Abstract
HASPIN has been identified as a nuclear Ser/Thr kinase specifically expressed in haploid germ cells. HASPIN kinase inhibitors were recently isolated, and their antitumor activity reported. Colorectal cancer occurs with high incidence worldwide. In this study, we examined whether HASPIN inhibitor CHR-6494 suppresses cancer progression in ApcMin/+ mice, a familial colon tumor disease model. Mice were treated by intraperitoneal injection of CHR-6494 for 50 days. Following the treatment period, intestinal polyps were counted and testosterone and spermatogenesis levels were observed. Intraperitoneal administration of CHR-6494 significantly inhibited intestinal polyp development and recovered body weight in ApcMin/+ mice. Although spermatogenesis was inhibited with increasing age in ApcMin/+ mice, CHR-6494 significantly improved blood testosterone levels and spermatogenesis. Our results suggest that HASPIN inhibitors may be useful as anti-cancer agents and for the treatment of hypogonadism in colorectal cancer patients.
Keywords: cancer, chemoprevention, infertility, testis, testosterone
Introduction
Mouse haspin (known as Gsg2) has been cloned as a gene encoding the nuclear Ser/Thr kinase-subtracted cDNA library, derived from W/Wv mutant testis cells of wild-type (WT) testis cDNAs (Tanaka et al., 1994; Tanaka et al., 1999). HASPIN has been suggested to phosphorylate histone H3 at threonine 3 in mitotic cells (Dai et al., 2005; Dai and Higgins, 2005). HASPIN is found in the centrosomes and spindles during mitosis, where it integrates the regulation of chromosome and spindle function during mitosis and meiosis (Yu et al., 2017; Cao et al., 2019). Recently, HASPIN was detected during phosphorylation of threonine 127 on TH2A, a germ cell-specific H2A variant, in condensed spermatids and mitotic early preimplantation mouse embryos (Hada et al., 2017). To clarify the role of Haspin, haspin gene-disrupted mice were generated, but no distinct phenotype was observed (Shimada et al., 2016). Microscopic observation of the motility of haspin-disrupted sperm showed that the sperm were not abnormal, although seminiferous tubules lacking germ cells were occasionally observed (Shimada et al., 2016). Inhibitors of HASPIN kinase activity have also been reported to suppress cancer growth (Huertas et al., 2012; Kim et al., 2017; Opoku-Temeng et al., 2018). Experiments using cultured cells and haspin-disrupted mice have indicated that HASPIN functions may be compensated by other molecules in normal cells; however, HASPIN may play an important role in cell division during the proliferation of male germ cells and cancer cells.
Human colorectal cancer (CRC) is a major cause of death worldwide (Jackstadt and Sansom, 2016); although treatments such as polyp excision have been established, the development of a more effective treatment is required. Apc is a tumor-suppressing adenomatous polyposis coli gene that is involved in the earliest stage of CRC development. ApcMin/+ mice are a familial colon cancer mouse model with a nonsense mutation at codon 850 in the Apc gene (Tomita et al., 2007). APC protein forms a complex with various other proteins and is involved in the control of signal transduction pathways (Tomita et al., 2007; Jackstadt and Sansom, 2016). APC may be a tumor-suppressing gene involved in the earliest stage of CRC development. In a previous study, approximately 25–75 adenomas (polyps) were found to develop in the small intestine in ApcMin/+ mice at 160–180 days of age, with cachexia and hypogonadism, gradually progressing during tumor development (White et al., 2013).
In this study, we observed polyp formation in ApcMin/+ mice following administration of HASPIN inhibitor CHR-6494 to determine whether it can prevent cancer growth in vivo.
Methods
Animals
ApcMin/+ (C57BL/6J) mice were developed by Jackson Laboratories (Bar Harbor, Maine, USA). In this study, C57BL/6J mice were purchased from Japan SLC (Shizuoka, Japan) and sacrificed by cervical dislocation immediately before experiments. All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation and Research Ethics Committee of Nagasaki International University. This article does not contain any experiments with human subjects performed by any of the authors. Mice were maintained under specific pathogen-free conditions in the animal experimentation facility at Nagasaki International University, with temperature and lighting controlled throughout the experimental period. Mice were provided with food and water ad libitum.
Administration of CHR-6494
The experimental procedure is schematically shown in Fig. 1. ApcMin/+ males were bred with WT females, and the tails of the pups were subjected to PCR by 3 weeks of age under the conditions described in a previous study (Mochida et al., 2003). The ApcMin/+ male pups were selected. Experiments were performed on 12 ApcMin/+ males, randomized into a control group and a treatment group. In the control group, six mice were treated with a 200-µl intraperitoneal injection of a vehicle solution of 10% dimethyl sulfoxide (DMSO) and 20% 2-hydroxypropyl-b-cyclodextrin (Sigma-Aldrich, Tokyo, Japan) beginning at the age of 5 weeks. In the treatment group, six mice were treated with a 200-µl intraperitoneal injection of 50 mg/kg CHR-6494 (Cayman Chemical, Ann Arbor, Michigan, USA) diluted with a final concentration of 10% DMSO and 20% 2-hydroxypropyl-b-cyclodextrin beginning at the age of 5 weeks. The CHR-6494 (5 µg/µl) was stored in 100% DMSO at −30°C. The injectable material included 200 µl of fresh 100% 2-hydroxypropyl-b-cyclodextrin and 100 µl of 5-µg/µl CHR-6494 adjusted to 1 ml with saline. The injection volume was adjusted according to the weight of each mouse. Treatments were performed for five cycles consisting of five consecutive injection days and five consecutive naive days over a period of 50 days. The treatments followed previous drug treatment procedures (Mochida et al., 2003; Huertas et al., 2012).
Fig. 1.
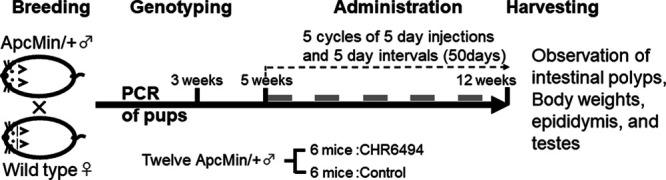
Schematic representation of the experimental procedure. Treatments were performed for five cycles consisting of five consecutive injection days (gray bars) and five consecutive naive days over a period of 50 days.
Counts of intestinal polyps
Intestinal polyps were counted according to previously published methods (Mochida et al., 2003). Freshly removed small intestines were each divided into five equal segments. These segments were incised longitudinally, washed with PBS, laid flat on filter paper, and fixed for 24 hours in 10% neutral-buffered formalin. Fixed intestinal segments were stained with 1% methylene blue and examined for tumors by gross inspection and light microscopy.
Histological observation of testes and epididymides
Bouin’s solution-fixed mouse testes were cut into 7-μm-thick sections and mounted on silane-coated slides. Slides were then stained and examined under a microscope.
Serum testosterone measurements
Serum testosterone levels were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods (Oriental Yeast Co., Ltd., Shiga, Japan).
Statistical analyses
Data are expressed as means ± SD. Means were compared between treatment groups using Student’s t-test; significant differences were determined at the level of P < 0.05.
Results
Intestinal polyps
The numbers of intestinal polyps observed in 5-week-old ApcMin/+ mice treated with and without CHR-6494 for 7 weeks are shown in Fig. 2a. Significantly fewer polyps were observed in the small intestines of mice treated with CHR-6494 (24.6 ± 13.8) than in those of control mice (58.5 ± 17.5). No special polyp, body weight, or testis phenotypes were observed in C57BL/6J WT mice treated with CHR-6494.
Fig. 2.
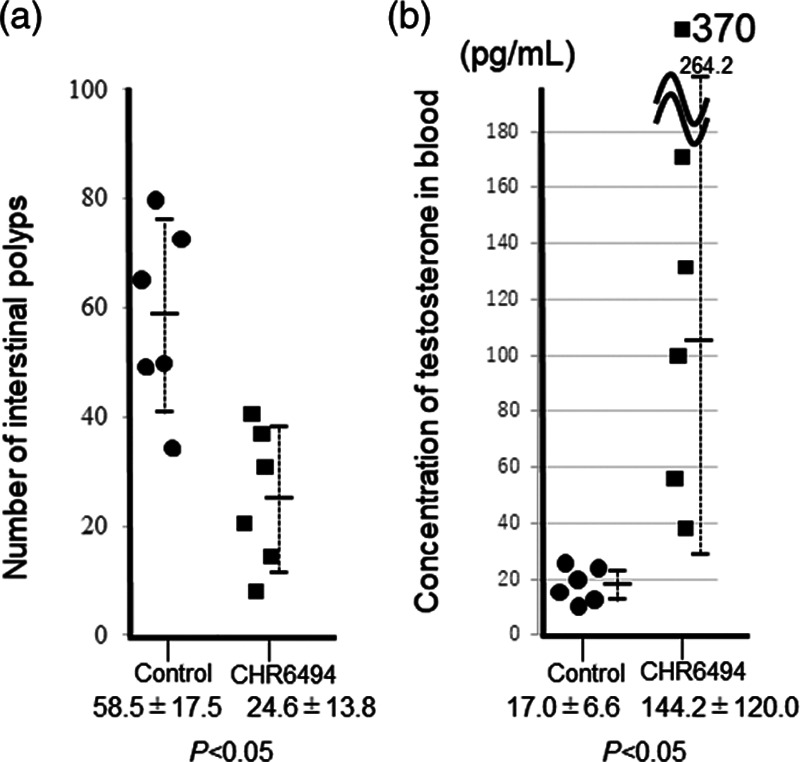
Numbers of intestinal polyps in Apcmin/+ mice treated with CHR-6496 (a) and Testosterone levels in blood from Apcmin/+ mice treated with CHR-6496 (b). Dots and squares indicate results in treated and untreated mice, respectively. Testosterone levels were examined using liquid chromatography-tandem mass spectrometry (LC-MS/MS). In control mice, the mean testosterone level was 221.0 ± 47.1 pg/ml (Steinfeld, 2018).
Body weights
ApcMin/+ mice developed cachexia during the treatment period (White et al., 2013). We measured body weight in 12-week-old mice at the end of the experiment. Body-weight was significantly recovered in mice treated with CHR-6494, at 23.6 ± 4.2 g, compared with 18.8 ± 1.7 g in control mice (Table 1).
Table 1.
Effect of CHR6496 treatment on body and testicular weights
| Wild type | ApcMin/+ | CHR6494a | |
|---|---|---|---|
| Body weight (g) | 26.4 ± 1.5 | 18.8 ± 1.7 | 23.6 ± 4.2 (0.03) |
| Testicular weight (g) | 0.09 ± 0.01 | 0.04 ± 0.03 | 0.08 ± 0.03 (0.006) |
| Epididymal weight (g) | 0.012 ± 0.0007 | 0.006 ± 0.0014 | 0.0097 ± 0.0012 (2.1E-06) |
The numbers of in parentheses indicated p values in ApcMin/+ and CHR6494.
CHR6494 mean ApcMin/+ min mice treated with CHR6494.
Observation of testes and epididymides
ApcMin/+ mice developed hypogonadism (Fig. 3a and b) (You et al., 2006; White et al., 2013). Testicular and epididymal weights were significantly recovered in mice treated with CHR-6494, at 0.08 ± 0.03 and 0.0097 ± 0.0012 g, respectively, compared with 0.04 ± 0.03 and 0.006 ± 0.0014 g, respectively, in control mice (Table 1). Histochemical observation showed that spermatogenesis was recovered from hypogonadism in ApcMin/+ mice treated with CHR-6494 (Fig. 3c and d). Testosterone levels were significantly recovered in 12-week-old mice treated with CHR-6494, at 142.6 ± 118.3 pg/ml, compared with 17.0 ± 6.6 pg/ml in control mice (Fig. 2b).
Fig. 3.
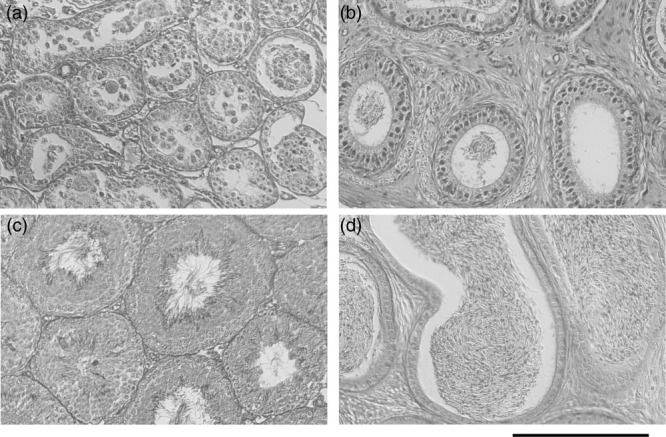
Sections of Bouin’s solution-fixed testes (a and c) and epididymides stained (b and d) with hematoxylin and eosin. Sperm were not included in testes (a) and epididymides (b) of Apcmin/+ mice. Spermatogenesis was fully recovered in the testes (a) and epididymides (b) of Apcmin/+ mice treated with CHR-6496. Bar = 200 μm.
Discussion
HASPIN has been identified as a nuclear serine-threonine kinase, and has been cloned in humans (Tanaka et al., 1999; Tanaka et al., 2001). HASPIN is expressed in trace amounts in various organs (Higgins, 2001a), and the function and evolution of haspin genes among different organisms is the subject of considerable research interest (Higgins, 2001b). Experiments with cultured cells have shown that phosphorylated histone H3 functions in chromosome distribution (Yu et al., 2017), whereas histone H2A is phosphorylated in germ cells (Hada et al., 2017). The generation of haspin-disrupted mice has demonstrated that germ cells and organisms are maintained even without haspin (Shimada et al., 2016). Seminiferous tubules with abnormal germ cells, which are not found in WT, are often found in haspin-disrupted mice (Shimada et al., 2016). Together, the findings of these previous studies suggest that HASPIN’s function is compensated by other molecules during normal cell differentiation and proliferation. Recently, HASPIN inhibitors have been shown to inhibit cancer cell growth (Huertas et al., 2012), suggesting that HASPIN’s function is not complemented during cell division in actively proliferating cells.
To investigate whether HASPIN inhibitor CHR-6494 can suppress cancer cell growth in vivo, we administered CHR-6494 to ApcMin/+ mice, a model of human CRC. Apcmin/+ mice developed intestinal polyps, cachexia, and hypogonadism with age (You et al., 2006; White et al., 2013; Jackstadt and Sansom, 2016). Our results indicate that CHR-6494 administration significantly suppressed intestinal polyp development, cachexia, and hypogonadism in Apcmin/+ mice. Intestinal polyp development involved extensive cell proliferation. Thus, HASPIN’s function, which is required for cell growth, was not complemented by other molecules in Apcmin/+ mice treated with CHR-6494, and intestinal polyp development was suppressed.
Although the cause of germ cell abnormality in Apcmin/+ mice is unknown, Leydig cells in Apcmin/+ mice are abnormal in that they do not develop tumors due to a decrease in testosterone. APC is involved in the Wnt signal (Tomita et al., 2007); testicular cells including Leydig cells are maintained by the Wnt/β-catenin signal (Qian et al., 2013). Disruption of the Wnt signaling network may disarrange testicular cells, although it remains unclear whether this effect is direct or indirect. That is, HASPIN may directly disturb signal transduction abnormalities in Apcmin/+ mouse testicular cells through avoidance behavior. Alternatively, testicular cells may cause dysfunction via abnormal cytoadhesin or humoral factors in Apcmin/+ mice.
In conclusion, although our results arise from a small sample, they indicate that HASPIN inhibitors may be useful as anti-cancer agents and for the treatment of hypogonadism in CRC. The significance of the results of the present study requires further validations in humans.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- Cao Z, Xu T, Tong X, Zhang D, Liu C, Wang Y, et al. HASPIN kinase mediates histone deacetylation to regulate oocyte meiotic maturation in pigs. Reproduction. 2019; 157:501–510 [DOI] [PubMed] [Google Scholar]
- Dai J, Sultan S, Taylor SS, Higgins JM. The kinase haspin is required for mitotic histone H3 thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005; 19:472–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Higgins JM. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle. 2005; 4:665–668 [DOI] [PubMed] [Google Scholar]
- Hada M, Kim J, Inoue E, Fukuda Y, Tanaka H, Watanabe Y, Okada Y. TH2A is phosphorylated at meiotic centromere by haspin. Chromosoma. 2017; 126:769–780 [DOI] [PubMed] [Google Scholar]
- Higgins JM. The haspin gene: location in an intron of the integrin alphae gene, associated transcription of an integrin alphae-derived RNA and expression in diploid as well as haploid cells. Gene. 2001a; 267:55–69 [DOI] [PubMed] [Google Scholar]
- Higgins JM. Haspin-like proteins: a new family of evolutionarily conserved putative eukaryotic protein kinases. Protein Sci. 2001b; 10:1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas D, Soler M, Moreto J, Villanueva A, Martinez A, Vidal A, et al. Antitumor activity of a small-molecule inhibitor of the histone kinase haspin. Oncogene. 2012; 31:1408–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R, Sansom OJ. Mouse models of intestinal cancer. J Pathol. 2016; 238:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida Y, Taguchi K, Taniguchi S, Tsuneyoshi M, Kuwano H, Tsuzuki T, et al. The role of P-glycoprotein in intestinal tumorigenesis: disruption of mdr1a suppresses polyp formation in apc(min/+) mice. Carcinogenesis. 2003; 24:1219–1224 [DOI] [PubMed] [Google Scholar]
- Kim JE, Lee SY, Jang M, Choi HK, Kim JH, Chen H, et al. Coumestrol epigenetically suppresses cancer cell proliferation: coumestrol is a natural haspin kinase inhibitor. Int J Mol Sci. 2017; 18:E2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku-Temeng C, Dayal N, Aflaki Sooreshjani M, Sintim HO. 3H-pyrazolo[4,3-f]quinoline haspin kinase inhibitors and anticancer properties. Bioorg Chem. 2018; 78:418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Liu S, Guan Y, Pan H, Guan X, Qiu Z, et al. Lgr4-mediated wnt/β-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development. 2013; 140:1751–1761 [DOI] [PubMed] [Google Scholar]
- Shimada M, Goshima T, Matsuo H, Johmura Y, Haruta M, Murata K, et al. Essential role of autoactivation circuitry on aurora B-mediated H2AX-ps121 in mitosis. Nat Commun. 2016; 7:12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld K, Beyer D, Mühlfeld C, Mietens A, Eichner G, Altinkilic B, et al. Low testosterone in apoe/LDL receptor double-knockout mice is associated with rarefied testicular capillaries together with fewer and smaller Leydig cells. Sci Rep. 2018; 8:5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Yoshimura Y, Nishina Y, Nozaki M, Nojima H, Nishimune Y. Isolation and characterization of cdna clones specifically expressed in testicular germ cells. FEBS Lett. 1994; 355:4–10 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yoshimura Y, Nozaki M, Yomogida K, Tsuchida J, Tosaka Y, et al. Identification and characterization of a haploid germ cell-specific nuclear protein kinase (haspin) in spermatid nuclei and its effects on somatic cells. J Biol Chem. 1999; 274:17049–17057 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Iguchi N, Nakamura Y, Kohroki J, de Carvalho CE, Nishimune Y. Cloning and characterization of human haspin gene encoding haploid germ cell-specific nuclear protein kinase. Mol Hum Reprod. 2001; 7:211–218 [DOI] [PubMed] [Google Scholar]
- Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, Hara A, et al. Development of gastric tumors in apc(min/+) mice by the activation of the beta-catenin/tcf signaling pathway. Cancer Res. 2007; 67:4079–4087 [DOI] [PubMed] [Google Scholar]
- White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male apcmin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. 2013; 2:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Ohmori M, Peña MM, Nassri B, Quiton J, Al-Assad ZA, et al. Developmental abnormalities in multiple proliferative tissues of apc(min/+) mice. Int J Exp Pathol. 2006; 87:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Jiang Y, Lu L, Cao M, Qiao Y, Liu X, et al. Aurora-A promotes the establishment of spindle assembly checkpoint by priming the haspin-aurora-B feedback loop in late G2 phase. Cell Discov. 2017; 3:16049. [DOI] [PMC free article] [PubMed] [Google Scholar]


