Endoluminal pulmonary banding and duct stenting are the basis for nonsurgical, transcatheter stage I in newborns with hypoplastic left heart syndrome (HLHS).
We report a novel, nonsurgical palliation for newborns with HLHS and variants.1 The aim of our transcatheter approach is to replace the Norwood and open-chest hybrid procedures,2 as well as complex neonatal biventricular repairs, to enable comprehensive stage II or biventricular repair with a lower overall risk by postponing surgeries to later infancy. Following institutional guidelines, this compassionate percutaneous transcatheter approach was performed with written parental consent and was approved by our ethics committee. Spontaneously breathing, sedated newborns received an endoluminal pulmonary artery (PA) branch banding with US Food and Drug Administration–approved, CE-marked Medtronic Micro Vascular Plug (MVP) devices, manually modified to a pulmonary flow restrictor (PFR), as previously described.3 After bilateral PFR placement, the arterial duct was stented, as is routine in the Giessen hybrid approach.4 Briefly, after local anesthesia, the femoral vein and artery were punctured, and 4F sheaths were placed. Heparin was administered intravenously in a single dose of 100 U/kg, followed by 50 U/kg after placement of PFRs.
On the basis of angiographically determined diameters of the left and right PAs, custom-made MVPs were chosen: MVP-5Q for a vessel size up to 5 mm, 7Q for up to 7 mm, and 9Q for up to 9 mm. Cineangiograms were performed in 20° to 25° cranial and 25° to 30° left oblique or 25° to 30° right oblique planes. Contrast medium was injected manually through a 4F Terumo Cobra shaped catheter positioned within pulmonary branches. The same diagnostic catheter was used for PFR placement. The thin polytetrafluoroethylene-covered, nitinol-framed, self-expandable MVPs were manually converted from a plug device to a flow restrictor. Considering that the MVP-5Q consists of 7 and the 7Q and 9Q consist of 10 covered segments, the covering of 1 or 2 segments was carefully removed by scalpel, generating perforations of ≈3 to 4.5 mm (Figure).
Figure.
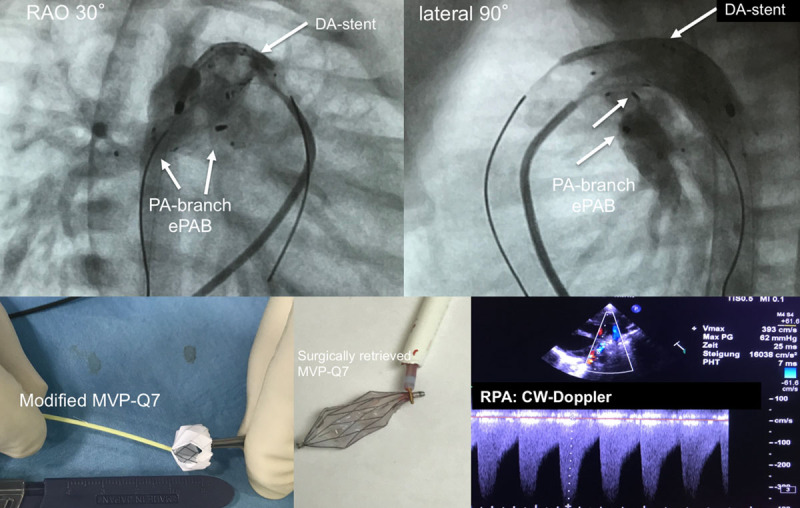
Transcatheter stage-I. Completion angiographies are shown in right anterior oblique (RAO 30°) and lateral 90° plane after placement of modified Micro Vascular Plug within the pulmonary branches and stented duct. Furthermore, a modified MVP-7Q is depicted after removal of polytetrafluoroethylene from 2 segments, as well as a device after intraoperative removal by the snare technique. In addition, a 2D echocardiographic short-axis plane is depicted, with continuous-wave (CW) Doppler tracing representing pulmonary systolic-diastolic flow profile of an effective pulmonary flow restrictor obtained from the right pulmonary artery (RPA). DA indicates arterial duct; and PA, pulmonary artery.
After loading of the modified MVP within the Cobra 4F (0.038-in) catheter, the PFRs were placed within the PA branches under fluoroscopy guidance. After bilateral PFR placement, the arterial duct was stented with a Formula balloon-expandable stent (COOK, Copenhagen, Denmark) for treating a narrowed duct and a self-expandable Sinus-Superflex-DS stent (OptiMed, Karlsruhe, Germany) for stenting a wide-open arterial duct.
Follow-up drug treatment consisted of continuous infusion of heparin (400 U·kg−1·d−1) for 2 days, followed by oral clopidogrel (0.2 mg·kg−1·d−1) and acetylsalicylic acid (1–2 mg·kg−1·d−1) together with low-dose bisoprolol/lisinopril/spironolactone.
Some additional transcatheter procedures were required during the interstage period. For the first treated patient with symptoms of overcirculation, replacement of the modified MVPs became necessary. Because of the brief time interval of only 3 weeks, removal of the flow restrictors was performed easily by the snare technique. A patient with HLHS with a barely visible and tiny ascending aorta was initially treated only by endoluminal PA-branch banding and continuous infusion of prostaglandin E1. Listed for cardiac transplantation, he received an arterial duct stent for a prostaglandin E1–resistant duct obstruction. Two patients received a Sinus-Superflex-DS stent for stenting of a restrictive atrial communication; one of them additionally received a second duct stent and a coronary stent treating a residual duct and aortic isthmus stenosis.
Six neonates have been treated without mortality regardless of whether they had transcatheter procedures or follow-up surgeries. A successful comprehensive stage II repair was performed in 4 patients at 4 months of age. A neonate with tricuspid atresia IIC (transposition of great arteries with unrestricted pulmonary blood flow), dextrocardia, and left persistent superior vena cava underwent the transcatheter approach when he was readmitted in congestive heart failure. During the comprehensive stage II surgery, the revised PFR was removed by the snare technique. Two patients with HLHS (mitral stenosis, aortic atresia) also underwent a successful comprehensive stage II repair. The PFRs were removed with a dissector when the main PA was cut. Attempted removal of the PFR from the left PA with a snare induced an intimal flap necessitating left PA stenting immediately after surgery. The sixth patient with congenitally corrected transposition of the great arteries and hypoplastic systemic right ventricle received also an uneventful comprehensive stage II. The patient with interrupted aortic arch, hypoplastic ascending aorta, subaortic stenosis, and aberrant right subclavian artery had biventricular repair at 5.4 months of age. The PFR from the right PA was removed with the snare technique, and the left PFR was removed with a dissector. A patient with HLHS had successful cardiac transplantation at 83 days of age. The bilateral PFRs were removed intraoperatively with the snare technique. To date, all infants have an excellent cardiac and gross examined neurological outcome. The patient who underwent heart transplantation, however, has developed syndrome-related liver dysfunction.
This transcatheter approach has the potential to allow broader treatment of complex congenital heart defects that currently require highly specialized surgical centers. The catheter-based treatments described here could be used widely as the first-stage intervention in neonates with HLHS and variants.
Acknowledgments
This research letter is dedicated to Professor Leonard Bailey, who passed away much too early.
Disclosures
None.
Footnotes
The data, analytical methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure
References
- 1.Barron DJ, Kilby MD, Davies B, Wright JG, Jones TJ, Brawn WJ. Hypoplastic left heart syndrome. Lancet. 2009;374:551–564. doi: 10.1016/S0140-6736(09)60563-8 [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Schranz D, D’Udekem Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation. 2016;134:1265–1279. doi: 10.1161/CIRCULATIONAHA.116.022816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AH, Hoskoppal D, Kumar TKS, Bird L, Allen K, Lloyd H, Knott-Craig CJ, Waller BR, Sathanandam S. Utility of the Medtronic microvascular plug™ as a transcatheter implantable and explantable pulmonary artery flow restrictor in a swine model. Catheter Cardiovasc Interv. 2019;93:1320–1328. doi: 10.1002/ccd.28162 [DOI] [PubMed] [Google Scholar]
- 4.Schranz D, Bauer A, Reich B, Steinbrenner B, Recla S, Schmidt D, Apitz C, Thul J, Valeske K, Bauer J, et al. Fifteen-year single center experience with the “Giessen hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015;36:365–373. doi: 10.1007/s00246-014-1015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


