ABSTRACT
The terminal sugars of Fc glycans can influence the Fc-dependent biological activities of monoclonal antibody therapeutics. Afucosylated N-glycans have been shown to significantly alter binding to FcγRIIIa and affect antibody-dependent cell-mediated cytotoxicity (ADCC). Therefore, in order to maintain and ensure safety and efficacy for antibodies whose predominant mechanism of action (MOA) is ADCC, afucosylation is routinely monitored and controlled within appropriate limits. However, it is unclear how the composition and levels of afucosylated N-glycans can modulate the biological activities for a recombinant antibody whose target is not a cell surface receptor, as is the case with ADCC. The impact of different types and varying levels of enriched afucosylated N-glycan species on the in vitro bioactivities is assessed for an antibody whose target is aggregated amyloid beta (Aβ). While either the presence of complex biantennary or high mannose afucosylated glycoforms significantly increased FcγRIIIa binding activity compared to fucosylated glycoforms, they did not similarly increase aggregated Aβ uptake activity mediated by different effector cells. These experiments suggest that afucosylated N-glycans are not critical for the in vitro phagocytic activity of a recombinant antibody whose target is aggregated Aβ and uses Fc effector function as part of its MOA.
KEYWORDS: Antibody, IgG1, antibody-dependent cell-mediated phagocytosis, ADCP, phagocytosis, Fc effector function, afucosylation, amyloid beta
Introduction
The Fc effector function of immunoglobulin G (IgG) and Fc-containing fusion proteins can be modulated by the composition of the N-glycans on their Fc domain.1–5 Different terminal sugars of Fc glycans may affect the safety, efficacy and pharmacokinetic (PK) properties of monoclonal antibodies (mAbs).2 Based on the link between specific N-glycan moieties and biological activities of Fc-containing proteins, many studies have been performed to manipulate the composition of N-glycans to either alter Fc effector function or to ensure that specific Fc-dependent bioactivities are maintained within appropriate pre-determined limits. For example, different production host cell lines can produce the same mAb with different N-glycan composition, and modifications to cell culture processes can yield different levels of N-glycans.3,4 Therefore, to understand how the composition and/or level of N-glycans may affect efficacy, safety and PK, it is critical to evaluate the impact of different N-glycan species on the in vitro properties of therapeutic mAbs.
The role of the terminal sugar fucose has been extensively studied for mAbs and Fc-containing fusion proteins.6–8 The absence of fucose, also known as afucosylation or nonfucosylation, can affect different properties of the antibody. Two major afucosylated forms are made up of complex biantennary and high mannose afucosylated glycoforms. Previously, it has been demonstrated that these two forms have both similar and different ways they can affect the biological properties of antibodies. For example, it has been shown that antibodies with high mannose glycans are cleared faster in vivo, whereas antibodies containing complex biantennary species have similar clearance to other N-glycan isoforms.4,9 Distinct afucosyl species have also been shown to differentially bind to different FcγRs for certain classes of antibodies, such as anti-CD20 antibodies.9 For example, both complex biantennary and high mannose afucosylated glycoforms increase binding to FcγRIIIa. In contrast, high mannose glycoforms have been reported to decrease binding to FcγRIIa, whereas complex biantennary afucosylated glycoforms have similar binding to FcγRIIa as fucosylated glycoforms.9 The mixture of these two species in a sample may have differential impacts on binding to FcγRIIa, especially since most binding activity assays, including the one presented herein, are measured in bulk.
In some cases, the stronger binding to FcγRIIIa in vitro results in higher in vivo potency in animal models. This correlation has been shown for several antibodies whose mechanism of action (MOA) is primarily natural killer (NK) cell-mediated ADCC, including anti-CD20 antibodies. In several cases, different alleles of FcγRIIIa that have higher affinity to such antibodies also lead to higher efficacy of therapeutics.10–12 However, for antibodies whose MOA is less well understood, or whose primary MOA is not ADCC, the impact of varying levels of afucosylated N-terminal glycans is also not well understood.
There are numerous candidate therapeutic antibodies targeting non-cellular antigens such as bacteria, viruses, and aggregated proteins.13–20 Antibodies that target the aggregated protein amyloid beta (Aβ) have been proposed to clear aggregated Aβ by antibody-dependent cell-mediated phagocytosis (ADCP), also known as Aβ uptake.15–17 Antibody binding to aggregated Aβ results in presentation of multivalent Fc domains that can bind to high and low affinity Fcγ receptors on patrolling immune cells, such as macrophages and microglia, and recruit them to specific areas. The clustering of Fc receptors results in membrane deformation around the target antigen, activation of intracellular signal transduction, and changes in actin cytoskeletal dynamics that ultimately lead to target antigen engulfment by phagocytic cells. ADCP by several of these antibodies has been shown to require Fc effector function.15–17 However, unlike anti-CD20 antibodies or glycoengineered anti-human epidermal growth factor receptor 2 (HER2) antibodies whose targets are cell-surface receptors and whose MOA is primarily driven by FcγRIIIa-dependent ADCC and levels of afucosylation, the molecular pathways and product quality attributes of antibodies that drive Fc-mediated uptake of Aβ are less well understood.18
In this study, we use the antibody aducanumab as a model to investigate the role of afucosylated N-glycans on the phagocytosis activity of an anti-Aβ molecule. Fc-mediated clearance of Aβ by microglia has been proposed as one potential MOA of anti-Aβ antibodies.16,17 While there are several studies that have shown that FcγRI and FcγRIIa are critical for antibody-mediated phagocytosis, there have been no studies to determine which are the most critical N-glycan isoforms for these MOAs.13,14 In this report, we address several fundamental questions regarding the MOA of anti-Aβ antibodies in vitro: 1) whether the levels of afucosylated N-glycans affect the anti-Aβ-mediated phagocytosis activity; 2) whether complex biantennary and high mannose N-glycoforms have similar effects on anti-Aβ-mediated phagocytosis activity; and 3) whether FcγRIIIa activity is critical for anti-Aβ-mediated phagocytosis activity.
Results
Generation of enriched afucosylated forms of aducanumab
MAbs made from standard mammalian cell lines that have not been specifically glycoengineered to alter Fc effector function typically produce antibodies with very low levels of afucosyl species.4 Therefore, to understand how the levels and/or composition of afucosylated N-glycans may affect the in vitro phagocytosis activity of aducanumab, we generated samples that consisted of different levels of enriched afucosylated species. We used three different approaches to create highly enriched afucosylated antibody: 1) FcγRIIIa chromatography generated enriched forms of afucosyl species containing both complex biantennary and high mannose containing glycoforms, herein designated as FcγRIIIa-afuc; 2) treatment of expression cells with the mannosidase inhibitor kifunensine generated predominantly high mannose glycoform species, herein designated as HM-afuc;4,9 and 3) treatment of expression cells with TriAc-FluoroFucose, a small molecule inhibitor of the fucose salvage pathway, produced antibodies with very high levels of afucosyl complex binantennary glycoform and no high mannose species, herein designated as CB-afuc.21 These samples allowed us to thoroughly dissect how different afucosyl species affect the in vitro ADCP activity of anti-Aβ molecules.
Biochemical characterization of afucosylated antibody
The N-glycan profiles of the standard and the three afucosylation-enriched antibodies were determined and are reported in Table 1. Compared to the standard sample, the FcγRIIIa-afuc sample resulted in approximately 14-fold higher levels of afucosyl species, composed of complexed biantennary and high mannose N-glycans. This enriched species resulted in 3.9-fold higher in vitro FcγRIIIa binding activity, compared to the standard, yet similar in vitro FcγRIIa binding activity compared to the standard.
Table 1.
The percentage of complex biantennary and high mannose afucosylated species and the potency of FcγRI, FcγRIIa, and FcγRIIIa, relative to the standard. The N-glycan profiles were analyzed on a HILIC column by UPLC. The binding affinity for FcγRI was determined by a TR-FRET assay, while AlphaScreen was used to assess FcγRIIa and FcγRIIIa binding. Full concentration response curves were performed and IC50 values were calculated by non-linear regression analysis. Each sample was run alongside the standard and the effect the afucosylated species had on the potency of aducanumab was calculated by dividing the IC50 of the standard by the IC50 of the samples. Each sample was tested in duplicate or triplicate and n = 2–3. Since a ratio was calculated, only mean is provided.
| % afucosylation |
Relative potency (IC50 std/IC50 sample) |
||||
|---|---|---|---|---|---|
| Sample | Complex Biantennary |
High mannose |
FcγRI | FcγRIIa | FcγRIIIa |
| Standard | 2 | 4 | 1 | 1 | 1 |
| FcγRIIIa-afuc | 15 | 29 | ND | 0.9 | 3.9 |
| HM-afuc | 0 | 67 | 0.5 | 0.9 | 8.1 |
| CM-afuc | 30 | 0 | 1.0 | 1.0 | 4.1 |
ND, not determined
One disadvantage of using FcγRIIIa chromatography to enrich for afucosylated species is that it results in a mixture of complex biantennary and high mannose species.4,9,22,23 However, the more homogeneous CB-afuc and HM-afuc samples allowed us to characterize the impact of these different glycan compositions on the in vitro bioactivities of aducanumab. The HM-afuc samples contained ~17-fold higher levels of high mannose glycoforms than the standard (Table 1), but no change in the amount of complex biantennary species. Like the FcγRIIIa-afuc sample, HM-afuc had much higher FcγRIIIa binding and slightly lower binding to FcγRIIa and FcγRI, compared to the standard. The CB-afuc sample also had higher affinity to FcγRIIIa, but in contrast to the HM-afuc sample, did not have reduced binding to FcγRIIa or FcγRI (Table 1). The FcγR binding profiles of these two samples with more homogeneous populations of afucosyl species are similar to what has been described for highly enriched afucosyl anti-CD20 antibodies generated using the kifunensine approach and a FUT8 deleted Chines hamster ovary (CHO) cell line.9
Afucosylated aducanumab phagocytosis activity in vitro
Microglial and monocyte cells can promote antibody-mediated uptake of Aβ aggregates in vivo and in vitro.16–20 Previously, the BV-2 microglia cell line has been used to measure antibody dose-dependent phagocytosis activity of Aβ fibrils in vitro.17,19,20 To determine whether high FcγRIIIa activity in these samples resulted in increased antibody-mediated phagocytosis activity, all three forms of enriched afucosylated samples were compared to a standard preparation of aducanumab in an in vitro Aβ phagocytosis assay using the BV-2 cell line as previously described.17 All three preparations of highly enriched afucosylated aducanumab had similar dose-dependent BV-2-mediated phagocytic activity as standard aducanumab, suggesting that while afucosylated glycans significantly increase FcγRIIIa binding activity, they do not increase in vitro phagocytic activity of aducanumab in the microglia BV-2 cell system (Figure 1a,b). This result is in contrast to antibodies, like anti-CD20 and anti-HER2, that mediate cytotoxicity of tumor cells via ADCC,7,24,25 and suggests that phagocytic properties of anti-Aβ antibodies may not be driven by FcγRIIIa activity or by immune effector cells whose dominant Fc gamma receptor is FcγRIIIa.
Figure 1.
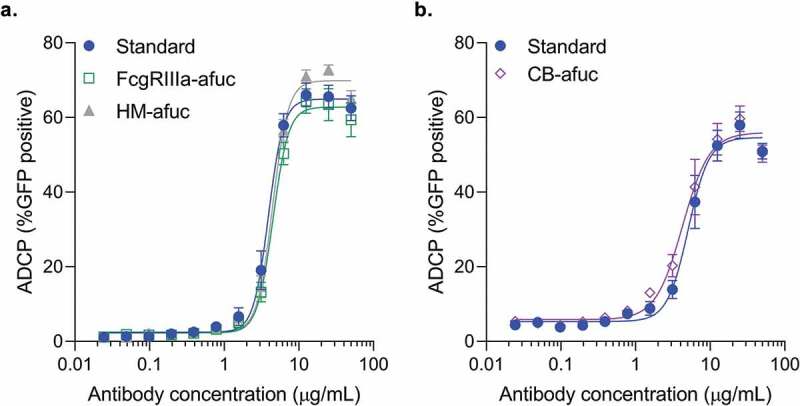
Comparison of the ADCP activity of different afucosylated forms to the aducanumab standard in the BV-2 cell line. Aβ-GFP phagocytosis was quantitated by flow cytometry, with the percent positive GFP cells of 1000 cells sampled per sample shown. Concentration response curves for each of the afucosylation samples were performed alongside the standard. Samples were run in duplicate or triplicate. Mean ± S.E.M. and non-linear regression curve fit analysis are shown. (a) FcγRIIIa enriched afucosyl isoforms (FcγRIIIa-afuc) and kifunensine-generated high mannose isoforms (HM-Afuc) have similar dose-dependent Aβ phagocytosis activity as the standard (two-way analysis of variance (ANOVA) for standard vs sample, p >.05; n = 4–7). (b) Complex biantennary afucosylated anti-Aβ (CB-Afuc) has similar dose-dependent Aβ phagocytosis activity as the standard (two-way ANOVA for standard vs sample, p >.05; n = 7).
A highly enriched afucosylated antibody may have similar phagocytosis activity to a standard antibody for two reasons: 1) murine effector cells (or the BV-2 cell line) may respond differently to human antibodies compared to human cells; or 2) different effector cells may express different types of FcγRs. To test these hypotheses, the highly afucosylated samples were compared to the standard in the phagocytosis assay mediated by different human cell line systems that express different FcγRs. The human monocyte THP-1 cell line and primary human monocyte-derived macrophages (MDM) both express human FcγRI, FcγRIIa and FcγRIIIa (Figure 2a-b). Previously, THP-1 cells have been used to measure ADCP activity of antibodies against the human immunodeficiency virus (HIV) and Aβ-coated beads.13,14,20 THP-1 and two different preparations of primary MDM cells had similar Fcγ receptor profiles with high levels of FcγRIIa, and lower levels of FcγRI and FcγRIIIa (Figure 2a). The relative Fcγ receptor expression level profiles were similar to what has previously been observed for THP-1 cells and macrophages.8,13,14 These cell types, like the BV-2 cell line, are capable of mediating ADCP of Aβ aggregates. Similar to the observation in the BV-2 cell line, enrichment of afucosylated anti-Aβ (HM-afuc) did not have any impact on ADCP activity mediated by human macrophages and the THP-1 cell line (Figure 2c and 2d respectively). These data suggest that afucosylation levels are not critical for anti-Aβ in vitro ADCP activity mediated by either murine or human effector cell lines.
Figure 2.
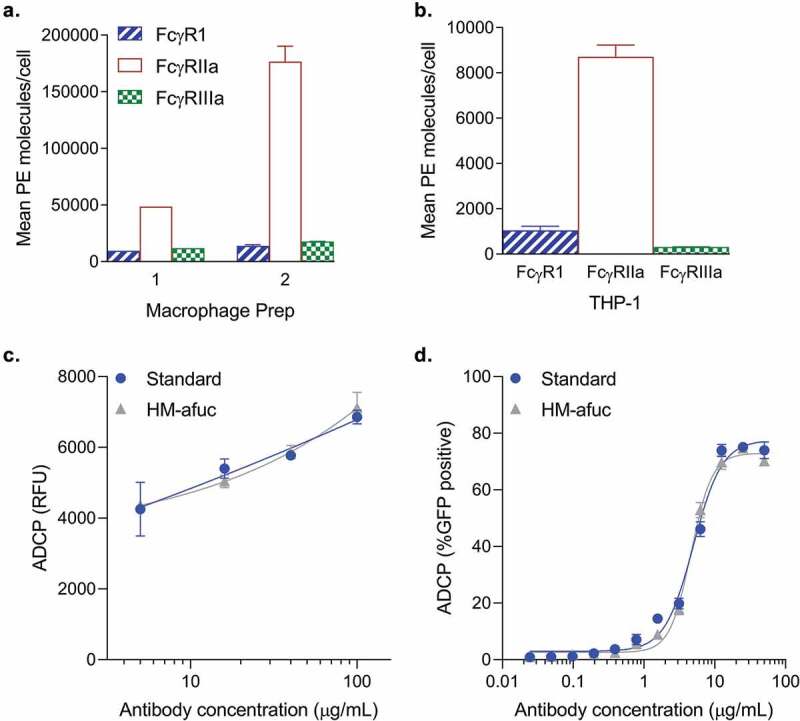
Antibody-mediated Aβ uptake in human cell systems. (a-c) Mean number of Fc Receptors determined using the QuantiBRITE PE kit on two different human macrophage preparations derived from human peripheral CD14+ monocytes (A, n = 1–2) and on the human THP-1 cell line (B, n = 2). Mean ± S.E.M. are provided. (c) Kifunensine high mannose sample (HM-Afuc) has similar dose-dependent Aβ phagocytosis activity as the standard mediated by primary human macrophages (two-way ANOVA for standard vs sample, p >.05; n = 3). Results are presented as the relative ADCP fluorescence unit (RFU) as measured using the trypan blue exclusion method versus antibody concentration. Mean ± S.E.M. and non-linear regression curve fit analysis are shown. (d) HM-Afuc has similar dose-dependent Aβ phagocytosis activity as the standard mediated by the THP-1 cell line (two-way ANOVA for standard vs sample, p >.05; n = 4). Samples were run in duplicate and Aβ-GFP phagocytosis was quantitated by flow cytometry. The mean ± S.E.M. of the percent positive GFP cells of 1000 cells sampled per sample and non-linear regression curve-fit analysis are shown.
Since FcγRIIa has been hypothesized to play a role in ADCP we developed an ADCP assay using a CHO cell line that stably expresses human FcγRIIa (CHO-FcγRIIa) as a surrogate model system to assess ADCP activity in vitro.13,26,27 Expression of human FcγRIIa is sufficient to render a non-phagocytic cell into a cell capable of mediating Fc-dependent uptake of opsonized particles and acidification of phagocytosed compartments.28,29 As expected, the CHO- FcγRIIa cell line was capable of mediating antibody-mediated uptake of fluorescent Aβ aggregates (Figure 3a). This surrogate cell line has several advantages over the use of either primary cells or a cell line like BV-2: 1) high expression of only a single Fc receptor; 2) more stable expression of the receptor over cell passage; and 3) as a result of 1) and 2), more consistent quantification of in vitro ADCP activity. This assay was used to determine whether increasing levels of complex biantennary afucosylation in aducanumab leads to higher ADCP activity. Several sample blends containing 7%, 10%, 15%, and 30% complex biantennary afucosylated antibody were created and tested in various binding assays and in the ADCP assay (Figure 3b). Only FcγRIIIa binding was affected by altered levels of complex biantennary afucosylated antibody, while FcγRI, FcγRIIa, and Aβ binding activity were not affected. Importantly, no impact of altered FcγRIIIa activity on ADCP was observed across this range of complex biantennary afucosylation. In contrast, high mannose afucosylated antibody had lower ADCP activity using this CHO-FcγRIIa cell line (data not shown), which is similar to what was measured for soluble FcγRIIa binding (Table 1). Like the BV-2 and human cell line systems, samples with very high FcγRIIIa activity do not have very high ADCP activity in the CHO- FcγRIIa cell line system.
Figure 3.
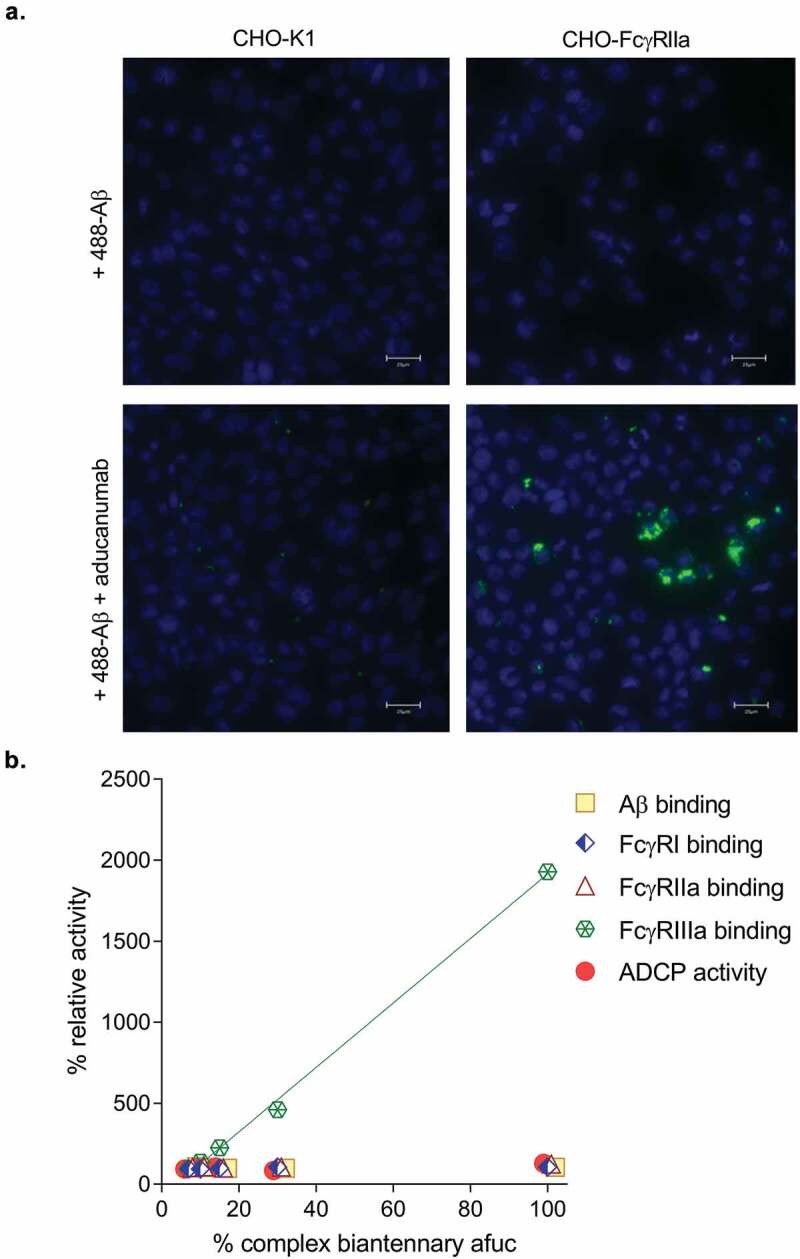
(a) Representative fluorescent images of aducanumab-mediated uptake of Alexa488-labeled Aβ (488- Aβ) aggregates. Following treatment with 488- Aβ with or without aducanumab, external fluorescence was quenched with trypan blue. No aducanumab-stimulated uptake was observable in wild-type CHO-K1 cells; however, 488- Aβ was internalized by aducanumab in cells expressing FcγRIIa (CHO-Fcγ-RIIa). No 488-Aβ was taken up into cells in the absence of aducanumab. Nuclei are stained with DAPI (scale bars represent 25 µm). (b) A 100% complex biantennary afucosylated sample was generated and then mixed with aducanumab standard to create blends with 7%, 10% 15% and 30% afucosylation. Each sample was tested in duplicate or triplicate for binding affinity for Aβ, FcγRI, FcγRIIa and FcγRIIIa and Aβ ADCP activity. The relative potency versus the antibody standard was calculated for each blend in each assay, and the results are plotted as a percent. FcγRIIIa binding was analyzed by linear regression analysis (R2 = 0.998).
Discussion
To our knowledge, this is the first study to demonstrate that high levels of afucosyl N-glycans and high FcγRIIIa activity do not significantly impact in vitro phagocytosis activity of an anti-Aβ antibodies. These structure-activity relationship studies are necessary for understanding which product quality attributes are critical to control in order to ensure similar safety and efficacy during the manufacturing process.
While there is a strong correlation between afucosylation levels, binding to FcγRIIIa and ADCC activity, there are conflicting data on how levels of afucosylation may affect the ADCP activity of antibodies.29,30 Using multiple approaches, we have started to build a basis for the understanding of how N-glycan composition may impact the antibody-mediated Aβ phagocytosis activity. In this case, using aducanumab as a model system, we show that high FcγRIIIa activity mediated by high levels of afucosyl N-glycans does not result in high ADCP activity in vitro using different effector cells. These data point to the role of other factors that may affect the assessment of ADCP activity of antibodies in vitro, such as the specific target and the FcγR expression level on the recruited immune cell, which corroborate similar findings.8 For example, unlike NK cells, which predominantly express FcγRIIIa, cells from the monocytic lineage such as macrophages and microglia express multiple FcγRs.8,31 Human macrophage colony-stimulating factor (M-CSF)-cultured macrophages have high expression of FcγRIIa and the high affinity FcγRI receptor, both of which may combine and more tightly bind both fucosylated and afucosylated antibodies and, in turn, overwhelm the specific, high-affinity interaction of afucosylated antibodies to bind FcγRIIIa on those cells. Our data corroborates studies on highly afucosylated IgG-mediated uptake of erythrocytes by macrophages.8 The results presented here demonstrate that it is critical to not generalize the impact of specific glycans, such as afucosylation levels, on the biological activity of all glycosylated antibodies since there are multiple factors that may influence cell mediated Fc effector function(s).
FcγR-mediated clearance of Aβ aggregate has been proposed for multiple anti-Aβ antibodies.16,17 While it has been shown that the Fc effector function is required for antibody-mediated clearance of Aβ aggregates, it remains unclear which Fcγ receptors are most critical for the Fc effector functionalities of these antibodies.15,16 Human microglia express all three activating FcγRs, FcγRI, FcγRIIa and FcγRIIIa.31 Expression of these FcγRs can be upregulated upon association with amyloid plaques and overexpression of the M-CSF receptor.8 While these studies do not definitively determine which FcγR(s) is the most critical for ADCP of Aβ aggregates, the results suggest that FcγRIIIa binding activity is not required for the ADCP of Aβ aggregates. In addition, this study showed that FcγRIIa is sufficient for ADCP of Aβ aggregates, which has been similarly shown for uptake of opsonized red blood cells.28 These results also corroborate previous studies that showed macrophage-mediated phagocytosis by anti-epithelial cell adhesion molecule (EpCAM) and anti-HIV also required FcγRI and FcγRIIa, but not FcγRIIIa,19 suggesting that the FcγRIIIa receptor may not be a dominant FcγR for ADCP activity of antibodies.13,14
Finally, since aggregated Aβ is not a cell surface receptor, it is unlikely that there will be potential cell-mediated cytotoxicity from very high levels of FcγRIIIa-dependent ADCC activity. Therefore, the general classification of afucosylation and/or FcγRIIIa-dependent ADCC activity for a glycosylated antibody as a critical product quality attribute is not always valid. The criticality of product quality attributes such as afucosylation and FcγRIIIa activity must be evaluated under the appropriate context, taking into the account the antibody target, the MOA of the antibody and the relevant immune effector cell types that are being recruited.
Materials and methods
Aducanumab was expressed in a recombinant CHO cell line and purified at Biogen. The soluble FcγRIIIa-Glutathione S-transferase (GST) and FcγRIIa-GST fusion proteins were made at Biogen as previously described.18
Unlabeled, biotinylated, fluorescein amidites-labeled (FAM), HiLyte488-labeled Aβ (1–42) were purchased from Anaspec. Streptavidin-Alexa488, phalloidin-Alexa594, 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Life Technologies. Fucoidan was purchased from Sigma. 7.5% bovine serum albumin (BSA) fraction V, glutamine and fetal bovine serum (FBS) were purchased from Gibco.
Generation of high mannose afucosylated species
An aducanumab-producing stable CHO cell line was cultured in a 3 L bioreactor for 7 days following the historical fed-batch processes. On day 7, the cells were split into multiple 1 L shake flasks (Corning Life Science) with 200 mL working volume supplemented with various concentrations of kifunensine and then further cultured another 3 days with a fed-batch shake flask mode.32 The feed amount was calculated using the same fixed percentage as bench-scale bioreactor process. Time-course samples were collected daily until day-3 harvest. Both filtered supernatant and cell pellets samples were retained for N-glycan analysis. The antibody was purified by Protein A chromatography.
Generation of complex biantennary afucosylated species
50 µM of Tri-O-acetyl-2-deoxy-2-L-fucose (Carbosynth Batch MT15919) was added to an aducanumab-producing stable CHO cell line in a 0.5 L shake flask. On day 5, an additional 50 µM of Tri-O-acetyl-2-deoxy-2-L-fucose was added to the cell culture. On day 14, the cell culture supernatant was harvested. The antibody was purified by Protein A chromatography.
Cell lines
Recombinant CHO cell lines were cultured in Biogen proprietary media. BV-2 cells were obtained from Dr. V. Bocchini (University of Perugia, Italy) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM)/10% FBS/glutamine. THP-1 cells were purchased from ATCC (TIB-202).
Differentiation of monocyte to macrophage
Human peripheral blood CD14+ monocytes (70035, Stem Cell Technologies) were differentiated to macrophages with recombinant M-CSF (216-MC, R&D Systems) using the following media (Advanced RPMI1640 medium, 5% FBS, Penicillin-Streptomycin 1x, L-Glutamine 2 mM, M-CSF 100 ng/ml). Media was changed every 2 days for 6–8 days.
Enrichment of afucosylated antibody by FcγRIIIa chromatography
FcγRIIIa -GST columns were used to enrich afucosylated IgG1 as previously described.18 In brief, 10 mL of antibody was loaded on to FcγRIIIa -GST column and eluted from the column with low pH buffer. The UV elution peaks were collected in polypropylene tubes containing 2 mL of 2 M Tris pH 7.0 to immediately neutralize the solution. The low pH eluted fractions were buffer exchanged by centrifugation with an Amicon Ultra 4 Centrifugal filters, 50,000 NMWL filter tube.
N-glycan analysis
The N-glycan profile of the mAb was analyzed using the Prozyme GlykoPrep Digestion Module (GS96-RX) and the Prozyme GlykoPrep Cleanup Module (GS96-CU) as previously described.19 Briefly, 50 µg of the mAb was used in each preparation. The N-glycans were removed by digestion with N-Glycanase for 1 h at 50°C, separated from the mAb with the RX tips supplied in the Digestion Module and labeled with 2-amino benzamide (2-AB). Excess 2-AB was removed by passing the reactions solution through the Clean Up tips supplied in the Cleanup Module. The labeled N-glycan samples were analyzed on a hydrophilic interaction liquid chromatography (HILIC) column (BEH Glycan Column, 2.1 mm x 150 mm, 186004742) on a Waters Acquity UPLC (Binary Solvent Manager) with a fluorescence detector. Samples were run on a 24-min gradient of 25% 0.1 M ammonium formate, 75% acetonitrile pH 4.5 to 100% 0.1 M ammonium formate pH 4.5 at 60°C with a flowrate of 0.4 mL/minute.
FcγR binding assays
To determine the relative affinity for FcγRIIIa and FcγRIIa binding, a competitive AlphaScreen assay was used as previously described.19 In brief, samples were diluted in FcγRIIIa assay buffer (phosphate-buffered saline (PBS), 0.01% Tween 20, 0.1% BSA) or FcγRIIa assay buffer (50 mM Tris, 25 mM NaCl, 0.01% Tween 20, 0.1% BSA) and added to the assay plate at a starting concentration of 200 µg/mL. Next, FcγR-GST was added to the plate at a final concentration of 0.17 µg/mL and GSH-coated donor beads and antibody-conjugated acceptor beads (donor and acceptor beads) from Perkin Elmer; IgG1-conjugated acceptor beads made for Biogen by Perkin Elmer were added to the plate at a final concentration of 3.3 µg/mL. After shaking the plate for 2 hours at 22°C ± 1°C, luminescence was read using a Perkin Elmer EnVision plate reader. The data were analyzed using SoftMax Pro, and IC50 values were used to determine relative binding activity.
To determine the relative affinity for FcγRI binding, a competitive time-resolved fluorescence resonance energy transfer (TR-FRET)-based assay was used. In brief, Europium (Eu) chelate-labeled IgG1 (Eu-IgG1) competes with unlabeled sample for binding to Cy5-labeled FcγRI. Fixed concentrations and volumes of Eu-IgG1 and Cy5-labeled FcγRI were added to the assay plate containing the sample. The plate was incubated for 1 h at ambient temperature with moderate agitation. The fluorescence signal, which is inversely proportional to FcγRI binding activity, was measured at a wavelength of 665 nm on a Perkin Elmer EnVision plate reader. The data were analyzed using SoftMax Pro, and IC50 values were used to determine relative binding activity.
Aβ (1-42) aggregate formation
Hexafluoro-2-propanol-treated unlabeled and Hilyte488-labeled Aβ or FAM-labeled Aβ were dissolved in dimethyl sulfoxide. For the ADCP assay, unlabeled and labeled Aβ were mixed at a 1:10 ratio. Aβ monomers were converted to Aβ aggregate in a tube at 37°C while shaking overnight as previously described.17,33
Aβ binding assay
Aβ(1–42) was coated onto 96-well immunoassay plates overnight at 4°C. The following day, the plates were blocked with blocking buffer (1X PBS, 3% BSA). A mixture of Europium-labeled IgG1 and unlabeled samples were added to the plate and were incubated at ambient temperature with agitation for 1–2 hours. Following this incubation, the plates were washed, and Europium enhancement solution was added to the plates. Time-resolved fluorescence was measured using a Perkin Elmer Envision plate reader. The data were analyzed using SoftMax Pro, and IC50 values were used to determine relative binding activity.
ADCP assays
The ADCP assay was performed as previously described with some modifications.17,33 BV-2 cells were trypsinized and seeded at 60,000 cells/well onto 96-well tissue culture plates and allowed to adhere overnight in a humidified CO2 incubator at 37°C. The following day, cells were washed with PBS and resuspended in ADCP assay diluent (DMEM/F12, 20 mM HEPES, 1% BSA). THP-1 and CHO-FcγRIIa were harvested on the day of the assay, washed with PBS and resuspended in ADCP assay diluent. For BV-2 experiments, 100 µg/mL Fucoidan, an inhibitor of the scavenger receptor, was added 30 minutes prior to the experiment. Antibodies and aggregated fluorescent Aβ were mixed and incubated at 37°C while shaking for 30–60 minutes. After the incubation, the antibody/Aβ suspension was added to cells and the mixture was incubated in a humidified CO2 incubator at 37°C for 60–90 minutes. After the incubation, BV-2 cells were washed twice with PBS to remove unassociated aggregated Aβ. Cells were treated with trypsin/ethylenediaminetetraacetic acid for 20 minutes at 4°C, transferred to a 96-well round bottom plate and washed twice with fluorescence-activated cell sorting (FACS) wash buffer (1X PBS with 2% BSA) by centrifugation at 500 x g for 5 minutes at 4°C. Cells were fixed for 20 minutes in FACS-Fix (PBS, 2% formaldehyde, 2% Glucose, 5 mM NaN3). The percent green fluorescent protein (GFP) positive in 1000 cells was determined on a Millipore Guava flow cytometer and the data were analyzed using a 4-parameter curve-fitting program (SoftMax Pro).
The procedure for analyzing ADCP by fluorescent microscopy is like the ADCP assay described above with the following modifications. CHO cells were plated at a density of 40,000 cells per well of a 96-well, black-walled, optically clear bottom tissue culture plate. After the ADCP was complete as described above, external fluorescence was quenched by 0.2% Trypan Blue solution. Cells were then fixed, permeabilized and incubated with DAPI. Fluorescent images were taken with an EVOS cell imaging system. A 40x objective was used with the settings consistent between imaging (Dapi: light = 2; exposure = 10, gain = 3.86; GFP: light = 22.21, exposure = 46.9, gain = 48.3).
Trypan blue exclusion method for ADCP
Macrophages were seeded on the collagen-coated 96-well plate at 150,000 cells per well. The cells were washed with PBS, the antibody-Aβ mixture was added to the cells, and the plate was incubated at 37°C. After 60 minutes, the media containing the antibody- Aβ mixture was removed, cells were washed gently with 200 µL PBS, and 0.2% Trypan Blue solution was added to quench extracellular fluorescence. After approximately 1 min at ambient temperature, the Trypan Blue solution was removed, and fluorescence was measured at 480 nm for FAM-Aβ.
FcγR quantification using BD Quantibrite phycoerythrin (PE) beads
PE-labeled anti-Fc receptor antibodies (Invitrogen Catalog # CD3204, MHCD1604, CD6404) were used to quantify Fc gamma receptors on cells. In brief, 5 µL of anti-CD32, anti-CD16, or anti-CD64 were added to 1 × 106 cells in 100 µL ADCP assay diluent. Cells were incubated for 1 h ± 15 minutes on an orbital plate shaker (speed 2) at 2°C–8°C. After the incubation was complete, cells were washed 2 times with FACS wash buffer by centrifugation at 250 g x 5 minutes. The cell pellet was resuspended in 100 µL wash buffer and run on a Millipore Guava flow cytometer. BD Quantibrite PE beads (BD Biosciences Catalog # 340495) conjugated with four levels of PE were run using the same settings to generate a standard curve. The mean fluorescence of the PE-antibody labeled cells is converted into the number of PE molecules per cell using this standard curve.
Data analysis
GraphPad Prism software (v. 8.0) was used for data and statistical analyses which are specifically described in the figure legends. All data are presented at the mean ± standard error of the mean (S.E.M.). Concentration response curves were fit to a non-linear regression (four parameter) model. The n for each experiment is indicated in the figure legends.
Acknowledgments
We thank Fang Qian for generation materials, Elise Levi and Suli Liu for providing testing support and Janine Ferrant, Paul Weinreb, Thierry Bussiere and Chris Ehrenfels for providing guidance and discussion.
Funding Statement
This work was sponsored by Biogen.
Disclosure of potential conflicts of interest
All authors were employees of Biogen and held stock in the company at the time of their contributions to this work.
References
- 1.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M.. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10:101–9. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 2.Reusch D, Tejada ML.. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology. 2015;25:1325–34. doi: 10.1093/glycob/cwv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu X, Marshall MJE, Cragg MS.Improving Antibody-Based CM. Cancer Therapeutics Through Glycan Engineering. BioDrugs Clin Immunother Biopharm Gene Ther. 2017;31:151–66. doi: 10.1007/s40259-017-0223-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhang A, Tsang VL, Markely LR, Kurt L, Huang YM, Prajapati S, Kshirsagar R. Identifying the differences in mechanisms of mycophenolic acid controlling fucose content of glycoproteins expressed in different CHO cell lines. Biotechnology and Bioengineering. 2016;113:2367–76. doi: 10.1002/bit.25995. [DOI] [PubMed] [Google Scholar]
- 5.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 2017;114:3485–90. doi: 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SHA, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 7.Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, Fong C, Lau W, Qiu ZJ, Shen A. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. mAbs. 2012;4(3):326–40. doi: 10.4161/mabs.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruggeman CW, Dekkers G, Bentlage AEH, Treffers LW, Nagelkerke SQ, Lissenberg-Thunnissen S, Koeleman CAM, Wuhrer M, van den Berg TK, Rispens T, et al. Enhanced effector functions due to antibody defucosylation depend on the effector cell Fc gamma receptor profile. J Immunol. 1950;2017(199):204–11. [DOI] [PubMed] [Google Scholar]
- 9.Webster SD, Galvan MD, Ferran E, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Antibody-mediated phagocytosis of the amyloid beta-peptide in microglia is differentially modulated by C1q. Journal of Immunology (Baltimore, Md. 1950;2001:7496–503. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ, Czuczman MS, Islam H, Klein C, Cairo MS.. Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haematol. 2015;171:763–75. doi: 10.1111/bjh.13764. [DOI] [PubMed] [Google Scholar]
- 11.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu –positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 12.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:3940–47. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87:5468–76. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–19. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 16.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32:9677–89. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin J, Masci A, Gronke RS, Bergelson S, Co C. A simple enzyme-substrate localized conjugation method to generate immobilized, functional glutathione S-transferase fusion protein columns for affinity enrichment. Anal Biochem. 2016;505:51–58. doi: 10.1016/j.ab.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics J Am Soc Exper NeuroTher. 2013;10:459–72. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, Wong A, Stephan JP, Bayer R. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. mAbs. 2012;4:475–87. doi: 10.4161/mabs.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippold S, Nicolardi S, Dominguez-Vega E, Heidenreich AK, Vidarsson G, Reusch D, Haberger M, Wuhrer M, Falck D. Glycoform-resolved FcRIIIa affinity chromatography-mass spectrometry. mAbs. 2019;11:1191–96. doi: 10.1080/19420862.2019.1636602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton GR, Ackerman ME, Boesch AW. Separation of nonfucosylated antibodies with immobilized FcgammaRIII receptors. Biotechnol Prog. 2013;29:825–28. doi: 10.1002/btpr.1717. [DOI] [PubMed] [Google Scholar]
- 24.Ulvestad E, Williams K, Matre R, Nyland H, Olivier A, Antel J. Fc receptors for IgG on cultured human microglia mediate cytotoxicity and phagocytosis of antibody-coated targets. J Neuropathol Exp Neurol. 1994;53:27–36. doi: 10.1097/00005072-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Subedi GP, Barb AW. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. mAbs. 2016;8:1512–24. doi: 10.1080/19420862.2016.1218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kute T, Stehle JR Jr., Ornelles D, Walker N, Delbono O, Vaughn JP. Understanding key assay parameters that affect measurements of trastuzumab-mediated ADCC against Her2 positive breast cancer cells. Oncoimmunology. 2012;1:810–21. doi: 10.4161/onci.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–27. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 28.Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–99. doi: 10.1182/blood.V86.12.4389.bloodjournal86124389. [DOI] [PubMed] [Google Scholar]
- 29.Downey GP, Botelho RJ, Butler JR, Moltyaner Y, Chien P, Schreiber AD, Grinstein S. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J Biol Chem. 1999;274:28436–44. doi: 10.1074/jbc.274.40.28436. [DOI] [PubMed] [Google Scholar]
- 30.Thomann M, Schlothauer T, Dashivets T, Malik S, Avenal C, Bulau P, Rüger P, Reusch D. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PloS One. 2015;10:e0134949. doi: 10.1371/journal.pone.0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, Jeffrey SC, Klussman K, Law C-L, Sussman D. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A. 2013;110(14):5404–09. doi: 10.1073/pnas.1222263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WC, Lu J, Kwiatkowski C, Yuan H, Kshirsagar R, Ryll T, Huang Y-M. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol Prog. 2014;30(3):616–25. doi: 10.1002/btpr.1884. [DOI] [PubMed] [Google Scholar]
- 33.Halle M, Tribout-Jover P, Lanteigne A-M, Boulais J, St-Jean JR, Jodoin R, Girouard M-P, Constantin F, Migneault A, Renaud F. Methods to monitor monocytes-mediated amyloid-beta uptake and phagocytosis in the context of adjuvanted immunotherapies. J Immunol Methods. 2015;424:64–79. doi: 10.1016/j.jim.2015.05.002. [DOI] [PubMed] [Google Scholar]


