Editor—The coronavirus 2019 disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing global health concern1 that has so far caused >29 million infections and resulted in more than 900 000 deaths worldwide. The clinical manifestations of COVID-19 range from asymptomatic infection to severe acute respiratory failure and multi-organ dysfunction (MOD) requiring organ supportive therapy, such as mechanical ventilation in the ICU. Once established, multi-organ dysfunction is associated with reduced patient survival and quality of life after ICU discharge.2 There is a pressing need to understand the disease mechanisms underlying COVID-19 in order to develop novel therapeutic strategies to improve patient survival.
Overwhelming immune activation resulting in a ‘cytokine storm’ and systemic hypoxaemia caused by pulmonary dysfunction may lead to cell death within vital organs, including brain, lung, kidney, liver, and gut, and are thought to contribute to multiple organ dysfunction and poor outcomes in COVID-19.2 Although a number of therapeutic approaches have been proposed or trialled to modulate the dysregulated immune response in COVID-19, thus far only dexamethasone, a potent glucocorticoid steroid with broad effects on innate and adaptive immunity, has been shown to improve patient survival. Accumulating data show that cell necrosis and necroptosis (programmed cell death) are key cell death mechanisms implicated in both acute organ injury and chronic inflammatory disease.3 When lung alveolar cells, lung macrophages, or both become infected with SARS-CoV-2, resultant cell death may lead to widespread immune cell activation through activation of pattern recognition receptors on innate immune cells. The therapeutic efficacy of antiviral therapies such as remdesivir,4 a nucleotide analogue that inhibits viral RNA polymerase, or lopinavir/ritonavir, a combination viral protease inhibitor combination used for human immunodeficiency virus 1 (HIV-1) treatment, remains to be verified. Targeting angiotensin-converting enzyme 2 (ACE2),5 the cell surface receptor whereby SARS-CoV-2 enters into cells for replication, or suppressing systemic inflammation with anti-tumour necrosis factor antibodies have also been suggested.
It might be advantageous to take a different perspective by targeting the cell death pathways involved in the development of multi-organ dysfunction during the infection. Strategies that inhibit upstream cell death pathways may prevent downstream immune activation implicated in COVID-19 associated multi-organ dysfunction. In addition, direct inhibition of alveolar cell death may preserve lung architecture and prevent some of the long-term sequelae such as the breathlessness experienced by patients who have recovered from COVID-19. Furthermore, preserving the alveolar capillary interface, which provides an anatomical barrier, may prevent secondary bacterial infection associated with SARS-CoV-2.
The potent and selective α2-adrenoceptor agonist dexmedetomidine exerts sedative and analgesic effects and has been widely used as an adjunct for anaesthesia, analgesia, and sedation in the ICU.6 In addition, dexmedetomidine has both cytoprotective and anti-inflammatory properties.7 In fact, its organoprotective effects against acute organ injury, such as brain,8 lung and kidney,9 have been well established in pre-clinical settings. Mice treated with dexmedetomidine exhibited reduced inflammation-induced cell death (pyroptosis) in astrocytes and in turn protected neurones in sepsis-induced brain injury.8 The underlying protective mechanisms of dexmedetomidine include increasing parasympathetic tone, dampening of the inflammatory response, prevention of cell death, and inhibition of oxidative stress.10 By increasing parasympathetic tone and decreasing sympathetic tone, dexmedetomidine appears to confer protective effects on immune function by effects on T cells and natural killer cells. Furthermore, its cholinergic anti-inflammatory mechanisms might suppress excessive inflammatory responses.10 A trial in the critical care setting has shown that dexmedetomidine was effective in attenuating the incidence of delirium in older patients.6 , 11
Based on these properties, we propose that dexmedetomidine may serve as a novel therapeutic strategy to attenuate the vital organ injuries in COVID-19 (Fig. 1 ), whilst simultaneously providing beneficial sedative effects to enable oxygen therapy via either noninvasive or invasive mechanical ventilation. ICU sedation with dexmedetomidine is associated with impaired ventilatory responses to hypoxia and hypercapnia to a similar extent as that associated with propofol sedation, indicating that ventilatory suppression by dexmedetomidine is likely caused by effects on both peripheral and central regulation of breathing.12 This attenuation of respiratory drive could be beneficial for patients with COVID-19 requiring ventilatory support in which hypoxaemia-associated hyperventilation and respiratory distress are significant problems.
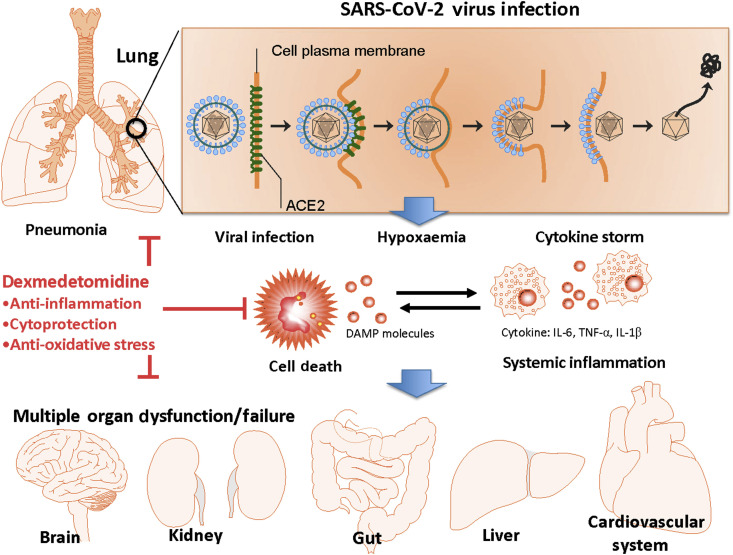
Fig 1.
Putative mechanisms of SARS-CoV-2 infection induced dysfunction or failure of multiple organs (MOD/MOF) and the protection afforded by dexmedetomidine (DEX) in COVD-19 patients. SARS-CoV-2 can bind with angiotensin-converting enzyme 2 (ACE2) to enter human cells for replication and cause a viral pneumonia. The subsequent cell death, cytokine storm and systemic hypoxaemia caused by pulmonary dysfunction/failure are considered to be the pathogenesis of MOD/MOF which includes neurological dysfunction, acute kidney and liver injury, myocardial dysfunction. DEX has potent protective effects through up-regulating protective proteins and attenuating cell death and systemic inflammation, and, thereby may protect vital organs from MOD/MOF in COVID-19 patients who require sedation and mechanical ventilation in the ICU. In addition, its cholinergic anti-inflammatory mechanisms can suppress excessive inflammatory responses and its anti-oxidative effects preserve cells from oxidative stress conferring additional benefits to COVID-19 patients irrespective of other supportive treatments currently available. IL, interleukin; TNF- α, tumour necrosis factor-alpha. DAMP: Damage-associated molecular pattern.
We propose that dexmedetomidine should be considered when sedation is required, during the early disease course to help prevent the onset or progression of multi-organ dysfunction in COVID-19. When deep sedation is required, dexmedetomidine may be used as a sedative adjunct together with other sedatives, such as propofol or midazolam. Its use as a single agent may also be considered to facilitate noninvasive ventilation or during liberation from invasive mechanical ventilation, although at high doses the risk of bradycardia and hypotension should be taken into consideration. In summary, there is a strong rationale for further clinical studies investigating the effects of dexmedetomidine on outcomes in ICU patients with COVID-19.
Declarations of interest
DM is a board member of the British Journal of Anaesthesia. The other authors have no competing interests.
Funding
Westminster Medical School Research Trust and BJA/RCoA project grant, London, UK.
References
- 1.Callaway E., Cyranoski D., Mallapaty S., Stoye E., Tollefson J. The coronavirus pandemic in five powerful charts. Nature. 2020;579:482–483. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- 2.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welz P.S., Wullaert A., Vlantis K., et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 4.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su X., Meng Z.T., Wu X.H., et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang K., Wu M., Xu J., et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123:777–794. doi: 10.1016/j.bja.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y.-B., Zhao H., Mu D.-L., et al. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis. 2019;10:167. doi: 10.1038/s41419-019-1416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J., Sun P., Zhao H., et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J., Chen Q., Li J., et al. Dexmedetomidine-mediated prevention of renal ischemia-reperfusion injury depends in part on cholinergic anti-inflammatory mechanisms. Anesth Analg. 2020;130:1054–1062. doi: 10.1213/ANE.0000000000003820. [DOI] [PubMed] [Google Scholar]
- 11.Duan X., Coburn M., Rossaint R., Sanders R.D., Waesberghe J.V., Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121:384–397. doi: 10.1016/j.bja.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Å Lodenius, Ebberyd A., Hårdemark Cedborg A., et al. Sedation with dexmedetomidine or propofol impairs hypoxic control of breathing in healthy male volunteers: a nonblinded, randomized crossover study. Anesthesiology. 2016;125:700–715. doi: 10.1097/ALN.0000000000001236. [DOI] [PubMed] [Google Scholar]