Abstract
Background:
Focal brain lesions can lend insight into the causal neuroanatomical substrate of depression in the human brain. However, studies of lesion location have led to inconsistent results.
Methods:
Five independent datasets with different lesion etiologies and measures of post-lesion depression were collated (N = 461). Each 3D lesion location was mapped to a common brain atlas. We used voxel lesion symptom mapping to test for associations between depression and lesion locations. Next, we computed the network of regions functionally connected to each lesion location using a large normative connectome dataset (N = 1000). We used these lesion network maps to test for associations between depression and connected brain circuits. Reproducibility was assessed using a rigorous leave-one-dataset-out validation. Finally, we tested whether lesion locations associated with depression fell within the same circuit as brain stimulation sites effective for improving post-stroke depression.
Results:
Lesion locations associated with depression were highly heterogeneous, and no single brain region was consistently implicated. However, these same lesion locations mapped to a connected brain circuit, centered on the left dorsolateral prefrontal cortex. Results were robust to leave one-dataset-out cross-validation. Finally, our depression circuit derived from brain lesions aligned with brain stimulation sites effective for improving post-stroke depression.
Conclusions:
Lesion locations associated with depression fail to map to a specific brain region but do map to a specific brain circuit. This circuit may have prognostic utility in identifying patients at risk for post-stroke depression and therapeutic utility in refining brain stimulation targets.
Keywords: depression, lesion, functional connectivity, network, functional MRI, imaging, stroke
Introduction:
Patients with focal brain lesions can yield insight into the causal neuroanatomical substrate underlying neuropsychiatric symptoms (1, 2). Several decades ago, an association between left frontal lesions and depression was reported for both stroke (3, 4) and brain tumors (5, 6). Subsequent work refined this association to lesions in the bilateral dorsolateral prefrontal cortex (DLPFC) (7). These lesion localization studies are important as depression is an independent predictor of morbidity and mortality post-stroke (8). These lesion studies are also important for the insight they provide into the neuroanatomy underlying primary depression, including identification of treatment targets. For example, the first trials of transcranial magnetic stimulation (TMS) to the left DLPFC for treatment of primary depression were motivated partly by lesion data (9, 10).
However, localization of depression based on focal brain lesions has been inconsistent. Even the early studies noted that most patients with post-stroke depression had lesions outside the left frontal cortex (3, 4). Work aimed at replicating this association found it only held true for specific time points (11), or not at all (12). Multiple meta-analyses have failed to find an association between left frontal lesions and depression (13–16). Studies using newer methods such as voxel-based lesion-symptom mapping have also failed to find lesion locations significantly associated with depression (17, 18).
One potential reason for these inconsistent findings is that lesions causing similar symptoms may localize to connected brain networks rather than individual brain regions (19). Similarly, symptoms caused by focal brain lesions can arise from brain regions connected to the lesion location rather than the lesion location itself, a phenomenon termed diaschisis (20, 21). A recently validated technique called lesion network mapping can better account for these factors and incorporate them into lesion-based localization (19, 22–25). This method uses lesion locations as seed regions in resting state functional connectivity analyses, taking advantage of connectome data from large cohorts of healthy subjects. By comparing the functional connectivity profiles of lesions associated with a particular symptom, this method can identify brain regions or networks underlying a symptom of interest. This technique has proven useful for understanding hallucinations (19), delusions (22), criminality (26), and even disorders of free will (27).
In this study, we analyzed the association of lesion location with depression across 5 independent lesion data sets and several lesion etiologies (ischemic stroke, intracerebral hemorrhage, and penetrating traumatic brain injury) (7, 17, 28–30). We hypothesized that lesion location alone would not be significantly associated with depression but that resting-state functional connectivity between the lesion location and other brain regions would be.
Methods and Materials:
Subjects and Lesions:
This study was approved by the institutional review board at Beth Israel Deaconess Medical Center (protocol number: 2018P000128). We performed a systematic literature search to identify lesion data sets containing depression assessments and wrote to investigators to request data (Supplementary Methods). Five independent lesion data sets totaling 461 patients were included with varied lesion etiologies, depression scales, and timing of depression assessment (Table 1, Figure 1, Supplementary Methods) (7, 17, 28–30). Our primary analysis focused on patients with moderate to severe depression (N = 58) versus patients with no depression (N = 300, referred to subsequently as ‘controls’ or ‘non-depressed controls’) using established cutoffs for each depression scale (Supplementary Methods, Table 1) (7, 17, 31–36). We used a binary contrast of “depressed” versus “non-depressed” for our main analysis for three reasons. First, this maintains consistency with the post-stroke depression literature, including studies whose findings we sought to replicate (7, 37, 38). Second, this enables combining datasets with different depression measures, each of which has established cutoffs for binary classification. Finally, because depression scales can be influenced by many factors, focusing on the extremes may be more likely to identify associations with lesion location. However, we also repeated our analyses on the full cohort of lesions (N = 461) treating depression as a continuous measure. In order to perform analyses across datasets, depression scores for subjects within each dataset were z-scored against other subjects within that same dataset, yielding a normalized continuous depression score for each subject.
Table 1.
Subject Demographics.
| Total Data | Naidech et al, 2016 (29) | Corbetta et al, 2015 (28) | Egorova et al, 2018 (30) | Gozzi et al, 2014 (17) | Koenigs et al, 2008 (7) | |
|---|---|---|---|---|---|---|
| Total Subjects for Primary Analysis (Depressed/Non-Depressed) | 358 (58/300) | 33 (10/23) | 87 (14/73) | 49 (5/44) | 45 (7/38) | 144 (22/122) |
| Mean age (stdev) | 59.3 (11.1) | 58.6 (14.4) | 53.8 (11.0) | 68.1 (14.3) | 63.5 (13.5) | 58.5 (3.4) |
| Sex (% M, % F) | 272 M (76%), 86 F (24%) | 18 M (55%), 15 F (45%) | 45 M (52%), 42 F (48%) | 36 M (73%), 13 F (27%) | 29 M (64%), 16 F (36%) | 144 M (100%) |
| Lesion etiology | Intracerebral hemorrhage | Stroke (ischemic or hemorrhagic) | Ischemic stroke | Stroke (ischemic or intracerebral hemorrhagic) | Penetrating traumatic brain injury | |
| Depression scale | Neuro-QOL depression scale (31) | Geriatric Depression Score Short Form (32) | Patient Health Questionnaire (PHQ-9) (33) | Hospital Anxiety and Depression Scale (34), followed by Mini-International Neuropsychiatric Interview (35) | Beck Depression Inventory II (36) | |
| Depression threshold | Neuro-QOL T score ≥ 59.9 (equivalent to PHQ-9 ≥ 10) | GDSS ≥ 11 | PHQ-9 ≥ 10 | HADS ≥ 11, major depression per structured interview | BDI-II score ≥ 20 | |
| Threshold for Non-depressed status | Neuro-QOL T score ≤ 50.5 (equivalent to PHQ-9 ≤ 4) | GDSS ≤ 5 | PHQ-9 ≤ 4 | HADS < 11 | BDI-II score ≤ 8 | |
| Time of Depression Assessment | Varied; 28 days, 3 months, or 12 months after hospital discharge | 3 months after stroke | 3 months after stroke | 1 month after stroke | 33–39 years following traumatic brain injury | |
| Total Subjects for Continuous Analysis | 461 | 51 | 100 | 63 | 51 | 196 |
| Mean age (stdev) | 59.3 (10.7) | 61.1 (14.3) | 53.6 (10.6) | 67.5 (13.4) | 62.6 (13.9) | 58.3 (3.1) |
| Sex (% M, % F) | 347 M (75 %) 114 F (25 %) | 28 M (55%), 23 F (45%) | 51 M (51%), 49 F (49%) | 41 M (65%), 22 F (35%) | 31 M (61%), 20 F (39%) | 196 M (100%) |
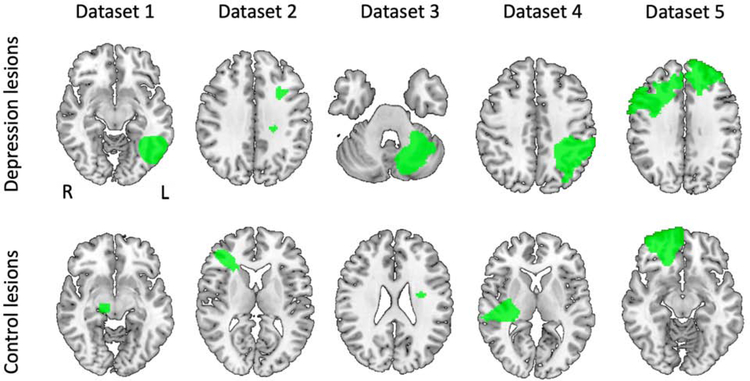
Figure 1:
Lesions from depressed and non-depressed subjects from each of our five datasets demonstrate heterogeneity in lesion location: Dataset 1: Naidech et al, 2016 (intracerebral hemorrhage); Dataset 2: Corbetta et al, 2015 (stroke); Dataset 3: Egorova et al, 2018 (stroke); Dataset 4: Gozzi et al, 2004 (stroke); Dataset 5: Koenigs et al, 2008 (penetrating traumatic brain injury).
Lesions were manually segmented based on CT (7, 29) or structural MRI images (17, 28, 30), spatially normalized to MNI152 atlas space, and binarized, such that voxels within the lesion carried a value of 1, and all other voxels carried a value of 0. Lesion masks were added together to create lesion overlap maps (Supplementary Figure S1).
Analysis of Lesion Location:
To identify any lesioned brain voxels associated with depression, we performed voxel-based lesion symptom mapping (VLSM) (1, 2) using the Matlab package NiiStat (https://github.com/neurolabusc/NiiStat), controlling for lesion size and dataset as covariates (39) and using standard settings and statistical cutoffs (Supplementary Methods) (1, 2). To maximize sensitivity, we focused our analysis on the bilateral dorsal lateral prefrontal cortex (DLPFC), defined using the Harvard Oxford atlas “middle frontal gyrus” (MFG) region and a cutoff of >0% probability. This focus was motivated by literature implicating the DLPFC in depression and its use as a treatment target for TMS. To ensure results were not dependent on our choice of ROI, we repeated our analysis using two other DLPFC ROIs used in prior studies of lesion location and depression (4, 7) (Supplementary Figure S2). To ensure that we did not miss important results outside the DLPFC, we repeated our analysis using no mask (i.e., including the whole brain). Finally, we repeated analyses treating depression as a continuous rather than a binary variable and including all lesions (N = 461).
In addition to the above voxel-wise analyses, we also replicated ROI-based analyses from prior studies that reported positive associations between lesion location and depression (4, 7) and tested for associations between depression and lesions to the left versus right hemisphere (Supplementary Methods, Supplementary Figure S2). In total, we tested seven a priori hypotheses regarding lesion intersection with these ROIs.
Lesion Network Mapping:
Using previously validated methods for lesion network mapping (19, 22), we tested whether lesions associated with depression mapped to a connected brain circuit. Functional connectivity between each lesion location and all other brain voxels was computed using resting state functional connectivity data from 1000 healthy subjects (40, 41) (Figure 2, Supplementary Methods).
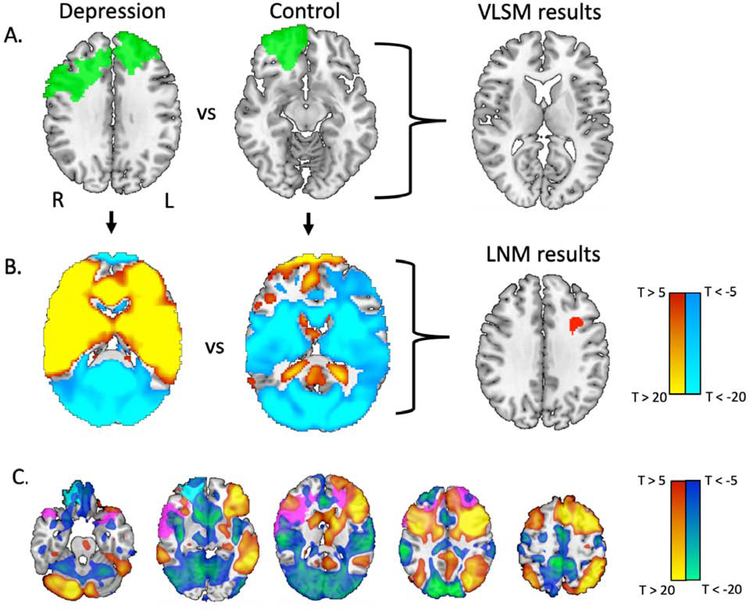
Figure 2:
Lesion locations associated with depression intersect a connected brain circuit, not an individual brain region. (A) A sample lesion from a depressed subject and a non-depressed subject are depicted in green. Standard voxel-based lesion-symptom mapping identified no lesioned voxels significantly associated with depression. (B) Functional connectivity of each lesion location to the rest of the brain was computed using resting state functional connectivity data from 1000 healthy controls. A focal region in the left dorsolateral prefrontal cortex showed greater functional connectivity to lesions of depressed subjects than to lesions of non-depressed subjects (depicted in red; voxel-level family-wise error [FWE] corrected p < 0.05). (C) Using normative connectome data, we examined the whole-brain functional connectivity of this region, generating a depression circuit. By definition, lesions from depressed subjects will intersect positive nodes of this network (sample lesion shown in violet) while lesions from non-depressed subjects will not (sample lesion shown in cyan). In panels B and C, the red-yellow coloration indicates regions positively connected to the region, while the blue-green coloration indicates regions negatively connected (anti-correlated) to the region. Network maps are thresholded at T = ±5 for ease of visualization (actual network maps were unthresholded). Z coordinates of slices in panel C: −25, −5, 15, 35, 55.
Unthresholded lesion network maps of depressed (N = 58) versus non-depressed control (N = 300) subjects were statistically compared using a general linear model and permutation testing (Permutation Analysis of Linear Models in FSL 3.2.0), including dataset and lesion size as covariates (42, 43). The location of each lesion was not excluded from the corresponding lesion network map. As in our VLSM analysis, we searched for significant results within the Harvard Oxford bilateral MFG mask. We used a conservative voxel-level family-wise error correction for multiple comparisons, correcting for all brain voxels within our mask (pc < 0.05). This is more stringent than the commonly used cluster-based correction which can be associated with false positives depending on the detection threshold (44). As with our VLSM analysis, we repeated our analysis using two other DLPFC masks and using no mask at all (Supplementary Methods).
Significant voxels from this analysis were extracted as a seed region of interest, and the functional connectivity of this region to the rest of the brain was computed using the normative connectome of 1000 healthy subjects. By definition this network map, which we term the “depression circuit”, best encompasses lesion locations associated with depression while avoiding lesion locations that are not.
We repeated this analysis using the subset of lesions with a common lesion etiology (ischemic stroke, hemorrhagic stroke, penetrating traumatic brain injury) and the subset of lesions with a confirmed lack of history of depression pre-lesion. Similarity to our primary circuit (N = 358) was assessed through spatial correlation (Supplementary Methods).
Leave-one-dataset-out cross-validation and network damage scores:
To ensure that our findings were not biased by any one of our five datasets, and to test whether our depression circuit could predict depression in independent datasets, we performed a leave-one-dataset-out validation. We statistically compared the lesion network maps of depressed and control subjects five times, each time leaving out one of the five datasets. Each time, voxels that survived voxel-wise FWE correction were extracted as regions of interest (Figure 3A).
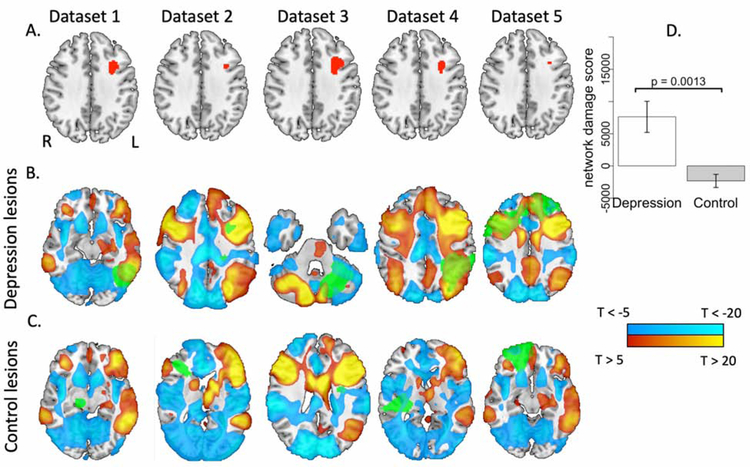
Figure 3.
Lesion locations associated with depression intersect a brain circuit derived from independent lesion datasets. (A) The analysis shown in figure 2B was repeated five times, each time leaving out one of the five datasets. In all five analyses, a similar region in the left dorsolateral prefrontal cortex was significantly more connected to lesions of depressed subjects than to lesions of non-depressed subjects (depicted in red; voxel-level family-wise error [FWE] corrected p < 0.05). (B) and (C) The functional connectivity of each of these regions to the remainder of the brain was computed using a normative connective of 1000 healthy subjects, generating five depression circuits. The red-yellow and blue-cyan coloration depict positive connectivity and negative connectivity (anti-correlation) to the region, respectively, while green coloration depicts sample lesion locations from the excluded dataset. Depression circuits are thresholded at T = ±5 for ease of visualization (actual depression circuits for analysis are unthresholded). (D) Network damage scores, representing intersection of each lesion with the depression circuit generated from the other lesion datasets, was significantly higher for depressed subjects than control subjects.
Then, we used each of these five regions of interest as seeds and computed their functional connectivity with the rest of the brain using our normative connectome dataset (N = 1000). This generated five different ‘depression circuit’ maps (Figure 3B and 3C). We then assigned each subject a ‘network damage score’ using the depression circuit that was generated from the other four datasets to which the lesion did not belong (Figure 3). Each subject’s ‘network damage score’ was calculated by summing the intensity (T values) of those voxels in the depression circuit that overlapped with that subject’s lesion. To avoid bias associated with a choice of threshold, network damage scores were computed using unthresholded depression circuit maps. To control for lesion size and dataset, we regressed this score against these variables and extracted the residuals to create an adjusted network damage score, which was used in all analyses.
First, we examined whether the network damage score differed between depressed and control subjects. Second, we examined whether subjects with higher network damage scores were more likely to have depression. Due to the network damage score’s non-normality, statistical significance for these analyses were calculated using permutation testing with one million permutations, a non-parametric procedure that can be used on non-normal data (45) (Supplementary Methods).
To ensure that results were not overly dependent on the cutoff values used to define control and depressed subjects, we performed additional analyses on the full cohort of subjects (N = 461) using the continuous depression score. We performed a Pearson’s correlation between this score and the network damage score, using permutation testing to determine statistical significance due to non-normality of both variables. We also examined whether lesion size predicted depression in binary and continuous models of depression.
We then divided subjects into three risk categories: low risk (network damage score < 2 SD below the mean), high risk ( network damage score > 2 SD above the mean), and medium risk (the remaining subjects). Mean depression scores were compared across risk categories using one-way analysis of variance (Figure 4A). Finally, we grouped subjects with mild or questionable depression along with control subjects, and evaluated whether the prevalence of depression differed among the three risk categories using a chi-squared test (Figure 4B).
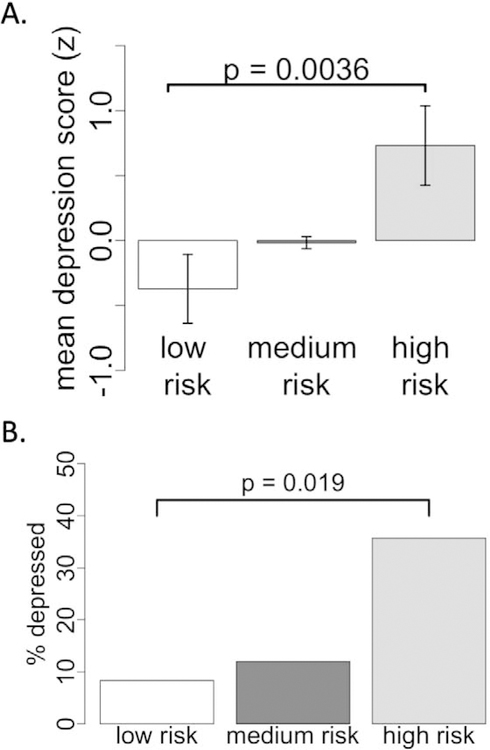
Figure 4.
Network damage score is associated with depression severity and prevalence. (A) All subjects were divided into three risk categories based on their network damage score. Mean depression score significantly differed across the risk categories and was highest in the high-risk group. (B) Prevalence of depression significantly differed across the risk categories and was highest in the high-risk group.
Post-stroke Depression Treatment Targets:
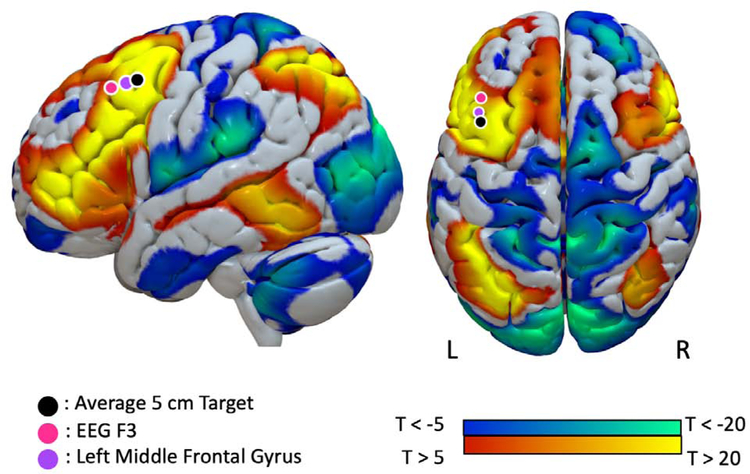
From the literature, we identified TMS targets that have successfully been used to treat depression following strokes (Supplementary Methods). We constructed 12 mm cone models of TMS activation for each TMS target and masked them against the MNI brain, as described in prior work (46). As a control, we also constructed a 12 mm cone model centered around the vertex (equivalent to Cz electrode location in the 10/20 EEG system), commonly used as a control target in trials of TMS and shown not to improve depression. Using normative connectome data, we assessed whether the successful TMS targets showed positive functional connectivity with the seed identified in our lesion network mapping analysis (‘LNM results’ in Figure 2B). We then assessed whether the successful TMS targets showed significantly greater functional connectivity to the results of our lesion network mapping analysis than the vertex using Hotelling’s t-test (Figure 5; (47)).
Figure 5.
Our lesion-based depression circuit aligns with TMS sites used to treat post-stroke depression. Spheres indicate the stimulation locations used by prior transcranial magnetic stimulation studies that have successfully treated post-stroke depression. Our depression circuit is displayed on the cortical surface and thresholded at T = ±5 for ease of visualization (actual network is unthresholded).
Results:
Analysis of Lesion Location
Across our five datasets (Table 1), lesions associated with moderate to severe depression and lesions associated with no depression occurred in various brain locations (Figure 1). Only 8 of 58 lesions associated with depression overlapped in any one location (Supplementary Figure S1A). Using voxel-based lesion-symptom mapping on the pooled data, no lesioned voxels were significantly associated with depression, either inside our DLPFC ROIs or in a whole brain analysis (1) (Supplementary Table S1, Figure 2A). There were also no significant voxel-based associations when treating depression as a continuous variable (N = 461), either inside or outside our DLPFC ROIs.
Finally, we found no significant association between lesion location and depression when repeating ROI-based analyses from prior papers (4, 7) or when performing laterality analyses (Supplementary Materials). Specifically, there was no difference in depression prevalence comparing left anterior versus left posterior lesions (p = 0.36), left anterior versus right anterior lesions (p = 0.32), right anterior versus right posterior lesions (p = 1), bilateral dorsal lateral prefrontal versus bilateral ventral medial prefrontal lesions (p = 1), or bilateral dorsal lateral prefrontal and non-prefrontal lesions (p = 0.76). There was also no difference in depression prevalence between left- and right-sided lesions in the cerebral hemispheres (p = 1), or between lesions falling within the Harvard Oxford left MFG and right MFG (p = 0.44) (Supplementary Table S2).
Lesion Network Mapping
Functional connectivity between each lesion location and the whole brain was computed using a normative connectome (Figure 2B). In contrast to analyses focused on lesion location alone, lesion connectivity was significantly associated with depression. Specifically, a focal region in the left DLPFC was significantly more connected to lesions of depressed subjects compared to non-depressed subjects (159 voxels surviving voxel-level family-wise error [FWE] correction pc < 0.05). The peak of this left DLPFC region was highly significant (pc = 0.005) and located at the grey-white matter junction (MNI coordinates: x = −32, y = 12, z = 36, Figure 2B). This result was independent of the specific DLPFC mask (Supplementary Figure S2) and the peak remained significant in a whole brain analysis (pc < 0.05). No other significant peaks outside the left DLPFC were identified.
By definition, functional connectivity with this DLPFC region defines a human brain circuit that encompasses lesion locations associated with depression while avoiding lesion locations that are not (Figure 2C; Supplementary Table S3). Lesion locations from patients with depression will intersect positively connected brain regions in this circuit, while lesions from non-depressed subjects will intersect neutral or negatively connected regions in this circuit. This lesion-based depression circuit was similar when restricted to cases of hemorrhagic stroke (r = 0.66), ischemic stroke (r = 0.52), penetrating traumatic brain injury (r = 0.95), or a documented lack of depression prior to the lesion (r = 0.53) (Supplementary Figures S3 and S4).
Leave-one-dataset-out cross-validation and network damage scores:
As a rigorous test of reproducibility, we repeated the above analysis 5 times, each time excluding one dataset. Lesions associated with depression were always more connected to a focal region in the left DLPFC (pc < 0.05) that was similar no matter which dataset was excluded (Figure 3A). Connectivity with this left DLPFC region of interest defined a brain circuit that was similar no matter which dataset was excluded (average spatial correlation r = 0.91, Figure 3B, Supplementary Table S4). Lesions associated with depression intersected this circuit to a significantly greater degree than control lesions (p = 0.0013), as demonstrated by a higher network damage score. The degree to which lesions intersected this circuit predicted depression status (OR = 1.62, p = 0.0035). Lesion size alone did not predict depression status (OR = 1.14, p = 0.31).
To ensure these results were not dependent on the cutoffs used to classify patients as “depressed” or “not depressed”, we repeated this analysis of circuit intersection on our full cohort of patients (n = 461) treating depression as a continuous rather than a binary variable. Intersection with our depression circuit (defined using the other four datasets) was a significant predictor of continuous depression severity (r = 0.13, p = 0.0040). Lesion size alone did not predict continuous depression severity (r = 0.061, p = 0.19).
Binning all 461 subjects into three risk categories based on circuit intersection revealed a significant difference in continuous depression scores (F(2,458) = 4.8, p = 0.0036, Figure 4A) and depression prevalence (X2 = 7.2, p = 0.019, Figure 4B). Prevalence of depression was four times higher (35.7%) in the high-risk category compared to the low-risk category (8.3%).
Post-stroke Depression Treatment Targets:
We identified three TMS targets with evidence of efficacy in treating post-stroke depression (Figure 5). These included the 5 cm target traditionally also used in treatment of primary depression (48, 49), the left F3 electrode location on the scalp using the 10/20 EEG coordinate system (50, 51), and the center of the left MFG (52). All three targets fell within our depression circuit derived from focal brain lesions, defined by positive connectivity to our node in the left DLPFC (r = 0.23 for the 5 cm target, r = 0.14 for the EEG F3 target, and r = 0.20 for the center of the MFG, all p < 0.00001). All three TMS targets were significantly more connected to this DLPFC node than our control site in the vertex (p < 0.0001 for all comparisons).
Discussion:
Our results define a depression circuit in the human brain based on brain lesions. First, we found that lesion location alone was not significantly associated with depression. Second, we showed that functional connectivity between lesion locations and the left DLPFC was strongly associated with depression, independent of lesion etiology, lesion size or dataset. Third, we validated our depression circuit by predicting depression status and depression severity in independent lesion cohorts. Finally, we showed that our circuit derived from brain lesions associated with depression aligns with brain stimulation sites that improve depression, suggesting therapeutic utility. Although we used binary cutoffs for our main analysis, our findings are not dependent on these cutoffs and were reproducible when treating depression as a continuous measure.
Lesion Location and Depression:
We did not find an association between lesion location and depression anywhere in the brain using either VLSM or when replicating prior positive analyses from the literature (4, 7). While it is possible that significant associations would have become apparent with more lesions and statistical power, this study is the largest VLSM analysis of depression to date, about twice the size of the next largest study (18). Other studies approaching the size of our study, but classifying lesion location based on rough anatomical descriptions rather than lesioned voxels, have also been negative (53), including multiple meta-analyses (12–15). Collectively, these results suggest that lesions associated with depression do not localize to any specific brain region.
Lesion Network Mapping, Frontal Connectivity and Depression:
In contrast to the negative results regarding lesion location, functional connectivity between lesion locations and the rest of the brain was a significant predictor of depression. This finding is consistent with a growing literature suggesting that symptoms localize to connected brain circuits, not single brain lesions (19, 22, 54–56).
Functional connectivity between lesion location and a region within the left DLPFC was higher in depressed than in non-depressed subjects, a finding that remained significant even in a conservative whole-brain analysis with voxel-wise FWE correction. Because this analysis compared lesions from depressed individuals with non-depressed individuals, we control for the possibility that the DLPFC is simply a hub connected to all lesion locations. These findings are consistent with lesion studies implicating the DLPFC in depression (4, 7). However, our findings also help explain why multiple lesion studies, including the present study, failed to see such relationships (12–15). Specifically, our results suggest that lesions located outside the DLPFC, but functionally connected to this area, can also cause depression.
Connectivity with the DLPFC defines a human brain circuit that best encompasses lesion locations associated with depression. This circuit was independent of lesion etiology, dataset, or scale used to measure depression. Intersection between lesion location and this circuit predicted prevalence and severity of depression in independent lesion cohorts. As such, this circuit might be used to identify patients who are at elevated risk of depression after a stroke or brain injury. Clinicians could examine the overlap between a patient’s lesion and our depression circuit, and patients at elevated risk could then be directed towards early psychiatric evaluation and treatment.
Our circuit derived from brain lesions associated with depression aligned with stimulation sites found to improve depression in post-stroke depression. Although previous work has suggested therapeutic relevance of lesion network mapping results (57–59), this is the first study to show that lesions causing a symptom are part of the same brain circuit as stimulation sites improving that symptom in lesion patients. While the peak of our lesion-based depression circuit is in the left DLPFC, TMS to secondary nodes in this circuit may also be beneficial, although this remains a testable hypothesis for future work. These results support the use of lesion network mapping to identify therapeutic brain stimulation targets for improving lesion-induced symptoms.
It is likely that our depression circuit has implications for understanding and treating primary depression not caused by focal brain lesions. While depression has not consistently been associated with changes in DLPFC activity (60, 61), increases in DLPFC activity have been associated with antidepressant response (62), especially improvement in the cognitive symptoms of depression (63). However, it is now widely recognized that primary depression is associated with circuit abnormalities that extend beyond the DLPFC (64, 65). Our circuit, based on causal brain lesions, may help refine circuit models of primary depression.
Finally, our results may help inform treatment targets in primary depression. The DLPFC region was initially chosen as a TMS target for primary depression based partly on lesion studies (66). Our current depression circuit may help refine this target and motivate new strategies for neuromodulation that target a brain circuit rather than a single brain region (67, 68).
Limitations and Future Directions:
There are several limitations. First, as previously discussed (22), lesion network mapping using a normative data set assumes that the patterns of connectivity in healthy individuals are approximately the same as for an individual patient prior to their brain lesion. This assumption appears reasonable given the success of lesion network mapping across numerous symptoms (19, 22–25) and the fact that prior work using a connectome age-matched to lesion patients had no effect on results (19). In similar work on TMS- or DBS-electrodes, disease-matching the connectomes also had no effect on results (25, 69). Second, the current study focused only on lesion location and connectivity to explain lesion-associated depression. We did not account for compensatory network adaptations that may occur following a lesion nor did we account for many other factors that contribute to post-lesion depression such as genetics, psychosocial situation, degree of disability, or co-morbid diagnosis (14). Third, lesions were treated as functionally homogenous units regardless of their size. A potential approach to address this limitation could involve deconstructing lesions into functionally homogenous seeds using an atlas, and then using these seeds to generate multiple lesion network maps for each subject. However, as this potential approach has not been standardized, we elected to pursue our previously validated approach.
Fourth, we did not have complete information about psychiatric comorbidities or pre-lesion history of depression. Substance dependence and post-traumatic stress disorder can be significant co-morbidities in veterans with penetrating traumatic brain injury, for example. Additionally, the time point of depression assessment relative to the lesion was variable across datasets, limiting our ability to make strong causal inferences. At least some patients in this study likely had depression that was unrelated to the location of their brain damage. However, a depression circuit created from the subset of individuals who had a documented lack of depression prior to their lesion was similar to our primary depression circuit (r = 0.53). Fifth, depression is a syndrome composed of many different symptoms with potentially distinct neuroanatomical correlates. Future work applying this technique to individual symptoms of depression is needed. Sixth, our results should not be interpreted as specific to the left DLPFC relative to the right DLPFC. Although connectivity to the left DLPFC was the only significant finding in our full cohort, some sub-cohorts showed similar findings in the right DLPFC (Supplementary Figures S3 and S4). Finally, there is certain to be some error in lesion tracing and atlas registrations. However, it is important to note that all of these limitations should bias us against the present findings, namely consistent localization of lesions associated with depression to a specific brain circuit across datasets and lesion etiologies.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Software; Algorithm | FSL (Permutation Analysis of Linear Models) | Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage, 2014;92:381–397 | https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM | |
| Software; Algorithm | NiiStat | Stark BC, Yourganov G, Rorden C (2018): User Manual and Tutorial for NiiStat. http://www.nitrc.org/projects/niistat. | https://github.com/neurolabusc/NiiStat), | |
| Software; Algorithm | Matlab 2015b | The MathWorks I (2015): MATLAB and Statistics Toolbox Release 2015b. Natick, Massachusetts. | ||
| Deposited Data; Public Database | 1000 healthy subject connectome data | Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. | ||
Acknowledgements:
Funding/Support: This work was supported in part by funding from the Sidney R. Baer, Jr. Foundation (to J.L.P., R.R.D., S.H.S., and M.D.F.), the Harvard Medical School Department of Psychiatry Dupont-Warren Award (to J.L.P.), the American Psychiatric Association Kempf Award (to J.L.P.), the Alzheimer’s Association Clinical Fellowship Program (to R.R.D.), the BrightFocus Foundation Alzheimer’s Disease Research Program (to R.R.D.), the Vanderbilt Faculty Research Scholars Award (to R.R.D.), the Academy of Finland grant #295580 (to J.J.), and the National Institutes of Health Grants R01MH113929 and K23NS083741 (to M.D.F.). All funding agencies had no role in the study design, collection, management, analysis, interpretation, preparation, review, interpretation, or submission of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary information is available at Biological Psychiatry’s website.
References:
- 1.Karnath HO, Sperber C, Rorden C (2018): Mapping human brain lesions and their functional consequences. Neuroimage. 165:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rorden C, Karnath HO, Bonilha L (2007): Improving lesion-symptom mapping. J Cogn Neurosci. 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 3.Robinson RG, Price TR (1982): Post-stroke depressive disorders: a follow-up study of 103 patients. Stroke; a journal of cerebral circulation. 13:635–641. [DOI] [PubMed] [Google Scholar]
- 4.Robinson RG, Kubos KL, Starr LB, Rao K, Price TR (1984): Mood disorders in stroke patients. Importance of location of lesion. Brain. 107 ( Pt 1):81–93. [DOI] [PubMed] [Google Scholar]
- 5.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J (2002): Predicting major depression in brain tumor patients. Psychooncology. 11:230–238. [DOI] [PubMed] [Google Scholar]
- 6.Belyi BI (1987): Mental impairment in unilateral frontal tumours: role of the laterality of the lesion. Int J Neurosci. 32:799–810. [DOI] [PubMed] [Google Scholar]
- 7.Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J (2008): Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 28:12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everson SA, Roberts RE, Goldberg DE, Kaplan GA (1998): Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med. 158:1133–1138. [DOI] [PubMed] [Google Scholar]
- 9.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. (2000): A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 48:962–970. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Leone A, Rubio B, Pallardo F, Catala MD (1996): Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 348:233–237. [DOI] [PubMed] [Google Scholar]
- 11.Narushima K, Kosier JT, Robinson RG (2003): A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci. 15:422–430. [DOI] [PubMed] [Google Scholar]
- 12.Kutlubaev MA, Hackett ML (2014): Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. 9:1026–1036. [DOI] [PubMed] [Google Scholar]
- 13.Wei N, Yong W, Li X, Zhou Y, Deng M, Zhu H, et al. (2015): Post-stroke depression and lesion location: a systematic review. Journal of neurology. 262:81–90. [DOI] [PubMed] [Google Scholar]
- 14.Ayerbe L, Ayis S, Wolfe CD, Rudd AG (2013): Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 202:14–21. [DOI] [PubMed] [Google Scholar]
- 15.Yu L, Liu CK, Chen JW, Wang SY, Wu YH, Yu SH (2004): Relationship between post-stroke depression and lesion location: a meta-analysis. Kaohsiung J Med Sci. 20:372–380. [DOI] [PubMed] [Google Scholar]
- 16.Rooney AG, Carson A, Grant R (2011): Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 103:61–76. [DOI] [PubMed] [Google Scholar]
- 17.Gozzi SA, Wood AG, Chen J, Vaddadi K, Phan TG (2014): Imaging predictors of poststroke depression: methodological factors in voxel-based analysis. BMJ Open. 4:e004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagnier S, Munsch F, Bigourdan A, Debruxelles S, Poli M, Renou P, et al. (2019): The Influence of Stroke Location on Cognitive and Mood Impairment. A Voxel-Based Lesion-Symptom Mapping Study. J Stroke Cerebrovasc Dis. 28:1236–1242. [DOI] [PubMed] [Google Scholar]
- 19.Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr., et al. (2015): Network localization of neurological symptoms from focal brain lesions. Brain. 138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, et al. (2010): Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 30:3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrera E, Tononi G (2014): Diaschisis: past, present, future. Brain. 137:2408–2422. [DOI] [PubMed] [Google Scholar]
- 22.Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD (2017): Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain. 140:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laganiere S, Boes AD, Fox MD (2016): Network localization of hemichorea-hemiballismus. Neurology. 86:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. (2016): A human brain network derived from coma-causing brainstem lesions. Neurology. 87:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. (2017): Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 82:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darby RR, Horn A, Cushman F, Fox MD (2018): Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A. 115:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darby RR, Joutsa J, Burke MJ, Fox MD (2018): Lesion network localization of free will. Proc Natl Acad Sci U S A. 115:10792–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, et al. (2015): Common behavioral clusters and subcortical anatomy in stroke. Neuron. 85:927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidech AM, Polnaszek KL, Berman MD, Voss JL (2016): Hematoma Locations Predicting Delirium Symptoms After Intracerebral Hemorrhage. Neurocrit Care. 24:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egorova N, Cumming T, Shirbin C, Veldsman M, Werden E, Brodtmann A (2018): Lower cognitive control network connectivity in stroke participants with depressive features. Transl Psychiatry. 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershon RC, Lai JS, Bode R, Choi S, Moy C, Bleck T, et al. (2012): Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 21:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke WJ, Roccaforte WH, Wengel SP (1991): The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 4:173–178. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB (2001): The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP (1983): The hospital anxiety and depression scale. Acta Psychiatr Scand. 67:361–370. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. (1998): The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Brown G (1996): Manual for the Beck Depression Inventory-II. San Antonion, TX: Psychological Corporation. [Google Scholar]
- 37.Zhang Y, Zhao H, Fang Y, Wang S, Zhou H (2017): The association between lesion location, sex and poststroke depression: Meta-analysis. Brain Behav. 7:e00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, et al. (2000): Depression after stroke and lesion location: a systematic review. Lancet. 356:122–126. [DOI] [PubMed] [Google Scholar]
- 39.Stark BC, Yourganov G, Rorden C (2018): User Manual and Tutorial for NiiStat. http://wwwnitrcorg/projects/niistat.
- 40.Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, et al. (2015): Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2:150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The MathWorks I (2015): MATLAB and Statistics Toolbox Release 2015b. Natick, Massachusetts. [Google Scholar]
- 44.Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curran-Everett D (2017): Explorations in statistics: the assumption of normality. Adv Physiol Educ. 41:449–453. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD, Liu H, Pascual-Leone A (2013): Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 66:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diedenhofen B, Musch J (2015): cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 10:e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanDerwerker CJ, Ross RE, Stimpson KH, Embry AE, Aaron SE, Cence B, et al. (2018): Combining therapeutic approaches: rTMS and aerobic exercise in post-stroke depression: a case series. Top Stroke Rehabil. 25:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Etribi A, El Nahas N, Nagy N, Nabil H (2010): Repetitive transcranial magnetic stimulation in post stroke depression. Current Psychiatry. 17:9–14. [Google Scholar]
- 50.Gu SY, Chang MC (2017): The Effects of 10-Hz Repetitive Transcranial Magnetic Stimulation on Depression in Chronic Stroke Patients. Brain stimulation. 10:270–274. [DOI] [PubMed] [Google Scholar]
- 51.Kim BR, Kim DY, Chun MH, Yi JH, Kwon JS (2010): Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil. 89:362–368. [DOI] [PubMed] [Google Scholar]
- 52.Jorge RE, Robinson RG, Tateno A, Narushima K, Acion L, Moser D, et al. (2004): Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry. 55:398–405. [DOI] [PubMed] [Google Scholar]
- 53.Snaphaan L, van der Werf S, Kanselaar K, de Leeuw FE (2009): Post-stroke depressive symptoms are associated with post-stroke characteristics. Cerebrovasc Dis. 28:551–557. [DOI] [PubMed] [Google Scholar]
- 54.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. (2010): Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 67:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M (2007): Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 53:905–918. [DOI] [PubMed] [Google Scholar]
- 56.Siegel JS, Ramsey LE, Snyder AZ, Metcalf NV, Chacko RV, Weinberger K, et al. (2016): Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A. 113:E4367–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox MD (2018): Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med. 379:2237–2245. [DOI] [PubMed] [Google Scholar]
- 58.Joutsa J, Horn A, Hsu J, Fox MD (2018): Localizing parkinsonism based on focal brain lesions. Brain. [DOI] [PMC free article] [PubMed]
- 59.Joutsa J, Shih LC, Horn A, Reich MM, Wu O, Rost NS, et al. (2018): Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 84:153–157. [DOI] [PubMed] [Google Scholar]
- 60.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong X, Pu W, Yao S (2016): Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: A meta-analysis of resting-state fMRI data. J Affect Disord. 206:280–286. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ (2006): An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 148:33–45. [DOI] [PubMed] [Google Scholar]
- 63.Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR (2001): Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry. 50:171–178. [DOI] [PubMed] [Google Scholar]
- 64.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. (1999): Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 156:675–682. [DOI] [PubMed] [Google Scholar]
- 65.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. (2005): Deep brain stimulation for treatment-resistant depression. Neuron. 45:651–660. [DOI] [PubMed] [Google Scholar]
- 66.Pascual-Leone A, Wassermann EM, Grafman J, Hallett M (1996): The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res. 107:479–485. [DOI] [PubMed] [Google Scholar]
- 67.Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, et al. (2017): Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage. 157:34–44. [DOI] [PubMed] [Google Scholar]
- 68.Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A (2014): Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 89:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. (2018): Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry. 84:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.