Abstract
Opioid-targeted vaccines represent an emerging treatment strategy for opioid use disorder. To determine whether concurrent vaccination against two commonly abused opioids (fentanyl and heroin) would confer broader spectrum opioid coverage, the current study evaluated dual fentanyl/heroin conjugate vaccine effectiveness using a warm water tail-withdrawal and a fentanyl/heroin-vs-food choice procedure in male and female rats across a 105-day observation period. Vaccine administration generated titers of high-affinity antibodies to both fentanyl and heroin sufficient to decrease the antinociceptive potency of fentanyl (25-fold), heroin (4.6-fold), and a 1:27 fentanyl/heroin mixture (7.5-fold). Vaccination did not alter the antinociceptive potency of the structurally dissimilar opioid agonist methadone. For comparison, continuous treatment with a naltrexone dose (0.032 mg/kg/h) shown previously to produce clinically relevant plasma-naltrexone levels decreased the antinociceptive potency of fentanyl, heroin, and the 1:27 fentanyl/heroin mixture by approximately 20-fold. Naltrexone treatment also shifted the potency of 1:27 fentanyl/heroin mixture in a drug-vs-food choice self-administration procedure 4.3-fold. In contrast, vaccination did not attenuate 1:27 fentanyl/heroin mixture self-administration in the drug-vs-food choice procedure. These data demonstrate that a vaccine can simultaneously attenuate the thermal antinociceptive effects of two structurally dissimilar opioids. However, the vaccine did not attenuate fentanyl/heroin mixture self-administration, suggesting a greater magnitude of vaccine responsiveness is required to decrease opioid reinforcement relative to antinociception.
Keywords: heroin, fentanyl, vaccine, antinociception, drug self-administration, choice
Graphical Abstract
INTRODUCTION
In response to the current opioid crisis, the National Institutes of Health has increased efforts toward the development of novel and more effective treatment strategies for opioid use disorder (OUD). Current Food and Drug Administration- approved treatments for OUD include the full mu opioid receptor (MOR) agonist methadone, the partial MOR agonist buprenorphine, and the MOR antagonist naltrexone.1 These medications decrease the risk of opioid-related fatality,2 and both methadone and buprenorphine are among the World Health Organization’s List of Essential Medicines.3 However, limitations in the clinical utility of these medications have also been identified. For example, MOR agonists (methadone, buprenorphine) can produce opioid dependence4 and possess abuse liability,5,6 leading to restrictions in their availability. Although MOR antagonist treatments (i.e., naltrexone) do not have dependence-inducing or abuse liability concerns, their use requires the patient to be fully detoxified from opioids, otherwise risking the precipitation of severe opioid-withdrawal signs.7 In addition, patient compliance for naltrexone in both oral and depot-injection formulations is low.8 Taken together, these limitations represent obstacles to OUD treatment and highlight the need for the development of novel OUD treatments that retain efficacy and minimize these clinical limitations.
Opioid-targeted vaccines are an emerging treatment strategy for OUD, and they can decrease opioid potency similarly to competitive MOR antagonists.9–11 Opioid-targeted vaccines are synthesized by conjugating a nonimmunogenic drug molecule that resembles the targeted opioid (e.g., fentanyl, heroin) to an immunogenic carrier protein (e.g., CRM197 or tetanus toxoid (TT)). Administration of this immunoconjugate results in the generation of antibodies that are intended to recognize and sequester the targeted molecule outside of the central nervous system and preclude reward pathway activation. One potential advantage of opioid-targeted vaccines over depot naltrexone formulations is that antiopioid antibodies have been shown to remain elevated for months following vaccination.12 This antibody longevity would be hypothesized to decrease the frequency at which a patient would need to be administered the medication relative to depot naltrexone formulations, which may increase patient compliance or retain therapeutic effectiveness longer following a missed dose. In addition, because antiopioid antibodies have not been shown to bind endogenous opioids,13 these antibodies should not impair endogenous opioid signaling that contributes to endocrine function or stress responsiveness. Finally, antiopioid antibodies have a high degree of targeted opioid selectivity13–17 and would afford clinical opportunities for the medicinal use of structurally dissimilar opioids for pain management or adjunctive OUD treatments. However, one potential disadvantage of this selectivity is that a vaccinated individual could circumvent the desired effect of the vaccine by consuming a structurally dissimilar opioid of abuse. To provide protection against a broader spectrum of abused opioids, vaccine research efforts have focused on combination vaccine development targeting multiple structurally dissimilar abused opioids.18–21
The present study evaluated the effectiveness of a dual fentanyl/heroin vaccine on two complementary behavioral end points in male and female rats. First, a warm water tail-withdrawal procedure was utilized to efficiently evaluate the time course of dual-vaccine effectiveness on fentanyl alone, heroin alone, and a fentanyl/heroin mixture. Second, a drug-vs-food choice self-administration procedure was utilized to assess vaccine effectiveness on opioid reinforcement.22,23 Preclinical drug self-administration procedures model clinical drug-taking behavior and have been valuable in the assessment of the efficacy of candidate substance use disorder medications (for review, see refs 24–26). Among these drug self-administration procedures, a drug-vs-food “choice” procedure was selected because candidate medication effects on the primary end point (i.e., behavioral allocation or “choice”) are more predictive of effects in humans than candidate medication effects in more conventional preclinical drug self-administration procedures that use rates of drug self-administration as the primary end point.10,27 In both the warm water tail-withdrawal and the drug-vs-food choice procedures, vaccine effectiveness was compared to continuous naltrexone treatment using a naltrexone dose shown to produce plasma naltrexone levels and opioid antagonist effects similar to those in humans using clinically available naltrexone formulations.28–31
RESULTS AND DISCUSSION
The current study determined the effectiveness of a dual fentanyl/heroin conjugate vaccine over a 105-day period. Previous research has illustrated the utility of preclinical nociceptive measures (e.g., tail or paw withdrawal from a noxious stimulus) in opioid-targeted vaccine development,9,12,15,32,33 and a decrease in the potency of the targeted opioid has been interpreted as evidence of vaccine effectiveness. A strength of this approach is that antinociceptive potency measures can be efficiently, repeatedly, and reliably evaluated within the same experimental subject over experimental timelines of weeks to months.12,28,34 In addition, this provides experimental opportunities to compare vaccine effects on the targeted opioid(s) relative to a structurally dissimilar opioid.28,34 Finally, we recently reported similar effectiveness of a fentanyl-targeted vaccine to attenuate the antinociceptive and reinforcing potency of fentanyl. These results provided evidence that vaccines may produce a similar magnitude of effectiveness across these procedures.12 Accordingly, an additional goal of the present study was to determine whether similar vaccine effectiveness on antinociceptive and drug self-administration end points was observed with a dual fentanyl/heroin vaccine.
Figure 1A shows the antinociceptive dose–effect functions for intravenous (IV) fentanyl and heroin in two cohorts of rats (12 total rats, 3 females and 3 males in each cohort, see Methods for details). Fentanyl and heroin produced full antinociception, and the antinociceptive potencies, expressed as ED50 (95% CL) values, were 5.3 μg/kg (4.1–6.5) and 143 μg/kg (105–182), respectively. Based on these antinociceptive ED50 values, the relative potency of fentanyl to heroin was calculated to be 1:27 (fentanyl/heroin). Thus, 1 mg/kg of fentanyl would be predicted to be functionally equivalent to 27 mg/kg of heroin, at least within this tail-withdrawal procedure. Subsequent antinociception and drug self-administration experiments utilized the same 1:27 fentanyl/heroin fixed-proportion mixture similar to previous drug interaction studies.35,36 Rats were then randomly assigned to opioid antinociception (Cohort 1) or opioid self-administration (Cohort 2) studies.
Figure 1.

Panel A shows the relative potency of fentanyl (upward-facing triangles) and heroin (downward-facing triangles) to produce thermal antinociception in the 12 rats of Cohorts 1 and 2 (6 females and 6 males). Both fentanyl and heroin produced “full” antinociceptive effects (i.e., 20 s of tail immersion) in each rat. Depicted are data points that span the 20–80% maximum possible effect level, as these data constitute the linear portion of the dose–effect function and were included in the linear regression analysis. Abscissa: Cumulative IV fentanyl or heroin dose in micrograms per kilogram. Ordinate: %MPE collected in a warm water tail-withdrawal procedure. Points represent mean ± SEM. The 14-day cycle of experimental conditions completed by Cohorts 1 and 2 are depicted in panel B. Panel C shows the timeline of the vaccination and blood collection schedule.
The antinociceptive effects of IV fentanyl, heroin, the 1:27 fentanyl/heroin mixture, and methadone are shown in Figure 2. All opioids produced full dose-dependent antinociception (i.e., maximum latency of 20 s was observed at highest tested dose), and the corresponding ED50 values are reported in Table 1. Next, continuous naltrexone treatment (0.032 mg/kg/h, subcutaneous) was evaluated as a positive control comparison. Our previously published experiments have shown that this naltrexone dose produces plasma concentrations of approximately 5 ng/mL in rats,28 similar to peak plasma concentrations achieved by clinically available naltrexone formulations in humans (i.e., 384 mg depot injection).29–31 Naltrexone treatment decreased the IV potencies of fentanyl, heroin, and the 1:27 fentanyl/heroin mixture by approximately 20-fold (Figure 2; Table 1). Although naltrexone decreased the antinociceptive potency of methadone by at least 13-fold (Figure 2D), a precise methadone ED50 value in the presence of naltrexone and associated potency change could not be calculated because of apparent MOR-independent toxicity of methadone doses exceeding 10 mg/kg (unpublished observations). More specifically, IV administration of larger methadone doses (18 or 32 mg/kg, cumulative dose) resulted in acute toxicity that was not sensitive to 10 mg/kg IV naltrexone in pilot studies. The acute IV methadone toxicity and insensitivity to opioid antagonist administration was consistent with previous results explicitly examining acute subcutaneously administered methadone toxicity with a reported LD10 value of 18.5 mg/kg and LD50 value of 54 mg/kg.37 Taken together, these results demonstrate an FDA-approved OUD pharmacotherapy decreases MOR agonist antinociceptive potencies alone or in a fentanyl/heroin mixture. Furthermore, these data provide an empirical foundation for interpreting subsequent vaccine effects.
Figure 2.

Panels A–D show opioid antinociceptive potency at baseline (open symbols), during continuous naltrexone (0.032 mg/kg/h, SC) treatment (gray-filled symbols), and at time of maximal fentanyl/heroin vaccine effect (black-filled symbols) for fentanyl, heroin, a 1:27 fentanyl heroin mixture, and methadone, respectively. With the exception of the “methadone + naltrexone” experiment, each opioid produced “full” antinociceptive effects (i.e., 20 s of tail immersion) in each rat. Depicted are data points that span the 20–80% maximum possible effect level, as these data constitute the linear portion of the dose–effect function and were included in the linear regression analysis. Abscissae: Cumulative IV opioid dose in μg/kg. Ordinates: %MPE collected in a warm water tail-withdrawal procedure. Points represent mean ± SEM for the six rats in Cohort 1 (3 females and 3 males).
Table 1.
Group Mean ED50 Values (95% CL) in Micrograms Per Kilogram and Fold Shift Relative to Baseline for Thermal Antinociception of Methadone, Heroin, Fentanyl, and a 1:27 Fentanyl/Heroin Mixture in Male and Female Rats of Cohort 1 (n = 3 Male, 3 Female)a
| Methadone | Heroin | Fentanyl | 1:27 Fentanyl/Heroin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | µg/kg ED50 (95% CL) | Fold Change | Condition | µg/kg ED50 (95% CL) | Fold Change | Condition | µg/kg ED50 (95% CL) | Fold Change | Condition | *µg/kg ED50 (95% CL) | Fold Change |
| Baseline | 896 (431–1320) | Baseline | 129 (67–177)) | Baseline | 3.7 (2.0–5.2) | Baseline | 2.7 (1.2–4.0) | ||||
| Naltrexone (0.032 mg/kg/h) | >11687 (4981–18708) | >13 | Naltrexone (0.032 mg/kg/h) | 2665 (1722–3536) | 25.6 | Naltrexone (0.032 mg/kg/h) | 81.3 (41–124) | 20.5 | Naltrexone(0.032 mg/kg/h) | 42.1 (29–56) | 25.7 |
| Day 1 | 487 (225–705) | 0.6 | Day 5 | 104 (60–138) | 1.0 | Day 7 | 11.3 (5.0–17) | 3.4 | Day 12 | 3.6 (2.5–4.7) | 1.8 |
| Day 14 | 775 (399–1103) | 1.0 | Day19 | 149 (95–192) | 1.4 | Day 21 | 39.6 (23–55) | 13.5 | Day 26 | 6.8 (4.2–9.1) | 3.4 |
| Day 28 | 1738 (800–2360) | 2.7 | Day 33 | 159 (100–205) | 1.5 | Day 35 | 37.9 (16–59) | 11.9 | Day 40 | 7.2 (3.4–10.3) | 3.3 |
| Day 42 | 837 (325–1243) | 1.7 | Day 47 | 142 (106–176) | 1.4 | Day 49 | 52.9 (44–62) | 19.7 | Day 54 | 14.9 (7.4–20) | 7.5 |
| Day 56 | 611 (317–888) | 0.7 | Day 61 | 200 (116–271) | 1.8 | Day 63 | 72.4 (30–104) | 19.1 | Day 68 | 10.3 (7.2–13) | 4.9 |
| Day 70 | 1117 (597–1601) | 1.6 | Day 75 | 269 (168–357) | 2.5 | Day 77 | 63.9 (38–86) | 20.2 | Day 82 | 12.8 (4.9–18) | 5.1 |
| Day 84 | 959 (632–1257) | 1.3 | Day 89 | 461 (214–670) | 4.6 | Day 91 | 85.1 (41–119) | 27.1 | Day 96 | 6.3 (2.7–9.1) | 2.4 |
| Day 98 | 974 (502–1404) | 1.3 | Day 103 | 329 (158–492) | 2.9 | Day 105 | 42.0 (20–63) | 11.4 | *ED50 reflects fentanyl dose | ||
Bolded and underlined conditions indicate that 95% confidence limits of ED50 values did not overlap with those of baseline conditions for a given opioid.
ED50 reflects fentanyl dose
Following naltrexone pump removal, Cohort 1 was administered a dual fentanyl/heroin conjugate vaccine at days 0, 14, 42, and 70 (see Figure 1C for experimental timeline). The antinociceptive potencies of IV fentanyl, heroin, the 1:27 fentanyl/heroin mixture, and methadone were each evaluated across 14-day testing cycles (see Figure 1B, Cohort 1), which was repeated throughout the 105-day testing period. The time course of vaccine effects on opioid antinociceptive potencies is summarized in Table 1. The vaccine produced a sustained decrease in the antinociceptive potency of fentanyl, as evidenced by nonoverlapping 95% confidence intervals with prevaccination ED50 values (Table 1). Peak vaccine effects for fentanyl (27-fold) was observed on experimental day 91 (Figure 2A; Table 1). These data are consistent with previous evaluations of a similar fentanyl-targeted vaccine on fentanyl antinociception in mice,14 rats,12 and rhesus monkeys.34 Vaccine effects on the antinociceptive potency of heroin were less robust, peaking (4.6-fold) on experimental day 89 (Figure 2B; Table 1). The magnitude of this heroin potency shift was consistent with previous reports evaluating a previous generation heroin-targeted vaccine on heroin antinociception in rats and mice,28,38 but less robust than reported in other studies.39–41 Overall, these results suggest the effectiveness of this heroin-CRM vaccine was more transient than a previously reported heroin-TT vaccine.28
The vaccine also decreased the antinociceptive potency of the 1:27 fentanyl/heroin mixture across multiple testing days, and significant decreases in potency were observed on days 26, 54, 68, and 82 (Table 1). The peak vaccine effect was 7.5-fold on experimental day 54 (Figure 2C; Table 1). Furthermore, the magnitude of this vaccine effect was greater than recent evaluations of dual fentanyl/heroin vaccines on the antinociceptive potency of a 1:10 fentanyl/heroin mixture in mice, which were 4-fold19 and 2-fold.21 The vaccine did not significantly alter the antinociceptive potency of methadone at any time point, providing in vivo evidence for vaccine-induced antibody selectivity (Figure 2D; Table 1). In addition, the consistent antinociceptive potency of methadone provides evidence that opioid tolerance did not occur across the experimental period in Cohort 1. Taken together, these data suggest that this particular dual vaccine formulation attenuated the antinociceptive potency of fentanyl > 1:27 fentanyl/heroin mixture > heroin > methadone.
Rat Cohort 2 (Figure 1B) was used to evaluate fentanyl/heroin vaccine effectiveness to reduce opioid self-administration using a procedure in which rats allocate behavior or “choose” between an IV 1:27 fentanyl/heroin mixture injections and liquid food (18% v/v vanilla flavor Ensure in tap water). Operant choice sessions were conducted on Monday–Thursday each week, and the antinociceptive potency of 1:27 fentanyl/heroin was determined each Friday to permit direct comparison of vaccine effects on reinforcement and antinociception. Under baseline conditions, food was chosen over the absence of an injection or small unit 1:27 fentanyl/heroin mixture doses (dashed lines; Figure 3A–C). As the fixed-proportion mixture dose increased, rats reallocated behavior away from food and toward the 1:27 fentanyl/heroin mixture-lever and the largest unit mixture dose (32 μg/kg/injection fentanyl and 86.4 μg/kg/injection heroin) maintained nearly 100% opioid choice. Additionally, the number of choices completed per component decreased as a function of increasing 1:27 fentanyl/heroin mixture dose (dashed lines; Figure 3D–F). The dual vaccine did not significantly alter fentanyl/heroin-vs-food choice over the 105-day experimental period (Figure 3A: fentanyl/heroin dose: F1.6, 7.9 = 167, p < 0.0001; time: F1.7, 8.3= 1.4, p = 0.30; interaction: F3.1, 14.7 = 1.2, p = 0.33). However, the vaccine attenuated the rate-decreasing effects of the 1:27 fentanyl/heroin mixture at weeks 3 (days 21–24), 6 (days 43–45), 9 (days 63–66), and 15 (days 105–108) (Figure 3D: fentanyl/heroin dose: F1.3, 6.6 = 309, p < 0.0001; time: F1.5, 7.4= 13.8, p = 0.004; interaction: F2.1, 10.0 = 10.5, p = 0.003). Moreover, vaccination effects on the antinociceptive potency of the 1:27 fentanyl/heroin mixture were not different between the two Cohorts (Figure 4A). The similarity in vaccine effectiveness despite different opioid exposure regimens between Cohort 1 and 2 provides further evidence that opioid tolerance was not the primary mediator of the observed potency shifts. Overall, this dual fentanyl/heroin conjugate vaccine formulation was behaviorally active on end points of both antinociception and rates of drug self-administration end points; however, the effectiveness was not robust enough to decrease the choice measure of fentanyl/heroin mixture self-administration.
Figure 3.

Effectiveness of a dual fentanyl/heroin vaccine, continuous naltrexone (0.032 mg/kg/h, SC), or saline substitution for fentanyl and/or heroin to attenuate IV fentanyl/heroin-vs-food choice. Abscissae: unit IV fentanyl/heroin dose in μg/kg (note: fentanyl and/or heroin absent in panels C and F depending on saline substitution condition). Top row ordinates: percentage of completed ratio requirements on the fentanyl/heroin-associated lever. Bottom row ordinates: number of choices completed per component. Panels A and D show selected time points following administration of a dual fentanyl/heroin vaccine in six Cohort 2 rats (3 female and 3 male), although one male rat lost catheter patency prior to the Days 105–108 evaluation and was excluded from that time point. Panels B and E show the last 3 days of 5-day continuous naltrexone (0.032 mg/kg/h, SC) treatment effects in six Cohort 3 rats (3 female and 3 male). Panels C and F show the last 3 days of 5-day saline substitution experiments that included the removal of fentanyl (upward-facing triangles), heroin (downward-facing triangles), or both fentanyl and heroin (saline substitution; squares) in six Cohort 3 rats (3 female and 3 male). Points represent mean ± SEM, and filled symbols denote significant difference relative to baseline, defined as p < 0.05.
Figure 4.
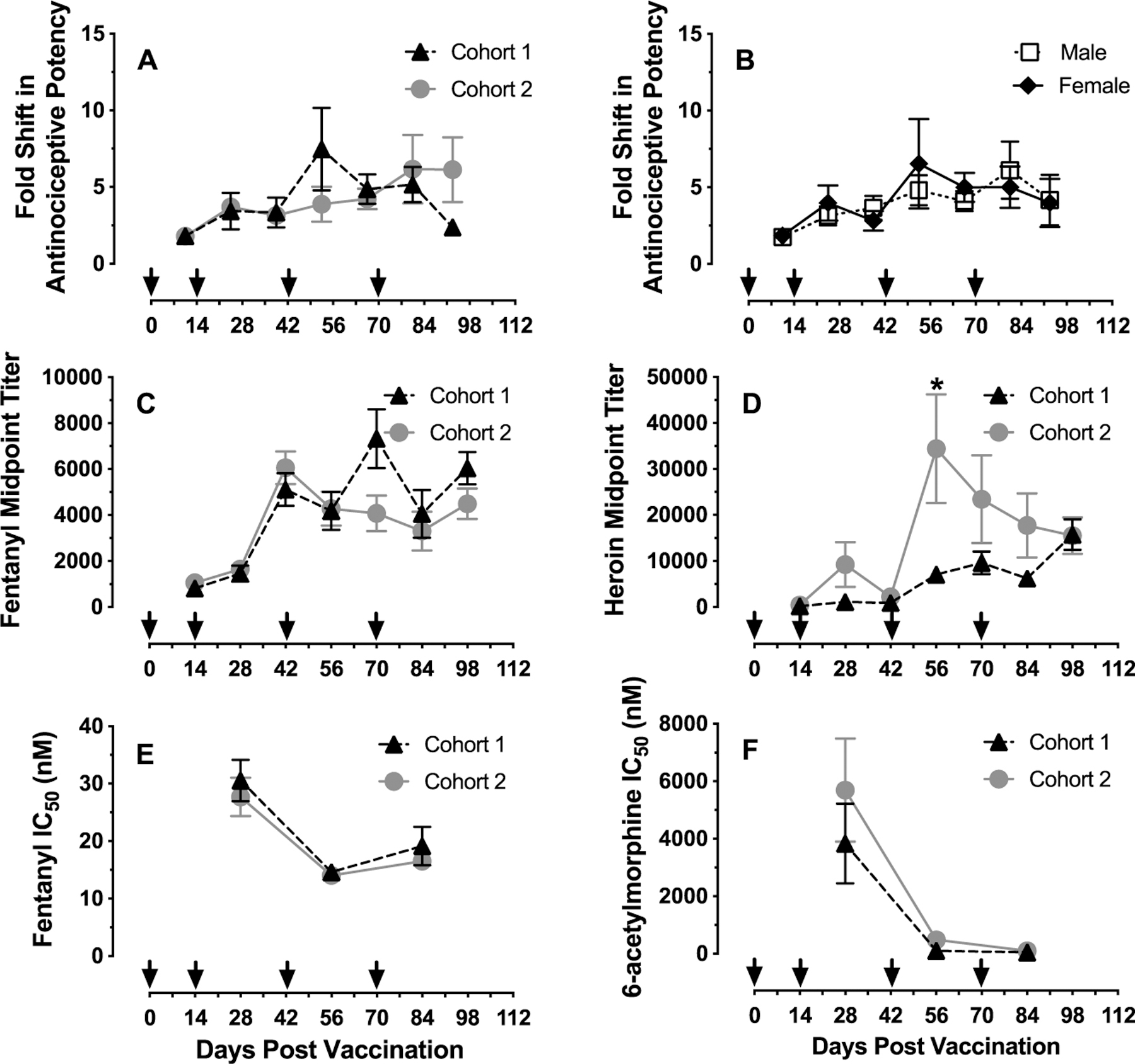
Time course of vaccine effectiveness, antibody midpoint titer levels, and affinities. Abscissae: experimental timeline with arrows indicating vaccination dates. Top ordinates: fold shift in antinociceptive potency of the 1:27 fentanyl/heroin mixture relative to prevaccination ED50 values. Middle ordinates: midpoint titer levels. Bottom ordinates: antibody IC50 values in nM determined from antiserum. Panel A shows vaccine effects on antinociceptive potency as a function of experimental day in Cohort 1 (n = 3 male, 3 female rats) and Cohort 2 (n = 3 male, 3 female rats). Panel B shows vaccine effects on antinociceptive potency as a function of experimental day in 12 rats (6 female and 6 male) from both Cohorts 1 and 2. Panels C and D show antifentanyl (left) and antiheroin (right) midpoint titers as a function of experimental day in Cohort 1 and Cohort 2. Panels E and F show antifentanyl (left) and anti-6-acetylmorphine (active heroin metabolite; right) antibody affinity (IC50 value) as a function of experimental day in Cohort 1 and Cohort 2. All points represent mean ± SEM. One male of Cohort 2 lost catheter patency before the Day 98 evaluation and was excluded from that time point. The asterisk in Panel D indicates significant difference in heroin midpoint titer levels between Cohorts 1 and 2 on Day 56, defined as p < 0.05.
To provide further context for interpretation of vaccine effects on fentanyl/heroin-vs-food choice, Cohort 3 (3 female, 3 male) was used to evaluate four control conditions: (1) 7-day continuous naltrexone treatment (0.032 mg/kg/h, subcutaneous), (2) 5-day removal of fentanyl from the fentanyl/heroin mixture, (3) 5-day removal of heroin from the fentanyl/heroin mixture, and (4) 5-day removal of both fentanyl and heroin (i.e., saline substitution). Continuous 0.032 mg/kg/h naltrexone delivery significantly decreased 1:27 fentanyl/heroin mixture self-administration when the choice was between 1 μg/kg/injection fentanyl/27 μg/kg/injection heroin and food (circles; Figure 3B: fentanyl/heroin dose: F2, 10.1 = 42.7, p < 0.0001; naltrexone: F1, 5 = 19.8, p = 0.007; interaction: F1.2, 5.6 = 6.0, p = 0.049). Continuous naltrexone treatment also significantly increased the number of choices completed per component relative to baseline (circles; Figure 3E: fentanyl/heroin dose: F1.2, 6 = 34.9, p = 0.0008; naltrexone: F1, 5= 106.4, p = 0.0001; interaction: F1.5, 7.5 = 44.6, p = 0.0001). An ED50 value for 1:27 fentanyl/heroin choice was not determined from data in Figure 3E because some rats failed to exceed 50% drug choice with this range of opioid unit doses; however, a follow-up study was conducted by increasing the unit-dose range by 0.5 log for 1 day. With these increased unit doses, all rats exceeded 50% drug choice, and an ED50 could be determined, indicating that naltrexone antagonism was fully surmountable and resulted in a 4.3-fold increase in ED50 for the 1:27 fentanyl/heroin mixture: (initial ED50 (95% CL): 0.60 (0.3–1.3) μg/kg/injection fentanyl, 16.3 (7.5–35.4) μg/kg/injection heroin); ED50 following naltrexone treatment: 2.6 (1.8–3.9) μg/kg/injection fentanyl, 70.6 (47.5–104.8) μg/kg/injection heroin). Notably, naltrexone effectiveness in the present rat study was similar to, but slightly less effective than, depot naltrexone effectiveness to shift heroin potency in a heroin-vs-money choice procedure in humans (≥8-fold).42 In addition, both continuous naltrexone treatment in rats and 384 mg depot naltrexone injection in humans result in naltrexone plasma levels of approximately 5 ng/mL.28–31 In summary, the current results provide empirical evidence for translational concordance of sensitivity of opioid-versus-nondrug choice procedures to naltrexone treatment between rats and humans.
The smaller effects of both the fentanyl/heroin vaccine and continuous naltrexone on fentanyl/heroin choice in comparison to fentanyl/heroin antinociception suggests that stimuli other than self-administered drug, such as visual cues or other environmental stimuli, may contribute to drug choice. To evaluate the role of nondrug cues in maintaining drug choice, we next examined experimental conditions where the fentanyl or heroin component of the mixture was removed for five consecutive days during the self-administration procedure, modeling a full vaccine-induced sequestration of each individual opioid. Removal of either fentanyl (Figure 3C, upward triangles) or heroin (Figure 3C, downward triangles) from the fentanyl/heroin mixture failed to significantly alter percent drug choice, a result similar to what was observed with the dual fentanyl/heroin vaccine. This result suggests that percent opioid choice was not sensitive to small potency shifts, as removal of either the fentanyl or heroin component of the mixture would be hypothesized to produce a two-fold rightward shift in the self-administration dose–effect function. In contrast to vaccine-induced attenuation of fentanyl/heroin mixture rate-decreasing effects, removal of either fentanyl (Figure 3F, upward triangles) or heroin (Figure 3F, downward triangles) failed to significantly alter the number of choices completed per component. However, when both fentanyl and heroin were removed from the self-administered solution (i.e., saline substitution), percent choice of the lever previously associated with the 1:27 fentanyl/heroin mixture was significantly decreased in the last two components of the behavioral sessions (squares; Figure 3C; unit dose: F1.8, 8.8 = 126.1, p < 0.0001; condition: F1.6, 7.8 = 26.6, p = 0.0005; interaction: F2.6, 13.2 = 14.6, p = 0.0002). In addition, saline substitution resulted in an increase in the number of choices completed in the last component, indicative of a behavioral reallocation toward the food-associated lever (squares; Figure 3F; unit dose: F1.5, 7.4 = 66.1, p < 0.0001; condition: F1.7, 8.6 = 28.6, p = 0.0002; interaction: F2.6, 12.9 = 21.4, p < 0.0001). Saline substitution was more effective than naltrexone treatment in attenuating responding on the drug mixture injection lever (unit dose: F1.8, 9.0 = 43.7, p < 0.0001; treatment: F1.6, 8.1 = 27.6, p = 0.0003; interaction: F1.7, 8.2 = 13.4, p = 0.003). Taken together, the present results suggest abuse-related end points measured with drug self-administration procedures are less sensitive than antinociceptive end points to opioid antagonists and vaccines. These results also highlight that robust antibody production to both vaccine components would be necessary for vaccines to decrease opioid-mixture choice and promote behavioral reallocation toward alternative nondrug reinforcers.
Figure 4 compares the time course of fentanyl/heroin vaccine effects in Cohorts 1 and 2 on fentanyl/heroin antinociception, antibody titers, and antibody affinities. The magnitude of fentanyl/heroin vaccine antagonism of 1:27 fentanyl/heroin antinociception was not different across cohorts (Figure 4A) or sexes (Figure 4B). Moreover, irrespective of cohort, fentanyl midpoint titer levels plateaued at approximately 1:5,000 by week 6 and remained constant for the experimental period (Figure 4C). Heroin midpoint titer levels peaked at 1:34 000 at week 8 in Cohort 1 and decayed to 1:15 000 at week 14 (Figure 4D). In Cohort 2, heroin midpoint titer levels appeared delayed and less robust, with titer levels gradually increasing throughout the experiment and peaking at 1:16 000 at week 14 (Figure 4D). Comparison of heroin midpoint titer levels between cohorts revealed a time by cohort interaction (time: F6, 58 = 10.3, p < 0.0001; cohort: F1, 10= 3.1, p = 0.12; interaction: F6, 58 = 3.5, p = 0.005), with a significantly higher titer level in Cohort 1 detected at week 8 (p = 0.002). Antibody affinities toward fentanyl improved following the third fentanyl/heroin vaccine administration and IC50 values stabilized between 14 and 19 nM (Figure 4E). Antibody affinities toward 6-acetylmorphine (active heroin metabolite) peaked around 50 nM at week 12 (Figure 4F). No cohort differences in fentanyl or heroin antibody affinities were detected. Overall, these results demonstrate the reproducibility of vaccine effects across several end points.
In contrast to our previous study demonstrating a fentanyl vaccine resulted in similar antinociceptive and drug self-administration potency shifts,12 the effectiveness of the dual fentanyl/heroin vaccine depended upon the behavioral end point. One interpretation of these findings is that antinociceptive end points are more sensitive than drug self-administration end points. Consistent with this interpretation, we found continuous naltrexone treatment shifted the antinociceptive potency of the 1:27 fentanyl/heroin mixture 20-fold compared to a 4.3-fold potency shift in the fentanyl/heroin-vs-food choice procedure. Previous preclinical research evaluating small molecules as candidate medications for substance use disorders has demonstrated the utility of drug-vs-food choice procedures in the therapeutic development process.27,43,44 Thus, we argue that candidate treatments should be as effective in decreasing drug self-administration as existing OUD treatments.10 To that end, the current formulation and administration regimen of this fentanyl/heroin vaccine, although improved over previous formulations and highly effective against fentanyl alone, would not be expected to confer similar effectiveness to depot naltrexone injection in users of a fentanyl/heroin mixture.
Some possible variables exist in current vaccine formulation that could explain the vaccine’s ineffectiveness to attenuate fentanyl/heroin mixture self-administration. First, the 27-fold greater potency of heroin relative to fentanyl leads to the requirement that the vaccine bind 27-fold greater molar quantities of heroin as 6-acetylmorphine over fentanyl. In anticipating this requirement, we administered a 2:1 ratio of heroin to fentanyl conjugate in the vaccine formulation. However, the heroin conjugate dose of 80 μg was insufficient for producing an immune response that could match the efficacy level produced by 40 μg of fentanyl conjugate. Second, the marginally lower hapten loading of the heroin hapten onto the CRM protein (12:1) compared to the fentanyl loading (18:1) could have impacted heroin vaccine effectiveness. In assessing both titer and antidrug affinity levels, the immunochemical response to heroin and 6-acetylmorphine was slightly greater than to fentanyl but was not sufficient to mitigate the behavioral effects of a 27-fold greater dose of heroin. Increasing the heroin conjugate dose and hapten loading are logical means for boosting anti-6-acetylmorphine responses in future iterations of this combination vaccine. Third, the relative proportion of fentanyl to heroin administered to a vaccinated subject would also be hypothesized to impact vaccine effectiveness. The 1:27 fentanyl/heroin mixture was based on the relative potency to produce thermal antinociception for experimental purposes, but the relative proportion of fentanyl to heroin in the clinical situation would be more variable. We hypothesize this dual vaccine would be more effective for a fentanyl/heroin mixture with a greater proportion of fentanyl (e.g., 1:10 fentanyl/heroin), but would be less effective for mixtures with a greater proportion of heroin. A fourth possibility could be the presence of one vaccine may have impacted the immunogenic responsiveness to the other vaccine. This possibility has been explicitly investigated by our group19,20 and others.45–48 However, our two recent publications do not provide empirical evidence supporting this interpretation.19,20 Other variables that contribute to vaccine immunogenicity and could be optimized in the future include the carrier protein and adjuvant, although it remains to be empirically determined how these variables would impact a combination opioid vaccine.
One potential limitation of the current experimental design was the absence of an unconjugated control vaccine condition. Inclusion of this control would empirically determine whether opioid tolerance developed during the 105-day experimental period. However, we propose three other experimental design features that address the potential confound of repeated opioid exposure, leading to tolerance to opioid behavioral effects that could have contributed to the present results. First, the antinociceptive potency of methadone (opioid not targeted by the fentanyl/heroin vaccine) was not significantly different from baseline across the entire experimental period. If opioid tolerance had developed, a decrease in methadone’s antinociceptive potency would have been expected. Second, the fentanyl/heroin vs food choice dose–response function did not change over the course of the experiment (Figure 3A). If opioid tolerance had developed, the fentanyl/heroin choice dose–effect function should have shifted rightward. Third, vaccination effectiveness for decreasing the antinociceptive potency of the 1:27 fentanyl/heroin mixture was similar between Cohort 1 and Cohort 2 despite different opioid exposures. Even in the absence of vaccine control group, our primary conclusion that this vaccine formulation and administration regimen was less effective than naltrexone to attenuate the antinociceptive and reinforcing effects of a fentanyl/heroin mixture is supported by the results.
METHODS
Drugs and Vaccine.
Fentanyl HCl, heroin HCl, and (–)-naltrexone HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD) and dissolved in sterile saline. (±)-Methadone HCl (M1426, Spectrum Chemicals, Gardena, CA) was dissolved in sterile water. All solutions were passed through a 0.22-μm sterile filter (Millex GV, Millipore Sigma, Burlington, MA) before administration. All drug doses were expressed as the salt forms listed above and delivered as micrograms per kilogram based on weights collected weekly. The fentanyl and heroin haptens were synthesized and conjugated to ecoCRM (Fina Biosolutions, Rockville, MD) as previously described14,40 and characterized by MALDI-TOF to reveal hapten to protein ratios of 12:1 for heroin-CRM and 18:1 for fentanyl-CRM. Conjugates were formulated with adjuvants at approximately 1.6–1.8 mg/mL protein in 1:3 glycerol/PBS and were stored fully formulated in liquid form at 4 °C as described previously.16,19 Each vaccine injection contained 80 μg of heroin-CRM, 40 μg of fentanyl-CRM, 50 μg of CpG ODN 1826 (Eurofins Genomics, Louisville, KY), and 170 μg of Alhydrogel (Invivogen, San Diego, CA).
Subjects.
A total of 18 Sprague–Dawley rats (9 male, 9 female) were acquired at 10 weeks of age (Envigo Laboratories, Frederick, MD, United States) and surgically implanted with vascular access ports (Instech, Plymouth Meeting, PA) and custom-made jugular catheters as described previously.49 Rats were divided into three cohorts. Cohort 1 (3 male, 3 female) was used in Experiments 1, 2, and 3. Cohort 2 (3 male, 3 female) was used in Experiments 1 and 4. Cohort 3 (3 male, 3 female) was used in Experiments 5 and 6. Rats were singly housed in a temperature- and humidity-controlled vivarium that was maintained on a 12-h light/dark cycle (lights off at 6:00 PM). Water and food (Teklad Rat Diet, Envigo) were provided ad-libitum in the home cage. Behavioral testing was conducted 5 days per week from approximately 2:00 PM to 4:00 PM unless otherwise noted. Catheter patency was verified periodically and at study end by instantaneous muscle tone loss following IV methohexital (0.5 mg) administration. Animal maintenance and research were conducted in accordance with the 2011 guidelines for the care and use of laboratory animals and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Experiment 1: Determination of the Relative Antinociceptive Potencies of Fentanyl and Heroin.
To determine the 1:1 fentanyl/heroin mixture proportion, the relative potency (ED50 value) of fentanyl and heroin to produce thermal antinociception was determined in Cohorts 1 and 2. Cumulative fentanyl and heroin alone dose–effects functions were determined in the warm water tail withdrawal procedure in a counterbalanced order and each dose–effect function was singly determined in each rat. Each session began by gently wrapping the rat in a towel, leaving the tail exposed. The distal 5 cm of the tail was immersed in heated water (50 ± 1 °C) in a water bath (Precision, 280 Series Water Bath, Winchester, VA), and the latency to fully remove the tail was recorded with a digital chronograph with a 0.01 s resolution (Sports Timer, Fisher Brand, Hampton, NH). If the rat did not remove its tail by 20 s, the tail was removed by the experimenter, and a latency of 20 s was assigned. Following baseline latency determination, IV saline or fentanyl was administered followed by a 0.1 mL saline flush, and tail-withdrawal latency was redetermined 120 s later. The pretreatment times were based on published data50 and our previous studies.12,28 Saline was always injected first, followed by increasing doses of fentanyl (1–1000 μg/kg) or heroin (10–10 000 μg/kg) such that each dose increased the total cumulative dose by 0.5 log dose increments. The interval between successive injections was 120 s. Testing continued until a maximum latency of 20 s was observed. At the conclusion of each test, catheters were flushed with 0.1 mL of gentamicin (0.4 mg) followed by 0.1 mL of heparinized saline (30 U/ml).
Experiment 2: Naltrexone Treatment Effects on Opioid-Induced Thermal Antinociception.
Continuous naltrexone treatment effects on opioid-induced thermal antinociception were determined as a positive control under nonvaccinated conditions in Cohort 1 rats. Following baseline antinociceptive potency determination of cumulative IV fentanyl (1–1000 μg/kg), heroin (10–10 000 μg/kg), 1:27 fentanyl/heroin mixture (0.32–100 μg/kg fentanyl; 8.64–2700 μg/kg heroin), and methadone (100–10 000 μg/kg) (i.e., Experiment 1), rats were aseptically implanted with a 2-week naltrexone (0.032 mg/kg/h) osmotic pump (Alzet, Model 2ML2, Cupertino, CA) in the midscapular subcutaneous region under isoflurane anesthesia on a Friday. Tail withdrawal test sessions occurred on Mondays and Fridays for 2 weeks to redetermine opioid agonist or opioid mixture antinociceptive potency under naltrexone treatment conditions. The testing order of methadone, heroin, fentanyl, and the 1:27 fentanyl/heroin mixture was counterbalanced across subjects (see Figure 1B, Cohort 1). At the conclusion of this two-week experiment, rats were again anesthetized with isoflurane before the pump was aseptically removed.
Experiment 3: Dual Fentanyl/Heroin Vaccine Effects on Opioid-Induced Antinociception.
Following Experiments 1 and 2, Cohort 1 rats received an intramuscular injection of the dual fentanyl/heroin vaccine on Monday of days 0, 14, 42, and 70 of the experiment under isoflurane anesthesia (see Figure 1C for timeline). Every 2 weeks following vaccination, tail vein blood was collected on Monday sunder isoflurane anesthesia into lithium heparin tubes (BD, Franklin Lakes, NJ). Tubes were immediately centrifuged at 3300 rpm for 15 min (Ample Scientific, Model E33–1, Norcross, GA), and plasma was pipetted into microcentrifuge tubes and stored at –80 °C until analyzed. Antifentanyl and antiheroin midpoint titer levels and affinity (i.e., IC50 values) measurements were performed as previously described.14 On Mondays when either vaccine administration or blood collection occurred, rats completed antinociceptive testing of methadone prior to isoflurane anesthesia and blood collection. Vaccinations occurred after blood was collected. As in Experiment 2, the antinociceptive potencies of methadone, heroin, fentanyl, and the 1:27 fentanyl/heroin mixture were evaluated every 14 days in the following testing order: Monday: methadone; Friday: heroin; Monday: fentanyl; Friday: fentanyl/heroin mixture (Figure 1B).
Experiment 4: Fentanyl/Heroin Vaccine Effects on Fentanyl/Heroin Mixture-vs-Food Choice.
Following Experiment 1, Cohort 2 rats were trained to respond for IV fentanyl/heroin mixture (1 μg/kg/infusion fentanyl, 27 μg/kg/infusion heroin) injections and liquid food alone in modular operant chambers located in sound-attenuating cubicles (Med Associates, St. Albans, VT) were equipped with two retractable levers, a set of three LED lights (red, yellow, green) mounted above each lever, and a retractable cup (0.1 mL) located between the levers for delivering diluted liquid food (18% v/v vanilla flavor Ensure in tap water; Abbott Laboratories, Chicago, IL). The IV fentanyl/heroin mixture was delivered by a syringe pump (PHM-100, Med Associates; located inside the sound-attenuating cubicle) to the rat through a single-channel fluid swivel and tether connected to the rat’s vascular access port. Rats were subsequently trained to respond under a drug-vs-food choice procedure as previously described.12,23 The behavioral session consisted of five 20 min response components each preceded by a 4 min “sample” component. Each sample component started with a noncontingent infusion of the unit fentanyl/heroin mixture dose available during the subsequent response component followed by a 2 min time out. Next, a 5-s presentation of liquid food was programmed followed by a 2 min time out. Following this second time out, the response component would begin. During each response component, both levers were extended, a red stimulus light above the left lever was illuminated to signal liquid food availability and a green stimulus light above the right lever was illuminated to signal IV fentanyl/heroin mixture availability. Response requirement (fixed-ratio; FR5) completion on the left lever resulted in a 5-s presentation of liquid food whereas response requirement (FR5) completion on the right lever resulted in the delivery of the IV fentanyl/heroin mixture dose available for that component. Responding on one lever reset the ratio requirement for the other lever. The liquid food concentration was held constant throughout the session. A different fentanyl/heroin mixture dose was available during each of the five successive response components as follows (doses in μg/kg/infusion): Component 1: no infusion; Component 2: 0.1 fentanyl +2.7 heroin; Component 3: 0.32 fentanyl + 8.64 heroin; Component 4: 1.0 fentanyl + 27 heroin; Component 5: 3.2 fentanyl + 86.4 heroin. The fentanyl/heroin mixture dose was varied by changing the infusion duration (e.g., 300 g rat: 0, 0.5, 1.56, 5, and 15.6 s during components 1–5, respectively), and the green light above the fentanyl/heroin mixture-lever flashed on and off in 3 s cycles (i.e., longer flashes associated with larger fentanyl/heroin mixture doses).
During each response component, rats could complete up to 10 total ratio requirements between the food- and fentanyl/heroin mixture-associated levers. Each ratio requirement completion initiated a 20-s time out, the retraction of both levers, and offset of the red and green stimulus lights. If all 10 ratio requirements were completed before 20 min had elapsed, then both levers retracted, and stimulus lights were turned off for the remainder of that component. Choice was considered stable when the smallest unit fentanyl/heroin mixture dose that maintained at least 80% of completed ratio requirements on the mixture-associated lever was within a 0.5 log unit of the running mean for three consecutive days with no upward or downward trends. Once these criteria were met, choice testing occurred Monday through Thursday each week unless otherwise specified. After each behavioral session, catheters were flushed with 0.1 mL of gentamicin (0.4 mg) and subsequently filled with 0.1 mL of heparinized saline (30 U/ml) to promote catheter patency.
Dual fentanyl/heroin vaccine injections occurred on Mondays on experimental days 0, 14, 42, and 70 (see Figure 1C for timeline) to match the vaccination schedule of Cohort 1. Every two weeks following vaccination, tail vein blood was collected on Monday under isoflurane anesthesia into lithium heparin tubes (BD, Franklin Lakes, NJ) and processed for midpoint titer and affinity of the antifentanyl and antiheroin vaccines as described above. Each week following vaccination, fentanyl/heroin mixture-vs-food choice sessions were conducted Monday through Thursday, and the antinociceptive potency of the 1:27 fentanyl/heroin mixture was determined on Friday. During weeks when the vaccine was administered, or blood was collected on a Monday, fentanyl/heroin mixture-vs-food choice testing was abbreviated to Tuesday through Thursday.
Experiment 5: Effect of Saline Substitution on Fentanyl/Heroin Mixture-vs-Food Choice.
Cohort 3 rats were trained to respond under a fentanyl/heroin mixture-vs-food choice procedure as described above. Once stable choice behavior was observed as described above, four test conditions were conducted in a counterbalanced order: (1) saline-vs-food choice (i.e., removal of both fentanyl and heroin from the mixture), (2) fentanyl-vs-food choice (i.e., removal of heroin from the mixture), (3) heroin-vs-food choice (i.e., removal of fentanyl from the mixture), and (4) the 1:27 fentanyl/heroin mixture-vs-food choice. Each condition was tested for five consecutive days and the last 3 days of each condition were used for subsequent analysis.
Experiment 6: Naltrexone Treatment Effects on Fentanyl/Heroin-vs-Food Choice.
Following Experiment 5 and once fentanyl/heroin-vs-food choice was stable, Cohort 3 rats were aseptically implanted on a Friday with a 2-week naltrexone (0.032 mg/kg/h) osmotic pump as in Experiment 2. Drug self-administration sessions resumed the following Monday through Friday, and the last 3 days were used for subsequent analysis. On the following Monday, rats were tested with a 0.5 log higher 1:27 fentanyl/heroin mixture unit dose range (0.32 fentanyl + 8.64 heroin to 10 fentanyl + 270 heroin; doses in μg/kg/infusion) for a single drug self-administration session.
Data Analysis.
Tail-withdrawal latencies were expressed as percent maximum possible effect (%MPE) using the following equation: %MPE = {(test latency – saline latency)/(20 s – saline latency)} × 100, where test latency was the latency in seconds after each opioid dose, and saline latency was the latency after saline administration at the start of each session. Log ED50 values (LCL-UCL) were calculated using linear regression when at least three points were above or below the 50% effect level or interpolation when only two data points were available. For analysis of the 1:27 fentanyl/heroin data, %MPE was calculated in terms of fentanyl dose. ED50 values were considered to be significantly different if 95% confidence limits did not overlap.
The two primary dependent measures for the drug-vs-food choice studies were (1) percent drug choice, defined as (number of ratio requirements, or “choices”, completed on the fentanyl/heroin mixture-associated lever/total number of choices completed on both levers) × 100, and (2) number of choices per component. For vaccine studies in Experiment 4, data were first averaged within a rat across M–Th (or T–Th, if blood collection or vaccine administration occurred) and then averaged between rats to yield group mean data. Results were then plotted as a function of the fentanyl/heroin mixture unit dose and analyzed using a mixed-model analysis (GraphPad Prism 8, La Jolla, CA) with experimental day and the 1:27 fentanyl/heroin mixture unit dose as the fixed main effects. For saline substitution and naltrexone-treatment experiments in Experiments 5 and 6, data from the last 3 days of each 5-day condition was averaged and analyzed using a mixed-model analysis with condition and the 1:27 fentanyl/heroin mixture unit dose as the fixed main effects. Comparisons of shifts in antinociceptive potency, titer levels, and antibody affinity between cohorts and sexes were analyzed using a mixed-model analysis, with experimental day and cohort/sex as the fixed main effects. In the presence of a significant main effect or interaction, a Dunnett or Tukey posthoc test was conducted as appropriate. Statistical significance was set a priori at the 95% confidence level.
ACKNOWLEDGMENTS
We acknowledge Kevin Costa for writing the original version of the behavioral program that was modified and used in the present drug-vs-food choice experiments. We acknowledge Dr. Bin Zhou for performing SPR-based opioid affinity determination of antiserum and Morgan Weaver for assistance with ELISA.
Funding
Research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Awards UH3DA041146 and F32DA047026. The National Institute on Drug Abuse had no role in study design, collection, analysis, or interpretation of the data; writing; or decision to submit the manuscript for publication. The manuscript content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. E.A.T., K.E.F., S.S.N., K.D.J., and M.L.B. declare their research has been funded by the National Institutes of Health.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acschemneuro.0c00064
The authors declare the following competing financial interest(s): P.T.B. is an employee at Cessation Therapeutics, and individual heroin and fentanyl vaccines are licensed to Cessation. The authors declare no other conflicts of interest.
Contributor Information
E. Andrew Townsend, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States.
Paul T. Bremer, Departments of Chemistry and Immunology and Microbial Science, Skaggs Institute for Chemical Biology, Worm Institute for Research and Medicine, The Scripps Research Institute, La Jolla, California 92037, United States
Kaycee E. Faunce, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States
S. Stevens Negus, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States.
Alaina M. Jaster, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States
Hannah L. Robinson, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States
Kim D. Janda, Departments of Chemistry and Immunology and Microbial Science, Skaggs Institute for Chemical Biology, Worm Institute for Research and Medicine, The Scripps Research Institute, La Jolla, California 92037, United States.
Matthew L. Banks, Department of Pharmacology and Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States.
REFERENCES
- (1).Blanco C, and Volkow ND (2019) Management of opioid use disorder in the USA: present status and future directions. Lancet 393 (10182), 1760–1772. [DOI] [PubMed] [Google Scholar]
- (2).Leshner AI, and Mancher M (2019) A consensus study report of the National Academies of Sciences, Engineering, Medicine; The National Academies Press: Washington, DC. [Google Scholar]
- (3).Herget G (2005) Methadone and buprenorphine added to the WHO list of essential medicines. HIV AIDS Policy Law Rev 10 (3), 23–4. [PubMed] [Google Scholar]
- (4).Negus SS (2006) Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J. Pharmacol. Exp. Ther 317 (2), 711–23. [DOI] [PubMed] [Google Scholar]
- (5).Daniulaityte R, Nahhas RW, Silverstein S, Martins S, Zaragoza A, Moeller A, and Carlson RG (2019) Patterns of non-prescribed buprenorphine and other opioid use among individuals with opioid use disorder: A latent class analysis. Drug Alcohol Depend 204, 107574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jones CM, Baldwin GT, Manocchio T, White JO, and Mack KA (2016) Trends in Methadone Distribution for Pain Treatment, Methadone Diversion, and Overdose Deaths - United States, 2002–2014. MMWR Morb Mortal Wkly Rep 65 (26), 667–71. [DOI] [PubMed] [Google Scholar]
- (7).Wightman RS, Nelson LS, Lee JD, Fox LM, and Smith SW (2018) Severe opioid withdrawal precipitated by Vivitrol(R). Am. J. Emerg Med 36 (6), 1128. [DOI] [PubMed] [Google Scholar]
- (8).Nunes EV, Bisaga A, Krupitsky E, Nangia N, Silverman BL, Akerman SC, and Sullivan MA (2020) Opioid use and dropout from extended-release naltrexone in a controlled trial: implications for mechanism. Addiction 115 (2), 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bremer PT, and Janda KD (2017) Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev 69 (3), 298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Banks ML, Townsend EA, and Negus SS (2019) Testing the 10 most wanted: a preclinical algorithm to screen candidate opioid use disorder medications. Neuropsychopharmacology 44 (6), 1011–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Pravetoni M, and Comer SD (2019) Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158, 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Townsend EA, Blake S, Faunce KE, Hwang CS, Natori Y, Zhou B, Bremer PT, Janda KD, and Banks ML (2019) Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology 44 (10), 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, Navarro HA, Langston TL, Runyon SP, Winston S, Pravetoni M, and Pentel PR (2017) Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One 12 (12), No. e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, and Janda KD (2016) Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem., Int. Ed 55 (11), 3772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sulima A, Jalah R, Antoline JFG, Torres OB, Imler GH, Deschamps JR, Beck Z, Alving CR, Jacobson AE, Rice KC, and Matyas GR (2018) A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J. Med. Chem 61 (1), 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hwang CS, Bremer PT, Wenthur CJ, Ho SO, Chiang S, Ellis B, Zhou B, Fujii G, and Janda KD (2018) Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol. Pharmaceutics 15 (3), 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Smith LC, Bremer PT, Hwang CS, Zhou B, Ellis B, Hixon MS, and Janda KD (2019) Monoclonal Antibodies for Combating Synthetic Opioid Intoxication. J. Am. Chem. Soc 141 (26), 10489–10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, and Pentel PR (2012) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30 (31), 4617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, and Janda KD (2018) Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: Antinociceptive Evaluation of Fentanyl-Contaminated Heroin. ACS Omega 3 (9), 11537–11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, and Janda KD (2018) Efficacious Vaccine against Heroin Contaminated with Fentanyl. ACS Chem. Neurosci 9 (6), 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Natori Y, Hwang CS, Lin L, Smith LC, Zhou B, and Janda KD (2019) A chemically contiguous hapten approach for a heroin-fentanyl vaccine. Beilstein J. Org. Chem 15, 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Thomsen M, Barrett AC, Negus SS, and Caine SB (2013) Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J. Exp. Anal. Behav 99 (2), 211–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Townsend EA, Negus SS, Caine SB, Thomsen M, and Banks ML (2019) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44 (12), 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mello NK, and Negus SS (1996) Preclinical Evaluation of Pharmacotherapies for Treatment of Cocaine and Opioid Abuse Using Drug Self-Administration Procedures. Neuropsychopharmacology 14 (6), 375–424. [DOI] [PubMed] [Google Scholar]
- (25).Haney M, and Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology 199 (3), 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Czoty PW, Stoops WW, and Rush CR (2016) Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol. Rev 68 (3), 533–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Townsend EA, Negus SS, and Banks ML (2020) Medications Development for Treatment of Opioid Use Disorder. Cold Spring Harbor Perspect. Med, a039263. [DOI] [PMC free article] [PubMed]
- (28).Schwienteck KL, Blake S, Bremer PT, Poklis JL, Townsend EA, Negus SS, and Banks ML (2019) Effectiveness and selectivity of a heroin conjugate vaccine to attenuate heroin, 6-acetylmorphine, and morphine antinociception in rats: Comparison with naltrexone. Drug Alcohol Depend 204, 107501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, and Fischman MW (2002) Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl) 159 (4), 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, and O’Brien CP (2006) Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry 63 (2), 210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bigelow GE, Preston KL, Schmittner J, Dong Q, and Gastfriend DR (2012) Opioid challenge evaluation of blockade by extended-release naltrexone in opioid-abusing adults: dose-effects and time-course. Drug Alcohol Depend 123 (1–3), 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, and Janda KD (2011) A vaccine strategy that induces protective immunity against heroin. J. Med. Chem 54 (14), 5195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Raleigh MD, Baruffaldi F, Peterson SJ, Le Naour M, Harmon TM, Vigliaturo JR, Pentel PR, and Pravetoni M (2019) A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther 368 (2), 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Tenney RD, Blake S, Bremer PT, Zhou B, Hwang CS, Poklis JL, Janda KD, and Banks ML (2019) Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 158, 107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Banks ML, Rice KC, and Negus SS (2010) Antinociceptive interactions between Mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: role of Mu agonist efficacy. J. Pharmacol. Exp. Ther 335 (2), 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Cornelissen JC, Steele FF, Rice KC, Nicholson KL, and Banks ML (2018) Additive and subadditive antiallodynic interactions between mu-opioid agonists and N-methyl D-aspartate antagonists in male rhesus monkeys. Behav. Pharmacol 29 (1), 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Janssen PA, and Jageneau AH (1958) A new series of potent analgesics: dextro 2:2-diphenyl-3-methyl-4-morpholinobutyrylpyrrolidine and related basic amides. II. Comparative analgesic activity, acute toxicity and tolerance development in rats for R875, morphine, pethidine and methadone. J. Pharm. Pharmacol 10 (1), 14–21. [DOI] [PubMed] [Google Scholar]
- (38).Hwang CS, Ellis B, Zhou B, and Janda KD (2019) Heat shock proteins: A dual carrier-adjuvant for an anti-drug vaccine against heroin. Bioorg. Med. Chem 27 (1), 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hwang CS, Smith LC, Wenthur CJ, Ellis B, Zhou B, and Janda KD (2019) Heroin vaccine: Using titer, affinity, and antinociception as metrics when examining sex and strain differences. Vaccine 37 (30), 4155–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hwang CS, Bremer PT, Wenthur CJ, Ho SO, Chiang S, Ellis B, Zhou B, Fujii G, and Janda KD (2018) Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol. Pharmaceutics 15 (3), 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Bremer PT, Schlosburg JE, Lively JM, and Janda KD (2014) Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Mol. Pharmaceutics 11 (3), 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sullivan MA, Vosburg SK, and Comer SD (2006) Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology (Berl) 189 (1), 37–46. [DOI] [PubMed] [Google Scholar]
- (43).Townsend EA, Negus SS, Poklis JL, and Banks ML (2020) Lorcaserin maintenance fails to attenuate heroin vs. food choice in rhesus monkeys. Drug Alcohol Depend 208, 107848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Brandt L, Jones JD, Martinez S, Manubay JM, Mogali S, Ramey T, Levin FR, and Comer SD (2020) Effects of lorcaserin on oxycodone self-administration and subjective responses in participants with opioid use disorder. Drug Alcohol Depend 208, 107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Cornish KE, de Villiers SH, Pravetoni M, and Pentel PR (2013) Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS One 8 (12), No. e82557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).de Villiers SH, Cornish KE, Troska AJ, Pravetoni M, and Pentel PR (2013) Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine 31 (52), 6185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, and Pentel PR (2013) Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem 56 (3), 915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, and Pentel PR (2012) An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J. Pharmacol. Exp. Ther 341 (1), 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, and Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 234 (4), 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, and Bagley J (1990) Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology 73 (6), 1227–35. [DOI] [PubMed] [Google Scholar]


