Abstract
Background
The coronavirus disease 2019 (COVID-19) is a recently emerged respiratory infectious disease with kidney injury as a part of the clinical complications. However, the dynamic change of kidney function and its association with COVID-19 prognosis are largely unknown.
Methods
In this multicenter retrospective cohort study, we analyzed clinical characteristics, medical history, laboratory tests, and treatment data of 12,413 COVID-19 patients. The patient cohort was stratified according to the severity of the outcome into three groups: non-severe, severe, and death.
Findings
The prevalence of elevated blood urea nitrogen (BUN), elevated serum creatinine (Scr), and decreased blood uric acid (BUA) at admission was 6.29%, 5.22%, and 11.66%, respectively. The trajectories showed the elevation in BUN and Scr levels, as well as a reduction in BUA level for 28 days after admission in death cases. Increased all-cause mortality risk was associated with elevated baseline levels of BUN and Scr and decreased levels of BUA.
Conclusions
The dynamic changes of the three kidney function markers were associated with different severity and poor prognosis of COVID-19 patients. BUN showed a close association with and high potential for predicting adverse outcomes in COVID-19 patients for severity stratification and triage.
Funding
This study was supported by grants from the National Key R&D Program of China (2016YFF0101504), the National Science Foundation of China (81630011, 81970364, 81970070, 81970011, 81870171, and 81700356), the Major Research Plan of the National Natural Science Foundation of China (91639304), the Hubei Science and Technology Support Project (2019BFC582, 2018BEC473, and 2017BEC001), and the Medical Flight Plan of Wuhan University.
Keywords: COVID-19, kidney function indicators, mortality, poor outcomes, BUN
Graphical Abstract

Context and Significance
Kidney injury is a common complication of coronavirus disease 2019 (COVID-19); however, the dynamic changes in kidney injury markers and their associations with COVID-19 prognosis remain largely uncertain. Here, using a multicenter retrospective cohort of 12,413 patients with COVID-19 from 17 hospitals in Hubei Province, China, Liu et al. reveal that elevated baseline levels of blood urea nitrogen (BUN) and serum creatinine (Scr), as well as a decreased baseline level of blood uric acid (BUA), are correlated with adverse outcomes of COVID-19. These results confer clinical evidence that kidney function markers may be simple tools for predicting adverse outcomes in COVID-19 patients for severity stratification.
Liu et al. show that among 12,413 cases of COVID-19, an elevation in baseline BUN and Scr levels and a decrease in baseline BUA level were associated with adverse outcomes in patients. These findings may assist in the risk stratification and triage of COVID-19 patients according to kidney function indicators.
Introduction
The coronavirus disease 2019 (COVID-19) is a recently emerged disease with unprecedented scale and high infectivity.1, 2, 3 There is accumulating evidence indicating that the respiratory system is not the only organ damaged by the virus. Kidney impairment has been reported to be a frequent complication resulting from this infection, especially among patients with severe symptoms.4 , 5 The latest data show that acute kidney injury (AKI) occurred in ∼37% of COVID-19 patients in New York6 and a history of kidney diseases and AKI during hospitalization were linked to an increased risk of mortality.7 However, the dynamic changes in kidney function after severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection and its predictive value for poor prognosis of COVID-19 have not yet been reported. To answer these fundamental questions, we conducted a large-scale analysis based on 12,413 COVID-19 cases from multiple centers in Hubei Province, China. We demonstrated the dynamic trajectories of kidney injury indicators in patients stratified based on disease severity and outcome, and uncovered that these kidney injury indicators, in particular blood urea nitrogen (BUN), were significantly associated with and had significant predictive properties for poor outcomes of COVID-19.
Results
Baseline Characteristics of the Study Cohort
We enrolled a total of 15,512 patients with COVID-19, of whom 12,413 (80.02%) met the eligibility criteria (Figure 1 ), including 8,441 non-severe cases, 3,202 severe (non-death) cases, and 770 deaths (Figure 1). The clinical and biochemical characteristics and preexisting complications of the study cohort at baseline, as well as treatment information during hospitalization, are described in Table 1 . The median age of the entire study cohort was 58 years (interquartile range, 46–67 years), and 48.22% were male. The median duration from the first symptom to hospitalization was 11 days for all of the patients. At admission, 764 (6.29%) had an elevated level of BUN, 633 (5.22%) patients had an elevated level of serum creatinine (Scr), and 1,422 (11.66%) had a decreased level of blood uric acid (BUA). Coexisting chronic diseases, including diabetes, hypertension, coronary heart disease, chronic obstructive pulmonary disease, cerebrovascular disease, and chronic liver disease had higher frequencies in the death group than in other groups. Of note, compared to the other groups, the patients from the death group had higher proportions of elevated BUN (37.86%), elevated Scr (18.64%), and decreased BUA (25.49%). During hospitalization, the highest incidence of AKI was observed in the patients who died of COVID-19 (death group: 29.22%, severe group: 1.59%, non-severe group: 0.49%). A total of 62 patients and 81 patients received renal replacement therapy (RRT) and continuous renal replacement therapy (CRRT), respectively. The patients in the death group had a significantly higher ratio to receive RRT and CRRT relative to other groups. There were still 73 patients who met indications but did not receive RRT or CRRT (death group: 9.09%, severe group: 0.03%, non-severe group: 0.02%).
Figure 1.
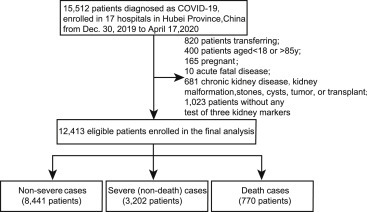
Flowchart of Patient Selection
Table 1.
Baseline Characteristics of Study Patients
| Parameters | All (N = 12,413) | Non-severe (n = 8,441) | Severe (n = 3,202) | Death (n = 770) | pa |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 58 (46–67) | 57 (44–66) | 60 (48–68) | 68 (62–76) | <0.001 |
| Male gender, n (%) | 5,986 (48.22) | 3,901 (46.21) | 1,581 (49.38) | 504 (65.45) | <0.001 |
| Heart rate, median (IQR) | 84 (78–96) | 82 (77–91) | 100 (80–109) | 88 (78–101) | <0.001 |
| Respiratory rate, median (IQR) | 20 (19–21) | 20 (19–20) | 20 (20–22) | 21 (20–25) | <0.001 |
| Days from symptom onset to hospitalization, median (IQR) | 11 (7–20) | 12 (7–21) | 11 (7–18) | 10 (6–14) | <0.001 |
| Symptoms and status upon admission, n (%) | |||||
| Fever | 9,192 (74.05) | 6,015 (71.26) | 2,559 (79.92) | 618 (80.26) | <0.001 |
| Cough | 7,846 (63.21) | 5,149 (61.00) | 2,198 (68.64) | 499 (64.81) | <0.001 |
| Fatigue | 3,852 (31.03) | 2,577 (30.53) | 999 (31.20) | 276 (35.84) | 0.009 |
| Dyspnea | 2,523 (20.33) | 1,347 (15.96) | 862 (26.92) | 314 (40.78) | <0.001 |
| Smoking status | 2,985 (24.05) | 2,274 (26.94) | 596 (18.61) | 115 (14.94) | <0.001 |
| Coexisting chronic diseases, n (%) | |||||
| Diabetes | 1,952 (15.73) | 1,163 (13.78) | 593 (18.52) | 196 (25.45) | <0.001 |
| Hypertension | 4,053 (32.65) | 2,511 (29.75) | 1,162 (36.29) | 380 (49.35) | <0.001 |
| Coronary heart disease | 1,030 (8.30) | 603 (7.14) | 279 (8.71) | 148 (19.22) | <0.001 |
| COPD | 132 (1.06) | 71 (0.84) | 38 (1.19) | 23 (2.99) | <0.001 |
| Cerebrovascular disease | 332 (2.67) | 193 (2.29) | 94 (2.94) | 45 (5.84) | <0.001 |
| Chronic liver disease | 253 (2.04) | 145 (1.72) | 83 (2.59) | 25 (3.25) | <0.001 |
| Lab tests on admission | |||||
| BUN increase, n (%) | 764 (6.29) | 292 (3.56) | 185 (5.83) | 287 (37.86) | <0.001 |
| Scr increase, n (%) | 633 (5.22) | 288 (3.50) | 205 (6.50) | 140 (18.64) | <0.001 |
| BUA decrease, n (%) | 1,422 (11.66) | 731 (8.86) | 497 (15.65) | 194 (25.49) | <0.001 |
| Leukocyte count, median (IQR) | 5.53 (4.32–7.09) | 5.38 (4.27–6.74) | 5.64 (4.33–7.48) | 8.19 (5.53–11.72) | <0.001 |
| Neutrophil count, median (IQR) | 3.47 (2.51–4.93) | 3.26 (2.42–4.45) | 3.76 (2.64–5.54) | 7.16 (4.43–10.46) | <0.001 |
| Lymphocyte count, median (IQR) | 1.25 (0.86–1.70) | 1.37 (0.98–1.80) | 1.07 (0.73–1.52) | 0.61 (0.42–0.88) | <0.001 |
| Complications, n (%) | |||||
| Acute kidney injury | 317 (2.55) | 41 (0.49) | 51 (1.59) | 225 (29.22) | <0.001 |
| ARDS | 1,963 (15.81) | 586 (6.94) | 678 (21.17) | 699 (90.78) | <0.001 |
| Septic shock | 366 (2.95) | 45 (0.53) | 70 (2.19) | 251 (32.6) | <0.001 |
| DIC | 107 (0.86) | 10 (0.12) | 19 (0.59) | 78 (10.13) | <0.001 |
| Intervention, n (%) | |||||
| Systemic corticosteroids | 2,116 (17.05) | 904 (10.71) | 914 (28.54) | 298 (38.7) | <0.001 |
| Oxygen therapy | 9,701 (78.15) | 5,894 (69.83) | 3,075 (96.03) | 732 (95.06) | <0.001 |
| Invasive ventilation | 443 (3.57) | 53 (0.63) | 87 (2.72) | 303 (39.35) | <0.001 |
| Noninvasive ventilation | 1,169 (9.42) | 328 (3.89) | 363 (11.34) | 478 (62.08) | <0.001 |
| RRT | 62 (0.50) | 11 (0.13) | 8 (0.25) | 43 (5.58) | <0.001 |
| CRRT | 81 (0.65) | 9 (0.11) | 19 (0.59) | 53 (6.88) | <0.001 |
| No. of patients with indications but did not receive RRT and CRRT | 73 (0.59) | 2 (0.02) | 1 (0.03) | 70 (9.09) | <0.001 |
| ECMO | 45 (0.36) | 9 (0.11) | 17 (0.53) | 19 (2.47) | <0.001 |
ARDS, acute respiratory distress syndrome; BUA, blood uric acid; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; RRT, renal replacement therapy; Scr, serum creatinine.
The p values were calculated by the Mann-Whitney U test for non-normally distributed continuous variable and Fisher’s exact test or χ2 test for categorical variables.
The Longitudinal Trajectories of Three Kidney Function Indicators within 28 Days after Admission
As depicted in Figure 2 , locally weighted scatterplot smoothing (Loess) was used to determine the distribution and trajectory of the three renal parameters in the three different severity groups. At admission, the baseline levels of kidney markers in non-survivors were the highest among all of the patients. Within 28 days after admission, there was a modest fluctuation of the three renal indicators over time in the non-severe-case and severe-case groups. In sharp contrast, however, the temporal specific pattern of the kidney parameters in the death group was markedly different. The plot linear fitting curve revealed a marked elevation in BUN and Scr levels, while a sustained downtrend followed by a plateau of BUA level was observed. A subgroup analysis demonstrated that males and females had comparable temporal dependent profiles; however, the trends in the males were more significant than those in the females (Figure S1). The dynamic trends suggested a potential association between levels of kidney function markers and the mortality of patients with COVID-19.
Figure 2.
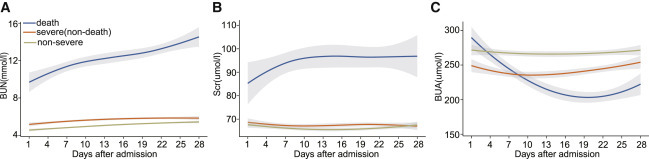
Loess Curves for Kidney Function Indicator Trajectories in COVID-19 Patients
Loess curves of mean BUN, Scr, and BUA by severity of the disease within 28 days after admission.
(A) BUN levels, (B) Scr levels, and (C) BUA levels. Yellow indicates non-severe cases, orange indicates severe (non-death) cases, and blue indicates death cases. The shaded regions represent 95% confidence intervals.
Association between Baseline Levels of Three Kidney Function Indicators and Poor Outcomes
Mixed-effects Cox regression analyses (Table 2 ) and Kaplan-Meier survival curves (Figure 3 ) were used to demonstrate the associations of BUN, Scr, and BUA with the mortality risk within the study cohort. Increased all-cause mortality risk was found to be associated with elevated levels of BUN (adjusted hazard ratio [aHR]: 6.27, 95% confidence interval [CI]: 5.29–7.42) and Scr (aHR: 2.65, 95% CI: 2.17–3.23) or decreased levels of BUA (aHR: 2.10, 95% CI: 1.75–2.51) at admission, by applying a mixed-effects Cox model treating hospital site as a random effect and adjusting for age, gender, and comorbidities. Among the three biomarkers, an elevated baseline BUN was associated with the highest risk of mortality. Figure 3 shows that patients with elevated baseline BUN, elevated baseline Scr, or decreased BUA had a markedly lower survival rate than those with normal levels of the three kidney function indicators.
Table 2.
Associations between Baseline Levels of Kidney Function Indicators with Mortality under the Mixed-Effects Cox Model
| Variables |
Crude |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | pb | HR | 95% CI | pb | |
| BUN | ||||||
| Normal | ref | |||||
| Elevated | 11.07 | (9.49–12.91) | <0.001 | 6.27 | (5.29–7.42) | <0.001 |
| Scr | ||||||
| Normal | ref | |||||
| Elevated | 4.72 | (3.90–5.72) | <0.001 | 2.65 | (2.17–3.23) | <0.001 |
| BUA | ||||||
| Normal | ref | |||||
| Decreased | 2.92 | (2.45–3.48) | <0.001 | 2.10 | (1.75–2.51) | <0.001 |
BUA, blood uric acid; BUN, blood urea nitrogen; CI, confidence interval; HR, hazard ratio; Scr, serum creatinine. “Normal” indicates within the reference range; “elevated” indicates above the upper limit of normal; “decreased” indicates below the lower limit of normal.
In the mixed-effects Cox model, adjusted variables included age, gender, and comorbidities (hypertension, coronary heart disease, diabetes), and hospital site was treated as a random effect.
The p values were calculated based on the mixed-effects Cox model.
Figure 3.
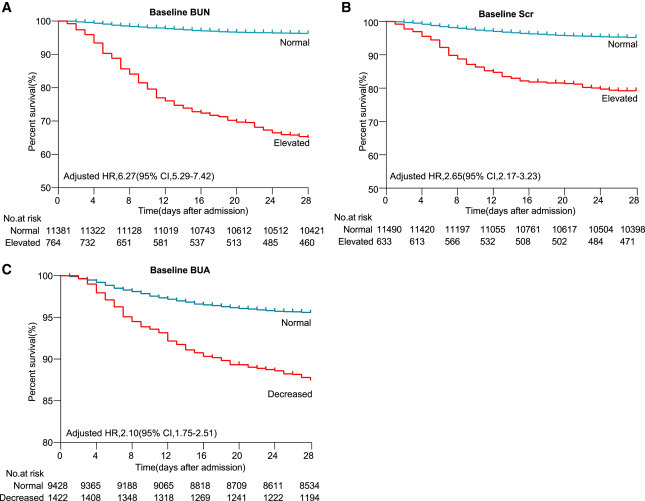
Kaplan-Meier Curves for Survival Probability of COVID-19 Patients with Different Baseline Levels of Kidney Function Indicators
The follow-up duration was 28 days. The blips indicate censoring. For Figure 3 A-C, P<0.001.
We further analyzed the associations between baseline levels of BUN, Scr, and BUA and secondary outcomes in COVID-19 patients. Mixed-effects Cox models demonstrated that the increased BUN and Scr levels, as well as the decreased BUA level at admission were also significantly associated with poor secondary outcomes in patients with COVID-19 (Table 3 ). Again, among the three markers, an elevated baseline BUN was associated with the highest risk of poor prognosis.
Table 3.
Associations between Baseline Levels of Kidney Function Indicators with Secondary Outcomes under the Mixed-Effects Cox Model
| Secondary outcomes | Elevated BUN |
Elevated Scr |
Decreased BUA |
|||
|---|---|---|---|---|---|---|
| HR (95% CI)a | pb | HR (95% CI)a | pb | HR (95% CI)a | pb | |
| Septic shock | 4.80 (3.74–6.17) | <0.001 | 2.26 (1.66–3.08) | <0.001 | 2.31 (1.79–2.98) | <0.001 |
| DIC | 4.49 (2.91–6.94) | <0.001 | 2.11 (1.24–3.57) | 0.006 | 2.10 (1.31–3.36) | 0.002 |
| ARDS | 3.11 (2.76–3.51) | <0.001 | 1.67 (1.45–1.94) | <0.001 | 2.17 (1.95–2.43) | <0.001 |
ARDS, acute respiratory distress syndrome; BUA, blood uric acid; BUN, blood urea nitrogen; CI, confidence interval; DIC, disseminated intravascular coagulation; HR, hazard ratio; Scr, serum creatinine. “Elevated” indicates above the upper limit of normal; “decreased” indicates below the lower limit of normal.
In the mixed-effects Cox model, adjusted variables included age, gender, and comorbidities (hypertension, coronary heart disease, diabetes), and hospital site was treated as a random effect.
The p values were calculated based on the mixed-effects Cox model.
Associations between Clinical Characteristics and Laboratory Indexes with Baseline Levels of Kidney Function Indicators
Logistic regression analyses were used to investigate the effects of baseline characteristics and laboratory indexes on the baseline levels of kidney function indicators in the study cohort (Table 4 ). The results showed that male gender, age, neutrophil count increase, lymphocyte count decrease, oxyhemoglobin saturation (SpO2) < 95, and diabetes were positively correlated with elevated BUN, elevated Scr, and decreased BUA. Among them, neutrophil count increase and lymphocyte count decrease were the most significant factors, indicating the potential association of inflammation with kidney markers after SARS-CoV-2 infection.
Table 4.
Associations between Clinical Characteristics and Laboratory Indexes at Admission with Baseline Levels of Kidney Function Indicators
| Parameters | Elevated BUN |
Elevated Scr |
Decreased BUA |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | pa | OR (95% CI) | pa | OR (95% CI) | pa | |
| Male sex | 2.25 (1.89–2.67) | <0.001 | 1.68 (1.41–1.99) | <0.001 | 1.73 (1.53–1.95) | <0.001 |
| Age | 1.05 (1.04–1.06) | <0.001 | 1.03 (1.03–1.04) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Neutrophil count increase | 4.01 (3.38–4.75) | <0.001 | 1.87 (1.53–2.28) | <0.001 | 1.74 (1.51–2.01) | <0.001 |
| Lymphocyte count decrease | 2.16 (1.81–2.59) | <0.001 | 1.27 (1.06–1.51) | 0.010 | 3.24 (2.85–3.68) | <0.001 |
| ALT increase | 1.26 (1.05–1.51) | 0.011 | 1.14 (0.94–1.38) | 0.196 | 1.41 (1.24–1.60) | <0.001 |
| SpO2 < 95 | 1.39 (1.12–1.71) | 0.003 | 1.34 (1.06–1.70) | 0.015 | 1.36 (1.18–1.57) | <0.001 |
| Hypertension | 1.35 (1.14–1.59) | 0.001 | 1.88 (1.57–2.25) | <0.001 | 0.58 (0.50–0.66) | <0.001 |
| Diabetes | 1.46 (1.22–1.76) | <0.001 | 1.32 (1.09–1.61) | 0.004 | 1.44 (1.24–1.67) | <0.001 |
| Coronary heart disease | 1.14 (0.91–1.43) | 0.248 | 1.14 (0.90–1.44) | 0.284 | 0.76 (0.62–0.95) | 0.014 |
ALT, alanine aminotransferase; BUA, blood uric acid; BUN, blood urea nitrogen; CI, confidence interval; OR, odds ratio; Scr, serum creatinine; SpO2, oxyhemoglobin saturation.
The p values were calculated based on the logistic model.
Discussion
To our knowledge, this is one of the largest cohorts to verify the relation between kidney function indicators and adverse outcomes in COVID-19 patients. From 12,413 cases, we found a remarkable increase in BUN and Scr and a reduction of BUA in the patients who died during hospitalization, but only modest fluctuation within normal ranges of the three kidney markers in the survivors with COVID-19. Furthermore, elevated baseline levels of BUN and Scr, as well as decreased baseline BUA, were associated with increased risks of adverse outcomes. This is the first report demonstrating that BUA is dramatically lower in COVID-19 patients with more severe symptoms and is significantly associated with a higher risk of COVID-19 death. Among the three indicators, an elevated baseline BUN was most significantly associated with the highest risk of adverse outcomes. These findings suggest that three kidney function indicators could reliably predict the risk of COVID-19 patients, among which BUN was the indicator with the most impact.
Our study illustrated the important role of kidney markers on admission in predicting the adverse outcomes of hospitalized patients with COVID-19. The results suggest that patients with elevated BUN and Scr or decreased BUA on admission should be monitored more carefully for early intervention, which may improve the survival rate of patients with COVID-19. These results are further corroborated by a study by Cheng et al.,7 which also reported that elevated BUN and elevated Scr increased the mortality risk in COVID-19 patients, based on a relatively small cohort of 701 cases. In addition, our findings linked a decrease in BUA with higher all-cause mortality, which is consistent with an observational study on SARS patients.8
Our results indicate that patients with an elevated baseline of BUN level are at a higher risk of all-cause death and poor outcomes than those with a normal range of BUN level. Thus, it is critically important that the BUN levels should be closely monitored. When the medical resources are limited, the BUN level on admission can provide a simple tool for early risk stratification among COVID-19 patients and guide physicians to reserve more medical resources to patients at higher risk to reduce the death rate. While an optimized management strategy is not the focus of this study, aggressive treatment with proper medication to reduce BUN level and related organ injury may contribute to mitigating the death risk and adverse outcome for COVID-19. However, due to the inherent limitations of a retrospective study, the present study cannot directly address whether increased BUN is a causal factor of poor prognosis and whether reducing BUN would benefit COVID-19 patients. These questions need to be addressed in future prospective studies and clinical trials.
The mechanism involved in the increase in BUN level after SARS-CoV-2 infection has not been fully elucidated. Given that angiotensin-converting enzyme 2 (ACE2) is the primary cellular receptor of SARS-CoV-2 and highly expressed in renal epithelial cells, it is possible that the viral infection may directly lead to an interaction of SARS-CoV-2 with its receptor in the kidney to reduce ACE2 expression, resulting in abnormal activation of the renin-angiotensin-aldosterone system (RAAS).9 , 10 The activated RAAS can significantly increase the absorption of water by kidney tubules while enhancing the resorption of urea, leading to elevated BUN levels.11 The elevation in BUN level is not only a kidney dysfunction indicator but it can also reflect inflammatory status, catabolism, nitrogen equilibrium, and renal hypoperfusion from hypovolemia, sepsis, or reduced cardiac output, many of which have been reported to be closely associated with the adverse outcomes in COVID-19 patients.11, 12, 13, 14, 15, 16, 17, 18 The coagulation status may progress and exacerbate along with the systematic inflammatory response and multiple organ injury. This is perhaps the reason why we observed a strong correlation between abnormal BUN with a broad spectrum of symptoms during pneumonia progression and why the high BUN level showed a more significant association with adverse outcomes than Scr, because the latter mainly represents a status of kidney injury and metabolic disturbance.19 Finally, the elevated BUN level may also be caused by cortisone therapy or an abnormal catabolic state. Nevertheless, findings from this retrospective study cannot provide a direct causal effect of BUN increase on all-cause mortality of COVID-19. Thus, the predictive value of BUN on the prognosis of COVID-19 still needs to be validated further by prospective studies and clinical trials, and more basic research is required to elucidate the underlying mechanisms.
In the present study, the level of BUA was much lower in the severe group than the non-severe group and was associated with increased risks of adverse outcomes in COVID-19 patients. Because uric acid is mainly dissolved in the blood, filtered through the kidneys, and expelled in the urine, it was commonly recognized as a kidney function marker.20 Under normal conditions, the reabsorption and excretion by kidney tubules of BUA are maintained in a balanced state. In the setting of SARS, decreased levels of BUA may as a result of defective tubular handling of uric acid and may be associated with cytokine storm.8 Because the dysfunction of kidney tubules was also observed after SARS-COV-2 infection,21 the lower uric acid level may be correlated with cytokine storm in the setting of COVID-19. Also, the decreased uric acid level may be a marker of kidney involvement severity (e.g., proximal tubular damage) in patients infected with SARS-COV-2. Except for kidney dysfunction, the reduced BUA may be related to the increase in decomposition by the intestinal tract, but the mechanism is unclear. It has been reported that the gut microbiome was disturbed in patients with COVID-19,22 , 23 which may lead to reduced uric acid in those patients.24, 25, 26 Thus, decreased BUA levels during SARS-CoV-2 infection may be due to an abnormal increase in BUA excretion by abnormal renal handling from inflammatory injury or hypoxemia, or an intensive uricolysis caused by enteric dysbacteriosis. However, these postulated mechanisms will still need to be investigated further.
Remarkably, not only can the baseline renal parameters predict adverse outcomes of COVID-19 but the dynamic changes of these renal parameters may also serve as warning signs for mortality in COVID-19 patients. In our study, we found that the dynamic changes in these kidney function indicators were associated with different severities and outcomes of COVID-19. Importantly, the sharp differences in the dynamic changes in the three markers for kidney function in non-survivors versus the relatively stable patterns observed in the survivors implied a significant association between the aggravation of kidney injury and the deterioration of the disease and death in the pathogenesis of COVID-19. Similar results were observed in male and female subgroups, with more significant trends in males than in females. It is known that estrogen affects ACE2 expression in the kidney, which could be one of the reasons for gender-related differences in the dynamic patterns of kidney parameters during hospitalization observed in our study.27, 28, 29
Limitations of Study
Several limitations of this study should be noted in interpreting the results. First, it was a retrospective study on a limited number of available kidney markers, and other biomarkers of kidney function, such as urine protein level and hematuria, were not investigated due to data availability. Second, smoking status and the medical history of chronic kidney diseases may not be collected sufficiently, especially from severely ill patients under urgent circumstances of COVID-19 surge. Third, we cannot determine the causal relationships between kidney function indicators and the severity as well as the mortality of COVID-19. Fourth, due to limited medical resources, an increase in Scr within 48 h was the only criterion for the diagnosis of AKI; thus, this may have resulted in an underdiagnosis of AKI in our population. A combination with a 7-day Scr and urine output criterion may increase the accuracy of AKI diagnosis. We noticed there were geographical differences in the incidence of AKI in patients with COVID-19. For instance, the AKI incidence in the United States was reported to be ∼37%,6 which is much higher than the 2.55% in our study and the 0.5% in another multicenter study in China.30 This discrepancy may be related to the difference in the occurrence of comorbidities, the severity of symptoms, and the exclusion of patients with chronic kidney diseases in our study. Therefore, the conclusions in this study require validation by prospective studies and randomized controlled trials (RCTs) in cohorts from extensive geographical regions. Fifth, the levels of kidney function indicators may be influenced by other factors. For example, the increased BUN may also be due to cortisone therapy or catabolic state, while the Scr level may be influenced by persistent muscle wasting in critically ill patients. Sixth, in our study cohort, the relatively small number and percentage of patients who received RRT may be related to the shortage of medical resources during the COVID-19 pandemic and the varied criteria of RRT initiation in different hospitals. Not all patients with indications of RRT received that therapy during hospitalization, which may induce potential confounders into the conclusion.
Conclusions
The dynamic changes in three markers of kidney function, elevated BUN, elevated Scr, and decreased BUA, were associated with different severity and mortality of patients with COVID-19. Among the three markers, an elevated baseline BUN was associated with the highest risk of adverse outcomes. The BUN level at admission may represent a sensitive factor for predicting death and adverse outcomes in COVID-19 patients and can be valuable for patient stratification and triage.
STAR★Methods
Key Resources Table
| RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| R-3.6.3 | R Foundation for Statistical Computing | https://www.r-project.org/ |
| SPSS statistics 23.0 | IBM Corporation | http://www.spss.com.hk/software/statistics/ |
| Adobe illustrator CC 2019 | Adobe company | https://www.adobe.com/cn |
| Coxme-2.2.16 | Therneau et al. | https://cran.r-project.org/web/packages/coxme/index.html |
| Tableone-0.11.1 | Kazuki Yoshida | https://github.com/kaz-yos/tableone |
| Survival-3.1-12 | Terry M Therneau et al. | https://cran.r-project.org/web/packages/survival/index.html |
| Ggplot-3.3.2 | Hadley Wickham et. al. | https://cran.r-project.org/web/packages/ggplot2/index.html |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Hongliang Li (lihl@whu.edu.cn).
Materials Availability
The study did not generate any new reagents or materials.
Data and Code Availability
Data and codes involved in this research are available from the corresponding author upon reasonable request. The research team will provide an email address for communication once the information sharing are approved. The proposal should include detailed aims, statistical plan, and other information/materials to guarantee the rationality of requirement and the security of the data. The related patient data will be shared after review and approval of the submitted proposal and any related requested materials. Of note, data with patient names, national identification number, and other identifiers cannot be shared.
Method Details
Study Design and Participants
This was a multicenter retrospective longitudinal cohort study, including 15,512 adults with confirmed COVID-19 who were diagnosed from December 30, 2019, to April 17, 2020, and admitted to 17 participating COVID-19 designated hospitals in Hubei province. The study procedures were approved by the institution ethic committee of Renmin Hospital of Wuhan University and Zhongnan Hospital of Wuhan University, and were accepted or approved by each local collaborating hospitals. This retrospective study just extracted and analyzed existing data without change of individuals’ intervention or welfare, and patient informed consent was thus waived by each ethics committee. The main inclusion and exclusion criteria for the present study were depicted in Figure 1. 820 cases transferred to other hospitals, 4040 patients aged > 85 or < 18 years, 165 cases with pregnancy, 10 cases with the acute fatal disease, 681 cases with a medical history of kidney diseases (599 with chronic kidney diseases of unknown causes, 16 with chronic glomerulonephritis, 3 with chronic pyelonephritis, 5 with diabetic nephropathy, 1 with hypertensive nephropathy, 4 with nephrotic syndrome, and 53 with kidney stones or cysts that might affect kidney function, kidney malformation, tumor, or transplant), and 1,023 patients without any test of three kidney markers during 28-day hospitalization were excluded. Patients with kidney stones or cysts but without renal dysfunction were not excluded. At last, 12,413 cases met the eligibility criteria and were enrolled in further analysis.
Data Collection and Definition
We extracted clinical characteristics from each patient’s clinical electronic medical records, nursing records, laboratory findings, and radiological examinations. A trained team of experienced physicians reviewed the data. The diagnosis and severity of COVID-19 were determined based on the guidelines for diagnosis and treatment of COVID-19 (trial fifth edition) published by the Chinese National Health Commission.31 The cases were stratified into non-severe, severe (non-death) and death groups. In details, patients with severe disease were defined as with fever or suspected respiratory infection, plus clinical manifestations as either: (1) respiratory rate > 30 breaths/min, or severe respiratory distress, or (2) SpO2 ≤ 93%, or (3) PaO2/FiO2 ≤ 300 mmHg on room air. Patients diagnosed as COVID-19 but without above mentioned symptoms were classified into the “non-severe” group. AKI was defined by an increase in serum creatinine by 26.5 mmol/l within 48 hours according to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group.32 The urine output was not applied for defining AKI. The initiation of RRT and CRRT was defined as KDIGO AKI stage 2 that was defined by a 2-fold increase in serum creatinine compared with baseline.33 , 34 Disseminated intravascular coagulation (DIC) was defined according to the criteria defined by the International Society on Thrombosis and Hemostasis (ISTH).35 Acute respiratory distress syndrome (ARDS) and septic shock were defined according to the World Health Organization (WHO) interim guideline.36 The increase of BUN, creatinine and uric acid was defined as their level higher than their upper limit of normal (ULN), while the decrease of those markers means lower than their lower limit of normal (LLN). The ULN and LLN of BUN, Scr, and BUA were provided in Table S1. The primary endpoint was defined as 28-day all-cause death of COVID-19, and the secondary endpoints included the occurrence of DIC, septic shock, and ARDS.
Quantification and Statistical Analysis
Categorical variables were presented as absolute numbers (percentages). Continuous variables were described as median (interquartile range, IQR). Analysis of Variance for comparison of means or Kruskal-Wallis test for comparison of medians was used for continuous variables and Fisher’s exact test for categorical variables. Dynamic trajectories of renal parameters were established using Loess. The proportional hazard assumptions were verified by correlation testing based on the Schoenfeld residuals. Mixed-effect Cox regression models were used to investigate the relationship between renal index levels and death among COVID-19 patients. Age, gender, coexisting chronic diseases (hypertension, coronary heart disease, and diabetes) were adjusted, and hospital site was treated as a random effect in this model. Logistic regression was conducted to assess the effect of clinical characteristics and laboratory indexes on the level of renal indicators. A two-sided P value of less than 0.05 was used as the threshold of statistical significance. All analyses were conducted using SPSS version 23.0 (IBM, Armonk, NY, USA) or R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgments
Author Contributions
Y.-M.L., J.X., M.-M.C., and Xiao Zhang designed the study, collected and analyzed the data, and wrote the manuscript. X.C., H.L., F.Z., J.-J.Q., and F.L. collected the data and performed the statistical analysis. Z.C., J.C., and X.-J.Z. wrote the manuscript and provided valuable suggestions for study. L.L., C.Y., W.M., G.C., H.L., X.X., D.W., X.L., J.Y., X.H., B.-H.Z., and Y.Y. collected and reviewed the clinical, laboratory, and radiological data. Y.W., Xin Zhang, Z.-G.S., and H.L. contributed equally, designed the project, edited the manuscript, and supervised the study. All of the authors approved the final version of the article.
Declaration of Interests
The authors declare no competing interests.
Published: October 2, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.medj.2020.09.001.
Supplemental Information
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Y.Y., Zheng Y., Cai G.Y., Chen X.M., Hong Q. Single-cell RNA sequencing data suggest a role for angiotensin-converting enzyme 2 in kidney impairment in patients infected with 2019-novel coronavirus. Chin. Med. J. (Engl.) 2020;133:1129–1131. doi: 10.1097/CM9.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The Novel Coronavirus 2019 Epidemic and Kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D., Northwell C.-R.C., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu V.C., Huang J.W., Hsueh P.R., Yang Y.F., Tsai H.B., Kan W.C., Chang H.W., Wu K.D., SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am. J. Kidney Dis. 2005;45:88–95. doi: 10.1053/j.ajkd.2004.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soleimani M. Acute Kidney Injury in SARS-CoV-2 Infection: Direct Effect of Virus on Kidney Proximal Tubule Cells. Int. J. Mol. Sci. 2020;21:3275. doi: 10.3390/ijms21093275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macedo E. Blood urea nitrogen beyond estimation of renal function. Crit. Care Med. 2011;39:405–406. doi: 10.1097/CCM.0b013e318205c33a. [DOI] [PubMed] [Google Scholar]
- 12.Cauthen C.A., Lipinski M.J., Abbate A., Appleton D., Nusca A., Varma A., Goudreau E., Cowley M.J., Vetrovec G.W. Relation of blood urea nitrogen to long-term mortality in patients with heart failure. Am. J. Cardiol. 2008;101:1643–1647. doi: 10.1016/j.amjcard.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 13.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudit G.Y., Pfeffer M.A. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur. Heart J. 2020;41:1818–1820. doi: 10.1093/eurheartj/ehaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren D., Ren C., Yao R.Q., Feng Y.W., Yao Y.M. Clinical features and development of sepsis in patients infected with SARS-CoV-2: a retrospective analysis of 150 cases outside Wuhan, China. Intensive Care Med. 2020;46:1630–1633. doi: 10.1007/s00134-020-06084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thum T. SARS-CoV-2 receptor ACE2 expression in the human heart: cause of a post-pandemic wave of heart failure? Eur. Heart J. 2020;41:1807–1809. doi: 10.1093/eurheartj/ehaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 20.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 21.Werion A., Belkhir L., Perrot M., Schmit G., Aydin S., Chen Z., Penaloza A., De Greef J., Yildiz H., Pothen L., et al. SARS-CoV-2 Causes a Specific Dysfunction of the Kidney Proximal Tubule. Kidney Int. 2020 doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo T., Liu Q., Zhang F., Lui G.C., Tso E.Y., Yeoh Y.K., Chen Z., Boon S.S., Chan F.K., Chan P.K., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A., Cheung C.P., Chen N., et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiaro T.R., Soto R., Zac Stephens W., Kubinak J.L., Petersen C., Gogokhia L., Bell R., Delgado J.C., Cox J., Voth W., et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017;9:eaaf9044. doi: 10.1126/scitranslmed.aaf9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z., Zhang J., Wang Z., Ang K.Y., Huang S., Hou Q., Su X., Qiao J., Zheng Y., Wang L., et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016;6:20602. doi: 10.1038/srep20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan L., Han P., Ma S., Peng R., Wang C., Kong W., Cong L., Fu J., Zhang Z., Yu H., et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sin. B. 2020;10:249–261. doi: 10.1016/j.apsb.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagnacci A. Age-related difference in the rate of COVID-19 mortality in women versus men. Am. J. Obstet. Gynecol. 2020;223:453–454. doi: 10.1016/j.ajog.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garovic V.D., August P. Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side. J. Am. Soc. Nephrol. 2016;27:2921–2924. doi: 10.1681/ASN.2016040454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Health Commission of China . National Health Commission of China; 2020. New Coronavirus Pneumonia Prevention and Control Program. [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- 33.Vinsonneau C., Allain-Launay E., Blayau C., Darmon M., Ducheyron D., Gaillot T., Honore P.M., Javouhey E., Krummel T., Lahoche A., et al. Renal replacement therapy in adult and pediatric intensive care: recommendations by an expert panel from the French Intensive Care Society (SRLF) with the French Society of Anesthesia Intensive Care (SFAR) French Group for Pediatric Intensive Care Emergencies (GFRUP) the French Dialysis Society (SFD) Ann. Intensive Care. 2015;5:58. doi: 10.1186/s13613-015-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarbock A., Kellum J.A., Schmidt C., Van Aken H., Wempe C., Pavenstädt H., Boanta A., Gerß J., Meersch M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 35.Gando S., Wada H., Thachil J., Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis (ISTH) Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS) J. Thromb. Haemost. 2013;11:826–835. doi: 10.1111/jth.12190. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and codes involved in this research are available from the corresponding author upon reasonable request. The research team will provide an email address for communication once the information sharing are approved. The proposal should include detailed aims, statistical plan, and other information/materials to guarantee the rationality of requirement and the security of the data. The related patient data will be shared after review and approval of the submitted proposal and any related requested materials. Of note, data with patient names, national identification number, and other identifiers cannot be shared.


