased appetite (20%, n = 77) Anemia (18%, n = 70) Diarrhea (18%, n = 70) Constipation (17%, n = 65) |
Total: 59%, n = 230 Neutropenia (14%, n = 54) Decreased neutrophil count (9%, n = 34) Febrile neutropenia (9%, n = 36) Hypertension (6%, n = 25) Anemia (6%, n = 24) Decreased platelet count (5%, n = 20) |
(63) |
| Durvalumab | II | NCT02087423 | 444 | 10 mg/kg, Q2W | Total: 58%, n = 256 Fatigue (11%, n = 50) Hypothyroidism (8%, n = 36) Asthenia (7%, n = 31) Nausea (6%, n = 28) Pruritus (6%, n = 28) Diarrhea (6%, n = 27) Vomiting (3%, n = 14) Anemia (2%, n = 9) |
Total: 9%, n = 40 Fatigue (<1%, n = 2) Vomiting (<1%, n = 2) Pneumonitis (1%, n = 4) Gamma-glutamyltransferase increased (1%, n = 4) |
(64) |
| Durvalumab | III | NCT02125461 | 475 | 10 mg/kg, Q2W | Total: 67.8%, n = 322 Fatigue (13%, n = 62) Hypothyroidism (11%, n = 65) Diarrhea (10%, n = 46) Pneumonitis (9%, n = 43) Rash (8%, n = 37) Pruritus (7%, n = 33) Hyperthyroidism (6%, n = 30) Asthenia (6%, n = 28) |
Total: 12%, n = 56 Pneumonitis (1%, n = 6) Asthenia (<1%, n = 3) Dyspnea (<1%, n = 3) |
(65) |
| Durvalumab +Tremelimumab | I | NCT02000947 | 102 | Durvalumab 10-20 mg/kg, Q4W + Tremelimumab 1-3 mg/kg, Q12W | Total: 80%, n = 82 Diarrhea (32%, n = 33) Colitis (12%, n = 12) Pruritus (21%, n = 21) Rash (15%, n = 15) Hypothyroidism (10%, n = 10) Pneumonitis (5%, n = 5) Rash maculopapular (4%, n = 4) |
Total: 42%, n = 43 Diarrhea (11%, n = 11) Colitis (9%, n = 9) Pneumonitis (4%, n = 4) Enteritis (1%, n = 1) Hypothyroidism (1%, n = 1) |
(66) |
| Avelumab | I | NCT01772004 | 184 | 10 mg/kg, Q2W | Total: 77%, n = 142 Fatigue (25%, n = 46) Infusion-related reaction (19%, n = 34) Nausea (13%, n = 23) Decreased appetite (7%, n = 13) Diarrhea (7%, n = 13) Chills (7%, n = 12) Hypothyroidism (6%, n = 11) |
Total: 13%, n = 23 Elevated lipase (2%, n = 3) Infusion-related reaction (1%, n = 2) Dyspnea (1%, n = 2) Elevated amylase (1%, n = 1) Autoimmune neutropenia (1%, n = 1) |
(67) |
| Avelumab | III | NCT02395172 | 393 | 10 mg/kg, Q2W | Total: 64%, n = 251 Infusion-related reaction (15%, n = 59) Decreased appetite (9%, n = 34) Fatigue (7%, n = 29) Asthenia (7%, n = 29) Diarrhea (6%, n = n = 24) Nausea (5%, n = 20) Myalgia (2%, n = n = 6) Mucosal inflammation (1%, n = 2) |
Total: 12%, n = 47 Infusion-related reaction (1%, n = 5) Lipase increased (1%, n = 3) Asthenia (<1%, n = 1) Fatigue (<1%, n = 1) |
(68) |
Comparison of the Toxicity Spectrum Between PD-1 and PD-L1 Inhibitors in the Treatment of NSCLC
At present, although various PD-1 and PD-L1 ICIs have shown activity in NSCLC, it is meaningful to analyze and compare the differences in their toxicity profiles (69). According to the results of a systematic meta-analysis by Pillai et al., there was no significant difference in the overall incidence of AEs between the PD-1 treatment group (n = 3284) and the PD-L1 treatment group (n = 2460) (69–71). However, any grade of irAEs in the PD-1 treatment group was slightly higher than the PD-L1 treatment group (16 vs. 11%; p = 0.07) (69). The most common AE of PD-1 and PD-L1 inhibitors is fatigue (19 vs. 21%, p = 0.4), while the most common irAE is hypothyroidism (6.7 vs. 4.2%; p = 0.07) (69). It was worth noting that in patients receiving PD-1 inhibitors, the incidence of pneumonitis was significantly higher than in the PD-L1 agents treatment group (4 vs. 2%; P = 0.01) (69, 70). Therefore, clinicians should be more alert to lung inflammation in NSCLC patients receiving PD-1 blockade therapy (69).
At present, there is no systematic study to analyze the differences in the toxic and side effects of PD-1/PD-L1 inhibitors alone or in combination with other therapies for NSCLC. However, the current clinical trial data seems to indicate that the overall incidence of AEs of PD-1/PD-L1 inhibitor monotherapy is lower than that of combination therapy. For example, any grade of TRAEs that occurred with pembrolizumab monotherapy was 70.9% (24), while pembrolizumab combined with chemotherapy showed a higher incidence of TRAEs (98.2%) (50). Several other clinical trials of PD-1/PD-L1 inhibitors that have been approved for the treatment of NSCLC also showed the same trend (54, 56).
Management of Organ-Specific Toxicities Caused by Anti-PD-1/PD-L1 Treatment
Skin-Related Adverse Events
Rash and pruritus are the most common skin irAEs that occur in NSCLC patients receiving anti-PD-1/PD-L1 immune checkpoint treatment (12). Skin-related irAEs usually occur after the second cycle of the patient's clinical course (72, 73). Other dermatological lesions include vitiligo, skin capillary hyperplasia (CCEP), lichenoid and bullous pemphigoid (74). Despite frequent reports of immune-related skin AEs, the incidence of skin AEs of grade III or higher is low, and life-threatening AEs are only occasionally reported, but still deserve attention (74). For PD-1/PD-L1 monotherapy, the incidence of treatment-related skin AEs of any grade is ~7–31%, and the incidence of grade III or higher AEs is lower. Existing clinical trial data showed that the incidence of skin-related AEs of anti-PD-1 monotherapy was slightly higher than that of anti-PD-L1 monotherapy (11–31 vs. 7–19%) (24, 53, 67, 75). In addition, the emergence of skin toxicity caused by pembrolizumab seems to be more frequent than other anti-PD-1/PD-L1 agents (24, 67, 75). However, there was no significant difference in the incidence of skin-related AEs between anti-PD-1/PD-L1 monotherapy and combination therapy (24, 47). Recent studies have shown that patients with complete/partial remission have a higher incidence of skin adverse reactions than patients with stable/progressive disease, suggesting that skin AEs may be a positive prognostic factor for patients, but more prospective studies are still needed to further verify this kind of association (76, 77). However, a basic skin examination is necessary for patients using ICIs, especially those with previous inflammatory skin diseases. Standard dermatological assessments include skin biopsies, kidney and liver function tests, serum tryptase as well as immunoglobulin E levels (74).
For mild (grade I–II) maculopapular patients, it may be managed successfully with moderate potency topical steroids to affected areas and/or oral prednisone 0.5–1 mg/kg/day (78). For grade III–IV maculopapular, immunotherapy may be temporarily held and patients should be treated with high potency topical steroids to affected areas and oral prednisone 0.5–1 mg/kg/day (increase dose up to 2 mg/kg/day if no improvement) (79). In addition, topical emollients, oral antihistamines and lidocaine patches are effective for pruritus. For patients with severe pruritus, the GABA agonists (gabapentin, pregabalin) are useful, and aprepitant or omalizumab can be used in refractory cases (79).
Respiratory System Related Adverse Events
Anti-PD-1/PD-L1 immunotherapy also frequently occurs respiratory system-related AEs, especially for patients with lung cancer, the incidence of such AEs seems to be higher (69). Among them, immune-related pneumonia is the most common. Pneumonia is defined as focal or diffuse inflammation of the lung parenchyma, including pulmonary sarcoidosis and organizing inflammatory pneumonitis (80). Once pneumonia occurs, it may endanger the life of the patient, so active interventions should be taken (12, 80). The incidence of pneumonia is generally 7.4–24.3 months after the start of treatment. The clinical symptoms are mainly dry cough, dyspnea, fever, and chest pain (12, 81). It is worth noting that the combination of ICIs and other drugs at risk of pneumonia will increase the incidence of pneumonia (82). Chen et al. (83) reported an unpredictable but relatively severe radiation recall pneumonitis (RRP), which was induced by anti-PD-1 inhibitor camrelizumab 2 years after radiotherapy. This indicated that previous radiotherapy combined with subsequent anti-PD-1 immunotherapy may result in overlapping damage to lung (83). Moreover, patients with other underlying lung diseases, such as COPD and pulmonary fibrosis, should be more alert to the occurrence of pneumonia (84, 85).
Chest CT is a key method for diagnosing pneumonia. The imaging features are ground-glass lesions and/or disseminated nodular infiltrates (12, 86). According to the management of the latest NCCN guidelines, any level of immune-related pneumonia should hold immunotherapy, and patients with severe pneumonia should permanently discontinue immunotherapy. Patients with mild (grade I) pneumonia need to re-evaluate arterial oxygen saturation (both resting and active) and repeat chest CT in 4 weeks or as clinically indicated for worsening symptoms (78, 87). For grade II or higher pneumonia should first rule out bacterial infections, such as nasal swab for potential viral pathogens, sputum culture, blood culture, and urine antigen test to detect pneumococcus and legionella (87). Additionally, bronchoscopy and bronchoalveolar lavage are necessary. If the infection cannot be completely ruled out, empiric antibiotics can be used. Management is guided by clinical symptoms, such that grade II pneumonia patients can be taken orally or intravenously prednisone/methylprednisolone 1–2 mg/kg/day (86, 87). Severe cases require hospitalization and intravenous methylprednisolone 1–2 mg/kg/day. Other forms of immunosuppression may be considered, such as infliximab, mycophenolate mofetil or intravenous immunoglobulin, if corticosteroids remain ineffective after 48 h of treatment (86, 87).
Digestive System Related Adverse Events
Colitis and diarrhea are the most common gastrointestinal toxicity during the treatment of anti-PD-1/PD-L1 immunotherapy (24). Other gastrointestinal adverse reactions include decreased appetite, nausea, vomiting, constipation (24). Colitis clinically involves clinical or imaging evidence of abdominal pain symptoms and colon inflammation, while diarrhea refers to an increase in stool frequency (72). In immune checkpoint blocking therapy, the incidence of gastrointestinal AEs with anti-CTLA-4 treatment is higher than that with anti-PD-1/PD-L1 therapy (72). Moreover, anti-PD-1/PD-L1 agents combined with chemotherapy drugs will increase the incidence of gastrointestinal AEs (any grade) (23, 56). In general, the incidence of grade III–IV colitis/diarrhea is about 5% and life-threatening cases are rarely reported (12). In clinical management of immune-related colitis and diarrhea AEs, stool evaluation should be performed to rule out any possible bacterial, viral pathogen, and parasitic infections (88). For mild diarrhea or colitis, it is useful to oral loperamide or diphenoxylate/atropine for 2–3 days and hydration (78, 88). Moderate or severe colitis/diarrhea should hold immunotherapy. Patients with grade 3 may consider re-use of anti-PD-1/PD-L1 therapy after toxicity has been relieved, but patients with grade IV should permanently discontinue immunotherapy (78). Patients with grade IV may be successfully managed by using prednisone/methylprednisolone (1–2 mg/kg/day). If the symptoms do not improve, consider adding infliximab or vedolizumab within 2 weeks. Severe cases should be hospitalized to provide supportive treatment (78).
Hepatic Toxicities
Among NSCLC patients receiving anti-PD-1/PD-L1 immunotherapy, the incidence of immune-related hepatitis is approximately 5%, while the incidence of severe hepatitis (grade III-IV) is <2% (89). The median time to onset is usually 6–14 weeks from the first taking of anti-PD-1/PD-L1 drugs, but may occur within a few months after starting treatment or even stopping treatment (89). Any asymptomatic elevations in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) enzymes levels should consider immune-related hepatitis (78, 90). Some patients occasionally observe elevated levels of bilirubin, usually without obvious symptoms. In addition, liver biopsy is the gold standard for diagnosing and evaluating the degree of autoimmune hepatitis and liver injury (90). The clinical symptoms of immune-mediated hepatitis include hepatomegaly, portal and periportal inflammation, lymphadenomegaly, and infiltrating eosinophils, lymphocytes as well as plasma cells (90). Before treatment of immune-related hepatitis, viral etiology (hepatitis A, hepatitis B or C, and emergency hepatitis E virus), disease-related liver dysfunction, and other drug-induced transaminase elevations should be excluded. Ultrasound or magnetic resonance cholangiopancreatography can be considered to rule out liver metastases or gallstones of cancer (78). For mild to moderate hepatitis (grade I–II), immunotherapy can be continued or suspended according to the patient's condition, and liver function tests (LFTs) are closely monitored. Patients with grade III–IV hepatitis should permanently discontinue immunotherapy and use prednisone/methylprednisolone 1–2 mg/kg/day. If the steroid treatment does not improve after 3 days, consider adding an additional immunosuppressant mycophenolates, but should not use infliximab as its potential hepatotoxicity (78).
Endocrine System Related Adverse Events
The endocrine system contains many important organs of the human body, such as hypothalamus, pituitary, thyroid, adrenal glands, and pancreas. The endocrine toxicity caused by PD-1/PD-L1 ICIs may affect any axis (12). Hypophysitis, thyroiditis, hypothyroidism, hyperthyroidism, and adrenal insufficiency are common immune-related endocrine diseases (44). Among patients with NSCLC, hypothyroidism is the most common endocrine toxicity, with an incidence of 5–15% (44). Since the clinical symptoms of immune endocrine disease are non-specific, such as fatigue, headache, and nausea. Cancer patients are often accompanied by such symptoms. Therefore, the diagnosis of immune-mediated endocrine toxicity is clinically challenging (12). Clinically, endocrine diseases such as central hypothyroidism and pituitary inflammation are diagnosed by evaluating biochemical indicators such as morning cortisol, ACTH (adreno-cortico-tropic-hormone), FSH (follicle-stimulating hormone), LH (luteinizing hormone), TSH (thyroid stimulating hormone), free T4, and DHEA-S (91). For patients with hypothyroidism, the thyroid hormone replacement therapy may be useful, and closely monitor the level of TSH is necessary (every 4–6 weeks) (78). If TSH > 10, levothyroxine should be used to make TSH reach the reference range or age-appropriate range. Patients with hyperthyroidism can be treated with standard antithyroid drugs. In addition, pituitary inflammation with obvious symptoms can be considered with prednisone/methylprednisolone 1–2 mg/kg/day for treatment (78). Primary adrenal insufficiency occurs less frequently in irAEs related to PD-1/PD-L1 blockade therapy, but in rare cases an adrenal crisis may occur (91). It should hold the immunotherapy and perform intravenous corticosteroid as well as supplement aggressive fluid and electrolyte when such AEs occur (91). Most endocrine-related toxicity is effective through hormone replacement therapy, without holding PD-1/PD-L1 immune checkpoint treatment.
Skeletal Muscle System Related Adverse Events
Some tumor patients receiving anti-immunity checkpoint treatment will also have skeletal muscle system-related AEs, but musculoskeletal symptoms are also present in the tumor patients themselves, therefore more attention should be paid to distinguishing (81). Overall, the majority of immune-related muscle AEs in patients with NSCLC are mild (grade I-II). The diagnosis of inflammatory arthritis is mainly by evaluating the degree of joint involvement, X-ray and joint ultrasound (78). Moreover, it is necessary to check the creation kinase/aldolase and troponin levels. NSCLC patients have the most reported immune-related muscle adverse reaction is myalgia (43). Patients with mild pain can continue immunotherapy and continuously monitor serial aldolase/creatine kinase levels, but moderate or severe pain should hold immunotherapy, using prednisone 1–2 mg/kg/day for treatment and considering muscle biopsy (72).
Management of Other Common Adverse Events
Fatigue
Fatigue widely occurs in patients with NSCLC who are treated with PD-1/PD-L1 immune checkpoint blockade (12). Overall, for NSCLC patients receiving anti-PD-1/PD-L1 monotherapy or combination therapy, ~6–71% of patients reported treatment-related fatigue (any grade), but the incidence of grade III/IV is low (<5%) (24, 45, 56). Compared with anti-PD-1/PD-L1 monotherapy, PD-1/PD-L1 ICIs combined with other therapies (chemotherapy, targeted therapy, anti-CTLA-4 therapy) significantly increased the incidence of fatigue side effects (6–33 vs. 13–71%) (47, 54, 75). However, it is worth noting that fatigue symptoms are sometimes caused by immune-related endocrine toxicity. For example, early symptoms of hypothyroidism can also cause fatigue (81). Therefore, the treatment of fatigue should consultation based on abnormalities, and the use of low-dose steroids is allowed (78). In addition, moderate physical activity and psychosocial intervention can also help relieve fatigue symptoms (72). For severe fatigue, consideration should be given to whether tumor disease progression or other medical diseases occur (78).
Pyrexia/Chills and Infusion Reactions
Anti-PD-1/PD-L1 immune checkpoint therapy may cause cytokine release and non-specific over-activation of the immune system, which may lead to symptoms of pyrexia, chill and infusion reactions in patients (81). Approximately 5–18% of patients with NSCLC develop immune-related pyrexia during treatment. It can be managed by using antipyretics, such as acetaminophen or non-steroidal anti-inflammatory drugs (78). For grade I–II infusion reactions, it can resume infusion or reduce the infusion rate after the symptoms disappear, and consider premedication with acetaminophen, famotidine, and diphenhydramine with future infusions. For grade III infusion reactions, the immunotherapy should be permanently discontinued, and intravenous antihistamine or corticosteroid drugs are required (74, 78).
Management of Rare But Serious Adverse Events
Immune-Related Encephalitis
Immune-related encephalitis is a rare and poorly understood irAE, with an incidence of <1% in cancer patients undergoing immune checkpoint blockade therapy, but it may be fatal (92). Therefore, it is necessary to increase its awareness for effective management. A multicenter cohort retrospectively analyzed the clinical, biological, and radiological characteristics of nine immune-related encephalitis in NSCLC patients undergoing anti-PD-1/PD-L1 treatment (40). The most common clinical symptoms of these patients include fever, confusion, and cerebellar ataxia (40). In addition, it was found that the levels of white blood cell increased, without any bacterial and viral infection. One patient's brain MRI examination showed that the limbic system is involved, which is fatal (40). The most important management of immune-related encephalitis is early treatment with corticosteroids (prednisone 1–2 mg/kg/day). Severe cases should permanently discontinue immunotherapy (78).
Myasthenia Gravis
The immune-related myasthenia gravis is also a rare but serious neurotoxicity caused by anti-PD-1/PD-L1 treatment (43, 91). The average onset time of the patient's symptoms appeared within 6 weeks of starting treatment (range 2–12 weeks) (93). Treatment-related reports of myasthenia gravis in NSCLC patients receiving PD-1 monoclonal antibodies seem to be more common than those receiving PD-L1 agents (41, 94, 95). A 63-year-old female patient with stage IV NSCLC adenocarcinoma, who failed conventional chemotherapy (disease progression) and subsequently used pembrolizumab, was diagnosed with myasthenia gravis after two cycles of treatment (41). The clinical symptoms are bilateral eyelid drooping, extraocular muscle paralysis, shortness of breath, and fatigue (41). Moreover, two patients with NSCLC who received nivolumab reported myasthenia gravis, and the onset time was within 2–3 cycles after the start of treatment (94, 95). Moderate and severe autoimmune myasthenia gravis should permanently discontinued immunotherapy, as well as oral pyridostigmine 30 mg TID and gradually increase to maximum of 120 mg four times a day as tolerated and based on symptoms (93). In addition, considering low-dose oral prednisone 20 mg daily and gradually increase the dose (not more than 100 mg/day) if necessary. Severe cases should use methylprednisolone 1–2 mg/kg/day and consider adding rituximab (375 mg/m2 weekly for 4 treatments or 500 mg/m2 every 2 weeks for 2 doses) if refractory to plasmapheresis or intravenous immunoglobulin (IVIG) (93).
Acute Renal Failure/Interstitial Nephritis
The main manifestation of kidney injury is elevated serum creatinine levels, and patients usually develop acute renal failure and interstitial nephritis (96). According to reports, the possible mechanism of kidney damage induced by ICIs is that drugs or drug metabolites activate circulating T cells, which binding to carrier proteins and form drug-carrier immune complexes to obtain immunogenicity (97). When these immune complexes are presented as a local antigen to the kidney, they trigger a hypersensitivity reaction through the release of cytokines, leading to the occurrence of kidney damage (97). In NSCLC patients, a phase I study (NCT01454102) of nivolumab combined with platinum-based dual chemotherapy reported 3 cases of grade 3 acute renal failure. In addition, Koda et al. (42) reported a 67-year-old stage IV acute tubulointerstitial nephritis caused by nivolumab monotherapy in patients with NSCLC. For the management of acute renal failure/interstitial nephritis, creatinine, and urine protein levels should be closely monitored (once every 3–7 days), and prednisone 0.5–1 mg/kg/day may be useful (42). Patients with severe kidney injury should permanently discontinue immunotherapy and use prednisone/methylprednisolone 1–2 mg/kg/day. Conduct renal biopsy and nephrology consultation if necessary. Moreover, add one of the following drugs, azathioprine, cyclophosphamide, cyclosporine, infliximab, and mycophenolate, if the symptoms still not improve after treated with steroids for more than 1 week (42).
Myocarditis
Immune-mediated cardiotoxicity, myocarditis, is a rare but serious side effect in NSCLC patients receiving anti-PD-1/PD-L1 immune checkpoint treatment, which needs to be recognized as soon as possible for better management (98–100). A case report showed that a 75-year-old NSCLC patient suffered a drug-induced AE of myocarditis during the ninth cycle of nivolumab treatment, and its clinical symptoms were dyspnea and acute chest pain (98). After treatment with ACE-inhibitors, β-blockers and diuretics as well as prednisolone (1 mg/kg/day), the cardiac function of patient was significantly improved (98). Similarly, Gibson et al. (101) reported that a 68-year-old female NSCLC patient receiving nivolumab developed autoimmune myocarditis. The patient's electrocardiogram showed sustained ventricular tachycardia and ectopic ventricular beats (101). In addition to the use of corticosteroids for the treatment of myocarditis, other immunosuppressive agents such as anti-thymocyte globulin, infliximab and mycophenolate can also be added if necessary (Figure 2).
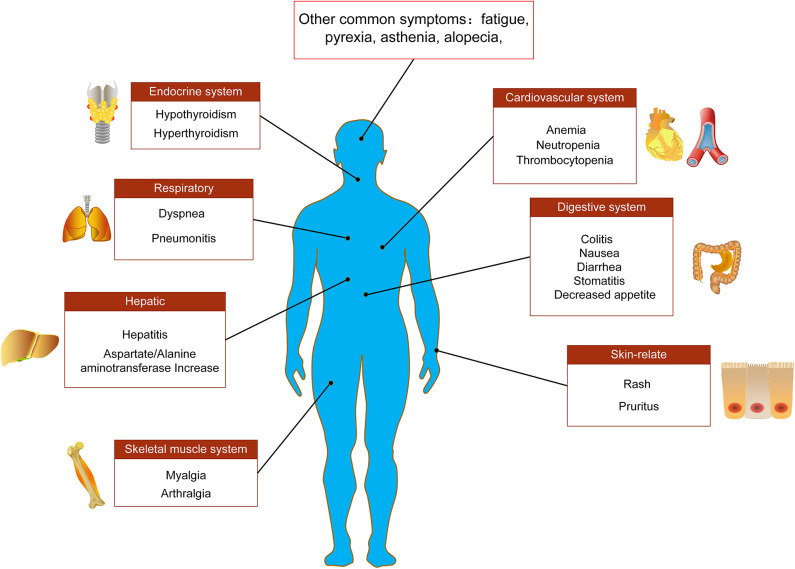
Figure 2.
Main adverse events of PD-1/PD-L1 immunotherapy. Adverse events associated with PD-1/PD-L1 immune checkpoint inhibitors in the treatment of NSCLC involve multiple tissues and organs, including endocrine system, respiratory system, digestive system, cardiovascular system, skeletal muscle system, liver, and skin-related adverse reactions.
Prevent or Reduce the Frequency of Adverse Events
Potential Predictive Biomarkers Related to Adverse Effects
The effective management strategy for irAEs is early detection and early intervention. Therefore, it is crucial to find biomarkers that can predict the occurrence of AEs during immunotherapy (102). Recently, a study performed by Kurimoto and his colleague found that serum thyroglobulin, thyroid autoantibodies and early changes in the levels of certain cytokines (increased levels of IL-1β, IL-2, and GM-CSF and decreased levels of IL-8, G-CSF, MCP-1) may indicate the development of autoimmune thyroiditis AEs (103). Similarly, thyroid peroxidase (TPO) and thyroglobulin antibody levels are associated with hypothyroidism in NSCLC patients receiving nivolumab treatment (104). Oyanagi et al. (105) reported that the increase in serum protein RANTES is a potential predictive biomarkers of the onset of irAEs in NSCLC patients who treated with nivolumab. In addition, the increase levels of serum C-reactive protein (CRP) are associated with a higher incidence of irAEs, but not with the severity of irAEs and the affected organ (106). For rare but severe immune-mediated myocarditis, several potential predictive biomarkers have also been found, such as serial troponin, miR-30c (107, 108).
Baseline Examination Before Immunotherapy Initiation
By comparing the changes of certain biochemical indicators and imaging features of tissues and organs before and after immunotherapy, it can help clinicians to quickly judge any irAEs that may occur (109). Routine baseline assessments include physical examination (height, weight, heart rate, blood pressure, and other general symptoms), imaging examination (chest CT, brain MRI) as well as laboratory tests (blood routine, blood biochemistry, blood glucose, total bilirubin, TSH, free T4, LH, FSH, testosterone, cortisol, ACTH, infectious disease screening, etc.) (109). In addition, carefully ask patient and family the history of autoimmune disease, infectious disease and organ specific diseases are necessary. Clinicians also need to inform patients of potential side effects of immune checkpoint blockade therapy, whether during or after treatment (73). Patients should also promptly feedback any new symptoms of discomfort.
Personalized Management
Tumor patients of different races, genders, and ages experience different irAEs profiles and severity, therefore precise care according to the patient's personal situation is conducive to reduce the incidence of AEs (110). Elderly people with lung cancer usually have comorbidities and polypharmacy, therefore adequate clinical monitoring is required (110). However, Hakozaki et al. (111) showed that polypharmacy was not associated with irAEs but was associated with higher rate of unexpected hospitalizations during anti-PD-1/PD-L1 treatment in early NSCLC patients (aged ≥ 65 years) in Japanese. Studies have also shown that immune-related fatigue is more common in elderly patients with lung cancer (aged ≥75 years) (49.1 vs. 40.2%), but no other differences in irAEs are observed, and it is not recommended to adjust the dosage of elderly patients (109, 110). Given the small number of elderly patients involved in most immune checkpoint blockade studies, the toxicity data for this group is limited and further studies are needed (112). PD-1/PD-L1 blockade may aggravate or reactivate certain existing viral infectious diseases, therefore patients with a history of chronic viral infections (such as HBV, HCV or HIV) should be excluded from clinical trials (109). Due to the ability of IgG to cross the placental barrier, ICI is not recommended for pregnant and lactating women unless the clinical benefit of the patient outweighs the potential risk (109). Most initial clinical trials of PD-1/PD-L1 blocking therapy are conducted in Caucasians or mix races (113). In recent years, more and more clinical trials of anti-PD-1/PD-L1 agents have been conducted in Asian populations (113). The analysis results of Yang et al. (113) showed that in cancer patients with PD-1/PD-L1 blockade therapy, the AEs of any grade with different prevalences between Asian populations and Western/international populations included fatigue, diarrhea, nausea, rash, vomiting, and hypothyroidism. Overall, we still need to develop more sophisticated medical tools in the future to achieve the best management strategy for irAEs in cancer patients.
Conclusion
The therapy based on PD-1/PD-L1 immune checkpoint blockade show a better tolerated than traditional standard chemotherapy in NSCLC patients, but the AEs of these drugs are different from traditional cytotoxic therapy. Therefore, it is necessary to increase awareness of these treatment-related toxic reactions for better management. These adverse reactions involved different tissues and organs in the human body, causing toxic reactions ranging from mild fatigue to severe, life-threatening liver and lung toxicity (115, 116). Compared with traditional chemotherapy, AEs caused by anti-PD-1/PD-L1 treatment were usually of low grade, with relatively good patient tolerance and fewer deaths. However, due to the rapid onset of AEs, so timely medical care was crucial, especially for the elderly patients, these toxic reactions should be more carefully monitored to prevent possible complications.
In conclusion, our review summarizes common and rare adverse reactions based on anti-PD-1/PD-L1 therapy in the treatment of NSCLC. Overall, adverse reactions caused by anti-PD-1/PD-L1 immunotherapy were usually low-grade and most patients were better tolerated. However, there were still some serious and even life-threatening adverse events related to treatment. Therefore, healthcare workers should be alert to the occurrence of such AEs to better monitor and manage these adverse reactions.
Author Contributions
CS, HW, and YL wrote the first draft of the manuscript. QG, LZ, JL, and WZ organized the structure of the manuscript. JZ, XZ, and YY contributed conception of the work. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by National Natural Science Foundation of China (81773888, U1903126, and 81902152) and Natural Science Foundation of Guangdong Province (2020A151501005 and 2020A1515010605).
References
- 1.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2018:A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. (2018) 68:297–316. 10.3322/caac.21446 [DOI] [PubMed] [Google Scholar]
- 2.Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. (2018) 52:103–9. 10.1016/j.semcancer.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W, Yan Y, Liu Y, Lin M, Ma J, Zhang W, et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin α2β1. Signal Transduct Target Ther. (2020) 5:39. 10.1038/s41392-020-0133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. (2018) 2018:6984948. 10.1155/2018/6984948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Knethen A, Brüne B. PD-L1 in the palm of your hand: palmitoylation as a target for immuno-oncology. Signal Transduct Target Ther. (2019) 4:18. 10.1038/s41392-019-0053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Fu L. Predictive efficacy biomarkers of programmed cell death 1/programmed cell death 1 ligand blockade therapy. Recent Pat Anticancer Drug Discov. (2016) 11:141–51. 10.2174/1574892811666160226150506 [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. (2015) 5:390–401. 10.1016/j.apsb.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets. (2015) 19:201–11. 10.1517/14728222.2014.980235 [DOI] [PubMed] [Google Scholar]
- 9.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. (2018) 175:313–26. 10.1016/j.cell.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haanen JBAG, Thienen Hv, Blank CU. Toxicity patterns with immunomodulating antibodies and their combinations. Semin Oncol. (2015) 42:423–8. 10.1053/j.seminoncol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. (2015) 26:2375–91. 10.1093/annonc/mdv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Pan X, Zhang W, Guo H, Cheng S, He Q, et al. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. (2020) 10:723–33. 10.1016/j.apsb.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousin S, Italiano A. Molecular pathways: immune checkpoint antibodies and their toxicities. Clin Cancer Res. (2016) 22:4550–5. 10.1158/1078-0432.CCR-15-2569 [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Guo W, Wu Y, Yang C, Zhong L, Deng G, et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. (2019) 9:304–15. 10.1016/j.apsb.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leventakos K, Mansfield AS. Advances in the treatment of non-small cell lung cancer: focus on nivolumab, pembrolizumab, and atezolizumab. BioDrugs. (2016) 30:397–05. 10.1007/s40259-016-0187-0 [DOI] [PubMed] [Google Scholar]
- 17.Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol. (2019) 234:1313–25. 10.1002/jcp.27172 [DOI] [PubMed] [Google Scholar]
- 18.Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. (2017) 22:81–8. 10.1634/theoncologist.2016-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. (2018) 6:39. 10.1186/s40425-018-0349-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. (2015) 41:868–876. 10.1016/j.ctrv.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Wei X. Deletion of the RNA-editing enzyme ADAR1A: new strategy to potentiate responses to PD-1 immune checkpoint blockade. Signal Transduct Target Ther. (2019) 4:6. 10.1038/s41392-019-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Long Y, Guo R, Liu X, Tang X, Rao J, et al. Multifunctional polymeric micelle-based chemo-immunotherapy with immune checkpoint blockade for efficient treatment of orthotopic and metastatic breast cancer. Acta Pharm Sin B. (2019) 9:819–831. 10.1016/j.apsb.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2015) 16:375–84. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 24.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 25.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist. (2017) 22:470–9. 10.1634/theoncologist.2016-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Kane GM, Labbé C, Doherty MK, Young K, Albaba H, Leighl NB. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. (2017) 22:70–80. 10.1634/theoncologist.2016-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. (2017) 214:895–904. 10.1084/jem.20160801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamurthy C, Godwin JL, Borghaei H. Immune checkpoint inhibitor therapy: what line of therapy and how to choose? Curr Treat Opt Oncol. (2017) 18:476. 10.1007/s11864-017-0476-y [DOI] [PubMed] [Google Scholar]
- 29.Escors D, Gato-Cañas M, Zuazo M, Arasanz H, García-Granda MJ, Vera R, et al. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. (2018) 3:26. 10.1038/s41392-018-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. (2017) 8:561. 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. (2018) 8:1307–16. [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. (2017) 8:2171–86. 10.18632/oncotarget.13895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and Its Ligands in Tolerance and Immunity. Ann Rev Immunol. (2008) 26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju X, Zhang H, Zhou Z, Wang Q. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. (2020) 10:1–11. [PMC free article] [PubMed] [Google Scholar]
- 35.Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol. (2018) 71:189–94. 10.1136/jclinpath-2017-204853 [DOI] [PubMed] [Google Scholar]
- 36.Cui C, Yu B, Jiang Q, Li X, Shi K, Yang Z. The roles of PD-1/PD -L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. (2018) 46:3–10. 10.1111/1440-1681.13028 [DOI] [PubMed] [Google Scholar]
- 37.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci. (2008) 105:20852–7. 10.1073/pnas.0810958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JY YY, Li JJ, Adhikari R Fu LW. PD-1/PD-L1 based combinational cancer therapy: icing on the cake. Front. Pharmacol. (2020) 11:722. 10.3389/fphar.2020.00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. (2019) 17:661–74. 10.1016/j.csbj.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchis-Borja M, Ricordel C, Chiappa AM, Hureaux J, Odier L, Jeannin G, et al. Encephalitis related to immunotherapy for lung cancer: analysis of a multicenter cohort. Lung Cancer. (2020) 143:36–9. 10.1016/j.lungcan.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 41.Lara MS, Afify A, Ellis MP, Phan CT, Richman DP, Riess JW. Immune checkpoint inhibitor-induced myasthenia gravis in a patient with advanced NSCLC and remote history of thymoma. Clin Lung Cancer. (2019) 20:e489–e91. 10.1016/j.cllc.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 42.Koda R, Watanabe H, Tsuchida M, Iino N, Suzuki K, Hasegawa G, et al. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. (2018) 19:48. 10.1186/s12882-018-0848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Auré K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. (2018) 91:e985–e94. 10.1212/WNL.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 44.Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. (2017) 28:874–81. 10.1093/annonc/mdx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 46.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 47.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 48.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. (2016) 17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 50.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 51.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. (2015) 16:257–65. 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. (2016) 34:2980–7. 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. (2017) 18:31–41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. (2016) 34:2969–79. 10.1200/JCO.2016.66.9861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. (2018) 19:694–704. 10.1016/S1470-2045(18)30148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. (2017) 35:2781–9. 10.1200/JCO.2016.71.9476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horn L, Gettinger SN, Gordon MS, Herbst RS, Gandhi L, Felip E, et al. Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer. (2018) 101:201–9. 10.1016/j.ejca.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 60.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spigel DR, Chaft JE, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol. (2018) 13:1733–42. 10.1016/j.jtho.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu SV, Camidge DR, Gettinger SN, Giaccone G, Heist RS, Hodi FS, et al. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur J Cancer. (2018) 101:114–22. 10.1016/j.ejca.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 63.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7:387–401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 64.Garassino MC, Cho B-C, Kim J-H, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. (2018) 19:521–36. 10.1016/S1470-2045(18)30144-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 66.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. (2016) 17:299–308. 10.1016/S1470-2045(15)00544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. (2017) 18:599–610. 10.1016/S1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. (2018) 19:1468–79. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 69.Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer. (2018) 124:271–7. 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spagnuolo A, Gridelli C. “Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer”: is there a substantial difference or not? J Thorac Dis. (2018) 10:S4065–8. 10.21037/jtd.2018.09.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Owen DH, Otterson GA. Do toxicity patterns vary between programmed death-1 and programmed death ligand-1 inhibitors? J Thorac Dis. (2018) 10:S4069–S72. 10.21037/jtd.2018.09.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. (2015) 76:76–83. 10.14694/EdBook_AM.2015.35.76 [DOI] [PubMed] [Google Scholar]
- 73.Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. (2016) 45:3. 10.1016/j.ctrv.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 74.Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. (2017) 8:730. 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 76.Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez-Almazor ME, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. (2018) 36:638–46. 10.1007/s10637-017-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. (2016) 22:886–94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. 10.1200/JOP.18.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balaji A, Verde F, Suresh K, Naidoo J. Pneumonitis from anti-PD-1/ PD-L1 therapy. Oncology. (2017) 31:739–46. [PubMed] [Google Scholar]
- 81.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. (2016) 54:139–48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 82.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. (2015) 373:288–90. 10.1056/NEJMc1505197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Huang Z, Xing L, Meng X, Yu J. Radiation recall pneumonitis induced by anti-PD-1 blockade: a case report and review of the literature. Front Oncol. (2020) 10:561. 10.3389/fonc.2020.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med. (2018) 197:325–36. 10.1164/rccm.201704-0795OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. (2019) 158:269–76. 10.1016/j.jtcvs.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. (2019) 10:108. 10.3389/fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bala-Hampton JE, Bazzell AF, Dains JE. Clinical management of pneumonitis in patients receiving anti-PD-1/PD-L1 therapy. J Adv Pract Oncol. (2018) 9:422–8. 10.6004/jadpro.2018.9.4.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology. (2017) 6:e1344805. 10.1080/2162402X.2017.1344805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vitale G, Lamberti G, Comito F, Di Nunno V, Massari F, Morelli MC, et al. Anti-programmed cell death-1 and anti-programmed cell death ligand-1 immune-related liver diseases: from clinical pivotal studies to real-life experience. Expert Opin Biol Ther. (2020) 20:1047–59. 10.1080/14712598.2020.1762562 [DOI] [PubMed] [Google Scholar]
- 90.De Martin E, Michot J-M, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. (2018) 68:1181–90. 10.1016/j.jhep.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 91.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Marchal C, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry. 91:772–8. (2020). 10.1136/jnnp-2020-323055 [DOI] [PubMed] [Google Scholar]
- 93.Makarious D, Horwood K, Coward JIG. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer. (2017) 82:128–36. 10.1016/j.ejca.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 94.Sciacca G, Nicoletti A, Rampello L, Noto L, Parra HJS, Zappia M. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve. (2016) 54:507–9. 10.1002/mus.25212 [DOI] [PubMed] [Google Scholar]
- 95.Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non-small-cell lung cancer. Muscle Nerve. (2016) 54:507. 10.1002/mus.25163 [DOI] [PubMed] [Google Scholar]
- 96.Xu J, Ma X, Yu K, Wang R, Wang S, Liu R, et al. Lactate up-regulates the expression of PD-L1 in kidney and causes immunosuppression in septic Acute Renal Injury. J Microbiol Immunol Infect. (2019). 10.1016/j.jmii.2019.10.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 97.Shingarev R, Glezerman IG. Kidney complications of immune checkpoint inhibitors: a review. Am J Kidney Dis. (2019) 74:529–37. 10.1053/j.ajkd.2019.03.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohé C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer. (2016) 99:117–9. 10.1016/j.lungcan.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 99.Matsuo K, Ishiguro T, Najama T, Shimizu Y, Kobayashi Y, Mutou M. Nivolumab-induced myocarditis successfully treated with corticosteroid therapy: a case report and review of the literature. Intern Med. (2019) 58:2367–72. 10.2169/internalmedicine.2596-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. (2016) 4:50. 10.1186/s40425-016-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. (2016) 2016:228. 10.1136/bcr-2016-216228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. (2017) 8:49 10.3389/fphar.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. (2020) 111:1468–77. 10.1111/cas.14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, et al. Predictive factors of nivolumab-induced hypothyroidism in patients with non-small cell lung cancer. In Vivo. (2017) 31:1035–9. 10.21873/invivo.11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oyanagi J, Koh Y, Sato K, Mori K, Teraoka S, Akamatsu H, et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer. (2019) 132:107–13. 10.1016/j.lungcan.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 106.Abolhassani A-R, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. (2019) 145:2625–31. 10.1007/s00432-019-03002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist. (2018) 23:936–42. 10.1634/theoncologist.2017-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou F, Lu X, Zhang X. Serum miR-30c level predicted cardiotoxicity in non-small cell lung cancer patients treated with bevacizumab. Cardiovasc Toxicol. (2018) 18:284–9. 10.1007/s12012-018-9457-z [DOI] [PubMed] [Google Scholar]
- 109.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. (2016) 27:559–74. 10.1093/annonc/mdv623 [DOI] [PubMed] [Google Scholar]
- 110.King-Kallimanis BL, Kanapuru B, Blumenthal GM, Theoret MR, Kluetz PG. Age-related differences in patient-reported outcomes in patients with advanced lung cancer receiving anti-PD-1/PD-L1 therapy. Semin Oncol. (2018) 45:201–9. 10.1053/j.seminoncol.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 111.Hakozaki T, Hosomi Y, Shimizu A, Kitadai R, Mirokuji K, Okuma Y. Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol. (2020). 10.1007/s00432-020-03252-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perrotta F, Rocco D, Vitiello F, De Palma R, Guerra G, De Luca A, et al. Immune checkpoint blockade for advanced NSCLC: a new landscape for elderly patients. Int J Mol Sci. (2019) 20:2258. 10.3390/ijms20092258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang J, He X, Lv Q, Jing J, Shi H. Management of adverse events in cancer patients treated with PD-1/PD-L1 blockade: focus on asian populations. Front Pharmacol. (2019) 10:726. 10.3389/fphar.2019.00726 [DOI] [PMC free article] [PubMed] [Google Scholar]