Abstract
Extrahepatic portal vein obstruction (EHPVO) is the most common cause of pediatric portal hypertension and can cause life-threatening variceal bleeding. Meso-Rex shunt (MRS) is a surgical procedure that restores physiological portal venous blood flow to the liver by using a graft to connect the superior mesenteric vein and the left portal vein within the Rex recess, and can relieve variceal bleeding and other complications associated with EHPVO. Although the MRS is regarded as an optimal and potentially curative treatment with good long-term patency, graft thrombosis or failure due to unknown causes is not rare, prompting the need for further research on the risk factors of graft failure or poor patency. Herein, we report two cases of EHPVO in patients with recurrent or uncontrolled variceal bleeding, one treated with the classic Rex shunt and the other with the modified Rex shunt, which resulted in a failure and success, respectively.
Keywords: Mesenteric venous thrombosis, Vascular grafting, Portal hypertension, Esophageal and gastric varices, Meso-Rex shunt
INTRODUCTION
Extrahepatic portal vein obstruction (EHPVO) is the most common cause of portal hypertension in children, which can lead to life-threatening variceal bleeding [1]. The classic Rex shunt, meso-Rex shunt (MRS), is an attractive treatment for EHPVO and uses an autologous internal jugular vein (IJV) graft to bypass the obstructed extrahepatic portal vein (PV) and direct blood from the superior mesenteric vein (SMV) to the intrahepatic left portal vein (LPV) within the Rex recess [2]. Recently, modified Rex shunts that use different conduits or venous inflows from those of the classic Rex shunt have been reported as well. Prosthetic and autologous grafts such as the external jugular vein and great saphenous vein (GSV) have been used as alternative grafts, and the coronary, splenic, or umbilical veins, as alternative venous inflow [3,4]. The MRS has been regarded as the most physiological method for decreasing PV pressure while restoring normal blood flow to the liver [5,6]. This report describes two cases of recurrent or uncontrolled variceal bleeding due to EHPVO, one treated with the classic MRS and the other by a modified technique using the GSV graft to connect between the coronary vein and the LPV.
CASE
1) Case 1
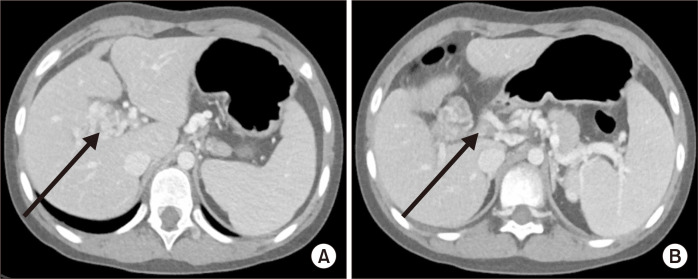
A 9-year-old girl presented with intermittent abdominal pain and melena for 10 months. She underwent an abdominal operation for choledochal cyst excision with Roux-en-Y hepatico-jejunostomy (HJ) 1 year before at another hospital. The bleeding focus of the melena could not be found even after various studies, so the patient was referred to our hospital for further evaluation and management. Review of the outside computed tomography (CT) revealed an extrahepatic PV thrombosis (PVT) that had occurred after surgery and newly formed varices around the HJ site, which caused the variceal bleeding (Fig. 1). To control the variceal bleeding and maintain the portal blood flow, MRS was planned.
Fig. 1.
Preoperative computed tomography scan showing a cavernous formation around the porta hepatis (arrow in A) and portal vein thrombosis (arrow in B).
Her liver function test results, including her coagulation profile, were normal. Owing to the continued variceal bleeding, her hemoglobin (Hb) level decreased to 6.9 g/dL, and 3 pints of red blood cell transfusions were administered for anemia correction, which increased the Hb level to 9.5 g/dL just before surgery.
Preoperative abdominal ultrasonography (USG) revealed a PVT, large cavernous transformation at the porta hepatis, and coarse increased echogenicity of the liver. Preoperative CT scans showed PVT of the main PV trunk, starting right before the division into the RPV and LPV, and extending to the confluence of the SMV and splenic veins.
Under general endotracheal anesthesia, an upper midline incision was made. The LPV at the Rex recess and the SMV at the mesenteric root were exposed. The left IJV was harvested for use as an autologous graft. Distal and proximal end-to-side anastomoses were performed to connect the IJV graft to the LPV, and the IJV graft to the SMV, respectively. A hand-held intraoperative Doppler examination revealed active flow through the graft. Then, a small incision was made on the jejunum near the HJ site, and multiple mucosal blood oozing was found. Although the active bleeding focus was not found, several suture ligations were performed at suspected oozing sites, followed by primary repair of the jejunum.
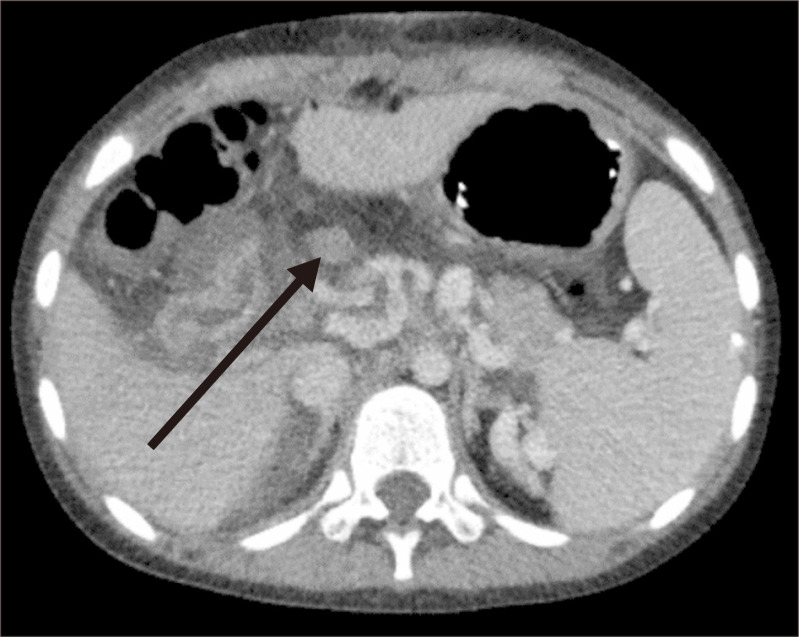
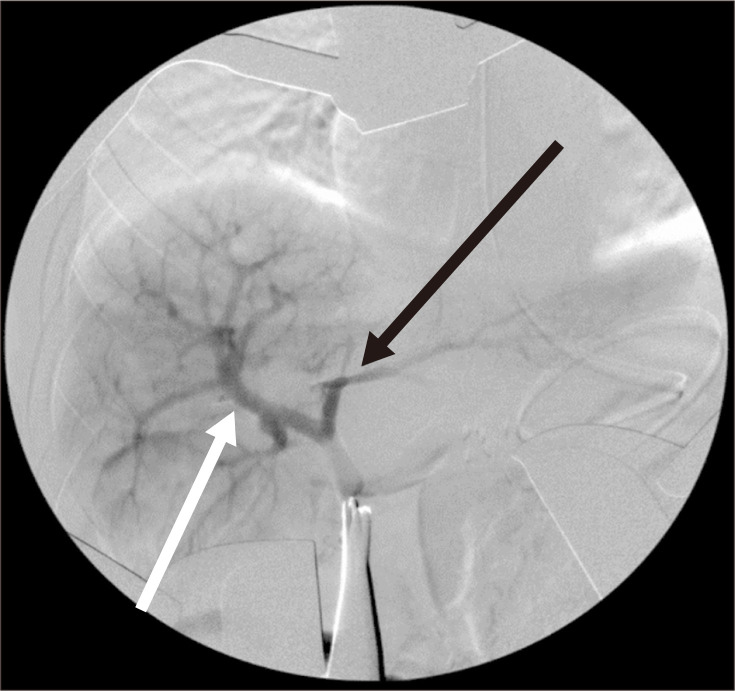
Postoperatively, conventional systemic heparinization was started to achieve an activated partial thromboplastin time between 60 and 95 seconds. However, the routine follow-up CT angiography performed on the second postoperative day revealed total occlusion of the IJV graft (Fig. 2). Emergent surgical thrombectomy was performed, revealing no kinking or twisting of the graft. Intraoperative angiography revealed that the right PV and its anterior and posterior branches were patent, but the LPV was patent only up to the main branch (Fig. 3). The conventional systemic heparinization was resumed postoperatively. Liver Doppler USG (DUS) after 3 days revealed a thrombosis of the SMV-LPV graft. After evaluating the risks and benefits, observation without further intervention or surgery was decided. When no evidence of gastrointestinal bleeding was found, the patient was discharged on the 12th postoperative day. During the 4-year follow-up, she had no complaint of abdomen pain or gastrointestinal bleeding. However, she underwent one elective endoscopic variceal ligation (EVL) procedure for prominent esophageal varices found in a routine endoscopic examination. The most recent follow-up abdominal USG revealed increased coarse liver parenchymal echogenicity that suggested liver cirrhosis and ascites production. The patient is currently receiving conservative treatment with propranolol 10 mg twice daily. Although her blood test results, including liver enzyme, bilirubin, and albumin levels, and coagulation profiles, are normal, the development of ascites and esophageal varices indicated the irreversible progression of liver cirrhosis. Thus, the patient was listed on the waiting list for deceased-donor liver transplantation.
Fig. 2.
Computed tomography scan on the second postoperative day showing a thrombosed internal jugular vein graft.
Fig. 3.
Intraoperative portal venogram showing a patent right portal vein (white arrow). However, only the main left portal vein is opacified without showing its branches (black arrow).
2) Case 2
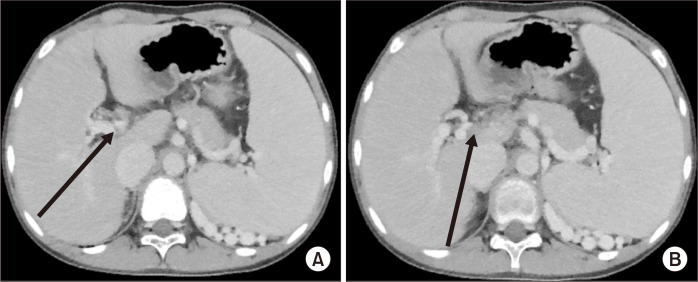
An 11-year-old boy presented with melena due to esophageal variceal bleeding. He was diagnosed as having esophageal varices 4 years earlier, and since then, he had been taking propranolol 10 mg bid (twice a day) and underwent a total of 4 EVL procedures for recurrent variceal bleeding. A CT scan showed esophageal and gastric varices with EHPVO and cavernous transformation of the PV (Fig. 4). As the varices, splenomegaly, and thrombocytopenia worsened, a meticulous workup was performed to assess whether the patient was suitable for a MRS. Preoperative USG revealed a relatively normal hepatic stiffness and echotexture without focal lesions. The PVT was confined only to the main PV trunk and was not occluding the confluence of the SMV and splenic veins, and the LPV seemed patent on the preoperative CT scan. Although the patient’s liver enzyme, bilirubin, and albumin levels were normal, his prolonged prothrombin time international normalized ratio (PT INR) was 1.46. His preoperative platelet count was 37,000/µL, and 2 pints of platelet concentrates were transfused to achieve a platelet count of 62,000/µL just before surgery. As no evidence of intrahepatic fibrosis was found and the LPV seemed patent based on the imaging findings, the patient was considered suitable for the MRS.
Fig. 4.
Preoperative computed tomography scan showing a cavernous formation around the porta hepatis (A) and portal vein thrombosis (B).
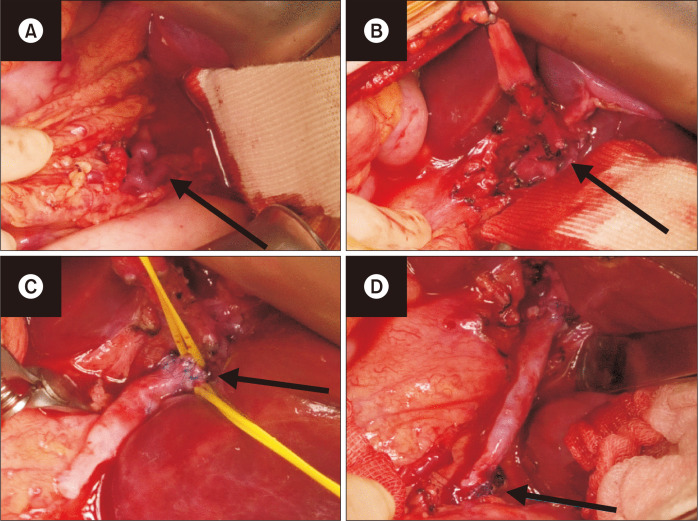
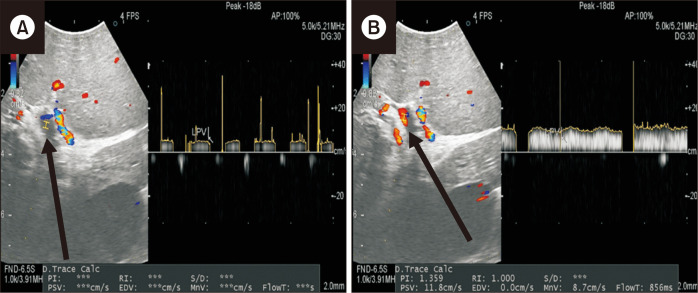
Under general anesthesia, an upper midline incision was made. After opening the lesser sac, the coronary vein was exposed, and so was the LPV at the Rex recess. The LPV branches were elastic without fibrosis. The coronary vein was chosen as the venous inflow instead of the SMV because of its easier access. Coronary vein transposition was also considered, but it was not possible because of the multiple branching of the coronary vein into small diameters. The left GSV was harvested for use as an autologous conduit because the diameters of the GSV and coronary vein were similar. A distal end-to-end anastomosis was performed to connect the GSV graft to the LPV; and a proximal end-to-side anastomosis, to connect the coronary vein from the portal side to the GSV graft (Fig. 5). The coronary vein from the stomach side was ligated. Intraoperative DUS was used to check the PV flow. Whereas no LPV flow was detected before the anastomosis, good flow was observed after the anastomosis (Fig. 6). The liver biopsy at the time of surgery revealed no portal fibrosis and minimal portal inflammation.
Fig. 5.
Intraoperative findings showing the procedure of the modified coronary vein-left portal vein (LPV) shunt. (A) Coronary vein isolation. (B) LPV isolation. (C) Distal end-to-end anastomosis of the great saphenous vein (GSV) graft to the LPV. (D) Proximal end-to-end anastomosis of the coronary vein to the GSV graft.
Fig. 6.
Intraoperative Doppler ultrasonography image showing no left portal vein flow during clamping (A) but showing active flow after the anastomosis (B).
After the surgery, 1 mg/kg enoxaparin was administered every 12 hours via subcutaneous injection until postoperative day 5 and was switched subsequently to aspirin 100 mg once daily. The routine follow-up CT on the fifth postoperative day revealed a patent MRS with visible flow in both the LPV and RPV. The patient’s postoperative course was uneventful, and he was discharged 7 days after the surgery. His coagulation profile after 2 weeks had improved with a PT INR of 1.21. The rest of his liver function test results were normal, and his platelet count had increased to 68,000/µL. The CT scan 3 months after surgery revealed a patent MRS, and the laboratory examination results, including liver function and platelet count, were all within the normal limits. Currently, the patient is doing well without any symptoms.
The characteristics of both patients are summarized in Table 1.
Table 1.
Characteristics of the patients in both cases
| Clinical characteristic | Case 1 | Case 2 |
|---|---|---|
| Patient characteristics (at surgery) | ||
| Age (y) | 9 | 11 |
| Sex | Female | Male |
| Weight (kg) | 40 | 39.9 |
| Height (cm) | 140 | 165.9 |
| BMI (kg/m2) | 20.4 | 13.4 |
| Weight percentile by age | 90th percentile | 50th percentile |
| BMI percentile by age | 85th percentile | <3rd percentile |
| Symptoms and signs | ||
| Abdominal pain | Yes | No |
| Gastrointestinal bleeding | Yes | Yes |
| Varices | Yes | Yes |
| Splenomegaly | Yes | Yes |
| Spleen size (cm) | 13.4 | 16.6 |
| Thrombocytopenia | No | Yes |
| Platelet count (/μL) | 131,000 | 62,000 |
| Cause of PVT | Choledochal cyst surgery | Idiopathic |
| Operation | ||
| Inflow | SMV | Coronary vein |
| Conduit | IJV | GSV |
| Outflow | LPV, mild sclerotic | LPV |
| Intraoperative liver biopsy | No | Yes, no cirrhosis on biopsy |
| Intraoperative DUS | No, only handheld Doppler | Yes |
| Intraoperative portography | Yes, after thrombectomy | No |
| Postoperative results | ||
| Graft patency | Thrombosed graft | Good patency |
| Clinical outcome | No variceal bleeding | No variceal bleeding |
| Worsening liver cirrhosis | Improved hypersplenism |
BMI, body mass index; PVT, portal vein thrombosis; SMV, superior mesenteric vein; IJV, internal jugular vein; GSV, great saphenous vein; LPV, left portal vein; DUS, duplex ultrasonography.
DISCUSSION
The etiology of EHPVO is diverse, but most cases are idiopathic. Risk factors include perinatal events such as umbilical catheterization, omphalitis, and perinatal sepsis, and the presence of thrombophilia resulting from prothrombin mutation or deficiency of protein C, protein S, or antithrombin III [1]. Clinically, EHPVO results in various complications such as variceal bleeding, hypersplenism, ascites, neurocognitive defects, poor somatic growth, portal biliopathy, and advanced liver disease [7]. EHPVO is diagnosed on the basis of clinical signs and symptoms of portal hypertension, and imaging findings of PV cavernous transformation and no evidence of significant parenchymal dysfunction [8]. In most cases, gastrointestinal hemorrhage is the first clinical manifestation, which is often life-threatening and needs appropriate treatment [9,10]. Nonsurgical treatments of EHPVO include prophylaxis with nonselective beta-blockers and endoscopic variceal obliteration or ligation for esophageal and gastric varices. However, recurrent bleeding episodes or uncontrolled varices despite medical and endoscopic treatments should be considered for surgical treatment [9].
Surgical procedures for EHPVO include non-shunt and shunt surgeries. Non-shunt surgeries include surgical ligation of varices, esophageal transaction and re-anastomosis, splenectomy, and splenic embolization. Shunt surgeries can be categorized as “nonphysiological,” diverting blood flow away from the liver, and “physiological,” restoring blood flow back to the liver. Portosystemic shunts (PSSs) are nonphysiological routes created to divert the portal and mesenteric blood flow into the systemic circulation, thereby resulting in reduced pressure and decompression of the portal venous system [9]. Distal spleno-renal shunts, the preferred procedure for children with portal hypertension in our center, are selective PSSs proven to be effective and reliable [10]. However, PSSs have the potential disadvantage of reduced portal liver perfusion, which may deteriorate liver function and increase the risk of hepatic encephalopathy.
The MRS, the most physiological shunt for EHPVO, was first described in 1992 by de Ville de Goyet et al. [11] and was originally designed to treat post-liver transplantation patients with PVT but have been successfully applied to nontransplant cases as well [5,11,12]. The operation consists of bypassing the area of EHPVO by creating a conduit between the mesenteric venous system and the LPV within the Rex recess, a part of the left portal system that runs sagittally within the umbilical scissure between Couinaud segments II, III, and IV. This shunt reduces portal pressure and restores the physiological portal flow toward the liver (hepatopetal) and, ultimately, can alleviate the side effects of either portal hypertension or portosystemic connections [2]. Studies have shown that it has additional metabolic benefits over PSSs by restoring normal portal venous circulation to the liver. It can reverse coagulopathy, hyperammonemia, hepatopulmonary syndrome, and encephalopathy, and improve neurocognitive ability, nutrition, and somatic growth [13-17].
The MRS is commonly considered to be most effective when all of the following three prerequisites are met: 1) no intrinsic liver disease, 2) a patent intrahepatic portal tree, and 3) an appropriate vein in the mesenteric circulation to function as a suitable venous inflow [2]. Careful preoperative assessment is important to evaluate the feasibility of the bypass, and this can be performed with imaging studies such as DUS, CT, and magnetic resonance imaging. The goal of imaging studies is to determine the extent of thrombosis in the PV and intrahepatic portal branches, and to assess the patency of the mesenteric veins and intrahepatic portal branches, especially around the Rex recess. Further assessments include evaluation of possible conduits for the MRS, together with a thorough hepatobiliary assessment [18]. In our first case, the shunt immediately failed despite of the good IJV conduit and good SMV inflow. Owing to the long course of the conduit via the retrocolic, retrogastric, and anterior routes to the previous HJ site, graft kinking or twisting was suspected at first, but surgical exploration proved that this was not the cause. The preoperative USG revealed a coarse liver parenchymal echogenicity, which may have been a sign of an existing liver disease. If an underlying liver disease or cirrhosis indeed existed and was undetected at the time, the blood inflow may have been poor to have ultimately caused the graft thrombosis. Routine examination of the intraoperative portal blood inflow may be a good decision-making factor because other options such as distal spleno-renal shunt can be good alternatives to MRS if the portal blood inflow is unsatisfactory.
The most common conduit for the MRS is the IJV, a long and large autologous vein that matches the length and diameter of the PV to create a rapid adequate flow and effectively decompress the portal system [2]. However, the operative trauma and large scar on the neck from harvesting the IJV has been a concern. Hence, alternative conduits such as the GSV, prosthetic grafts, and adjacent veins, including the splenic, coronary, and umbilical veins, have been used [3,4]. Previous studies showed that the IJV conduit had a patency of 84% to 96%, while those of the GSV and adjacent veins were approximately 71% and 67% to 80%, respectively [3,6,16,19]. However, modified Rex shunts also showed good patency, and the best conduit is still controversial [20].
After the failure of the first classic MRS, the patient in the second case was treated with a modified version, using the GSV graft to create a bypass between the coronary vein and the LPV, which resulted in great success. The critical factor in deciding the success and failure of MRS is still unknown. More factors may influence graft patency besides conduit type and venous inflow selection. Despite the reported success of the MRS throughout the years, still no consensus has been reached on the feasibility, indications, timing, and optimal technique. The MRS seems to be the generally accepted treatment for EHPVO in patients with intractable complications, but the indications are shifting toward early evaluation of the feasibility of a bypass and early surgical treatment in a more preemptive and prophylactic manner [2]. Further research on appropriate indications, adequate type of surgical shunt, and careful preoperative assessment to evaluate the feasibility of a bypass is needed to avoid unnecessary treatment and bypass failure.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: MYO, SKM. Analysis and interpretation: MYO, HKK. Data collection: MYO, HKK. Writing the article: MYO, SKM. Critical revision of the article: AH, NJY, SKM. Final approval of the article: all authors. Statistical analysis: none. Obtained funding: none. Overall responsibility: SKM.
REFERENCES
- 1.Weiss B, Shteyer E, Vivante A, Berkowitz D, Reif S, Weizman Z, et al. Etiology and long-term outcome of extrahepatic portal vein obstruction in children. World J Gastroenterol. 2010;16:4968–4972. doi: 10.3748/wjg.v16.i39.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.di Francesco F, Grimaldi C, de Ville de Goyet J. Meso-Rex bypass--a procedure to cure prehepatic portal hypertension: the insight and the inside. J Am Coll Surg. 2014;218:e23–e36. doi: 10.1016/j.jamcollsurg.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Bhat R, Lautz TB, Superina RA, Liem R. Perioperative strategies and thrombophilia in children with extrahepatic portal vein obstruction undergoing the meso-Rex bypass. J Gastrointest Surg. 2013;17:949–955. doi: 10.1007/s11605-013-2155-z. [DOI] [PubMed] [Google Scholar]
- 4.Shinkai M, Ohhama Y, Honda S, Kitagawa N, Mochizuki K, Take H, et al. Recanalized umbilical vein as a conduit for mesenterico/porto-Rex bypass for patients with extrahepatic portal vein obstruction. Pediatr Surg Int. 2011;27:315–319. doi: 10.1007/s00383-010-2742-y. [DOI] [PubMed] [Google Scholar]
- 5.Bambini DA, Superina R, Almond PS, Whitington PF, Alonso E. Experience with the Rex shunt (mesenterico-left portal bypass) in children with extrahepatic portal hypertension. J Pediatr Surg. 2000;35:13–18. discussion 18–19. doi: 10.1016/S0022-3468(00)80005-6. [DOI] [PubMed] [Google Scholar]
- 6.Sharif K, McKiernan P, de Ville de Goyet J. Mesoportal bypass for extrahepatic portal vein obstruction in children: close to a cure for most! J Pediatr Surg. 2010;45:272–276. doi: 10.1016/j.jpedsurg.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Superina R, Shneider B, Emre S, Sarin S, de Ville de Goyet J. Surgical guidelines for the management of extra-hepatic portal vein obstruction. Pediatr Transplant. 2006;10:908–913. doi: 10.1111/j.1399-3046.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, Sarin SK. Non-cirrhotic portal hypertension- diagnosis and management. J Hepatol. 2014;60:421–441. doi: 10.1016/j.jhep.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Alberti D, Colusso M, Cheli M, Ravelli P, Indriolo A, Signorelli S, et al. Results of a stepwise approach to extrahepatic portal vein obstruction in children. J Pediatr Gastroenterol Nutr. 2013;57:619–626. doi: 10.1097/MPG.0b013e31829fad46. [DOI] [PubMed] [Google Scholar]
- 10.Moon SB, Jung SE, Ha JW, Park KW, Seo JK, Kim WK. The usefulness of distal splenorenal shunt in children with portal hypertension for the treatment of severe thrombocytopenia and leukopenia. World J Surg. 2008;32:483–487. doi: 10.1007/s00268-007-9356-0. [DOI] [PubMed] [Google Scholar]
- 11.de Ville de Goyet J, Clapuyt P, Otte JB. Extrahilar mesenterico-left portal shunt to relieve extrahepatic portal hypertension after partial liver transplant. Transplantation. 1992;53:231–232. [PubMed] [Google Scholar]
- 12.de Ville de Goyet J, Alberti D, Clapuyt P, Falchetti D, Rigamonti V, Bax NM, et al. Direct bypassing of extrahepatic portal venous obstruction in children: a new technique for combined hepatic portal revascularization and treatment of extrahepatic portal hypertension. J Pediatr Surg. 1998;33:597–601. doi: 10.1016/S0022-3468(98)90324-4. [DOI] [PubMed] [Google Scholar]
- 13.Lautz TB, Keys LA, Melvin JC, Ito J, Superina RA. Advantages of the meso-Rex bypass compared with portosystemic shunts in the management of extrahepatic portal vein obstruction in children. J Am Coll Surg. 2013;216:83–89. doi: 10.1016/j.jamcollsurg.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Mack CL, Zelko FA, Lokar J, Superina R, Alonso EM, Blei AT, et al. Surgically restoring portal blood flow to the liver in children with primary extrahepatic portal vein thrombosis improves fluid neurocognitive ability. Pediatrics. 2006;117:e405–e412. doi: 10.1542/peds.2005-1177. [DOI] [PubMed] [Google Scholar]
- 15.Chiu B, Superina RA. Encephalopathy caused by a splenorenal shunt can be reversed by performing a mesenteric-to-left portal vein bypass. J Pediatr Surg. 2006;41:1177–1179. doi: 10.1016/j.jpedsurg.2006.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Stringer MD. Improved body mass index after mesenterico-portal bypass. Pediatr Surg Int. 2007;23:539–543. doi: 10.1007/s00383-007-1920-z. [DOI] [PubMed] [Google Scholar]
- 17.Lautz TB, Sundaram SS, Whitington PF, Keys L, Superina RA. Growth impairment in children with extrahepatic portal vein obstruction is improved by mesenterico-left portal vein bypass. J Pediatr Surg. 2009;44:2067–2070. doi: 10.1016/j.jpedsurg.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Carollo V, Marrone G, Cortis K, Mamone G, Caruso S, Milazzo M, et al. Multimodality imaging of the Meso-Rex bypass. Abdom Radiol (NY) 2019;44:1379–1394. doi: 10.1007/s00261-018-1836-1. [DOI] [PubMed] [Google Scholar]
- 19.Luoto T, Pakarinen M, Mattila I, Rintala R. Mesoportal bypass using a constructed saphenous vein graft for extrahepatic portal vein obstruction--technique, feasibility, and outcomes. J Pediatr Surg. 2012;47:688–693. doi: 10.1016/j.jpedsurg.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JS, Li L, Cheng W. The optimal procedure of modified Rex shunt for the treatment of extrahepatic portal hypertension in children. J Vasc Surg Venous Lymphat Disord. 2017;5:805–809. doi: 10.1016/j.jvsv.2017.02.011. [DOI] [PubMed] [Google Scholar]