Abstract
In a post hoc analysis, the effect of pimavanserin on anxious depression was determined from CLARITY, a randomized, double-blind, placebo-controlled study in patients with major depression and an inadequate response to previous therapy. Patients were randomized in a 3:1 ratio to placebo or pimavanserin 34 mg daily added to ongoing antidepressant therapy. At 5 weeks, placebo nonresponders were rerandomized to placebo or pimavanserin for an additional 5 weeks. Mean change from baseline to week 5 for the Hamilton depression rating scale (HAMD) anxiety/somatization (AS) factor was examined for all patients and those with a score ≥7 at baseline. Least squares (LS) mean [standard error (SE)] difference between placebo and pimavanserin for the AS factor score was −1.5 (0.41) [95% confidence interval (CI) −2.4 to −0.7; P = 0.0003; effect size: 0.634]. Among patients with an AS factor score ≥7 at baseline, LS mean (SE) difference was −2.2 (0.66) (95% CI −3.5 to −0.9; P = 0.0013; effect size: 0.781). Response rates (≥50% reduction in HAMD-17 from baseline) were 22.4 and 55.2% (P = 0.0012) and remission rates (HAMD-17 total score <7) were 5.3 and 24.1% (P = 0.0047), respectively, with placebo and pimavanserin among patients with a baseline AS factor score ≥7. Among patients with anxious major depressive disorder at baseline, adjunctive pimavanserin was associated with a significant improvement.
Keywords: anxious depression, controlled study, pimavanserin, randomized
Introduction
The frequency of comorbid anxiety disorders and/or higher levels of anxiety symptoms in people with major depressive disorder (MDD) is approximately 50% (Kessler et al., 2003; Fava et al., 2004; Ionescu et al., 2013; Dold et al., 2017; Gasperz et al., 2018). Residual anxiety symptoms, high baseline levels of anxiety, or the presence of a comorbid anxiety disorder are known to be risk factors for relapse or recurrence of MDD (Wilhelm et al., 1999; Parker et al., 2000; Dombrovski et al., 2007; Fava et al., 2008; Yang et al., 2010). Anxious depression also is associated with impaired functioning, higher rates of unemployment, and a greater risk of suicidality (Fava et al., 2006; Nelson, 2008; Farabaugh et al., 2012). A recent study found that the severity of anxiety at baseline adversely affected depression severity at 12 months and that a reduction of anxiety within the first 3 months of antidepressant treatment led to additional improvements in symptoms of depression (Bair et al., 2013). Antidepressant use may actually worsen symptoms in a minority of patients (Jha et al., 2018). A standard definition of anxious depression is a Hamilton depression rating scale (HAMD-17; Hamilton, 1960) anxiety/somatization factor subscale score of ≥7 (Fava et al., 2008). This definition of anxious depression has been used in previous studies, including secondary analyses of the Sequenced Treatment Alternatives to Relieve Depression trial (Fava et al., 2008; Farabaugh et al., 2012; Thase et al., 2014; Lyndon et al., 2019). Based on previous studies, the management of anxious depression should be aimed at treating symptoms of both depression and anxiety (Ionescu et al., 2014).
Pimavanserin is a potential 5-hydroxytryptamine2A (5-HT2A) receptor antagonist/inverse agonist with less activity as a 5-HT2C antagonist/inverse agonist and no appreciable activity at adrenergic, dopaminergic, histaminergic, or muscarinic receptors (Vanover et al., 2006). Pimavanserin is approved in the United States by the Food and Drug Administration for treating hallucinations and delusions in patients with Parkinson’s disease psychosis. In a phase 2, randomized, placebo-controlled study (CLARITY), adjunctive pimavanserin demonstrated a significant improvement of depressive symptoms in patients with MDD and an inadequate response to previous treatment (Fava et al., 2019). This was a post hoc analysis of CLARITY was undertaken to evaluate the effects of adjunctive pimavanserin vs. placebo in a subgroup of patients with MDD and a baseline score ≥7 on the six-item HAMD-17 anxiety/somatization factor.
Materials and methods
The CLARITY study was conducted in accordance with the International Council of Harmonization guidelines and followed the principles of Good Clinical Practice derived from the Declaration of Helsinki. The study protocol and amendments and informed consent forms were reviewed and approved by an independent ethics committee or institutional review board. Prior to any study procedures, all patients were informed of the risks and benefits of the study and provided written informed consent. The CLARITY study was registered at clinicaltrials.gov: NCT03018340.
Study design
The detailed study methodology for CLARITY was previously published (Fava et al., 2019). In brief, this was a multicenter, randomized, double-blind, placebo-controlled study in patients with MDD. Following an 8–21-day screening period, patients entered a 10-week double-blind treatment period, followed by a 30-day safety period to assess safety. The study used a two-stage Sequential Parallel-Comparison Design (Fava et al., 2003). In stage 1, eligible patients were randomized in a 3:1 ratio to placebo or pimavanserin 34 mg once daily added to background treatment with a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) for 5 weeks. Nonresponders to placebo after 5 weeks (HAMD-17 total score >14 and <50% reduction in score from baseline) were rerandomized in a 1:1 ratio to placebo or pimavanserin 34 mg once daily in addition to an SSRI or SNRI for an additional 5 weeks. All patients assigned to pimavanserin in stage 1 continued treatment with pimavanserin in stage 2, whereas responders to placebo in stage 1 remained on placebo in stage 2.
Patient selection
Men or women at least 18 years of age with a BMI of 19–35 kg/m2 were eligible if they had a primary diagnosis of MDD and a current Major Depressive Episode defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and confirmed by the Structured Clinical Interview for DSM-5, Clinical Trials Version (First et al., 2016). A history of MDD for ≥1 year prior to screening, a Montgomery–Åsberg Depression Rating Scale (Montgomery and Asberg, 1976) total score >20, a Clinical Global Impression – Severity scale (Guy, 1976) score ≥4 (moderately ill or worse) at screening and baseline visits and a history of inadequate response to one or two adequate treatment trials with an SSRI or SNRI antidepressant during the current depression episode were required. Inadequate treatment response was determined with the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire, and this was confirmed by independent Massachusetts General Hospital Clinical Trials Network and Institute raters during the SAFER [State versus trait, Assessability, Face validity, Ecological validity, and Rule of three Ps (pervasive, persistent, and pathological)] interview. Patients with eating disorder, obsessive-compulsive disorder, attention deficit hyperactivity disorder, panic disorder, acute stress disorder, or posttraumatic stress disorder, according to DSM-5 criteria were excluded.
Study assessments
Clinic visits occurred weekly from weeks 1 to 10 (end of study). The HAMD-17 was administered at baseline and weekly during the study. Safety and tolerability were assessed from adverse events, physical examination, vital signs, and clinical laboratory testing.
Statistical analysis
In this post hoc analysis, anxious depression was defined as a score ≥7 on the six-item HAMD-17 anxiety/somatization factor, which consisted of the sum of items 10 (psychic anxiety), 11 (somatic anxiety), 12 (gastrointestinal somatic symptoms), 13 (general somatic symptoms), 15 (hypochondriasis), and 17 (insight) (Ionescu et al., 2014; Farabaugh et al., 2010). Mean change from baseline to week 5 for the HAMD-17 anxiety/somatization factor was examined for the entire study population. Second, mean change from baseline to week 5 was examined for the subgroup of patients with a baseline score ≥7 for the HAMD-17 anxiety/somatization factor. An analysis was conducted of the mean change from baseline to week 5 among a subgroup with severe MDD at baseline (HAMD-17 total score ≥24) and a HAMD-17 anxiety/somatization factor score ≥7 at baseline. Finally, the effect of a baseline HAMD-17 anxiety/somatization factor score ≥7 on response (≥50% improvement in the HAMD-17 total score from baseline) and remission (HAMD-17 total score ≤7) was examined. Efficacy data were analyzed for the full analysis set for each of the two stages, comprising all randomized patients who received ≥1 dose of blinded study drug and who had a baseline value and at least one postbaseline value for the HAMD-17 total score within each stage.
Least squares (LS) mean [standard error (SE)] was determined from a stage-specific mixed models for repeated measures (MMRM) analysis with change from baseline as the outcome; treatment group, visit, treatment-by-visit interaction, baseline HAMD-17 anxiety/somatization factor score, and baseline HAMD-17 anxiety/somatization factor score-by-visit interaction as the factors. An unstructured covariance matrix is used to model the within-subject errors. The denominator degrees of freedom were estimated using the Kenward–Roger approximation. A two-sided P-value was reported for treatment difference from the stage-specific MMRM analysis. Cohen’s d effect size was calculated for comparisons between treatments. For response and remission, mean difference for pimavanserin vs. placebo was determined. A Newcombe 95% confidence interval (CI) was calculated on the difference, and P values were calculated from a stage-specific Pearson’s chi-square test.
Results
In the efficacy population, 152 patients were randomized to placebo and 51 to pimavanserin in stage 1, and 29 patients each were rerandomized to treatment with pimavanserin or placebo in stage 2 (Fava et al., 2019). At week 5 of stage 1, the least squares (LS) mean difference for pimavanserin vs. placebo was −4.0 for the HAMD-17 (P = 0.0003).
In the post hoc analysis, pimavanserin produced a significantly greater effect on the HAMD-17 anxiety/somatization factor score vs. placebo in stage 1 (P = 0.0003) and across stages 1 and 2 using prespecified weighting (P = 0.0166), but not in stage 2 (P = 0.980) (Table 1). At week 5 in stage 1, the LS mean (SE) difference between placebo and pimavanserin for the anxiety/somatization factor score was −1.5 (0.41) (95% CI −2.4 to −0.7; P = 0.0003; Cohen’s d effect size: 0.634) (Fig. 1).
Table 1.
Mean baseline and least squares mean change and treatment difference between pimavanserin and placebo for study outcomes
| Stage 1 | Stage 2 | |||
|---|---|---|---|---|
| Placebo | Pimavanserin | Placebo | Pimavanserin | |
| HAMD anxiety/somatization factor at baseline | N = 152 | N = 51 | N = 29 | N = 29 |
| Baseline mean (SE) | 6.6 (0.18) | 6.9 (0.36) | 6.0 (0.43) | 6.5 (0.49) |
| Change from baseline to week 5 | ||||
| LS mean (SE)a | −2.1 (0.21) | −3.7 (0.36) | −0.6 (0.35) | −0.6 (0.33) |
| 95% CI of LS mean | (−2.5 to −1.7) | (−4.4 to −3.0) | (−1.3 to 0.1) | (−1.3 to 0.0) |
| LS mean (SE) difference (pimavanserin 34 mg-placebo) | −1.5 (0.41) | 0 (0.48) | ||
| 95% CI of difference | (−2.4 to −0.7) | (−1.0 to 1.0) | ||
| P-valueb | 0.0003 | 0.980 | ||
| Effect size (Cohen’s d) | 0.634 | −0.007 | ||
| Overall treatment comparison at week 5 (linear combination test) | ||||
| Weighted difference in LS mean (SE) | −0.8 (0.32) | |||
| 95% CI of weighted difference | (−1.4 to −0.1) | |||
| P-valueb | 0.0166 | |||
| HAMD anxiety/somatization factor ≥7 at baseline | N = 76 | N = 29 | N = 18 | N = 19 |
| Baseline mean (SE) | 8.5 (0.15) | 8.8 (0.28) | 7.1 (0.54) | 7.5 (0.59) |
| Change from baseline to week 5 | ||||
| LS mean (SE)a | −2.8 (0.35) | −5.0 (0.56) | −1.5 (0.49) | −1.3 (0.44) |
| 95% CI of LS mean | (−3.5 to −2.1) | (−6.1 to −3.8) | (−2.5 to −0.5) | (−2.3 to −0.4) |
| LS Mean (SE) difference (pimavanserin 34 mg-placebo) | −2.2 (0.66) | 0.1 (0.66) | ||
| 95% CI of difference | (−3.5 to −0.9) | (−1.2 to 1.5) | ||
| P-valueb | 0.0013 | 0.847 | ||
| Effect size (Cohen’s d) | 0.781 | −0.067 | ||
| Overall treatment comparison at week 5 (linear combination test) | ||||
| Weighted difference in LS mean (SE) | −1.0 (0.47) | |||
| 95% CI of weighted difference | (−2.0 to −0.1) | |||
| P-valueb | 0.027 | |||
| HAMD total score ≥24 and HAMD anxiety/somatization factor ≥7 at baseline | N = 36 | N = 17 | N = 8 | N = 10 |
| Baseline mean (SE) | 27.6 (0.41) | 27.6 (0.70) | 24.0 (1.20) | 22.0 (1.90) |
| Change from baseline to week 5 | ||||
| LS mean (SE)a | −9.3 (1.40) | −17.4 (1.97) | −1.3 (1.68) | −3.7 (1.43) |
| 95% CI of LS mean | (−12.1 to −6.5) | −21.4 to −13.4) | (−4.9 to 2.3) | −6.8 to −0.6) |
| LS mean (SE) difference (pimavanserin 34 mg-placebo) | −8.1 (2.42) | −2.4 (2.22) | ||
| 95% CI of difference | (−13.0 to −3.2) | (−7.2 to 2.3) | ||
| P-valueb | 0.0018 | 0.295 | ||
| Effect size (Cohen’s d) | 1.037 | 0.536 | ||
| Overall treatment comparison at week 5 (linear combination test) | ||||
| Weighted difference in LS mean (SE) | −5.2 (1.64) | |||
| 95% CI of weighted difference | −8.5 to −2.0) | |||
| P-valueb | 0.0014 | |||
ANCOVA, analysis of covariance; CI, confidence interval; HAMD, Hamilton depression rating scale; LS, least squares; SE, standard errors.
LS mean from the stage-specific ANCOVA analysis with the change from baseline as the outcome, treatment group as a factor, and the corresponding baseline value as a covariate.
Two-sided P-value for treatment difference from the stage-specific ANCOVA analysis.
Fig. 1.
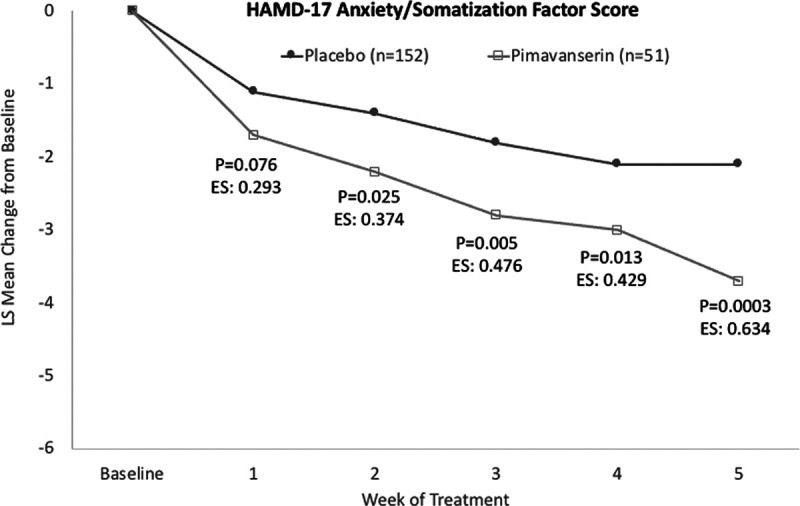
LS mean change from baseline for HAMD-17 anxiety/somatization factor score. HAMD, Hamilton depression rating scale; LS, least squares.
For the subgroup of patients (placebo 75, pimavanserin 29) with a baseline HAMD-17 anxiety/somatization factor score ≥7, pimavanserin significantly reduced the HAMD-17 anxiety/somatization factor score in stage 1 (P = 0.0013) and across stages 1 and 2 using prespecified weighting (P = 0.027), but not in stage 2 (P = 0.847) (Table 1). In stage 1, among patients with a baseline HAMD anxiety/somatization factor score ≥7, the LS mean (SE) difference at week 5 was −2.2 (0.66) (95% CI −3.5 to −0.9; P = 0.0013; Cohen’s d effect size: 0.781) (Fig. 2).
Fig. 2.
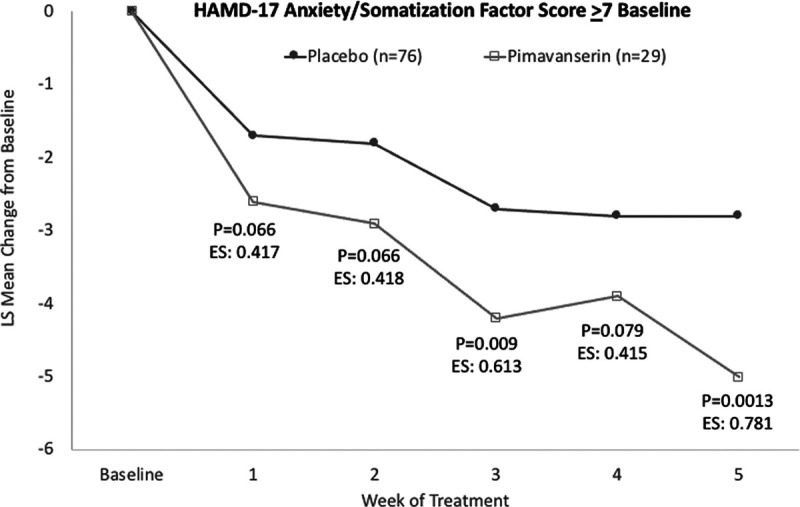
LS mean change from baseline for patients with a HAMD-17 anxiety/somatization factor score ≥7 at baseline. HAMD, Hamilton depression rating scale; LS, least squares.
Among the subgroup of patients with a baseline HAMD-17 total score ≥24 indicating severe depression AND a HAMD-17 anxiety/somatization factor score ≥7, pimavanserin significantly reduced the HAMD-17 anxiety/somatization factor score in stage 1 (P = 0.0018) and across stages 1 and 2 using prespecified weighting (P = 0.0014), but not in stage 2 (P = 0.295) (Table 1). In stage 1, the LS mean (SE) difference at week 5 was −8.1 (2.42) (95% CI −13.0 to −3.2; P = 0.0018; Cohen’s d effect size: 1.037) (Fig. 3).
Fig. 3.
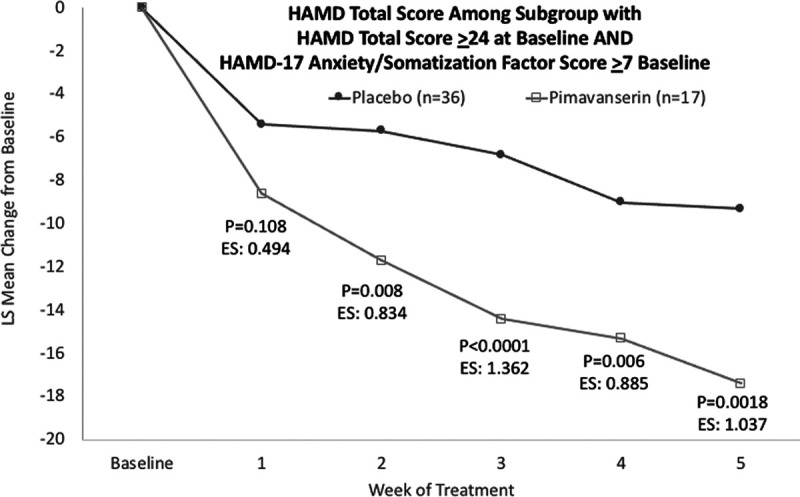
LS mean change from baseline for the HAMD-17 total score among patients with a baseline HAMD-17 total score ≥24 and a HAMD-17 anxiety/somatization factor score ≥7. HAMD, Hamilton depression rating scale; LS, least squares.
At week 5, response rates (≥50% reduction in HAMD-17 from baseline) were 22.4 and 55.2% (P = 0.0012) and remission rates (HAMD-17 total score <7) were 5.3 and 24.1% (P = 0.0047), respectively, with placebo and pimavanserin, respectively, among patients with a baseline HAMD anxiety/somatization factor score ≥7 (Fig. 4).
Fig. 4.
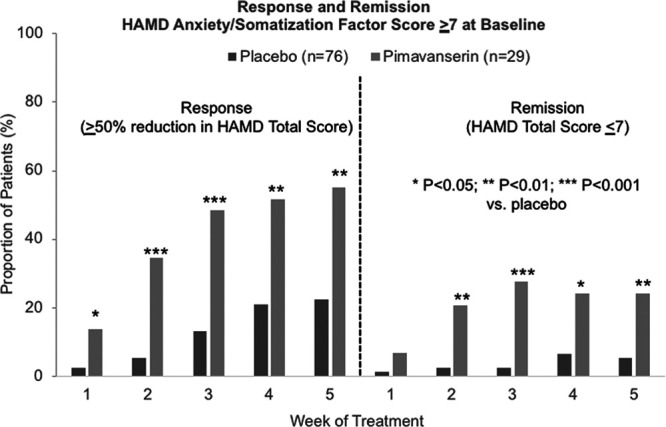
Response and remission rates among patients with a HAMD-17 anxiety/somatization factor score ≥7 at baseline. HAMD, Hamilton depression rating scale.
Anxiety was reported as an adverse event in two (1.3%) patients in the placebo group and 1 (1.9%) in the pimavanserin group during stage 1. No anxiety as an adverse event was reported in stage 2.
Discussion
This post hoc analysis of patients with MDD enrolled in the phase 2 CLARITY study found reductions in the HAMD-17 total score as well as the HAMD anxiety/somatization factor score with adjunctive pimavanserin compared with placebo. Significant differences from placebo were observed with adjunctive pimavanserin for the HAMD anxiety/somatization factor score overall and among patients with a baseline HAMD anxiety/somatization factor score ≥7, with effect sizes >0.6 at week 5. Among the subgroup with a baseline HAMD anxiety/somatization factor score ≥7 and a HAMD-17 total score ≥24, representing patients with severe and anxious depression, the HAMD-17 total score was markedly reduced from baseline to week 5 with adjunctive pimavanserin vs. placebo. Of note, significant differences from placebo were observed as early as week 2 with pimavanserin. The lack of significant differences between pimavanserin and placebo in stage 2 may be attributed to the small sample sizes. Among the subgroup of patients with a baseline HAMD anxiety/somatization factor score ≥7, significant improvement in the HAMD-17 response rate was observed at week 1 and the HAMD-17 remission rate at week 2 was observed with pimavanserin vs. placebo. Further, response and remission rates were higher with pimavanserin and lower with placebo in this subgroup of patients with anxious depression compared with the overall study population (Fava et al., 2019).
Anxious depression is reported to occur in approximately 50% or more of patients with MDD (Wiethoff et al., 2010; Papakostas and Larsen 2011). Patients with anxious depression are more likely to experience poor outcomes including increased rates of treatment failure and treatment resistance (Souery et al., 2007; Papakostas and Larsen 2011; Papakostas et al., 2012; Farabaugh et al., 2012; Ionescu et al., 2014; Dold et al., 2017; Gaspersz et al., 2018; Kautzky et al., 2019). Previous studies have found that patients with comorbid anxiety and MDD were less responsive to antidepressant treatment (Souery et al., 2007; Fava et al., 2008; Wiethoff et al., 2010; Kautzky et al., 2019). However, others have found an improved response with SSRI or SNRI antidepressants in depressed patients with anxiety symptoms or anxious depression at baseline (Thase et al., 2014; Lyndon et al., 2019). In this analysis, patients were enrolled who had an inadequate response to SSRI or SNRI treatment, and those with anxious depression experienced a robust response to adjunctive pimavanserin. Thus, consideration should be given to the impact of anxiety symptoms and anxious depression when treating patients with major depression and the potential role of adjunctive therapy in inadequate responders to SSRI or SNRI antidepressants.
A limited number of studies have been published describing the use of antidepressants for treating anxious depression. Greater improvement in symptoms of MDD was observed among those with anxious depression at baseline (Thase et al., 2014; Lyndon et al., 2019), together with higher response and remission rates (Lyndon et al., 2019). However, the majority of studies of patients with comorbid anxiety or anxious depression and MDD found less benefit from antidepressant therapy among patients with anxiety compared with those without anxiety (Nelson, 2008).
Limitations of this analysis include its post hoc design that was not specified a priori in the original analyses, where patients with comorbid anxiety were not prospectively identified, the small sample size, especially among patients with severe MDD at baseline in stage 2, and the short 5-week duration of follow-up in stages 1 and 2. However, limited clinical data are available where antidepressant therapy has been studied in patients with anxious depression. The lack of significant differences between pimavanserin and placebo in stage 2 likely was a result of small sample sizes. Thus, these results add valuable information about anxious depression in patients with MDD.
In summary, these results from a post hoc analysis demonstrated a marked response among patients with anxious depression to adjunctive pimavanserin compared with placebo. Statistically significant and clinically meaningful improvements were observed with pimavanserin vs. placebo within 2 weeks. Reductions in anxiety measured by the HAMD anxiety/somatization factor were associated with significant improvement in response and remission on the HAMD-17 total score. Ongoing phase 3 studies, with adjunctive pimavanserin in patients with MDD, will provide a larger population of patients in which the occurrence of anxious depression and the response to pimavanserin can be examined further. Future studies may also assess whether pimavanserin is specifically efficacious in anxious MDD compared with other subtypes and whether it may be considered to treat comorbid anxiety disorders, as well as anxious symptoms, in the context of a major depressive episode.
Acknowledgements
The authors acknowledge the editorial assistance of Richard S. Perry, PharmD, in the preparation of this manuscript, which was supported by ACADIA Pharmaceuticals Inc., San Diego, CA.
This study was supported by ACADIA Pharmaceuticals Inc., San Diego, CA, USA. The funder of the study had a role in study design, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and had full responsibility for the content of the manuscript for publication. The corresponding author was responsible for the final review and had final responsibility for the decision to submit for publication. This study was supported by Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clarus Funds; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; Wyeth-Ayerst Laboratories.
Presented at the 2019 American Psychiatric Association Annual Meeting, 18–22 May 2019, San Francisco, CA, USA.
Advisory board/consultant: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; Boehringer Ingelheim; Boston Pharmaceuticals; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Navitor Pharmaceuticals, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite LLC.; PharmoRx Therapeutics; Praxis Precision Medicines; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Purdue Pharma; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Relmada Therapeutics, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex; Teva Pharmaceuticals; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Usona Institute, Inc.; Vanda Pharmaceuticals, Inc.; Versant Venture Management, LLC; VistaGen.
Speaking/publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories.
Stock/other financial options: Equity Holdings: Compellis; PsyBrain, Inc.
Royalty/patent, other income: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691).
Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd.
Conflicts of interest
M.P.F.: advisory/consulting – Alkermes, Otsuka, Janssen, Sage, JDS Therapeutics, Sunovion. Independent Data Safety and Monitoring Committee – Janssen (Johnson & Johnson). Medical editing – GOED newsletter. Research support through MGHg – National Pregnancy Registry for Atypical Antipsychotics, Alkermes Biopharmaceuticals, AstraZeneca Pharmaceuticals, Otsuka Pharmaceuticals, Forest/Actavis Pharmaceuticals, Ortho-McNeil Janssen, Sunovion Pharmaceuticals, Inc. Other research support – Takeda Pharmaceuticals, JayMac Pharmaceuticals. As an employee of MGH. M.P.F. works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and NIMH. M.F. – Disclosures (lifetime) (updated: September 2018). All disclosures can be viewed online at http://mghcme.org/faculty/faculty-detail/maurizio_fava. G.I.P.: consulting: Abbott Laboratories, Acadia Pharmaceuticals, Inc*, Alkermes Inc, AstraZeneca PLC, Avanir Pharmaceuticals, Axsome Therapeutics*, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Genentech, Inc*, Genomind, Inc*, GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Janssen Global Services LLC*, Jazz Pharmaceuticals, Johnson & Johnson Companies*, Methylation Sciences Inc, Mylan Inc*, Novartis Pharma AG, One Carbon Therapeutics, Inc*, Osmotica Pharmaceutical Corp.*, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceutical Co, Ltd, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc; Received honoraria (for lectures or consultancy) from: Abbott Laboratories, Acadia Pharmaceuticals, Inc, Alkermes Inc, Asopharma America Cntral Y Caribe, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, Forest Pharmaceuticals, GlaxoSmithKline, Inflabloc Pharmaceuticals, Grunbiotics Pty LTD, Jazz Pharmaceuticals, H. Lundbeck A/S, Medichem Pharmaceuticals, Inc, Meiji Seika Pharma Co. Ltd, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer, Pharma Trade SAS, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc; Research support (paid to hospital): AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, Neuralstem, Inc*, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, Tal Medical, and Theracos, Inc.; Served (not currently) on the speaker’s bureau for BristolMyersSquibb Co and Pfizer, Inc. *Denotes activity undertaken on behalf of Massachusetts General Hospital. R.C.S. – grants: National Institutes of Health, Patient-Centered Outcomes Research Institute, Acadia Pharmaceuticals, Alkermes, Inc., Allergan Inc., Avanir Pharmaceuticals, Cerecor, Inc., Genomind, Intracellular Therapies, Janssen Pharmaceutica, Myriad Genetics, Navitor Pharmaceuticals Inc., NeuroRx Inc., Novartis Inc., Otsuka Pharmaceuticals, Nestle’ Health, Novartis Inc., Takeda Pharmaceuticals; consulting: Acadia Pharmaceuticals, Allergan Inc., Cerecor, Inc., Clintara, LLC, Janssen Pharmaceutica, Lundbeck A/S, Medtronic, Inc., MSI Methylation Sciences, Inc., Naurex, Inc., Nestle’ Health, Pfizer, Inc., Takeda Pharmaceuticals. M.J. has received contract research grants from ACADIA Pharmaceuticals and Janssen Research & Development and honoraria for CME presentations from North American Center for Continuing Medical Education and Global Medical Education. M.T. – Disclosure information (past 3 years). Advisory/consultant: ACADIA Pharmaceuticals, Akili, Inc., Alkermes PLC, Allergan, Inc. (Forest, Naurex), Cerecor, Inc., Fabre-Kramer Pharmaceuticals, Inc., Gerson Lehrman Group, Inc., Guidepoint Global, LLC, Johnson & Johnson (Janssen, Ortho-McNeil); H. Lundbeck, A/S, Moksha8 Pharmaceuticals, Inc., Nestlé (PamLab), Neuralstem, Novartis International AG, Otsuka Pharmaceutical Company, Ltd.; Pfizer, Inc., Sage Pharmaceuticals, Sunovion. M.T. received grant support from ACADIA Pharmaceuticals, Agency for Healthcare Research and Quality (AHRQ), Alkermes PLC, Allergan, Inc. (Forest, Naurex), AssureRx Health, Inc., Avanir, Axsome, Inc., Intracellular Therapies, Inc., Janssen Pharmaceuticals, Inc., Johnson & Johnson (Janssen, Ortho-McNeil), National Institutes of Health/National Institute of Mental Health (NIMH), Otsuka Pharmaceutical Company, Ltd., Patient-Centered Outcomes Research Institute (PCORI), Takeda. Speakers Bureau: None. Royalties: American Psychiatric Foundation, Guilford Publications, Herald House, W.W. Norton & Company, Inc. Spouse’s employment: Peloton Advantage, which does business with most major pharmaceutical companies. M.H.T.: Advisor/consultant and received fees from Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., Shire, and Takeda. M.H.T. has received grants/research support from the National Institute of Mental Health (NIMH) and the National Institute on Drug Abuse (NIDA). B.D., K.L., and S.S. are employees of ACADIA Pharmaceuticals, San Diego, CA.
References
- Bair MJ, Poleshuck EL, Wu J, Krebs EK, Damush TM, Tu W, Kroenke K. Anxiety but not social stressors predict 12-month depression and pain severity. Clin J Pain. 2013; 29:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold M, Bartova L, Souery D, Mendlewicz J, Serretti A, Porcelli S, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders - results from a European multicenter study. J Psychiatr Res. 2017; 91:1–13 [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, et al. Residual symptoms and recurrence during maintenance treatment of late-life depression. J Affect Disord. 2007; 103:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh A, Alpert J, Wisniewski SR, Otto MW, Fava M, Baer L, et al. Cognitive therapy for anxious depression in STAR(*) D: what have we learned? J Affect Disord. 2012; 142:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh AH, Bitran S, Witte J, Alpert J, Chuzi S, Clain AJ, et al. Anxious depression and early changes in the HAMD-17 anxiety-somatization factor items and antidepressant treatment outcome. Int Clin Psychopharmacol. 2010; 25:214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004; 34:1299–1308 [DOI] [PubMed] [Google Scholar]
- Fava M, Dirks B, Freeman MP, Papakostas GI, Shelton RC, Thase ME, et al. A Phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019; 80:19m12928. [DOI] [PubMed] [Google Scholar]
- Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003; 72:115–127 [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008; 165:342–351 [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Carmin CN, Balasubramani GK, Wisniewski SR, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006; 51:823–835 [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV). 2016, Arlington, VA: American Psychiatric Association [Google Scholar]
- Gaspersz R, Nawijn L, Lamers F, Penninx BWJH. Patients with anxious depression: overview of prevalence, pathophysiology and impact on course and treatment outcome. Curr Opin Psychiatry. 2018; 31:17–25 [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publ No. ADM 76-338). 1976, Rockville, MD: US Department of Health, Education, and Welfare Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 218–222 [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Niciu MJ, Henter ID, Zarate CA. Defining anxious depression: a review of the literature. CNS Spectr. 2013; 18:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Niciu MJ, Richards EM, Zarate CA., Jr Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord. 2014; 16:PCC.13r01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, South C, Rush AJ, Trivedi MH. Worsening anxiety, irritability, insomnia, or panic predicts poorer antidepressant treatment outcomes: clinical utility and validation of the concise associated symptom tracking (CAST) scale. Int J Neuropsychopharmacol. 2018; 21:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky A, Dold M, Bartova L, Spies M, Kranz GS, Souery D, et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr Scand. 2019; 139:78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sampson NA, Berglund P, Gruber MJ, Al-Hamzawi A, Andrade L, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015; 24:210–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndon GJ, Prieto R, Wajsbrot DB, Allgulander C, Bandelow B. Efficacy of venlafaxine extended release in major depressive disorder patients: effect of baseline anxiety symptom severity. Int Clin Psychopharmacol. 2019; 34:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979; 134:382–389 [DOI] [PubMed] [Google Scholar]
- Nelson JC. Anxious depression and response to treatment. Am J Psychiatry. 2008; 165:297–299 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fan H, Tedeschini E. Severe and anxious depression: combining definitions of clinical sub-types to identify patients differentially responsive to selective serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2012; 22:347–355 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Larsen K. Testing anxious depression as a predictor and moderator of symptom improvement in major depressive disorder during treatment with escitalopram. Eur Arch Psychiatry Clin Neurosci. 2011; 261:147–156 [DOI] [PubMed] [Google Scholar]
- Parker G, Wilhelm K, Mitchell P, Gladstone G. Predictors of 1-year outcome in depression. Aust N Z J Psychiatry. 2000; 34:56–64 [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, et al. ; Group for the Study of Resistant Depression. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry. 2007; 68:1062–1070 [DOI] [PubMed] [Google Scholar]
- Thase ME, Chen D, Edwards J, Ruth A. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014; 29:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N’-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006; 317:910–918 [DOI] [PubMed] [Google Scholar]
- Wiethoff K, Bauer M, Baghai TC, Möller HJ, Fisher R, Hollinde D, et al. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German Algorithm Project. J Clin Psychiatry. 2010; 71:1047–1054 [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Parker G, Dewhurst-Savellis J, Asghari A. Psychological predictors of single and recurrent major depressive episodes. J Affect Disord. 1999; 54:139–147 [DOI] [PubMed] [Google Scholar]
- Yang H, Chuzi S, Sinicropi-Yao L, Johnson D, Chen Y, Clain A, et al. Type of residual symptom and risk of relapse during the continuation/maintenance phase treatment of major depressive disorder with the selective serotonin reuptake inhibitor fluoxetine. Eur Arch Psychiatry Clin Neurosci. 2010; 260:145–150 [DOI] [PubMed] [Google Scholar]


