SEVERE RESPIRATORY syndrome coronavirus-2 is responsible for coronavirus disease 2019 (COVID-19), which may lead to acute respiratory failure. In its most severe manifestation including refractory hypoxemia and/or hypercapnia, it can require escalation to extracorporeal membrane oxygenation (ECMO). Rates of thromboembolic complications are elevated in COVID-19 patients despite deployment of thromboprophylaxis.1 Among the sickest COVID-19 patients requiring ECMO, much less is known regarding hemostatic derangements, as well as the resulting risk profile for the development of both thromboembolic and hemorrhagic complications. Thus, defining the ideal pharmacologic anticoagulant approach (agent, intensity, monitoring) for these critically ill patients remains a challenge. A recent case series by Usman et al. highlighted safety issues encountered in a cohort of 10 COVID-19 patients cannulated for venovenous ECMO (VV-ECMO) and subsequently anticoagulated with unfractionated heparin (three patients) or argatroban (one patient).2 Of these, Usman et al. reported hemorrhagic strokes in four patients despite modest activated partial thromboplastin time (aPTT) reflective of typical levels of anticoagulation for maintenance of mechanical support (mean aPTT without stroke 41.8 seconds v 52.8 seconds with stroke, p = 0.09) and, in all affected individuals, platelet counts greater than 130,000 per µL with fibrinogen counts >250 mg/dL. Similarly, systolic blood pressures appeared largely within normal limits preceding the diagnosis of intracranial hemorrhage (ICH). ICH in COVID-19 previously has been reported with a yet-to-be determined incidence or underlying etiology.3 , 4 The high cerebrovascular event rate seen by Usman et al. exceeded the incidence previously reported in non–COVID-19 ECMO patients, suggestive of unique aspects in this population that elevate the risk of this dreaded complication.5
COVID-19 is understood to cause endothelial dysregulation leading to thrombosis, but also an increased risk of bleeding in certain situations. The pathogenesis initially requires mechanisms of entry and action of the virus mediated by the binding of a surface glycoprotein, known as spike, to angiotensin-converting enzyme two.6 Subsequent entry of the virus can cause cytopathologic changes at the epithelial alveolo-capillary interface, including disruption of intercellular junctions, cellular swelling, and detachment from the basal membrane.7 Exposure of potent activators of the hemostatic cascade produces downstream activation of thrombin and, secondarily, the production of fibrin. Catalyzing the propagation of clot generation is the thrombin receptor–mediated activation of platelets and degranulation of dense granules containing polyphosphates. It may be at this juncture that the linkage between the coagulation cascade and the immune system exacerbates the hyper-immune state of COVID-19. Under physiologic conditions, the endothelium will use mechanisms to counteract clot generation, including prostacyclin and tissue plasminogen activator secretion, to prevent vascular thrombus formation. In contrast, pathophysiologic conditions may perturb this delicate balance through the secretion of plasminogen activator inhibitor-1 by endothelial cells, leading to the opposite effect of anti-fibrinolysis.8 Thus, anticoagulation strategies in COVID-19 patients frequently are deployed as chemoprophylaxis against the evolution of these interdependent cascades.
In those critically ill patients requiring extracorporeal support, thromboembolic events, in addition to high rates of hemorrhagic complications, have been reported, including those delineated by Usman et al. These findings underscored the complex clinical dilemmas encountered in this vulnerable patient population and may point to an unknown interaction between the hypercoagulability of COVID-19 and the ECMO circuitry. As Usman et al. suggested, viscoelastic testing (thromboelastography [TEG], rotational thromboelastometry) has emerged as a useful monitoring adjunct for the detection of coagulation abnormalities, including fibrinolysis, in patients requiring ECMO.9 , 10 Abnormalities in coagulation metrics, as identified by TEG, are common. A recent retrospective study by Yuriditsky et al. of 64 COVID-19 patients in New York City assayed with TEG (Haemonetics, Boston, MA) identified rates of a clotting index in the hypercoagulable range in 50% of the patients and 31% encountered thromboembolic events.11 Subtle laboratory value disturbances may precede critical clinical events, including intracranial hemorrhage, and viscoelastic evidence of hyperfibrinolysis may even be present in patients with numerically normal fibrinogen levels.12
At the time of this writing, in the authors’ center's admittedly small cohort of 7 COVID-19 patients requiring VV-ECMO with a combined total of 154 ECMO days, hyperfibrinolysis was detected (reference range Lysis30 >4.8% on TEG) in four individual patients, generating 11 discrete occurrences (nine of whom had Lysis30 >8). All pulsatile ECMO patients at the authors’ center routinely are anticoagulated with bivalirudin, including all COVID-19 patients to date.13 All episodes responded to escalations in anticoagulation intensity (up titration of bivalirudin infusion to target an aPTT of 70-90 seconds) except one patient who required the administration of an anti-fibrinolytic agent, in this case tranexamic acid (TXA). TXA reduces the conversion of plasminogen to plasmin, thereby preventing fibrin degradation and preserving the fibrin matrix structure (Fig 1 ). Dosing for TXA leveraged an existing ECMO-specific antifibrinolytic therapy protocol (Fig 2 ) involving a bolus of one g followed by initiation of a continuous infusion at 2 mg/kg/h. In all patients, the remainder of coagulation studies remained within normal parameters barring the expected elevation in aPTT while systemically anticoagulated. In one patient, elevated Lysis30 preceded low fibrinogen levels by one day. Importantly, no patients developed evidence for thromboembolic or hemorrhagic complications, including intracranial hemorrhage or deep vein thrombosis/pulmonary embolism. Further, throughout all the patients’ ECMO runs thus far, no need has manifested for targeted circuit interventions including oxygenator change out, circuit exchange, or circuit reconfiguration. Interpretation of these findings must be tempered given the small cohort, absence of uniform investigative studies (such as computerized tomography scans, Doppler ultrasounds), and the lack of control groups (examples of which would include an anticoagulant strategy based on unfractionated heparin or a subgroup foregoing routine TEG monitoring). Fortunately, clinically significant thromboembolic or hemorrhagic complications did not manifest in any of these patients. Given the inability to provide causation in this context, the clinical significance of the isolated elevation in Lysis30 remains unclear. Other variables may have contributed to these findings, including variations in anticoagulation strategy and intensities, co-administered antiplatelet agents, immunosuppressive therapies, and individual patient risk factors.
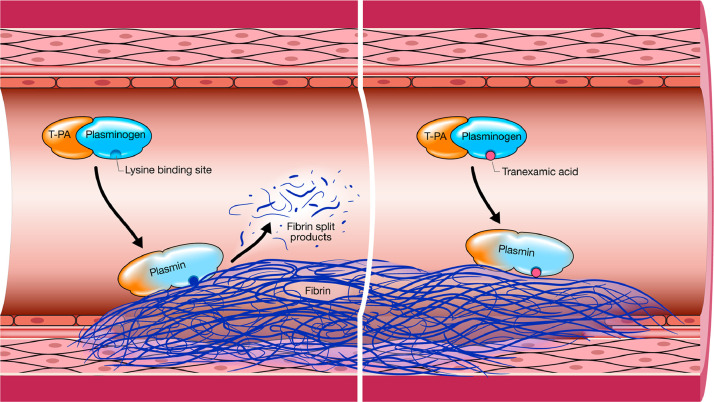
Fig 1.
Depiction of the intravascular cleavage of insoluble fibrin by plasmin thereby releasing fibrin split products, of which D-dimer is one type. The mechanism of action of intravascular TXA is also depicted on the right panel. TXA is a synthetic analog of lysine that reversibly binds to the lysine receptor site on plasminogen to decrease the conversion of plasminogen to plasmin. Used with permission of Mayo Foundation for Medical Education and Research.
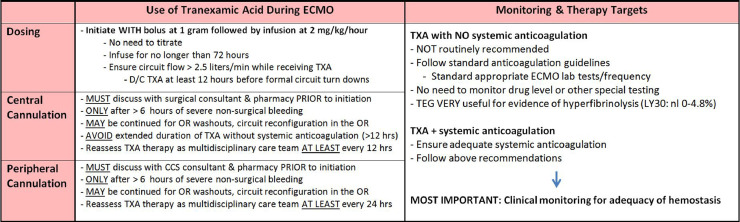
Fig 2.
Excerpt from the Mayo Clinic Adult ECMO Tranexamic Acid Dosing Guideline. Used with permission of Mayo Foundation for Medical Education and Research.
The agent selected to provide systemic anticoagulation may represent a relevant therapeutic intervention in the COVID-19 era. Clot-bound thrombin autocatalysis furthers deposition of fibrin through feedforward amplification pathways, thereby yielding clot expansion over time. Direct thrombin inhibitors, such as bivalirudin, abate the action of thrombin in circulating and clot-bound sites. Acute respiratory distress syndrome, including that as induced by severe respiratory syndrome coronavirus-2, can result in the excessive creation of thrombin,14 thereby upregulating the previously discussed interplay between the immune and thrombotic cascades. This critical step can be moderated by a titrated dose of a direct thrombin inhibitor and represents, in the authors’ view, a postulated clinical advantage warranting further investigation.
To expand on the genesis and importance of the concept of fibrinolysis as it relates to ECMO, further explanation is herein provided. As discussed, COVID-19 induces significant endothelial dysfunction, resulting in a prothrombotic and anti-fibrinolytic state, the latter of which is due to increased plasminogen activator inhibitor-1 secretion.8 Although anticoagulation may have a therapeutic role in COVID-19 patients per se, the addition of ECMO complicates the overall picture and might tip the scale toward hyperfibrinolysis in select circumstances. It is accepted that all patients will be met with varying rates of fibrinolysis, which is present as an obligatory homeostatic mechanism often conceptualized as a “housekeeping” requirement to mitigate progressive thrombotic deposition that can complicate activation of the hemostatic cascade. Simply put, it counteracts progressive accumulation of fibrin clot through enzymatic cleavage into fibrin degradation products (FDP). Reflective of the regulatory nature of these cascades is the presence of modestly elevated D-dimer levels in adult ECMO patients.15 Intriguingly, the COVID-19 literature has within it numerous reports of elevated D-dimers, which represents the most frequently assayed FDP, and are generated during enzymatic fibrin cleavage by plasmin.1 , 16 D-dimer levels in COVID-19 patients serve as biomarkers, with elevated levels being associated with increased disease severity and higher in-hospital mortality.17
Implicit in the identification of FDPs, including D-dimers, is the action of the fibrinolytic cascade without which the generation of FDPs would be impossible. Given the frequently long ECMO runs required in COVID-19 patients superimposed on the underlying prothrombotic state18 and, despite appropriate systemic anticoagulation, it is plausible that fibrinolysis may advance in intensity to the point of hyperfibrinolysis during mechanical support in this population. This is reflected in the cohort of COVID-19 patients on ECMO anticoagulated with bivalirudin infusion whereby hyperfibrinolysis was detected in 7% of all routine daily assays. This rate was similar to the 5% recently reported by Yuriditsky et al. in COVID-19 patients without ECMO receiving enoxaparin or unfractionated heparin thromboprophylaxis.11
Routine viscoelastic monitoring may help detect subclinical coagulation abnormalities before the development of adverse events. This simple statement conceals the true advantage of this diagnostic approach. Of the various coagulation derangements as they relate to viscoelastic testing, in contemporary clinical practice fibrinolysis is unique because it may be ascertained by laboratory evaluation in no other readily available manner. An indirect and less-precise assessment of fibrinolysis can be made using D-Dimers, as recently reported by Besser et al.19 Once clinically significant hyperfibrinolysis is identified, the subsequent deployment of treatment strategies to mitigate either the cause or to abate the enzymatic cascade with antifibrinolytic pharmacotherapies, represents a potentially powerful strategy to optimize care for this high-risk population. The authors’ center previously reported on the use of point-of-care TEG to identify hyperfibrinolysis during ECMO and, perhaps more interestingly, to titrate TXA dosing to effect as defined by laboratory (resolution of elevated Lysis30) and clinical (denoted by abatement of hemorrhage) findings.20
Given the authors’ positive experience with regard to the routine use of TEG in COVID-19 patients receiving VV-ECMO therapy, they support Usman et al. in their call for the integration of viscoelastic testing as a routine practice in these patients. It is the authors’ perspective that it is through the integration of diagnostic assessment of viscoelastic testing with dedicated management schemes, up to and including the titration of antifibrinolytic therapies, that unmasks the capacity of this technology to enhance clinical outcomes. In addition, the authors encourage further research leveraging direct thrombin inhibitors during ECMO given its effective and predictable anticoagulation performance,21, 22, 23 as well as the high incidence of heparin-induced thrombocytopenia in these patients.24 Additionally, ideally, prospective studies examining the incidence and risk factors for the manifestation of hyperfibrinolysis, as well as the ideal anticoagulation and monitoring strategy as it relates to COVID-19 patients on ECMO, will be needed to define best practice approaches for this challenging population.
Conflict of Interest
All authors declare no conflicts of interest.
Footnotes
No funding or sponsorship was received for this manuscript, including manuscript design or any process in manuscript creation.
Standard Health Insurance Portability and Accountability Act documentation and consent for use of the patient's medical record for research purposes were obtained from the patients in accordance with Mayo Clinic policy.
References
- 1.Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. doi: 10.1097/CCM.0000000000004548. Accessed September 7, 2020. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 2.Usman A.A., Han J., Acker A. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2020;34:3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sy E., Sklar M.C., Lequier L. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care. 2017;39:87–96. doi: 10.1016/j.jcrc.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: Causative or coincidental? New Microbes New Infect. 2020;35 doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas J., Kostousov V., Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018;44:20–29. doi: 10.1055/s-0037-1606179. [DOI] [PubMed] [Google Scholar]
- 6.Huertas A., Montani D., Savale L. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koster A., Huebler S., Potapov E. Impact of heparin-induced thrombocytopenia on outcome in patients with ventricular assist device support: Single-institution experience in 358 consecutive patients. Ann Thorac Surg. 2007;83:72–76. doi: 10.1016/j.athoracsur.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 10.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuriditsky E., Horowitz J.M., Merchan C. Thromboelastography profiles of critically ill patients with coronavirus disease 2019. Crit Care Med. 2020;48:1319–1326. doi: 10.1097/CCM.0000000000004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster A., Ljajikj E., Faraoni D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann Cardiothorac Surg. 2019;8:129–136. doi: 10.21037/acs.2018.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seelhammer TG, Rowse P, Yalamuri S. Bivalirudin for maintenance anticoagulation during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. doi: 10.1053/j.jvca.2020.06.059. Accessed September 7, 2020. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 14.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: Cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 15.Mazzeffi M., Strauss E., Meyer M. Coagulation factor levels and underlying thrombin generation patterns in adult extracorporeal membrane oxygenation patients. Anesth Analg. 2019;129:659–666. doi: 10.1213/ANE.0000000000004275. [DOI] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y., Cao J., Wang Q. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization COVID-19 interim guidelines. ASAIO J. doi: 10.1097/MAT.0000000000001193. Accessed July 29, 2020. [e-pub ahead of print]. [DOI]
- 19.Besser V., Albert A., Sixt S.U. Fibrinolysis and the influence of tranexamic acid dosing in cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34:2664–2673. doi: 10.1053/j.jvca.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Seelhammer T.G., Mangla J., Demirci O. The use of thromboelastography to titrate tranexamic acid therapy for abatement of lysis-induced hemorrhagic complications during venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2019;33:1059–1062. doi: 10.1053/j.jvca.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Hamzah M., Jarden A.M., Ezetendu C. Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;21:827–834. doi: 10.1097/PCC.0000000000002384. [DOI] [PubMed] [Google Scholar]
- 22.Pieri M., Agracheva N., Bonaveglio E. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vasc Anesth. 2013;27:30–34. doi: 10.1053/j.jvca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Ranucci M., Ballotta A., Kandil H. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011;15:R275. doi: 10.1186/cc10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parzy G., Daviet F., Puech B. Venous thromboembolism events following venovenous extracorporeal membrane oxygenation for severe acute respiratory syndrome coronavirus 2 based on CT scans. Crit Care Med. 2020;48:e971–e975. doi: 10.1097/CCM.0000000000004504. [DOI] [PMC free article] [PubMed] [Google Scholar]