Abstract
Procyanidins are polymeric flavan-3-ones occurring in many plants with antioxidant and other beneficial bioactivities. Composed of catechin and epicatechin monomeric units connected by single carbon-carbon B-type linkages or A-type linkages containing both carbon-carbon and carbon-oxygen-carbon bonds. Their polymeric structure makes analysis of procyanidin mixtures always difficult. Evaluation of procyanidins according to degree of polymerization (DP) using HPLC is time consuming and at best has resolved polymeric families up to DP-17. To expedite studies of procyanidins, the utility of positive ion electrospray ion mobility-mass spectrometry (IM-MS) was investigated for the rapid separation and characterization of procyanidins in mixtures. Applying IM-MS to analyze structurally defined standards containing up to 5 subunits, procyanidins could be resolved in less than 6 ms not only by degree of polymerization but also by linkage type. A-type procyanidins could be resolved from B-type and both could be at least partially resolved from mixed-type procyanidins of the same DP. IM-MS separated higher order procyanidins with DP of at least 24 from extracts of cranberry. As DP increased, the abundances of multiply charged procyanidins also increased. During IM-MS of ions of similar m/z, the ion drift times decreased inversely with increasing charge state. Therefore, IM-MS was shown to separate mixtures of procyanidins containing at least 24 interconnected subunits in less than 16 ms, not only according to DP, but also according to linkage type between subunits, and charge state.
Keywords: procyanidin, ion mobility, mass spectrometry
INTRODUCTION
Procyanidins, a subgroup of the proanthocyanidins which constitute the second largest group of natural products after lignans (1), occur naturally in plant-based foods such as fruits, vegetables, nuts and grains (2-4). In addition to antioxidant activity, procyanidins have been reported to have antiviral, antibacterial, anti-inflammatory, and anticarcinogenic therapeutic benefits (5-11). Composed of catechin and epicatechin monomeric units, procyanidins are polymeric flavan-3-ols with two linkage types, the common B-type and the less common A-type, between monomeric units (Figure 1). While B-type linkages consist of a single carbon-carbon bond, the A-type linkage consists of a carbon-carbon bond in addition to a carbon-oxygen bond between adjacent subunits
Figure 1.
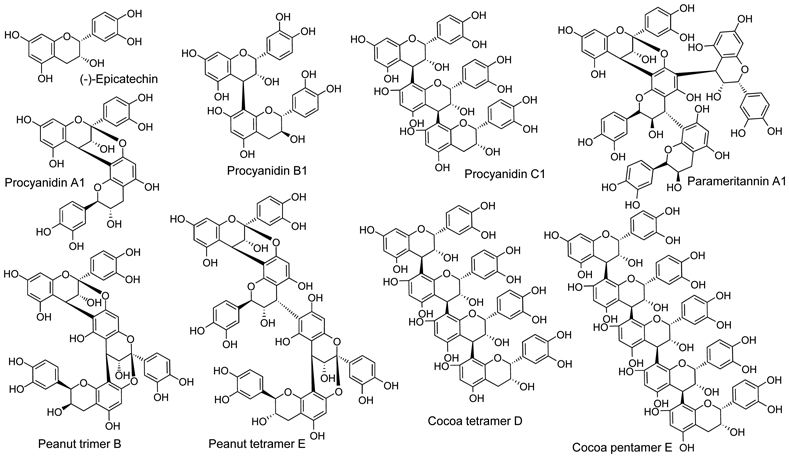
Chemical structures of procyanidin standards analyzed using IM-MS.
Mixed-type procyanidins contain combinations of B-type and A-type linkages between monomeric units (12,13). Furthermore, the polymeric chains of procyanidins are usually linear but can be branched. The incorporation of A-type linkages can add rigidity and stability to the compound (14). Due to the complexity of these molecules and their occurrence in mixtures, analysis can be challenging and separation using chromatographic approaches is nearly always time consuming.
Current approaches for procyanidin analysis include several modalities of liquid chromatography for separation followed by electrospray or MALDI mass spectrometry and NMR for structure elucidation (15-22). The primary limitations of these methods have been the time required for chromatographic isolation of procyanidins as well as the large amount of material required for NMR compared with mass spectrometry. Taking advantage of the selectivity, speed and sensitivity of mass spectrometry, we investigated the feasibility of using ion mobility spectroscopy instead of liquid chromatography for faster procyanidin separation coupled on-line with electrospray mass spectrometric analysis.
During ion mobility, separation of ions moving through an inert gas in a weak electric field depends on their mass, charge and shape (23-25). Separations can be achieved in milliseconds with ions of small collision cross sections traveling faster than larger ions and multiply charged ions travelling faster than ions of lower charge states. Different ion mobility techniques are available (26-28), and traveling wave ion mobility was utilized during this investigation (29,30). In addition to speed, another advantage of ion mobility is the ability to separate and distinguish isomers with similar mass spectra (31,32), which can be useful in studies of polymeric procyanidins. Rapid analysis and high sensitivity can make IM-MS ideal for the analysis of procyanidins.
MATERIALS AND METHODS
Materials
Procyanidin standards, which had been purified from cocoa, cinnamon, peanut skins, cranberries, and crab apples were obtained from Planta Analytica (New Milford, CT). The chemical structures of procyanidin standards were determined using mass spectrometry and NMR. Procyanidin mixtures, which had been partially purified from cranberry, were also obtained from Planta Analytica. All solvents were HPLC-grade and were purchased from Thermo Fisher (Pittsburgh, PA).
Instrumentation
Positive ion electrospray ion mobility mass spectrometry was carried out using a Waters (Milford, MA) Synapt G1 quadrupole time-of-flight mass spectrometer equipped with travelling wave ion mobility. Individual procyanidins or mixtures were infused into the ion source in methanol/water (50:50, v/v) containing 0.1% formic acid using a syringe pump at a flow rate of 10 μL/min. Solutions of procyanidin standards and procyanidin mixtures from cranberry were prepared at 1 μg/μL. Instrument parameters were as follows: capillary voltage, 3.6 kV; sample cone voltage, 26 V; extraction cone voltage, 4 V; desolvation gas (nitrogen) flow, 500 L/h; trap collision energy, 6 V; transfer collision energy, 4 V; trap gas (argon) flow, 5 mL/min; source temperature, 120 °C; and desolvation temperature, 200 °C. The traveling wave velocity was held constant at 300 m/s while the wave height was increased from 10 – 30V. The IM gas was nitrogen at a flow rate of 20 mL/min. Data were acquired using Waters MassLynx software (version 4.1) and DriftScope software (version 2.1).
RESULTS AND DISCUSSION
Positive ion electrospray IM-MS separation of procyanidins based on degree of polymerization was investigated using equimolar mixtures of linear B-type procyanidins (Figure 2). Epicatechin dimers, trimers, tetramers, and pentamers were each separated by up to a millisecond, while the drift time of the monomer was only slightly less than that of the dimer. Specifically, the drift times of monomeric epicatchin and dimeric procyanidin B1 were 1.10 ms and 1.13 ms, respectively, while the drift times of trimeric procyanidin C1, cocoa tetramer D, and cocoa pentamer E were 1.71 ms, 2.62 ms and 3.60 ms, respectively. Although the epicatechin monomer and B-type procyanidin dimer B1 were only partially resolved by drift time using these IM-MS conditions optimized for higher order procyanidins, they could be distinguished easily by mass. Note that alternative IM-MS parameters could resolve epicatechin and procyanidin dimer B1 (data not shown), but these conditions were not optimum for resolving the higher order procyanidins. The most abundant signals for all procyanidins during positive ion electrospray corresponded to their sodium adducts, [M+Na]+, of m/z 313 (epicatechin), m/z 601 (procyanidin B1), m/z 889 (procyanidin C1), m/z 1177 (cocoa tetramer D)), and m/z 1465 (cocoa pentamer E).
Figure 2.
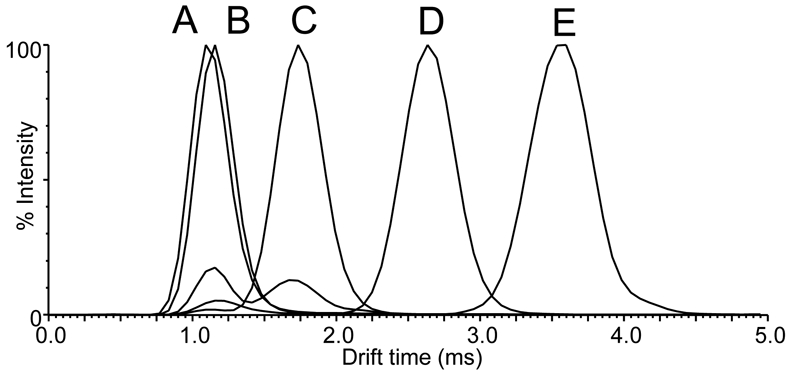
Positive ion electrospray ion mobility drift time distributions based on degree of polymerization for a mixture of B-type procyanidins. A) epicatechin (monomer); B) procyanidin B1 (dimer); C) procyanidin C1 (trimer); D) cocoa tetramer D; and E) cocoa pentamer E. Note that these procyanidins were detected as sodium adducts, [M+Na]+.
To assess IM-MS separation of procyanidins of different linkage types as well as different degrees of polymerization, a mixture of six compounds including peanut and cocoa dimers, trimers, and tetramers were analyzed (Figure 3). These compounds included A-type (procyanidin A1 and peanut trimer B), B-type (dimeric procyanidin B1, trimeric procyanidin C1, and cocoa tetramer D), and mixed A and B-type linkages (linear peanut tetramer E containing two A-type bonds and one B-type linkage) (Figure 1). All 6 procyanidins were resolved in less than 6 ms. B-type procyanidin dimers, trimers and tetramers were each separated by more than 1 ms, while A-type and B-type procyanidins of equal degrees of polymerization were partially resolved. For example, the B-type procyanidin cocoa trimer C1 had a shorter drift time (2.9 ms) than did the A-type peanut trimer B (3.4 ms), and the linear B-type procyanidin cocoa tetramer D had a shorter drift time (4.1 ms) than did the mixed type peanut tetramer E (4.6 ms). The drift times of the linear B-type procyanidins were always smaller than the linear A-type and mixed type procyanidins of identical degree of polymerization (Figure 3). The extra bond between subunits of A-type procyanidins increased their rigidity compared with B-type procyanidins and thereby increased collision-cross section and ion mobility drift time.
Figure 3.
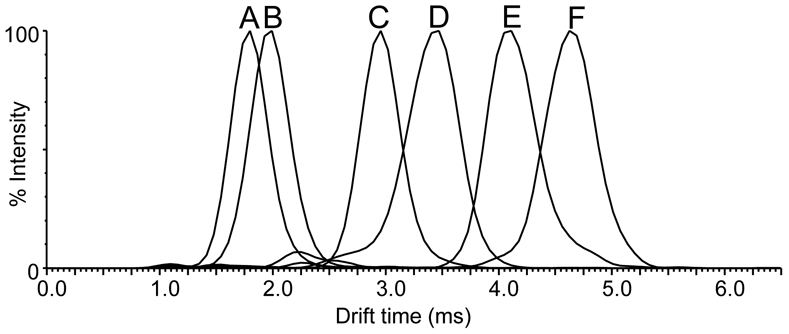
Positive ion electrospray IM-MS drift time distributions of A-type and B-type procyanidins by degree of polymerization as well as by linkage type. A) procyanidin B1 (B-type dimer), m/z 601; B) procyanidin A1 (A-type dimer), m/z 599 C) procyanidin C1 (B-type trimer), m/z 889 D) peanut trimer B (A-type), m/z 885; E) cocoa tetramer D (B-type), m/z 1177; and F) peanut tetramer E (mixed type), m/z 1173. Each procyanidin signal is shown as the singly-charged sodium adduct.
One branched procyanidin, parameritannin A1, containing 4 epicatechin subunits and one A-type linkage (Figure 1), was evaluated using IM-MS in a mixture of linear B-type and mixed-type procyanidin tetramers (data not shown). The drift time of branched parameritannin was ~0.1 ms less than that of the linear B-type cocoa tetramer D, which was 0.5 ms less that A-type peanut tetramer E (Figure 3). Although the compact branched structure of parameritannin resulted in a shorter drift time than those of linear procyanidins of comparable mass, the presence of one A-type linkage added rigidity to the structure resulting in a drift time only slightly shorter than the linear B-type cocoa tetramer D.
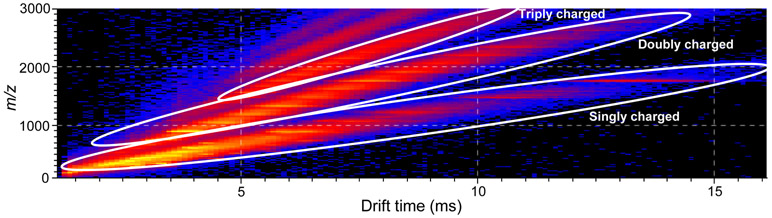
As an application of IM-MS to a complex botanical extract, a procyanidin-rich fraction of a cranberry extract was analyzed, and a two-dimensional heat map plot of m/z value vs drift time is shown in Figure 4. Singly-charged procyanidins were detected with degrees of polymerization up to 8 with drift times from 2 to 16 ms. Examination of the corresponding positive ion electrospray mass spectra indicated that the procyanidins in this fraction were predominantly mixed-type and contained only one A-type linkage per molecule (Figure 5). For example, the singly-charged procyanidin at a drift time of 12.6 ms was detected as a sodium adduct of m/z 1751 corresponding to a hexamer containing 4 B-type single bonds and 1 A-type linkage (Figure 5B).
Figure 4.
Positive ion electrospray IM-MS analysis of a cranberry extract plotted using DriftScope showing m/z value (y) vs drift time (x). Groups of abundant ions are indicated that were separated by charge state, [M+Na]+, [M+2Na]2+ and [M+3Na]3+.
Figure 5.
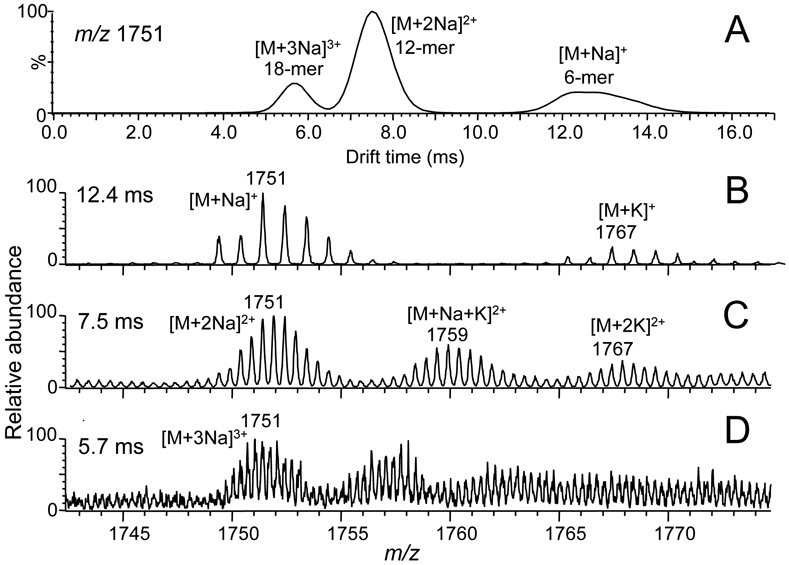
Computer-reconstructed selected ion drift time profile and corresponding mass spectra from the IM-MS analysis of the cranberry extract shown in Figure 4. A) Drift time profile for ions of nominal mass m/z 1751; B) mass spectrum of a singly charged procyanidin hexamer obtained at a drift time of 12.6 ms; C) doubly-charged procyanidin 12-mer at a drift time of 7.5 ms; and D) triply-charged procyanidin 18-mer detected at a drift time of 5.6 ms.
Plotting m/z 1751 vs drift time revealed 3 distinct peaks at 5.6, 7.5 and 12.6 ms (Figure 5A). The peak with a drift time of 12.6 ms corresponded to a singly-charged mixed-type procyanidin hexamer. Based on differences of 0.5 Da (Figure 5C) and 0.3 Da (Figure 5D) between adjacent ions, the ions of m/z 1751 at drift times of 7.5 ms and 5.6 ms corresponded to doubly-charged and triply-charged procyanidins with degrees of polymerization of 12 and 18, respectively. Although singly charged procyanidins were not observed for molecules with degrees of polymerization >8, electrospray formed doubly and triply-charged ions of higher degrees of polymerization up to at least 24 in this cranberry preparation. Furthermore, singly, doubly and triply-charged procyanidins of the same nominal mass were completely resolved from each other during ion mobility. Groups of singly, doubly and triply-charged procyanidins are indicated in the 2-D plot in Figure 4.
CONCLUSIONS
Procyanidin standards were used to demonstrate that IM-MS can resolve procyanidins by degree of polymerization as well as by linkage type. Furthermore, IM-MS may be used to separate and characterize procyanidins in milliseconds compared with minutes for LC-MS. In general, the higher the degree of polymerization of the procyanidin, the longer is the drift time; and B-type procyanidins have shorter drift times than do A-type procyanidins of comparable degrees of polymerization. During electrospray, higher order procyanidins form multiply charged ions, and IM-MS drift times become shorter as charge state increases. The use of high-resolution mass spectrometry facilitates the determination of charge states of procyanidin ions during electrospray IM-MS analysis of complex mixtures.
Acknowledgments
FUNDING INFORMATION
Funding for this research was provided by National Institutes of Health grant R01 AT007659 from the National Center for Complementary and Integrative Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagwat S, Haytowitz D. USDA Database for the Proanthocyanidin Content of Selected Foods. Release 2 2015, pp. 1–33. [Google Scholar]
- 3.Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. J. Nutr 2000, 130, 2086S–2092S. [DOI] [PubMed] [Google Scholar]
- 4.Hellström JK, Törrönen AR, Mattila PH. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem 2009, 57, 7899–906. [DOI] [PubMed] [Google Scholar]
- 5.Martin MA MA, Goya L L, Ramos S S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem Toxicol. 2013;56:336–51. [DOI] [PubMed] [Google Scholar]
- 6.Gentile C, Allegra M, Angileri F, Pintaudi AM, Livrea MA, Tesoriere L. Polymeric proanthocyanidins from sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. Eur. J. Nutr 2012, 51, 353–63. [DOI] [PubMed] [Google Scholar]
- 7.Benavente-García O, Castillo J, Marin FR, Ortuno A, Del Río JA. Uses and properties of citrus flavonoids. J. Agric. Food Chem 1997, 45, 4505–15. [DOI] [PubMed] [Google Scholar]
- 8.Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, Raul F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 2005, 26, 1291–5. [DOI] [PubMed] [Google Scholar]
- 9.Faria A, Calhau C, de Freitas V, Mateus N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem 2006, 54, 2392–7. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci 2007, 8, 950–88. [Google Scholar]
- 11.Fine AM. Oligomeric proanthocyanidin (OPCs). Altern. Med. Rev 2003, 8, 442–50. [PubMed] [Google Scholar]
- 12.Esatbeyoglu T, Jaschok-Kentner B, Wray V, Winterhalter P. Structure elucidation of procyanidin oligomers by low-temperature 1H NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 62–9. [DOI] [PubMed] [Google Scholar]
- 13.Khan ML, Haslam E, Williamson MP. Structure and conformation of the procyanidin B-2 dimer. Mag. Res. Chem 1997, 35, 854–8. [Google Scholar]
- 14.Xie D-Y, Dixon RA. Proanthocyanidin biosynthesis - still more questions than answers? Phytochemistry 2005, 66, 2127–44. [DOI] [PubMed] [Google Scholar]
- 15.Montagut G, Bladé C, Blay M, Fernández-Larrea J, Pujadas G, Salvadó MJ, Arola L, Pinent M, Ardévol A. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J. Nutr. Biochem 2010, 21, 961–7. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira N, Azevedo J, Mateus N, de Freitas V. Proanthocyanidin screening by LC-ESI-MS of portuguese red wines made with teinturier grapes. Food Chem. 2016, 190, 300–7. [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Kelm MA, Hammerstone JF, Zhang Z, Beecher G, Holden J, Haytowitz D, Prior RL. Liquid chromatographic/ electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom 2003, 38, 1272–80. [DOI] [PubMed] [Google Scholar]
- 18.Sarnoski PJ, Johnson JV, Reed KA, Tanko JM, O’Keefe SF. Separation and characterisation of proanthocyanidins in Virginia type peanut skins by LC-MSn. Food Chem. 2012, 131, 927–39. [Google Scholar]
- 19.Gu L, Kelm M, Hammerstone JF, Beecher G, Cunningham D, Vannozzi S, Prior RL. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem 2002, 50, 4852–60. [DOI] [PubMed] [Google Scholar]
- 20.Hellenbrand N, Sendker J, Lechtenberg M, Petereit F, Hensel A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [DOI] [PubMed] [Google Scholar]
- 21.Karonen M, Loponen J, Ossipov V, Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–12. [Google Scholar]
- 22.Hellström J, Sinkkonen J, Karonen M, Mattila P. Isolation and structure elucidation of procyanidin oligomers from saskatoon berries (Amelanchier alnifolia). J. Agric. Food Chem 2007, 55, 157–64. [DOI] [PubMed] [Google Scholar]
- 23.Tsao R, McCallum J. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability. Eds: de la Rosa LA, Alvarez-Parrilla E, Gonzalez-Aguilar GA), Ames, Iowa: Wiley-Blackwell; 2010, pp. 131–154 [Google Scholar]
- 24.Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev 2013, 32, 43–71. [DOI] [PubMed] [Google Scholar]
- 25.Gabelica V, Marklund E. Fundamentals of ion mobility spectrometry. Curr. Opin. Chem. Biol 2018, 42, 51–9. [DOI] [PubMed] [Google Scholar]
- 26.Schneider BB, Nazarov EG, Londry F, Vouros P, Covey TR. Differential mobility spectrometry/mass spectrometry history, theory, design optimization, simulations, and applications. Mass Spectrom. Rev. 2016, 35, 687–737. [DOI] [PubMed] [Google Scholar]
- 27.Lanucara F, Holman SW, Gray CJ, Eyers CE. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem 2014, 6, 281–94. [DOI] [PubMed] [Google Scholar]
- 28.Michelmann K, Silveira JA, Ridgeway ME, Park MA. Fundamentals of trapped ion mobility spectrometry. J. Am. Soc. Mass Spectrom 2014, 26, 14–24. [DOI] [PubMed] [Google Scholar]
- 29.Richardson K, Langridge D, Giles K. Fundamentals of travelling wave ion mobility revisited: I. Smoothly moving waves. Int. J. Mass Spectrom 2018, 428, 71–80. [Google Scholar]
- 30.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL, Bateman RH. Applications of a travelling wave-based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom 2004, 18, 2401–14. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Bendiak B, Siems WF, Gang DR, Hill HH. Determining the isomeric heterogeneity of neutral oligosaccharide-alditols of bovine submaxillary mucin using negative ion traveling wave ion mobility mass spectrometry. Anal. Chem 2015, 87, 2228–35. [DOI] [PubMed] [Google Scholar]
- 32.Yassine MM, Dabek-Zlotorzynska E. Investigation of isomeric structures in a commercial mixture of naphthenic acids using ultrahigh pressure liquid chromatography coupled to hybrid traveling wave ion mobility-time of flight mass spectrometry. J. Chromatogr. A 2018, 1572, 90–9. [DOI] [PubMed] [Google Scholar]