Background
In December 2019, the novel COVID-19 virus spread from a cluster of pneumonia cases in Wuhan, China, to every corner of the globe, creating a worldwide pandemic pushing hospital systems past capacity and bringing economies worldwide to a halt. The COVID-19 pandemic is unique in comparison to prior coronavirus epidemics in its superior ability to be spread by asymptomatic and presymptomatic patients, allowing the virus to silently evade traditional symptoms-based screening approaches. Countries have implemented cutting-edge digital solutions to enhance traditional contact-tracing methodologies in combination with novel testing strategies to combat the virus, with variable levels of success. Despite having one of the most advanced and expensive health care systems in the world, the United States (U.S.) response is arguably one of the world’s largest failures, as it leads the globe in case number as well as deaths. Until a successful vaccine can be broadly distributed, it is imperative that the U.S. curb the viral spread by rapidly developing a framework implementing both enhanced tracing and testing strategies balancing the needs of public health while respecting individual liberties. This review will explore the role of technology-augmented contact-based surveillance in tracking the outbreak in select countries in comparison to the current U.S. approach. It will evaluate barriers in the U.S. to implementing similar technologies, focusing on privacy concerns and a lack of unified testing and tracing strategy. Finally, it will explore strategies for rapidly scaling testing in a cost-effective manner.
Keywords: COVID-19, COVID-19 testing, Infection surveillance, Infection prevention, Public health, Contact tracing, Digital health
Key Findings.
-
•
A digitally enabled testing and contact-tracing system combined with mask wearing, proper sanitation, and social distancing can be utilized successfully to help control COVID-19 transmission.
-
•
A successful testing strategy will rely on a combination of low-cost screening tests with at least moderate accuracy as well as focused, highly accurate diagnostic tests and novel at-scale community testing techniques, for example sewage testing, for early identification of disease presence.
-
•
The United States falls behind other countries that have used technology to augment traditional testing and contact-tracing strategies in the COVID-19 response as a result of privacy concerns and the absence of a coordinated national strategy.
Introduction
In late December 2019, a cluster of pneumonia cases emerged in Wuhan, Hubei, China, of unknown etiology.1 On January 7, 2020, the World Health Organization (WHO) identified the novel coronavirus SARS-CoV-2, or COVID-19, as the cause of this outbreak.2 By the end of the month, they declared the Chinese outbreak of COVID-19 a Public Health Emergency of International Concern, as the new virus infected individuals across the globe.3 In the first 3 months alone, nearly 1 million people were infected and 50,000 died. Hospital systems were pushed past capacity and health care providers faced shortages in essential supplies such as ventilators and even personal protective equipment. Entire economies were brought to a halt as large segments of the world’s population were ordered to shelter in place. Global trading routes were compromised as sovereign borders were shut, with 3 billion people now living in countries enforcing complete border closures.4 By the middle of September, 30 million cases and 950,000 deaths had been reported in the world.5
COVID-19 is a member of the coronavirus family, which are single-stranded RNA viruses that can cross species barriers and cause illnesses ranging from the common cold to respiratory failure and septic shock. In comparison to prior coronavirus epidemics, such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), where patients typically shed the virus when symptomatic, COVID-19 patients can be contagious without displaying symptoms, accelerating its transmissibility. Over 50% of infections can be attributed to asymptomatic and presymptomatic spread.6 Furthermore, the infectiousness level of asymptomatic people is estimated at 75% relative to those who are symptomatic.7 The mortality rate of COVID-19 is much lower than MERS and it requires hospitalization less frequently, increasing its spread throughout the community, leading to a global pandemic and a larger overall morbidity and mortality than seen in prior coronavirus outbreaks. The estimated basic reproduction number of COVID-19 is 2.5,7 whereas that of SARS is 1.7–1.9 and MERS is <1.8 Successful containment of COVID-19 will rely on a combination of proper hygiene, mask utilization, and, importantly, the capability to rapidly identify individuals at risk for COVID-19 through robust contact tracing and adequate, timely testing. Traditional screening methods that evaluate only for symptoms are thus inadequate, necessitating a novel approach to track the spread of this virus. New methods should include identifying COVID-19 patients without symptoms, as noted in revised Centers for Disease Control (CDC) and WHO guidelines.9,10 This review will evaluate the role of rapid technology-augmented contact-based surveillance in identification of exposed individuals. It will discuss the strategies and technologies used in select countries to successfully combat COVID-19 and compare this to the current U.S. approach. Finally, it will describe different types of viral testing and explore strategies for rapidly scaling testing in a cost-effective manner.
Digital augmentation of traditional contact-tracing methodologies
Strategies proposed to limit the spread of COVID-19 include early detection, isolation, prompt treatment, and the implementation of a robust system to trace contacts. Contact tracing has been instrumental in decades of response to disease outbreaks. The eradication of smallpox was not achieved by universal immunization, but rather by exhaustive contact tracing to find all those infected.11 Contact tracing was used to quell Ebola and is still performed today in the U.S. to manage outbreaks of tuberculosis.12,13 Public health officials manually contact identified cases both to provide education and support, such as directions for self-isolation and food services, and to further identify at-risk contacts, who are then referred for appropriate testing and quarantine. However, the silent transmission and sheer number of COVID-19 cases has overwhelmed the existing infrastructure of contact tracing across the world, leading countries to look to technology solutions to augment traditional contact-tracing infrastructure.
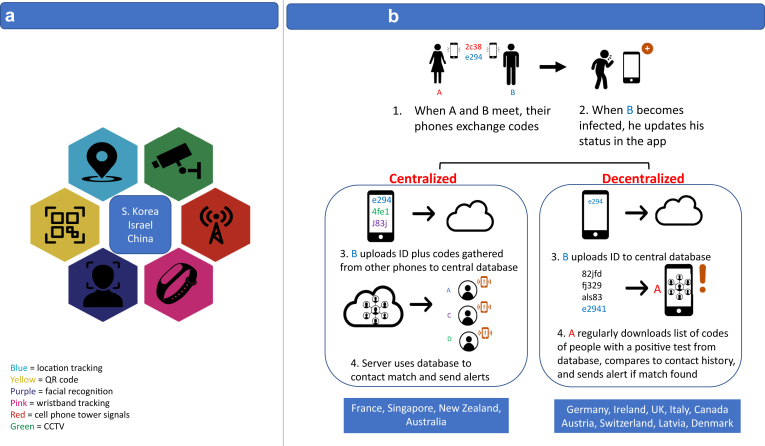
From real-time mass monitoring to smartphone-based contact-tracing apps, technology can expedite and streamline the work of contact-tracing teams. Automating the process of retracing a person’s movements and finding people they may have infected ensures earlier notification for isolation at scale. Reducing delays in contact tracing is imperative for its effectiveness; however, contact tracing is inherently invasive and what is gained in tracking capability through advancements in technologies can come at a loss of privacy. Mass surveillance utilizes technology, such as camera surveillance, tracking bands, and geolocation data collected from cellphones, to provide contact tracers continuous monitoring capabilities at a scale and finite level never before possible (Figure 1a); however, privacy regulations in countries such as the U.S. do not permit systematic mass surveillance. Instead, 2 models of voluntary smartphone-based apps with varying data-sharing capabilities have been proposed to augment traditional contact-tracing strategies: centralized and decentralized (Figure 1b). In a centralized design, the user’s phone’s anonymous ID code plus those gathered from other phones are deposited in a central server where all contact tracing, analysis, and alerts are generated. The database is operated by the government and protected by cybersecurity measures. In the decentralized architecture, only the individual user’s ID is sent to a centralized database, and the phone pulls the data down and performs all contact matching and risk analysis locally. Proponents of the centralized approach argue this method enables health officials to view networks of contacts and better identify superspreaders and hot spots. Privacy advocates argue this method is at risk for data breaches and potential overreach, as the data enable the future possibility of state surveillance.14,15
Figure 1.
Digital contact-tracing strategies. a: Mandatory Centralized Mass Surveillance: South Korea, Israel, and China have employed mandatory centralized mass surveillance techniques to contact trace. If an individual is infected, officials use location data to map where he visited and evaluate who he may have exposed before being diagnosed. Location data can be obtained by CCTV footage, facial recognition cameras, location-tracking bracelets, and cell phone tower signals. QR codes can also be used to track one’s location, as people must scan them on their smartphones to enter public areas. Citizens obtain a code by entering personal details and possible history of COVID-19 symptoms or exposure. They are assigned different color codes (green, yellow, red) based on their likelihood of infection. Green code holders are allowed to travel, yellow code holders cannot travel, and red code holders must quarantine.119b: Voluntary Contact-Tracing Applications: Apps can be divided into 2 categories based on whether data are collected in a centralized or decentralized manner. When 2 people come into contact, their phones exchange anonymized key codes. When B becomes infected, he updates his status in the app. In a centralized approach, B uploads both his ID code and all the codes of people he has been in contact with to a central server. The central server, usually run by a public health authority, uses the codes to do contact matching and sends alerts to those exposed to B. In a decentralized approach, B only uploads his ID to the central server and keeps his contact history on his phone. All other phones regularly download codes from the cloud that are uploaded from positive cases. Each phone then compares the downloaded codes with its contact history and if there is a match, the phone alerts the user of exposure. Countries like France, Singapore, New Zealand, and Australia have centralized apps. Germany, Ireland, the U.K., Italy, Canada, Austria, Switzerland, Latvia, and Denmark have decentralized apps.
Smartphone-based contact-tracing apps, in both centralized and decentralized models, utilize different tracking methods with varying levels of data privacy. These methods can be done via personal smartphones or wearable devices such as wristbands.16,17 GPS and Wi-Fi track the location of mobile phones, following the exact movements of infected individuals, which additionally aids in identification of COVD-19 hot spots. Bluetooth technology, on the other hand, logs each encounter a person makes and does not track location, providing added security and privacy and making it an ideal choice for the decentralized model. When phones come into contact, each phone generates a random numerical ID that it broadcasts to nearby phones. Bluetooth and GPS can incorrectly register interactions between people in different rooms when signals pass through walls or ceilings. Ultrasound technology may offer better proximity detection by utilizing soundwave technology to augment Bluetooth.18 This technique also preserves anonymity; however, for both Bluetooth and ultrasound technology, the app must remain open to work, significantly limiting utility.19 Experts predict that future devices will use ultra-wideband radio signals,20 Wi-Fi RTT (Round Trip Time) ranging signals to Wi-Fi access points,21 Bluetooth v5.0 with an angle-of-arrival direction-finding feature,22 and fingerprints of sensor measurements to enhance proximity detection and distinguish if 2 devices are in the same airspace or separated by a pane of glass or wall.23
Third-party smartphone-based mobile apps that leverage Bluetooth technology for contact tracing have experienced technical difficulties on Apple devices, most specifically battery drain, greatly decreasing their utility and popularity. Singapore developed 1 of the first Bluetooth-based apps (TraceTogether), which, while initially gathering worldwide excitement, only gained 17% use by the population after 1 month. Such low uptake was secondary to both the requirement for the app to be constantly running in the foreground on iPhones, causing significant battery drain, and public backlash over privacy concerns.24 In May 2020, Apple and Google released an exposure notification application-programming interface (API) to better enable Bluetooth.25 This toolkit implements cryptographic functions to generate and process the pseudonyms directly into operating systems, preserving battery by allowing apps to collect data in the background.14 To provide extra security, the toolkit prohibits third-party apps from collecting location data, and it only works with decentralized apps that store data on phones, rather than on central servers. Unlike other developer frameworks, this API was only available for use by public health authorities at first; however, in September 2020, Apple released iOS 13.7 to allow users to opt in to the Exposure Notification system without having to download an official public health app.26 This update still requires users to live in an area where such an app exists. While decentralized apps remedy concerns about users’ social networks being vulnerable to hacking or exploitation, they can hinder efficient contact tracing. By keeping contact networks anonymous, health officials will not know if the right people are getting notifications or if they have the resources they need to self-quarantine. These restrictions have led some developers to continue to make separate centralized apps in order to better enable health departments to track the spread of the virus. Additionally, large segments of the private sector are individually developing workplace-, school-, and consumer-focused apps. This has resulted in a fragmented system of unconnected applications deploying different technologies, making it near impossible to achieve the level of adoption of 60% suggested to be required27 to make such technology successful.25
Global examples of digital contact tracing
Globally, countries have employed a variety of methods for contact tracing to assure that individuals exposed or infected are complying with quarantine recommendations. Cultural norms are a large determinant of population compliance and willingness to be tracked. In addition, many governmental emergency measures to track populations, especially with smartphone contact tracing, have subsequently been challenged in court systems. As the pandemic persists, many populations are less willing to accept blanket government surveillance.28 Sharing outbreak data has created public discrimination and prevented people from getting tested in South Korea,29 and Israelis have started using cellphone holsters to block location tracking.30 With a small, homogenous population and relatively isolated location, Iceland has reduced COVID-19 transmission with a combination of traditional contract tracing and use of digital technologies. Despite starting off with one of the highest infection rates in Europe, its screening and contact-tracing system was so efficient that it virtually eliminated COVID-19.31 Iceland augmented the traditional contact-tracing methodology with a mobile tracking app, Ranking C-19, to more efficiently track COVID-19 transmission over time.32
Early in the pandemic, South Korea, Israel, and China deployed mass surveillance contact-tracing systems to identify people potentially exposed to the virus and successfully suppress viral spread (Figure 1a). In South Korea, exposure to a prior epidemic served to educate and prime the population for a high level of government surveillance. Following the MERS pandemic in 2015, the government was criticized for its lack of transparency in communicating information and responded by sending emergency phone alerts with intimate details of new cases and their movements to people in proximity. This system was accepted in response to COVID-19, with citizens familiar with and willing to wear masks, cooperate with contact tracers, and accept that the tradeoff was privacy. The population also accepts that those who violate quarantine will be punished with a location-tracking bracelet and possibly incarceration. Epidemiological intelligence officers monitor data from GPS, CCTV footage, credit card transaction data, and travel information to assure that those with infections and those under ordered quarantine comply.33, 34, 35 Israel’s Security Agency (The ISA) was initially granted wide permissions to share the name, ID number, cellphone number, internet browsing history, and every voice call and text message of confirmed COVID-19 patients with the Health Ministry in order to identify who came in close contact and to enforce quarantine.36,37 Though this was very successful in controlling viral spread, concerns developed related to privacy and improper identification of infected individuals. The program was suspended and then reauthorized as the spread of COVID-19 increased, but with more constraints related to sharing of data, types of data identified, and duration of data collection and storage.38 Additionally, the smartphone-based program has limited efficacy in the ultra-Orthodox community, owing to the low penetration of smartphones. The government has employed wearable devices for contact tracing in this populous community, who often congregate in large gatherings.36,37 China has adopted a variety of policies and tools to contain COVID-19, with very little consideration for individual privacy rights. China mandated the use of a mobile smartphone application, Health Code, which generates a rating indicating the likelihood of an individual’s being infected or exposed to the virus and grants permission as to whether they can walk around freely.39 Additionally, there are hundreds of millions of cameras installed in China’s physical infrastructure equipped with facial recognition, enabling contact tracing and identification of quarantine violations. Although heavy-handed and associated with severe penalties for violation, China’s surveillance program, combined with frequent mass testing, has been effective in preventing a second wave of infections.
In contrast to China, Israel, and South Korea, Europe has some of the most protective privacy laws concerning personal digital data, specifically the European Union General Data Protection Regulation (GDPR). European countries have taken the lead in developing contact-tracing apps with privacy in mind and have thus deployed either centralized or decentralized voluntary tracing applications to augment increased testing and manual contact-tracing capabilities (Figure 2b). Germany and Ireland pursued decentralized models incorporating Apple and Google’s privacy-focused, Bluetooth API early on, with strong adoption. Germany’s “Corona-Warn-App” had 6.5 million downloads within 24 hours of launch.40 Ireland’s app, COVID Tracker, reached roughly 37% of the country’s adult population in 1 week and has started to work with other countries and U.S. states to retool the app for themselves.41 Italy, Denmark, Latvia, Austria, and Switzerland have similarly also released decentralized apps. Norway developed a centralized model that relied on smartphone-based GPS tracking data in addition to Bluetooth proximity data, but it was suspended as an inappropriate encroachment on privacy, according to a ban notification from the Norwegian Data Protection Authority.28 Similarly, the United Kingdom (U.K.) government initially released a centralized contact-tracing mobile app but later switched to a decentralized model to address privacy and battery concerns. France remains the only major holdout in Europe to favor a centralized app.42 Its centralized app has been limited by user uptake, as it continues to experience battery drain, collects more personal information than advertised, and is not interoperable with neighbors’ decentralized apps.43,44 As of July 14, 2020, it had only been downloaded by 3% of the population.45 The perfect balance of effective technology-based contact tracing and protection of individual privacy remains a challenge.
Figure 2.
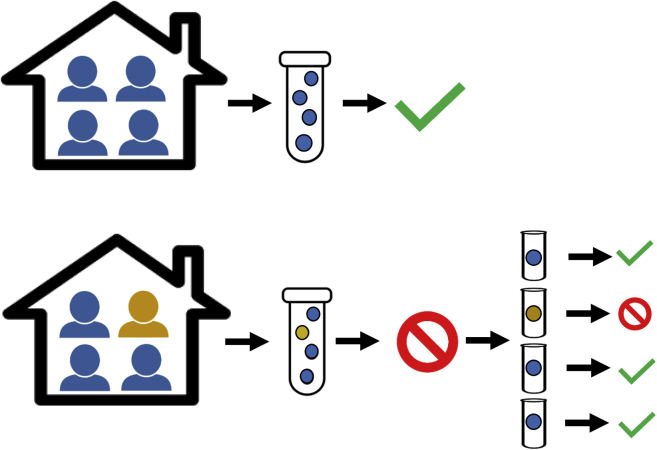
Pooled testing: Sample pooling allows multiple people to be tested at once. Swab samples are collected from each individual. Half of each sample is combined in a pool with the others, and half is set aside in case a retest is needed to confirm positivity. If the pool is negative, then all samples in that pool are cleared as negative. If the pool is positive, it means that 1 or more of the individuals in that pool are positive, so each sample is tested again individually.92
State of contact tracing in the U.S. and barriers to implementation of digital contact-tracing techniques
Basic infection control measures to COVID-19 in the U.S., such as social isolation and mask wearing, have become politicized and face significant resistance from subsets of the population. Thus, it is not surprising that there have also been significant problems associated with effective contact-tracing solutions.46 Tracing efforts lag in the U.S., where COVID-19 cases hit record highs in the middle of September 2020, leading the world with over 6.5 million infections and 200,000 deaths.47 The country has no national strategy for contact tracing, requiring states to take their own approaches. Experts estimate that, using traditional public health nondigital contact tracing, 100,000–300,000 contact tracers are needed for adequate tracing based on reported exposures of people testing positive for COVID-19 in the U.S.48; yet only 41,000 tracers had been hired by the end of July.49 Though states are rushing to expand contact-tracing capabilities, most are woefully behind in both contact-tracing and testing capabilities in comparison to European counterparts.49, 50, 51, 52
In the absence of unified U.S. federal strategy, a number of states have turned to contact-tracing apps to supplement deficiencies in traditional contact-tracing capabilities. Virginia was the first to launch COVIDWISE on the Apple-Google API, followed by 8 more states: Alabama, Arizona, Delaware, Nevada, North Carolina, North Dakota, Pennsylvania, and Wyoming.53,54 In total, at least 20 states are developing apps to cover almost half the U.S. population.55 In preparation, the Association of Public Health Laboratories partnered with Apple, Google, and Microsoft to build a cloud-based national server that can securely store COVID-19 Exposure Notification data.56 Instead of storing user contact-tracing keys on multiple, unlinked servers run by state agencies, it utilizes a national server to eliminate duplication and enable notifications across state borders. However, several states have released contact-tracing apps without the Exposure Notification framework, like Utah and South Dakota.27 Many states refuse to develop a contact-tracing app at this time, given privacy concerns and the need to further develop manual contact-tracing and testing capabilities. These gaps at the national and state level have led counties, cities, and even private corporations such as Uber and university systems to consider developing their own contact-tracing applications or strategies (ie, smart bands) to aid return to work and school; unfortunately, this will only serve to further fragment the system and inhibit the Department of Health’s ability to combat COVID-19.
To supplement smartphone-based contact tracing, several private companies have repurposed their existing products and digital platforms and data to help track COVID-19 transmission. Palantir, a data analytics company, released HHS Protect Now to pull data from across the federal, state, and local governments, healthcare facilities, and colleges to track spread and predict the required health response.57 However, a number of lawmakers are concerned Palantir is not well positioned to ensure privacy of sensitive medical records, given its work with national security agencies and immigration offices.58 To determine social distancing, Uncast, a location-based data broker, is using GPS data from smartphone apps, and Facebook is sharing movement data with infectious disease researchers. Clearview AI has pitched to use its facial recognition services on surveillance cameras. Uber is working to provide driver and user data to public health officials to track cases.59 While consumers may consent to existing data-collection protocol from these companies, they should be aware of possible data sharing and know precisely what their data will be repurposed for. These technologies eerily mirror techniques deployed by mass surveillance states and are concerning for the rise of surveillance capitalism in the absence of a strong, privacy-focused, central government solution.
The U.S. public is significantly wary of loss of privacy and civil liberties secondary to the adoption of digital contact tracing–aiding technologies, despite the possible public health gains. In a June 2020 survey from Avira, 71% of Americans did not plan to download a contact-tracing application, citing privacy as a primary concern,60 and there is an ideological opposition by some to any form of tracing.61 Much of this stems from concern about data safety and long-term use, as well as the possibility to use such data to gain insight into the individual’s daily activities. Furthermore, immigrant populations and communities of color, who are particularly at risk of adverse outcomes from COVID-19, are rightfully wary of discrimination based on personal data tracking. Additionally, vulnerable under-resourced populations and older individuals are the least likely to have access to smartphones, leading to an exacerbation of health disparities.62,63 The fragmented implementation of contact-tracing apps, a public wary over privacy concerns, and continued shortages of both testing and manual contact-tracing capabilities call to question whether the U.S. will successfully be able to employ the technology produced by its own technology giants.
COVID-19 testing strategies
Contact tracing alone is insufficient to control COVID-19 transmission without complementary large-scale testing to identify COVID-19 carriers. The availability of widespread testing has been a key component in successfully suppressing the disease in countries across the world, such as South Korea and China. Currently, tests are prioritized for those with symptoms or those who have been in contact with COVID-19-positive patients, given a limited availability of tests. The CDC estimates that the U.S. is only identifying and quarantining as little as 10% of cases.64 The current number of daily tests in the U.S. is at only 52% of the level considered necessary to mitigate the spread of the virus.65 The demand for testing is so high that it can take up to 7 days for most commercial labs to get results to patients. Even if all contacts are successfully traced, a delay of 3 days or more between symptom onset and testing will not reduce further spread of the virus, making improvements in contact-tracing capabilities unserviceable.66
Without a vaccine, the frequency of testing is the most powerful variable that businesses and universities can control to return to normal operations. The National Basketball Association (NBA) has been a model of success for return to play, currently testing players, including asymptomatic individuals, every day and relying on rapid return of results in less than 24 hours.67 However, this has come at a significant cost and will require an estimated 17,000 tests by October 13, 2020, for players alone.68 Tyson Foods has proposed weekly scheduled testing of all employees, and other private corporations are beginning to evaluate frequent testing strategies to enable return to work.69,70 Experts have modeled testing strategies to reopen universities and have found, similar to the NBA, that frequent testing of every individual every 2 days could allow schools to safely be reopened.71 However, testing every other day and even weekly would require testing at a mass scale that would be both cost prohibitive to the general population and technically not feasible without significant investment and expansion of current testing infrastructure.
Current diagnosis of active COVID-19 infection relies on tests that detect either viral RNA or viral antigens. The reverse transcription polymerase chain reaction (RT-PCR) is the gold-standard COVID-19 test that is highly sensitive and specific but requires expensive equipment, certified laboratories, and hours for results. Rapid point-of-care PCR-based and low-complexity molecular tests have since been created that deliver results in as little as 5–15 minutes.72,73 Antigen testing detects viral proteins with rapid results (20 minutes)74 but is less accurate.75, 76, 77 Antibody tests are also being developed by numerous companies, but since antibody responses do not form until up to 3 weeks after infection,78 their primary use is to document previous exposure and/or immunity to the virus (Table 1).
Table 1.
COVID-19 testing strategies74
| Test | Target | Use | Speed | Sensitivity | Specificity | PPV | NPV | Manufacturer(s)† |
|---|---|---|---|---|---|---|---|---|
| RT-PCR | Viral RNA | Active virus | 2–12 hours | Gold standard (100%) | Gold standard (100%) | 100% | 100% | Roche, Abbott, and LabCorp120, 121, 122 |
| Isothermal nucleic acid amplification test | Viral RNA | Active virus | 5–15 minutes | >94.7% | >98.6% | >78.1% | >99.7% | Abbott123,124 |
| Viral antigen test | Antigen | Active virus | 15 minutes | 80% | 100% | 100%‡ | 99%‡ | Quidel125 |
| Lateral flow assay | Antigen | Active virus | 15 minutes | 97.1% | 98.5% | 77.3% | 99.8% | Abbott126 |
| Lateral flow assay | Total binding antibodies | Immunity | <1 hour | 93.8-100% | 96%–98.8% | 55.2%–80.8% | 99.7%–100% | Cellex and Assure127,128 |
| Chemiluminescence immunoassay | Total binding antibodies | Immunity | 30 minutes | 97.6%–100% | 99% | 84%–88% | 100% | Abbott and DiaSorin129,130 |
| Enzyme-linked immunoabsorbent assay | Total binding antibodies | Immunity | <1 hour | 92.5% | 99%–100% | 100% | 99.6% | Mount Sinai and InBios131,132 |
| Surrogate virus neutralization test | Neutralizing antibodies | Immunity | 1 hour | 95%–100% | 100% | 100%‡ | 99.9%‡ | GenScript Biotech133 |
NPV = negative predictive value; PPV = positive predictive value; RT-PCR = reverse transcription polymerase chain reaction.
Not an exhaustive list.
Assuming 5% prevalence.
The distribution and turnaround time of widespread testing may be impeded by the emphasis on perfect sensitivity, requiring complex equipment that renders tests more expensive, less accessible, and slower. By putting a premium on the perfect accuracy of tests, the U.S. fails to identify a majority of cases and also introduces delays that undermine its ability to quarantine individuals in a timely manner and thus reduce spread. Although some may deem it irresponsible to use a test that might miss positive cases, the high cost and slow analysis from the gold-standard PCR test may undermine its high sensitivity to use. Increasing the frequency of testing would improve accuracy while less expensive equipment would expand its distribution to identify more cases, thus accelerating the speed of results required to prompt individuals to quarantine without delay. Experts propose a strategy of increasing the production and distribution of RT-PCR testing, implementing screening protocols for the public as well as workplaces, and supporting the development of rapid, easy-to-use, affordable testing screening tools.79 In the absence of a coordinated federal response, 7 states have banded together to combine purchasing power to fund the production of over 500,000 rapid antigen tests.80 Researchers have proposed the idea of a $1 test that could be mass-produced and provided freely to everyone to enable at-home daily testing.81 However, the over-the-counter tests that currently exist do not meet the sensitivity standards of the U.S. Food and Drug Administration (FDA); and, without supervision, cases may not be reported or contact traced. The newest antigen test, BinaxNOW, is administered by a health professional and has the capability to scale up testing without significantly compromising accuracy, as it quickly delivers results in 15 minutes, costs $5, and has 97.1% sensitivity and 98.5% specificity.82 The Trump Administration has purchased 150 million of these tests from Abbott, to be deployed to schools and other special needs populations.83 With an increased supply of screening tests, PCR tests with higher sensitivity can be preserved for high-risk settings such as nursing homes, where missing the detection of an infected person may lead to serious consequences. Since BinaxNOW is only authorized to test people with COVID-19 symptoms, pooled testing may present an opportunity to test more people with fewer tests, thus reducing labs’ backlog and allowing results to be returned more quickly.
Pooled testing
Pooled testing has been used since World War II to test soldiers for syphilis, to screen for chlamydia and gonorrhea, and to test donated blood for HIV, Zika virus, and hepatitis B and C.84 It has been deployed successfully in other countries to rapidly scale testing capabilities for COVID-19.85,86 Pooled testing is a cost-effective approach to test small groups, or pools, of people using only 1 test (Figure 2). Rather than testing 1 person at a time, samples from multiple individuals are mixed together. If the test comes back negative, everyone in the pool is cleared. If positive, each member of the pool is retested individually using their original sample, which can be done quickly in an automated manner. The need for retests may even be eliminated, as a research group in Israel recently developed a single-step method effective in groups of 48 saliva samples, refined by utilizing each individual’s sample in multiple pools.87 Although experts worry that pooling will dilute the samples and reduce the test sensitivity, studies have shown that pool sizes of 8 samples are as accurate as individual testing.88 Even if there is sensitivity loss on a single test, it will be counteracted by an overall increase in sensitivity from more frequent testing. Others are concerned that when infection prevalence is too high, pooled testing will be inefficient and require too many retests; but even at a prevalence of 10%, cost-savings can reach up to 40%.89 Machine learning can also improve efficiency at high prevalence rates by identifying who is likely to test positive and keeping them out of large pools.90 Dr Deborah Birx, the White House coronavirus response coordinator, claimed that pooled testing could allow for people to return to schools and work with the ability to test on a frequent basis.91 In July 2020, the FDA issued an emergency use authorization to Quest Diagnostics to authorize its RT-PCR test for use with pooled samples containing up to 4 specimens.92 Recent data show the second-highest source of infection after nursing homes is multigenerational households, which questions how effective such a small pool size will be in terms of both cost-savings and increasing capacity.93 To prepare for college classes resuming in the fall, the governor of New York granted The State University of New York approval to undertake pooled surveillance testing of groups of 10–25 people using saliva.94
Novel testing techniques
Alternative techniques for cost-efficient widespread testing are being widely explored. In Barcelona, Spain, virologists found traces of coronavirus in wastewater collected in March 2019, 9 months before the disease was identified in China.95 Because there are large quantities of coronavirus in stool that reaches sewage, water-based epidemiology presents a potential tool for early detection of viral circulation in a community, which can then serve as a signal to implement testing at the individual level. Such monitoring could prove beneficial with large numbers of those infected not displaying clinical signs and living in close proximity, as in college dorms. The University of Arizona possibly prevented an outbreak on campus by quickly testing a dorm with a positive wastewater sample and identifying 2 asymptomatic students who tested positive.96 In Israel, a start-up company developed a nanoscale, sensor-based smelling system that can identify a coronavirus patient via nasal exhalation.97 If successful, it could be used for mass 30-second screening of people at stores, hospitals, airports, and border entries. In Chile, Germany, and the U.K., police dogs are being trained to sniff volatile organic compounds released from humans with COVID-19.98,99 A dog can sniff 250 people in an hour, which could aid in reopening stadiums, schools, businesses, and restaurants. While daily testing is infeasible owing to cost and supply shortage, strategies like pooled testing, sentinel surveillance of sewage, NanoScent technology, and “bio-detection” dogs could expand its scale. Testing more frequently can lower the prevalence rate by containing infections, thereby requiring fewer tests and reducing costs in the long run. Regular testing also allows companies to use cheaper tests with lower sensitivity, as an individual patient is unlikely to have multiple false-negatives in a row.100 However, even with rigorous testing strategies, it is imperative that people comply with mask wearing, social distancing, sanitation, and contact tracing.101
Summary
The U.S. and the world have invested significant amounts of capital into vaccine development, and researchers are on their way to producing a vaccine at record speed. However, it is unlikely that the vaccine will be the panacea that the U.S. is hoping for, as, in reality, it will take time to vaccinate the entire population and vaccine hesitancy may hinder the vaccine’s ability to gain sufficient levels of protection for herd immunity.102 In the meantime, Americans are banned from travel to all but a few countries in the world, as the country accounts for over a fifth of the world’s confirmed COVID-19 cases, despite making up just 4% of the world’s population.5,103 Essential workers, frequently minorities, are unable to work remotely and become vectors of transmission within their homes and communities, exacerbating socioeconomic and racial health disparities.104,105 Persistent school closures force parents to be both educators and providers, and also perpetuate achievement disparities across income levels, sex, and races, with potential long-term negative effects on the entire U.S. economy.106,107 Furthermore, it is becoming increasingly evident that COVID-19 has long-term health effects not originally appreciated, including significant residual lung injury,108,109 as well as cardiac involvement,110, 111, 112, 113, 114, 115, 116, 117 even in mildly symptomatic or asymptomatic patients.
The sooner we can understand how to create an effective COVID-19 surveillance and containment strategy, the sooner people can get back to work, kids can get back to school, economies can reopen, and supply chains can be reinstated. The tremendous scale at which COVID-19 has spread throughout the globe has overwhelmed human tracing capabilities, necessitating digitization of health surveillance. Technology used in countries to successfully combat COVID-19 is beginning to surface in the U.S., but privacy concerns hinder adequate uptake. In this pandemic that has shut down economies and killed hundreds of thousands of people, the public health benefits justify a prudently designed surveillance system. Because the success of digital contact tracing is directly proportional to user uptake, the government must be transparent in its policies and enact legislation to regulate data collection and use.118 With mass testing, technology provides an opportunity to strengthen the fight against this virus and return to normalcy. It is imperative for the response to be unanimous and united to live freely again.
Sources of Funding
None.
Disclosures
All authors have no conflicts of interest to disclose.
References
- 1.WHO | Novel Coronavirus – China. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Available at.
- 2.WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 Available at.
- 3.NIH: National Institute of Allergy and Infectious Diseases. COVID-19, MERS & SARS. 2020. https://www.niaid.nih.gov/diseases-conditions/covid-19 Available at.
- 4.Connor P. 91% of world population lives in countries with restricted travel amid COVID-19. Pew Research Center. https://www.pewresearch.org/fact-tank/2020/04/01/more-than-nine-in-ten-people-worldwide-live-in-countries-with-travel-restrictions-amid-covid-19/ Available at.
- 5.COVID-19 Coronavirus Pandemic. Worldometer website. Available at https://www.worldometers.info/coronavirus/. Accessed August 3, 2020.
- 6.Moghadas S.M., Fitzpatrick M.C., Sah P., et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A. 2020;117:17513–17515. doi: 10.1073/pnas.2008373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. COVID-19 Pandemic Planning Scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html Available at.
- 8.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Overview of Testing for SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Fclinical-criteria.html#changes Available at.
- 10.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. July 9, 2020. https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations Available at.
- 11.WHO Frequently asked questions and answers on smallpox. 2016. http://www.who.int/csr/disease/smallpox/faq/en/ Available at.
- 12.Kasolo F.C., Lukoya C.O., Wamala J.F., et al. World Health Organization, ed. Disease Surveillance and Response Programme Area Disease Prevention and Control Cluster. WHO Regional Office for Africa; Brazzaville, Republic of Congo: 2014. Contact tracing during an outbreak of ebola virus disease. [Google Scholar]
- 13.WHO Contact investigation. 2020. http://www.who.int/tb/areas-of-work/laboratory/contact-investigation/en/ Available at.
- 14.Zastrow M. Coronavirus contact-tracing apps: can they slow the spread of COVID-19? Nature. 2020 May 19 doi: 10.1038/d41586-020-01514-2. [DOI] [PubMed] [Google Scholar]
- 15.Criddle C., Kelion L. Coronavirus contact-tracing: World split between two types of app. BBC News. May 7, 2020. May 7, 2020 https://www.bbc.com/news/technology-52355028 Available at. [Google Scholar]
- 16.Zakrzewski C. The Technology 202: Buzzing bracelets could become a workplace accessory in the coronavirus era. The Washington Post. May 14, 2020. May 14, 2020 https://www.washingtonpost.com/news/powerpost/paloma/the-technology-202/2020/05/14/the-technology-202-buzzing-bracelets-could-become-a-workplace-accessory-in-the-coronavirus-era/5ebc46fd88e0fa17cddfa4c0/ Available at. [Google Scholar]
- 17.gov.sg. Seniors to receive their first batch of TraceTogether Tokens from 28 June 2020. https://www.gov.sg/article/seniors-to-receive-their-first-batch-of-tracetogether-tokens-from-28-june-2020 Available at.
- 18.NOVID. https://www.novid.org/ Available at.
- 19.Payne E. NOVID Is the Most Accurate App for Contact Tracing. Carnegie Mellon University. June 30, 2020. https://www.cmu.edu/news/stories/archives/2020/june/novid-update.html Available at.
- 20.Jaggi A. Ultra Wideband Technology for COVID-19. The IOT Magazine. June 17, 2020. June 17, 2020 https://theiotmagazine.com/ultra-wideband-technology-f9ba3d7e04c8 Available at. [Google Scholar]
- 21.Android Developers Wi-Fi location: ranging with RTT. June 22, 2020. June 22, 2020. https://developer.android.com/guide/topics/connectivity/wifi-rtt Available at.
- 22.Lehtimäki S. Bluetooth Angle Estimation for Real-Time Locationing. Silicon Labs. https://www.silabs.com/whitepapers/bluetooth-angle-estimation-for-real-time-locationing Available at.
- 23.Faragher R. What Proximity Detection For Contact Tracing Apps Is Going To Look Like In 2021. Forbes. June 16, 2020. June 16, 2020 https://www.forbes.com/sites/ramseyfaragher/2020/06/16/what-proximity-detection-for-contact-tracing-apps-is-going-to-look-like-in-2021/#34db57c16fa2 Available at. [Google Scholar]
- 24.Cho H., Ippolito D., Yu Y.W. Contact Tracing Mobile Apps for COVID-19: Privacy Considerations and Related Trade-offs. March 2020. http://arxiv.org/abs/2003.11511 Available at.
- 25.Privacy-Preserving Contact Tracing - Apple and Google. https://www.apple.com/covid19/contacttracing Available at.
- 26.Owen M. Apple releases iOS 13.7, iPadOS 13.7 with Exposure Notification update. Appleinsider. https://appleinsider.com/articles/20/09/01/apple-releases-ios-137-ipados-137-with-exposure-notification-update Available at.
- 27.Digital contact tracing can slow or even stop coronavirus transmission and ease us out of lockdown. Research | University of Oxford. April 16, 2020. https://www.research.ox.ac.uk/Article/2020-04-16-digital-contact-tracing-can-slow-or-even-stop-coronavirus-transmission-and-ease-us-out-of-lockdown Available at.
- 28.NIPH stops collection of personal data in Smittestopp. Norwegian Institute of Public Health. June 15, 2020. https://www.fhi.no/en/news/2020/niph-stops-collection-of-personal-data-in-smittestopp/ Available at.
- 29.Kim M.S. Seoul’s Radical Experiment in Digital Contact Tracing. The New Yorker. April 17, 2020. April 17, 2020 https://www.newyorker.com/news/news-desk/seouls-radical-experiment-in-digital-contact-tracing Available at. [Google Scholar]
- 30.Jean C. Israelis use special phone case to avoid Shin Bet’s coronavirus tracking. The Jerusalem Post. July 9, 2020. July 9, 2020 https://www.jpost.com/israel-news/israelis-use-special-phone-case-to-avoid-shin-bets-coronavirus-tracking-634452 Available at. [Google Scholar]
- 31.Kolbert E. How Iceland Beat the Coronavirus. The New Yorker. June 2020. https://www.newyorker.com/magazine/2020/06/08/how-iceland-beat-the-coronavirus Available at.
- 32.Information on COVID-19 screening and infections at Iceland's borders. The Icelandic Directorate of Health & Department of Civil Protection and Emergency Management. August 3, 2020. https://www.covid.is/announcements Available at.
- 33.Oh J., Lee J.K., Schwarz D., Ratcliffe H.L., Markuns J.F., Hirschhorn L.R. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020;6:e1753464. doi: 10.1080/23288604.2020.1753464. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.I., Lee J.Y. Walk-through screening center for COVID-19: An accessible and efficient screening system in a pandemic situation. J Korean Med Sci. 2020;35:e154. doi: 10.3346/jkms.2020.35.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park O., Park Y.J., Park S.Y., et al. Contact transmission of Covid-19 in South Korea: Novel investigation techniques for tracing contacts. Osong Public Health Res Perspect. 2020;11:60–63. doi: 10.24171/j.phrp.2020.11.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amit M., Kimhi H., Bader T., Chen J., Glassberg E., Benov A. Mass-surveillance technologies to fight coronavirus spread: the case of Israel. Nat Med. 2020;26:1167–1169. doi: 10.1038/s41591-020-0927-z. [DOI] [PubMed] [Google Scholar]
- 37.Hershkowitz R.A., Altshuler T.S. How Israel’s COVID-19 mass surveillance operation works. July 6, 2020. https://www.brookings.edu/techstream/how-israels-covid-19-mass-surveillance-operation-works/ Available at.
- 38.Cahane A. Israel Reauthorizes Shin Bet’s Coronavirus Location Tracking. Lawfare. July 3, 2020. https://www.lawfareblog.com/israel-reauthorizes-shin-bets-coronavirus-location-tracking Available at.
- 39.Scarr S., Bhandari A. Reopening a megacity. Reuters Graphics. June 4, 2020. June 4, 2020 https://graphics.reuters.com/HEALTH-CORONAVIRUS/WUHAN/rlgpdkxzavo/index.html Available at. [Google Scholar]
- 40.German coronavirus tracing app downloaded 6.5 million times. Reuters. https://www.reuters.com/article/us-health-coronavirus-germany-app/german-coronavirus-tracing-app-downloaded-65-million-times-idUSKBN23O13G Available at.
- 41.Hamilton I.A. Ireland’s contact-tracing app has done so well that US states want to use it. Business Insider. July 22, 2020. July 22, 2020 https://www.businessinsider.com.au/nearform-ireland-covid-19-contact-tracing-app-approached-us-states-2020-7 Available at. [Google Scholar]
- 42.Kissick C., Setzer E., Schulz J. What Ever Happened to Digital Contact Tracing? Lawfare. July 21, 2020. July 21, 2020 https://www.lawfareblog.com/what-ever-happened-digital-contact-tracing Available at. [Google Scholar]
- 43.L’application StopCovid collecte plus de données qu’annoncé. Le Monde. June 16, 2020. https://www.lemonde.fr/pixels/article/2020/06/16/l-application-stopcovid-collecte-plus-de-donnees-qu-annonce_6043038_4408996.html Available at.
- 44.Bagchi K, Bannan C, Bradford Franklin S, et al. COVID-19 Rapid Response Impact Initiative. Digital Tools for COVID-19 Contact Tracing: Identifying and Mitigating the Equity, Privacy, and Civil Liberties Concerns. Cambridge, MA. 2 July 2020. Available at https://ethics.harvard.edu/files/center-for-ethics/files/22civilliberties.pdf. Accessed August 3, 2020.
- 45.Only 3.1% of French people have downloaded the StopCovid application, according to a study | World Today News. July 17, 2020. http://www.world-today-news.com/only-3-1-of-french-people-have-downloaded-the-stopcovid-application-according-to-a-study/ Available at.
- 46.Bosman J. Republicans Urge Masks for Coronavirus Despite Trump’s Resistance. The New York Times. July 1, 2020. July 1, 2020 https://www.nytimes.com/2020/07/01/us/coronavirus-masks.html Available at. [Google Scholar]
- 47.CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#cases Available at.
- 48.NACCHO Position Statement: Building COVID-19 Contact Tracing Capacity in Health Departments to Support Reopening American Society Safely. Washington, DC. 16 April 2020. Available at https://www.naccho.org/uploads/full-width-images/Contact-Tracing-Statement-4-16-2020.pdf. Accessed August 3, 2020.
- 49.Peltz J. ‘Are you doing OK?’: On the ground with NYC contact tracers. The Washington Post. August 16, 2020. August 16, 2020 https://www.washingtonpost.com/health/are-you-doing-ok-on-the-ground-with-nyc-contact-tracers/2020/08/17/311f9456-e048-11ea-82d8-5e55d47e90ca_story.html Available at. [Google Scholar]
- 50.Leventhal R. Reports: Massachusetts Significantly Scales Back Contact Tracing Efforts. Healthcare Innovation. July 10, 2020. July 10, 2020 https://www.hcinnovationgroup.com/covid-19/news/21145690/reports-massachusetts-significantly-scales-back-contact-tracing-efforts Available at. [Google Scholar]
- 51.Sun-Times staff Coronavirus live blog, July 22: Pritzker promises millions to expand COVID-19 contact tracing in Illinois. Chicago Sun-Times. July 22, 2020. July 22, 2020 https://chicago.suntimes.com/essential-coronavirus-news/2020/7/22/21333966/latest-coronavirus-news-live-updates-chicago-illinois-2020 Available at. [Google Scholar]
- 52.Otterman S., NYC Hired 3,000 Workers for Contact Tracing. It’s Off to a Slow Start. The New York Times. June 21, 2020. June 21, 2020 https://www.nytimes.com/2020/06/21/nyregion/nyc-contact-tracing.html Available at. [Google Scholar]
- 53.Virginia touts nation’s first contact tracing app with Apple-Google tech. Reuters. https://www.reuters.com/article/us-health-coronavirus-apps-virginia/virginia-touts-nations-first-contact-tracing-app-with-apple-google-tech-idUSKCN2512UU Available at.
- 54.Which U.S. states are using Apple’s Exposure Notification API for COVID-19 contact tracing? 9to5Mac. https://9to5mac.com/2020/09/22/covid-19-exposure-notification-api-states/ Available at.
- 55.Burke D. An update on Exposure Notifications. Google. July 31, 2020. July 31, 2020 https://blog.google/inside-google/company-announcements/update-exposure-notifications Available at. [Google Scholar]
- 56.Bringing COVID-19 exposure notification to the public health community. APHL Lab Blog. July 17, 2020. July 17, 2020 https://www.aphlblog.org/bringing-covid-19-exposure-notification-to-the-public-health-community/ Available at. [Google Scholar]
- 57.Glaser A. Palantir’s pandemic contracts stir concern ahead of IPO. July 22, 2020. https://www.nbcnews.com/tech/tech-news/palantir-s-pandemic-contracts-stir-concern-ahead-ipo-n1234537 Available at.
- 58.Albergotti R. HHS coronavirus data collection initiative may violate privacy. Warren and other lawmakers say. The Washington Post. July 1, 2020. July 1, 2020 https://www.washingtonpost.com/technology/2020/07/01/warren-hhs-data-collection/ Available at. [Google Scholar]
- 59.Bellon T. Uber offers COVID-19 contact tracing help amid chaotic U.S. response. Reuters. July 20, 2020. July 20, 2020 https://www.reuters.com/article/us-health-coronavirus-uber-focus/uber-offers-covid-19-contact-tracing-help-amid-chaotic-u-s-response-idUSKCN24L17X Available at. [Google Scholar]
- 60.COVID Contact Tracing App Report. Avira. 2020. https://www.avira.com/en/covid-contact-tracing-app-report Available at.
- 61.Banks G. Steven Hotze takes on Gov. Abbott and the constitutionality of contact tracing. HoustonChronicle.com. June 17, 2020. https://www.houstonchronicle.com/news/houston-texas/houston/article/Steve-Hotze-takes-on-Gov-Abbott-and-the-15344889.php Available at.
- 62.Landau S., Lopez C.E., Moy L. The Importance of Equity in Contact Tracing. Lawfare. May 1, 2020. May 1, 2020 https://www.lawfareblog.com/importance-equity-contact-tracing Available at. [Google Scholar]
- 63.Ipsos Mori. The Health Foundation COVID-19 Survey: A Report of Survey Findings. London, UK. June 2020. Available at https://www.health.org.uk/sites/default/files/2020-06/Health-Foundation-2020-COVID-19-Polling-v2.pdf. Accessed August 5, 2020.
- 64.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed]
- 65.Collins K. Is Your State Doing Enough Coronavirus Testing?. The New York Times. August 30, 2020. https://www.nytimes.com/interactive/2020/us/coronavirus-testing.html Available at.
- 66.Kretzschmar M.E., Rozhnova G.,J., Bootsma M.C., et al. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heid M. NBA Bubble - How Does It Work? Science Behind the NBA Bubble. Popular Mechanics. August 25, 2020. https://www.popularmechanics.com/science/health/a33796756/nba-bubble/ Available at.
- 68.Golden J., Tirrell M., Miller L. NBA adding coronavirus testing resources, not taking them away. July 15, 2020. https://www.cnbc.com/2020/07/15/nba-adding-coronavirus-testing-resources-not-taking-them-away.html Available at.
- 69.Trump Administration Announces New Resources to Protect Nursing Home Residents Against COVID-19. CMS. July 22, 2020. Available at. https://www.cms.gov/newsroom/press-releases/trump-administration-announces-new-resources-protect-nursing-home-residents-against-covid-19
- 70.Bhattarai A., Denham H. Tyson Foods will test employees for coronavirus weekly. The Washington Post. July 22, 2020. Available at. https://www.washingtonpost.com/business/2020/07/30/tyson-foods-coronavirus-tests/
- 71.Paltiel A.D., Zheng A., Walensky R.P. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3:e2016818. doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Japsen B. Demand For Abbott Labs Covid-19 Tests Soars Past 40 Million As Pandemic Cases Surge. Forbes. July 16, 2020. Available at. https://www.forbes.com/sites/brucejapsen/2020/07/16/demand-for-abbott-labs-covid-19-tests-surges-past-40m-as-pandemic-lingers/#68e0bafe667d
- 73.Xpert Xpress SARS-CoV-2 https://www.fda.gov/media/136314/download Available at.
- 74.Weissleder R., Lee H., Ko J., Pittet M.J. COVID-19 Diagnostics In Context. 2020. https://csb.mgh.harvard.edu/covid Available at. [DOI] [PubMed]
- 75.Shyu D., Dorroh J., Upendran A., Kannan R. Laboratory Tests for COVID-19: A Review of Peer-Reviewed Publications and Implications for Clinical Use. https://www.researchgate.net/publication/342242124 Available at. [PMC free article] [PubMed]
- 76.Lollar R.H. Sofia 2 SARS Antigen FIA - Letter of Authorization. 8 May 2020. https://www.fda.gov/media/137886/download Available at.
- 77.Fiechtner M.A. BD Veritor System for Rapid Detection of SARS-CoV-2 - Letter of Authorization. 2 July 2020. https://www.fda.gov/media/139752/download Available at.
- 78.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 79.Covid-19 National Testing & Tracing Action Plan. The Rockefeller Foundation. https://www.rockefellerfoundation.org/national-covid-19-testing-and-tracing-action-plan/ Available at.
- 80.Maryland, Virginia, Michigan, Ohio, Louisiana, Massachussetts form pact for rapid coronavirus tests. The Washington Post. https://www.washingtonpost.com/coronavirus/coronavirus-state-testing-compact/2020/08/04/8b73bed8-d66f-11ea-9c3b-dfc394c03988_story.html Available at.
- 81.Kotlikoff L.J., Mina M. Opinion | Coronavirus Testing the Cheap, Simple Way. The New York Times. July 3, 2020. July 3, 2020 https://www.nytimes.com/2020/07/03/opinion/coronavirus-tests.html Available at. [Google Scholar]
- 82.Abbott’s Fast, $5, 15-Minute, Easy-to-Use COVID-19 Antigen Test Receives FDA Emergency Use Authorization; Mobile App Displays Test Results to Help Our Return to Daily Life; Ramping Production to 50 Million Tests a Month. August 26, 2020. https://abbott.mediaroom.com/2020-08-26-Abbotts-Fast-5-15-Minute-Easy-to-Use-COVID-19-Antigen-Test-Receives-FDA-Emergency-Use-Authorization-Mobile-App-Displays-Test-Results-to-Help-Our-Return-to-Daily-Life-Ramping-Production-to-50-Million-Tests-a-Month Available at.
- 83.Trump Administration Will Deploy 150 Million Rapid Tests in 2020. HHS.gov. https://www.hhs.gov/about/news/2020/08/27/trump-administration-will-deploy-150-million-rapid-tests-in-2020.html Available at.
- 84.Infectious Disease Testing Red Cross Blood Services. https://www.redcrossblood.org/biomedical-services/blood-diagnostic-testing/blood-testing.html Available at.
- 85.Lohse S., Pfuhl T., Berkó-Göttel B., et al. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect Dis. 2020:0. doi: 10.1016/S1473-3099(20)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan W. Wuhan Tests Nine Million People for Coronavirus in 10 Days. WSJ. May 25, 2020. https://www.wsj.com/articles/wuhan-tests-nine-million-people-for-coronavirus-in-10-days-11590408910 Available at.
- 87.Shental N., Levy S., Wuvshet V., et al. Efficient high-throughput SARS-CoV-2 testing to detect asymptomatic carriers. Sci Adv. 2020:eabc5961. doi: 10.1126/sciadv.abc5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ben-Ami R., Klochendler A., Seidel M., et al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin Microbiol Infect. 2020;26:1248–1253. doi: 10.1016/j.cmi.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lakdawalla D., Keeler E., Goldman D., Trish E. USC Schaeffer Center for Health Policy & Economics; Los Angeles, CA: 2020. Getting Americans Back to Work (and School) with Pooled Testing. [Google Scholar]
- 90.Augenblick N., Kolstad J.T., Obermeyer Z., et al. Group Testing in a Pandemic: The Role of Frequent Testing, Correlated Risk, and Machine Learning. 2020 http://www.nber.org/papers/w27457.ack Available at. [Google Scholar]
- 91.Acosta J., Fossum S. Coronavirus testing: Anthony Fauci says task force “seriously considering” new strategy. CNNPolitics. June 26, 2020. Available at. https://www.cnn.com/2020/06/26/politics/anthony-fauci-testing-coronavirus-task-force/index.html
- 92.Coronavirus (COVID-19) Update: FDA Issues First Emergency Authorization for Sample Pooling in Diagnostic Testing. FDA. July 18, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-authorization-sample-pooling-diagnostic Available at.
- 93.Lovett I., Frosch D., Overberg P. Covid-19 Stalks Large Families in Rural America. WSJ. June 7, 2020. https://www.wsj.com/articles/covid-19-households-spread-coronavirus-families-navajo-california-second-wave-11591553896 Available at.
- 94.Governor Cuomo Announces New Testing Initiatives to Improve COVID-19 Detection & Control Across New York State. August 14, 2020. https://www.governor.ny.gov/news/governor-cuomo-announces-new-testing-initiatives-improve-covid-19-detection-control-across-new Available at.
- 95.Chavarria-Miró G., Anfruns-Estrada E., Guix S., et al. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. medRxiv June. 2020;2020 06.13.20129627. [Google Scholar]
- 96.Peiser J. University of Arizona used wastewater testing to detect cases of coronavirus in a dorm. The Washington Post. August 28, 2020. https://www.washingtonpost.com/nation/2020/08/28/arizona-coronavirus-wastewater-testing/ Available at.
- 97.NanoScent – making scents readable. https://nanoscentlabs.com/ Available at.
- 98.Arias T., Ulloa C. Could Covid-19 sniffer dogs help reopen public spaces in Chile? CNN. July 22, 2020. https://www.cnn.com/2020/07/22/health/dogs-coronavirus-sniff-public-spaces-intl/index.html Available at.
- 99.Rogers I. Dogs Can Sniff Out Coronavirus Infections, German Study Shows. Bloomberg. July 24, 2020. https://www.bloombergquint.com/coronavirus-outbreak/dogs-can-sniff-out-coronavirus-infections-german-study-shows Available at.
- 100.Paltiel A.D., Zheng A., Walensky R.P. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16818. e2016818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Overview of Testing for SARS-CoV-2 (COVID-19) | CDC. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. 17 July 2020. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed August 5, 2020.
- 102.Cohen E. Covid-19 vaccine might not get us the herd immunity if too many people refuse to get it, Fauci says. CNN. June 28, 2020. https://www.cnn.com/2020/06/28/health/fauci-coronavirus-vaccine-contact-tracing-aspen/index.html Available at.
- 103.Yong E. Why the Pandemic Is So Bad in America. The Atlantic. August 4, 2020. August 4, 2020 https://www.theatlantic.com/magazine/archive/2020/09/coronavirus-american-failure/614191/ Available at. [Google Scholar]
- 104.Health Equity Considerations and Racial and Ethnic Minority Groups.| CDC. Available at. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
- 105.Center for Employment Equity, UMass Amherst. How COVID Exposes Healthcare Deficits for Black Workers. https://www.umass.edu/employmentequity/how-covid-exposes-healthcare-deficits-black-workers Available at.
- 106.Achievement gap and coronavirus. McKinsey. https://www.mckinsey.com/industries/public-and-social-sector/our-insights/covid-19-and-student-learning-in-the-united-states-the-hurt-could-last-a-lifetime Available at.
- 107.The Importance of Reopening America’s Schools this Fall. CDC. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/reopening-schools.html#fn12 Available at.
- 108.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Inui S., Fujikawa A., Jitsu M., et al. Chest CT Findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020;2:e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart—is heart failure the next chapter? JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.3575. [DOI] [PubMed]
- 111.Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed]
- 112.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed]
- 113.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323 doi: 10.1001/jama.2020.2648. 1239–1242. [DOI] [PubMed] [Google Scholar]
- 114.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed]
- 115.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed]
- 116.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1286 [DOI] [PubMed]
- 117.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. https://doi.org/10.1001/jama.2020.12839. [DOI] [PubMed]
- 118.Exposure Notification Privacy Act, S. 2D, 116th Congress, 2020. https://www.cantwell.senate.gov/imo/media/doc/Exposure%20Notification%20Privacy%20Bill%20Text.pdf Available at.
- 119.In Coronavirus Fight, China Gives Citizens a Color Code, With Red Flags. New York Times. March 1, 2020. https://www.nytimes.com/2020/03/01/business/china-coronavirus-surveillance.html Available at.
- 120.Qualitative assay for use on the cobas® 6800/8800 Systems. https://www.fda.gov/media/136049/download Available at.
- 121.FDA. Alinity m SARS-CoV-2 AMP Kit. 2020. https://www.fda.gov/media/137979/download Available at.
- 122.FDA. Accelerated Emergency Use Authorization (EUA) Summary COVID-19 RT-PCR Test (Laboratory Corporation of America). 2020. https://www.fda.gov/media/136151/download Available at.
- 123.Abbott I.D. Now COVID-19. May 2020. https://www.fda.gov/media/136525/download Available at.
- 124.Abbott Releases Interim Clinical Study Data on ID NOW COVID-19 Rapid Test Showing Strong Agreement to Lab-Based Molecular PCR Tests. May 21, 2020. https://abbott.mediaroom.com/2020-05-21-Abbott-Releases-Interim-Clinical-Study-Data-on-ID-NOW-COVID-19-Rapid-Test-Showing-Strong-Agreement-to-Lab-Based-Molecular-PCR-Tests Available at.
- 125.Gever J. New Type of COVID-19 Test OK’d. MedPage Today. May 9, 2020. May 9, 2020 https://www.medpagetoday.com/infectiousdisease/covid19/86419 Available at. [Google Scholar]
- 126.BinaxNOW COVID-19 Ag CARD. https://www.fda.gov/media/141570/download Available at. [DOI] [PMC free article] [PubMed]
- 127.Cellex Cellex qSARS-CoV-2 IgG/IgM Rapid Test. https://www.fda.gov/media/136625/download Available at.
- 128.FDA Assure COVID-19 IgG/IgM Rapid Test Device. https://www.fda.gov/media/139792/download Available at.
- 129.Bryan A., Pepper G., Wener M.H., et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence testing in Idaho. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00941-20. e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.FDA LIAISON ® SARS-CoV-2 S1/S2 IgG ([REF] 311460) https://www.fda.gov/media/137359/download Available at.
- 131.EUA Authorized Serology Test Performance. FDA. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance Available at.
- 132.FDA SCoV-2 Detect IgM ELISA - Instructions for Use. https://www.fda.gov/media/139730/download Available at.
- 133.Tan CW, Chia WN, Chen MI, et al., A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction. Nat Biotechnol [published online ahead of print July 23, 2020]. https://doi.org/10.21203/rs.3.rs-24574/v1. [DOI] [PubMed]