Abstract
Eosinophils are typically associated with unique inflammatory settings, including allergic inflammation and helminth infections. However, new information suggests that eosinophils contribute more broadly to inflammatory responses and participate in local immune regulation and the tissue remodeling/repair events linked with a variety of diseases. Eosinophilic infiltration has long been a histologic hallmark of bullous pemphigoid (BP), a subepidermal autoimmune blistering disease characterized by autoantibodies directed against basement membrane protein BP180. However, the exact role of eosinophils in disease pathogenesis remains largely unknown. We show here that eosinophils are necessary for IgE autoantibody mediated BP blister formation in a humanized IgE receptor mouse model of BP. Disease severity is IgE dose dependent and correlates with the degree of eosinophil infiltration in the skin. Furthermore, IgE autoantibodies fail to induce BP in eosinophil-deficient mice, confirming that eosinophils are required for IgE mediated tissue injury. Thus, eosinophils provide the cellular link between IgE autoantibodies and skin blistering in this murine model of BP. These findings suggest a role for eosinophils in autoimmune disease and have important implications for the treatment of BP as well as other antibody mediated inflammatory and autoimmune diseases.
Keywords: IgE, eosinophils, bullous pemphigoid, autoimmune disease
INTRODUCTION
Eosinophil-mediated activities have generally been known to contribute to specific disease pathologies, most notably allergic conditions and parasitic infections (Liao et al., 2016; Rosenberg et al., 2013). However, the scope of eosinophil effector functions is expanding beyond these simple links with asthma and parasitic defense and now includes novel roles in local immune regulation and tissue remodeling and repair (Jacobsen et al., 2012; Lee et al., 2010). In this way, eosinophils represent an important cellular link between the innate and adaptive immune responses and have been shown to have more broad roles in diverse diseases such as inflammatory bowel disease, muscular dystrophy, and cancer, among others (Jacobsen et al., 2012). In cutaneous disease, peripheral eosinophilia and eosinophil infiltration of the skin are hallmarks of hypersensitivity reactions, allergic conditions, and some autoimmune blistering skin disorders suggesting a role for eosinophils in the pathogenesis of these diseases as well (Long et al., 2016).
Bullous pemphigoid (BP) is the most common antibody mediated autoimmune blistering disease of the skin. Clinically, the disease is characterized by tense bullae and urticarial type plaques. Histologically, lesions of BP show subepidermal clefting and a significant dermal infiltration of eosinophils and neutrophils (Lever, 1965). Peripheral eosinophilia is also a common finding present in over 50% of untreated patients (Bernard et al., 1987; Bushkell and Jordon, 1983; van Beek et al., 2016). Direct immunofluorescence studies typically reveal IgG and complement deposition along the basement membrane zone (dermal epidermal junction) (Jordon et al., 1975a; Jordon et al., 1975b); however, some patients show IgE deposition at the BMZ as well (Provost and Tomasi, 1974; Yayli et al., 2011). Circulating IgE directed against the BMZ has also been detected by indirect immunofluorescence (Parodi and Rebora, 1992; Soh et al., 1993).
Indeed, it has been well documented that BP is mediated by autoantibodies that recognize BMZ protein BP180 (also termed type XVII collagen), a transmembrane glycoprotein that is located in the hemidesmosome and critical for adhesion of the basal keratinocytes to the dermis (Diaz et al., 1990; Giudice et al., 1992; Nishizawa et al., 1993). The noncollagenous 16A (hNC16A) region of the BP180 ectodomain contains the major pathogenic epitopes recognized by autoantibodies from BP sera (Dresow et al., 2009; Giudice et al., 1993; Zillikens et al., 1997). As the hNC16A domain is poorly conserved between humans and mice, the development of the humanized hNC16A mice has allowed for a more precise understanding of how autoantibodies from BP patients induce disease in vivo (Liu et al., 1993; Liu et al., 2008). Passive transfer of BP IgG induces complement fixation, neutrophil infiltration, and blister formation in hNC16A mice, but not wildtype mice, confirming the importance of the hNC16A domain in pathogenicity of disease (Liu et al., 2008; Nishie et al., 2007). Most animal studies have focused on the pathogenicity of BP IgG autoantibodies and the role of neutrophil mediated tissue damage in BP pathogenesis, but have not demonstrated the classic eosinophil infiltration so commonly seen in BP patients. Thus, the role of eosinophils in disease remains poorly understood.
Several recent studies suggest a potential pathogenic role for IgE autoantibodies and a possible link between these IgE autoantibodies and eosinophil infiltrate seen histologically in BP (Fairley et al., 2007; Zone et al., 2007). Passive transfer of BP IgE to human skin grafted onto athymic, nude mice resulted in tissue infiltration of neutrophil, eosinophils and mast cells as well as a histologic subepidermal split. These studies have revealed that eosinophils may be involved in disease pathogenesis and are potentially related to IgE autoantibodies.
The goal of this study was to investigate the role of eosinophils and IgE autoantibodies in BP pathogenesis and the connection between them. We provide clear evidence that anti-hNC16A IgE purified from BP sera are pathogenic in hNC16A mice in an eosinophil-dependent manner. Thus, eosinophils represent the cellular link between IgE autoantibodies and BP blister formation. These findings firmly establish a role for eosinophils in human autoimmune disease and provide an animal model to further dissect specific eosinophil mediators of tissue injury and test new therapies.
RESULTS
Anti-hNC16A IgE bind to hNC16A of BP180 but do not induce BP in neonatal hNC16A mice
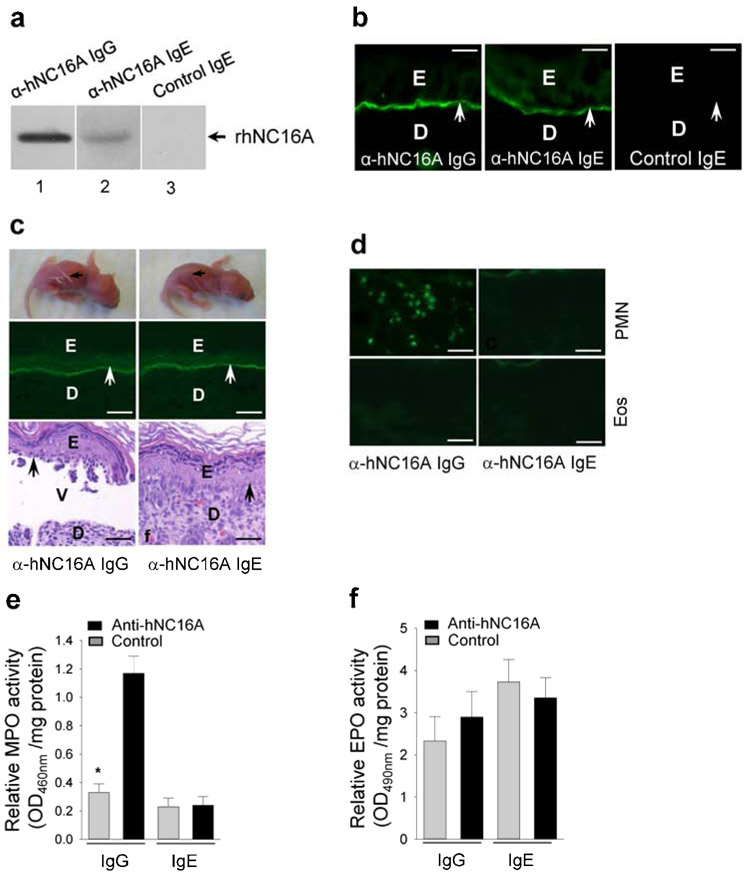
Like purified anti-hNC16A IgG, purified anti-hNC16A IgE, but not control IgE, recognized recombinant hNC16A by immunoblotting (Figure 1a, lane 2) and stained the basement membrane zone (BMZ) of hNC16A mouse skin sections by indirect immunofluorescence (Figure 1b). To determine whether anti-hNC16A IgE are able to bind hNC16A in vivo and trigger skin disease, anti-hNC16A IgE at a pathologically relevant dose (100ng/g body weight) was injected intradermally to neonatal hNC16A mice. While mice injected with anti-hNC16A IgG developed blister formation both clinically and histologically with IgG deposition at the BMZ (Figure 1c), those injected with anti-hNC16A IgE showed IgE deposition at the BMZ, but did not develop blisters (Figure 1c). Immunostaining identified infiltrating neutrophils only in the skin of anti-hNC16A IgG-injected mice (Figure 1d) and no infiltrating eosinophils in the skin of mice injected with either anti-hNC16A IgG or IgE (Figure 1d). MPO (neutrophil cell marker) enzymatic assays confirmed significantly increased neutrophil infiltration in mice injected with anti-NC16A IgG but not anti-hNC16A IgE (Figure 1e). Neither anti-hNC16A IgG nor anti-hNC16A IgE induced eosinophil infiltration as determined by EPO (eosinophil cell marker) enzymatic assay (Figure 1f). Mice injected with anti-hNC16A IgE up to 500ng/g body weight still failed to develop skin disease, ruling out the possibility that the dose of 100ng IgE/g body weight was below the threshold for pathogenicity. These results demonstrate that anti-hNC16A IgE autoantibodies are not pathogenic in neonatal hNC16A mice.
Figure 1. Anti-hNC16A IgE do not induce BP in neonatal hNC16A mice.
Anti-hNC16A IgE recognized recombinant hNC16A by immunoblotting (a, lane 2), stained the BMZ of hNC16A mouse skin sections by indirect IF (b), but failed to induce BP clinically and histologically in neonatal hNC16A mice at 48 hours post i.d. injection (c). (d) Immunostaining identified only neutrophils (PMN) in anti-hNC16A IgG-injected mouse skin; but no eosinophils were present in the skin of all anti-hNC16A antibody-injected mice. MPO and EPO enzymatic assays revealed significantly increased PMN in the anti-NC16A IgG-injected skin (e) but no eosinophil infiltration in the both anti-NC16A IgG- and IgE-injected skin 48 h post injection (f). Scale bars = 50 μm for panel b, scale bars = 100 μm for panels c, d. *p<0.05, n=6. E, epidermis; D, dermis; V, vesicle. Arrow, BMZ.
Anti-hNC16A IgE induce eosinophil infiltration but do not induce BP in adult hNC16A mice
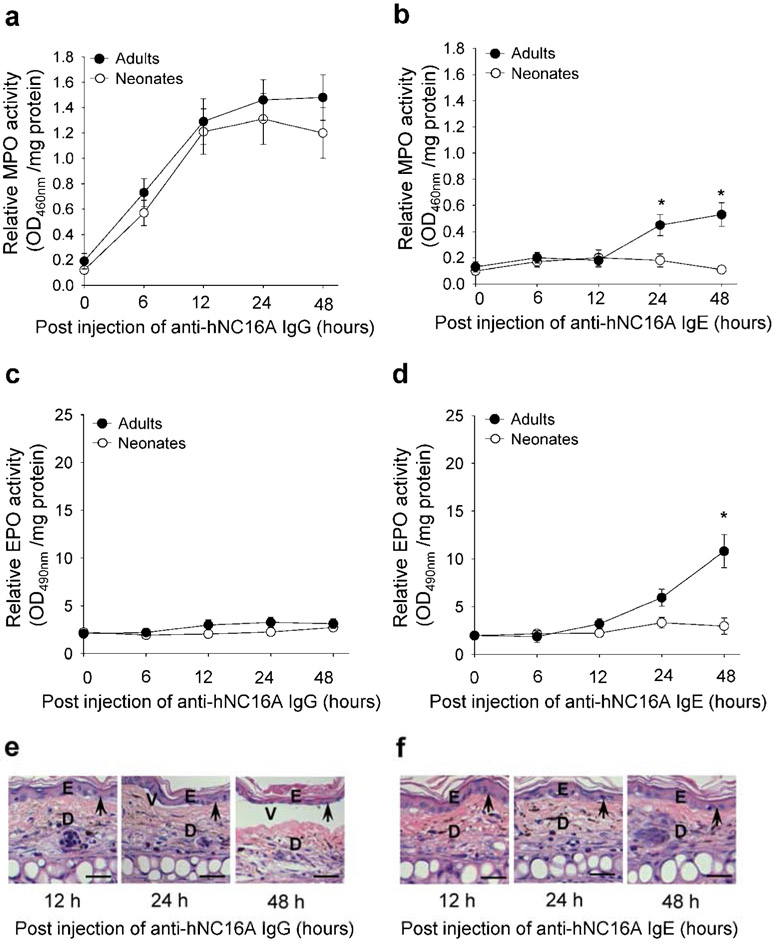
Though the classic experimental animal model for the study of BP involves passive transfer to neonatal hNC16A mice, it is possible that eosinophil migration differs between neonatal and adult mice. Thus, adult hNC16A mice were injected with anti-hNC16A IgG and IgE intradermally in the ear pinna and examined for infiltration of eosinophils and neutrophils and subepidermal blistering at 0-48 h post injection. Anti-hNC16A IgG induced similar degree of neutrophil infiltration in neonatal and adult mice (Figure 2a), but did not induce eosinophil infiltration in either adult or neonatal mice (Figure 2c). Anti-hNC16A IgG induced typical dermal-epidermal junction separation in adult hNC16A mice (Figure 2e), similar to the neonatal mouse model. As expected, passive transfer of anti-hNC16A IgE did not induce neutrophil or eosinophil infiltration in neonatal mice (Figure 2b and 2d). In adult hNC16A mice, anti-hNC16A IgE induced a low but statistically significant increase in neutrophil infiltration at the 24 and 48 hour post injection time points (Figure 2b). However, anti-hNC16A IgE induced markedly increased eosinophil infiltration in adult hNC16A mice at the 24 hour post injection time point and even more significantly at the 48 hour time point (Figure 2d). ELISA assay revealed that NC16A specific IgE level was 907 index units in the injected hNC16A mice. Despite anti-hNC16A IgE induced eosinophil infiltration in the tissue of adult hNC16A mice, no clinical or histologic evidence of blister formation was detected (Figure 2f).
Figure 2. Anti-hNC16A IgE induce Eos infiltration but do not induce BP in adult hNC16A mice.
Adult (8 week old) hNC16A mice were injected in the ear pinna and neonatal (24-36 h old) hNC16A mice were injected i.d. at dorsal back with anti-hNC16A IgE (100 ng/site) or anti-hNC16A IgG (100 μg/g body weight). Anti-hNC16A IgG induced infiltration of neutrophils (a) but not eosinophils (c) in both neonatal and adult mice and also triggered derma-epidermal separation in the IgG-injected ear (e). Anti-hNC16A IgE induced no neutrophil nor eosinophil infiltration in neonatal mice but increased neutrophil and eosinophil infiltration in adult mice at 24 and 48 time points (b, d). However, anti-hNC16A IgE-injected ear skin showed no derma-epidermal separation (f). Scale bars = 100 μm. 2-Way ANOVA, *p<0.01, n=6 for each group.
Anti-hNC16A IgE induce BP in adult double humanized hFcεRI/hNC16A mice
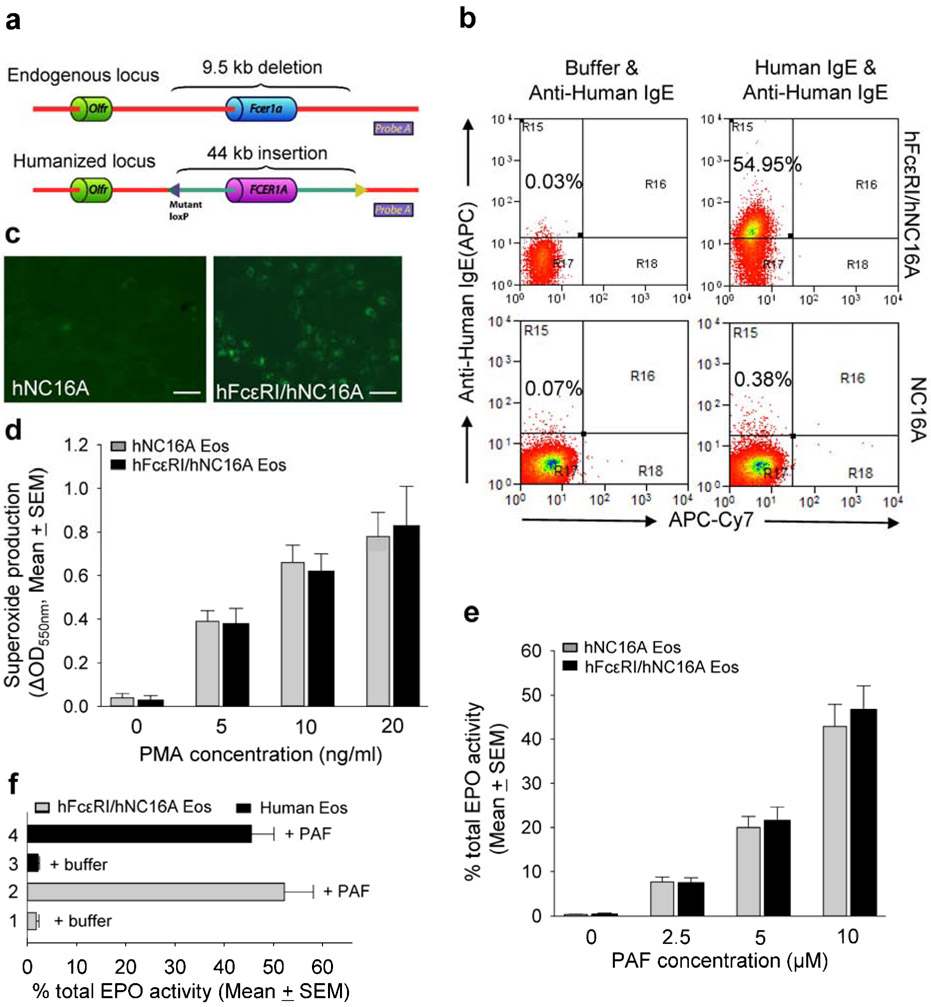
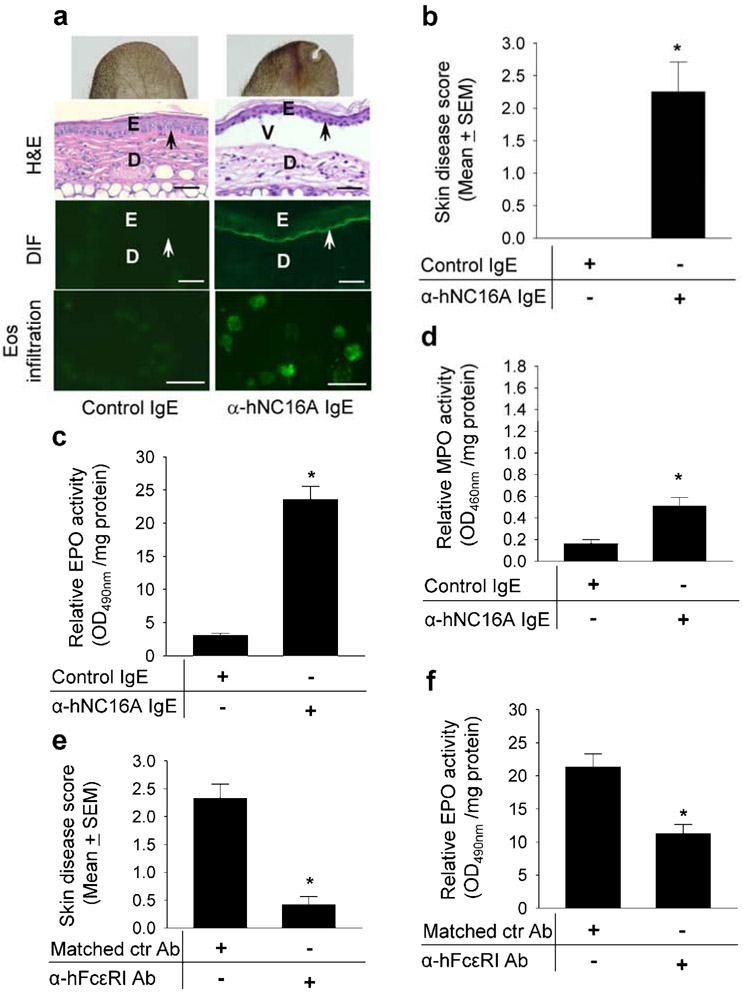
Anti-hNC16A IgE trigger eosinophil infiltration and fail to induce subepidermal blistering in hNC16A adult mice. Therefore, we hypothesized that the human high-affinity IgE receptor FcεRI on eosinophils is required for pathogenicity of anti-hNC16A IgE in hNC16A mice. To address this issue, we generated humanized hFcεRI mice, in which the mouse Fcer1a locus was replaced with the syntenic human FCER1A locus driven under its own promoter elements (Figure 3a). The hFcεRI mice were then crossed with hNC16A mice to generate double humanized hFcεRI/hNC16A strain. Eosinophils from hFcεRI/hNC16A mice expressed hFcεRI by flow cytometry (Figure 3b) and indirect immunofluorescence (Figure 3c) and remained functional. Their ability to undergo oxidative burst as measured by superoxide production in response to PMA stimulation (Figure 3d) and to degranulate as measured by EPO release in response to PAF stimulation (Figure 3e) was comparable to eosinophils from hNC16A mice. Similarly, human eosinophils show the same degree of degranulation as hFcεRI/hNC16A eosinophils in response to PAF stimulation (Figure 3f). More importantly, passive transfer of anti-hNC16A IgE into the ear pinna of adult hFcεRI/hNC16A mice induced a subepidermal split (Figure 4a) accompanied by IgE deposition at the BMZ by DIF (Figure 4a) and infiltration of eosinophils by immunostaining with anti-mouse MBP antibody (Figure 4a). ELISA assay revealed that NC16A specific IgE level was 712 index units in the injected hNC16A mice. Skin disease activity (Figure 4b) and dermal infiltration of eosinophils as measured by EPO activity assay (Figure 4c) were significantly higher in hFcεRI/hNC16A mice that received anti-hNC16A IgE compared to control IgE. MPO activity assay revealed an elevated dermal infiltration of neutrophils in hFcεRI/hNC16A mice that received anti-hNC16A IgE compared to control IgE (Figure 4d), which was similar to anti-hNC16A IgE-injected adult hNC16A mice (Figure 2b) but much lower than that seen in anti-hNC16A IgG-injected hNC16A mice (Figure 2a). These results suggest that anti-hNC16A IgE-induced BP in adult hFcεRI/hNC16A mice requires hFcεRI-expressing eosinophils.
Figure 3. Eosinophils in hFcεRI/hNC16A mice express hFcεRI and can be activated in vitro.
Homologous recombination is used to generate a 9.5 kb deletion of mouse Fcer1a, replaced with the corresponding 44 kb human FCER1A driven under its own promoter elements. Probe A indicates the location of the DNA probe to verify the humanization of the Fcer1a locus (a). Purified Eos from hFcεRI/hNC16A but not hNC16A mice expressed hFcεRI as determined by incubating with human IgE followed by flow cytometry using APC-conjugated anti-human IgE with APC-Cy7 used as a display channel (b) and by indirect IF (c). Eos (5x105 cells/ml) from hNC16A (gray bar) and hFcεRI/hNC16A mice (black bar) were stimulated with PMA for superoxide production measured by reduction of cytochrome c (oxidative burst assay) or stimulated with PAF for Eos degranulation (EPO release assay). 0.1% DMSO treatment was control. Eos from NC16A and hFcεRI/hNC16A mice show comparable superoxide production (d) and EPO release (e) in response to stimulation with PMA and PAF, respectively. Purified human eosinophils show similar degree of EPO release in response to stimulation with PAF (f). Scale bars = 100 μm. n=6 for each group.
Figure 4. Anti-hNC16A IgE induce BP in adult hFcεRI/hNC16A mice.
Eight week old hFcεRI/hNC16A mice were injected at ear pinna with anti-hNC16A IgE or control IgE (100 ng/g body weight) and examined 48 h post injection. (a) Anti-hNC16A IgE-injected mice showed increased erythema and developed dermal-epidermal separation associated with human IgE deposition at the BMZ by direct IF (DIF) and skin infiltrating Eos using antibody specific for the Eos marker major basic protein MBP. (b) Anti-hNC16A IgE-injected mice developed more severe disease than control IgE-injected mice. EPO and MPO activity assays showed significantly increased infiltrating Eos (c) and PMN (d) in the lesional skin of anti-hNC16A IgE-injected mice (bar 2) compared to control group (bar 1). Human FcεRI blockade (5 μg/ear) significantly reduced disease severity (e) and eosinophil infiltration (f) in hFcεRI/hNC16A mice injected with anti-NC16A IgE. Scale bars = 100 μm for panel a (DIF), scale bars = 25 μm for panel a (eos infiltration). *p<0.01, n=9 for each group.
We further confirm that hFcεRI is required in anti-NC16A IgE induced BP in hFcεRI/hNC16A mice by treating the mice with hFcεRI neutralizing antibody. hFcεRI blockade significantly reduced BP disease activity triggered by pathogenic anti-NC16A IgE accompanied with reduced eosinophil infiltration (Figure 4e, 4f).
Anti-hNC16A IgE induced BP in hFcεRI/hNC16A mice is independent of neutrophils
Anti-hNC16A IgE induce predominant eosinophil infiltration with a small, but significant increase in neutrophil infiltrate in hFcεRI/hNC16A mice. To rule out the possibility that this small amount of neutrophil infiltration was contributing to anti-hNC16A IgE induced disease, adult hFcεRI/hNC16A mice were pretreated with neutrophil depleting antibody or isotype control followed by ear pinna injection of anti-hNC16A IgE. At 48 hours post IgE transfer, hFcεRI/hNC16A mice pretreated with neutrophil depleting antibody developed similar subepidermal split (Supplementary Figure S1a) and disease activity to those pretreated with isotype control antibody or mice that were not pretreated (Supplementary Figure S1b). These results demonstrate that eosinophils and not neutrophils are required for anti-hNC16A IgE-induced BP in hFcεRI/hNC16A mice.
Disease severity of anti-hNC16A IgE induced BP in adult hFcεRI/hNC16A mice is dose dependent and correlates with the degree of eosinophil infiltration
If anti-hNC16A IgE are pathogenic, BP disease activity should correlate to anti-hNC16A IgE levels. To address this hypothesis, adult hFcεRI/hNC16A mice were treated with different doses of anti-hNC16A IgE injected into the ear pinna. As expected, there was a direct correlation between disease severity (disease score) and anti-hNC16A IgE dose (Figure 5a). Furthermore, eosinophil infiltration also correlated with higher anti-hNC16A IgE dose (Figure 5b). These results suggest that anti-hNC16A IgE mediated disease pathogenesis is directly related to levels of anti-hNC16A IgE and eosinophil infiltration.
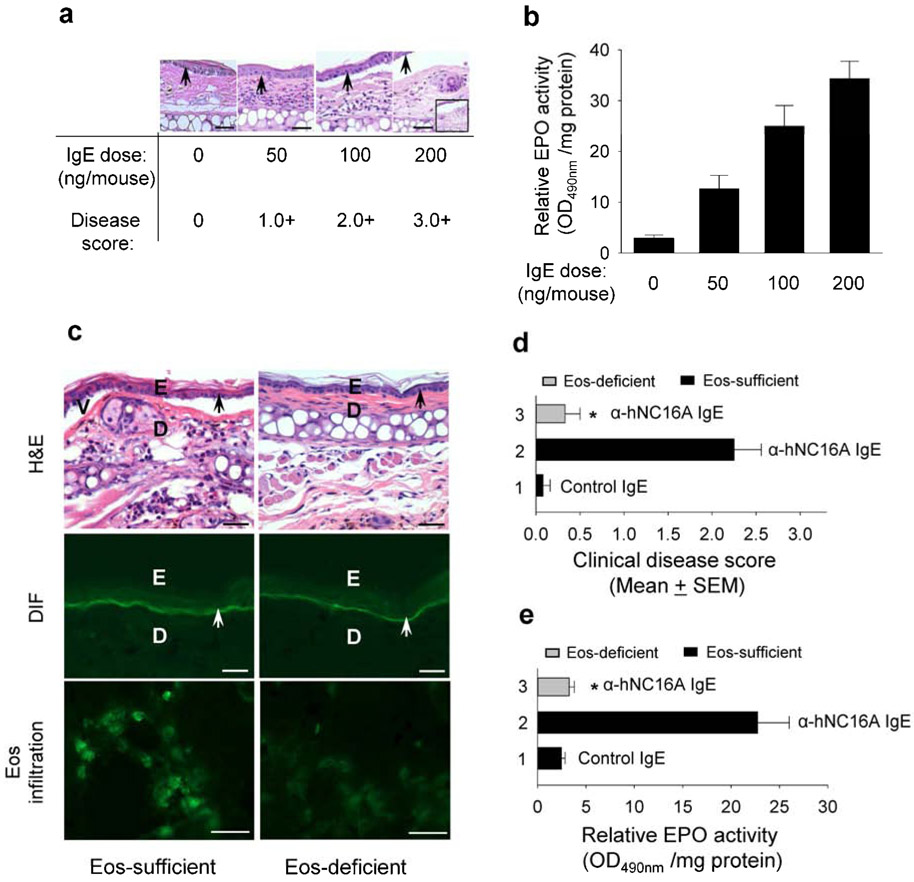
Figure 5. Anti-hNC16A IgE induce BP in a dose-dependent fashion and fail to induce BP in eosinophil-deficient mice.
Eight week old hFcεRI/hNC16A (Eos-sufficient) and eosinophil-deficient hFcεRI/hNC16A (Eos-deficient) mice were injected at the ear pinna with anti-hNC16A IgE or control IgE (0-200 ng/ear for a, b and 100 ng/g body weight for c-d) and examined 48 h post injection. (a) IgE dosing and disease scoring. H/E staining revealed anti-hNC16A IgE dose-dependent separation between epidermis and dermis. Inset shows a lower magnification image of dermal-epidermal separation. (b) Eos infiltration. Eosinophil peroxidase (EPO) activity assay shows direct correlation between level of infiltration Eos and amount of injected anti-hNC16A IgE. (c) Anti-hNC16A IgE-injected eosinophil-sufficient mice and not eosinophil-deficient mice developed dermal-epidermal separation associated with human IgE deposition at the BMZ by direct IF and skin infiltrating Eos by indirect IF using anti-MBP antibody. Anti-hNC16A IgE-injected eosinophil-sufficient mice developed more severe skin disease (d) and significantly increased Eos infiltration (e) than eosinophil-deficient mice compared to eosinophil-deficient mice (bar 3). Scale bars = 100 μm for panel c (H&E, DIF), scale bars = 25 μm for panel c (Eos infiltration). *p<0.01, bar 2 vs. bar 3, n=8 for each group.
Anti-hNC16A IgE fail to induce BP in eosinophil-deficient mice
To further confirm our hypothesis that anti-hNC16AIgE induced BP is directly dependent on infiltrating eosinophils, adult eosinophil deficient hFcεRI/hNC16A mice were injected with anti-hNC16A IgE or control IgE and examined 48 hours post IgE injection. While both eosinophil sufficient hFcεRI/hNC16A mice and eosinophil deficient hFcεRI/hNC16A mice (i.e., ΔdblGATA/hFcεRI/hNC16A) showed IgE deposition at the BMZ following passive transfer (Figure 5c), only eosinophil sufficient hFcεRI/hNC16A mice showed subepidermal clefting following passive transfer of anti-hNC16A IgE (Figure 5c), thus confirming that eosinophils are required for anti-hNC16A IgE mediated pathogenesis. As expected, infiltrating eosinophils were seen in the skin of eosinophil sufficient but not eosinophil deficient hFcεRI/hNC16A mice by immune staining with anti-mouse MBP antibody (Figure 5c). Anti-hNC16A IgE injected eosinophil sufficient hFcεRI/hNC16A mice exhibit significantly higher skin disease activity (Figure 5d) and eosinophil infiltration compared to eosinophil deficient hFcεRI/hNC16A mice (Figure 5e).
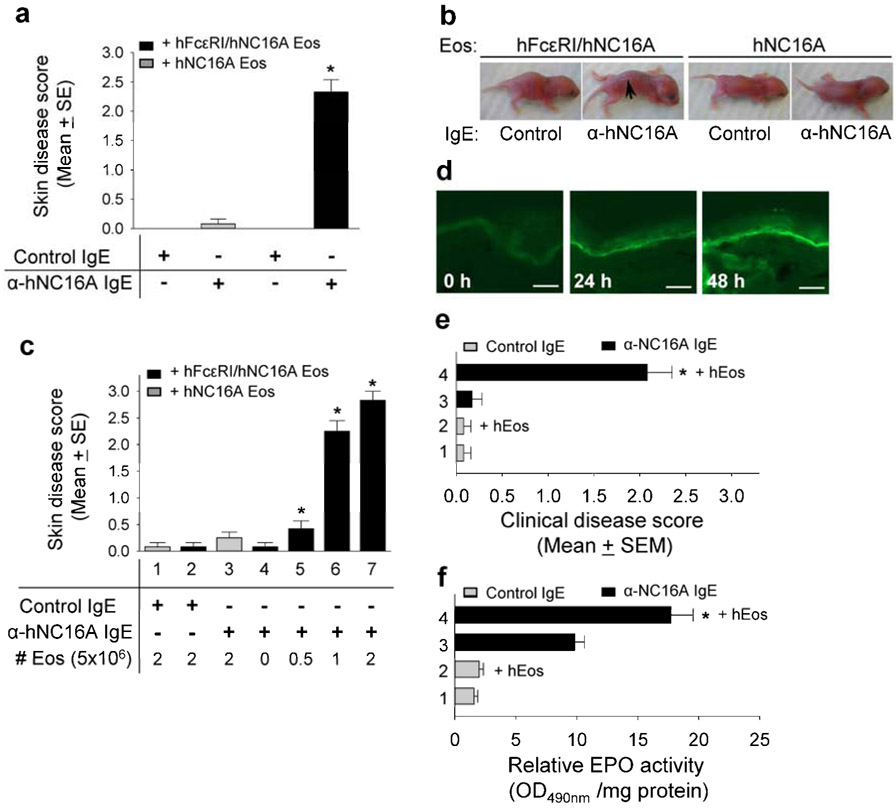
Reconstitution of hFcεRI-expressing eosinophils restores BP in hNC16A mice
Our results suggested that both expression of hNC16A in basal keratinocytes and eosinophils expressing hFcεRI are required for anti-hNC16A IgE mediated BP. Thus, reconstitution of NC16A mice with eosinophils expressing hFcεRI should allow for BP development following passive transfer with anti-hNC16A IgE. As shown in Figure 6a, adult hNC16A mice reconstituted intravenously with hFcεRI/hNC16A eosinophils had significantly higher skin disease scores following transfer of anti-hNC16A IgE compared to those receiving control IgE and those reconstituted with eosinophils from hNC16A mice. Similarly, neonatal NC16A mice developed clinical blisters after local intradermal reconstitution with hFcεRI/hNC16A eosinophils and passive transfer of anti-hNC16A IgE compared to neonatal hNC16A mice reconstituted with hNC16A eosinophils or mice receiving control IgE (Figure 6b). The severity of disease in neonatal hNC16A mice following passive transfer of anti-hNC16A IgE directly correlated to the number of hFcεRI/hNC16A eosinophils provided during local reconstitution (Figure 6c). To rule out the possibility that local injection (i.d.) of anti-NC16A IgE and/or mouse eosinophils artificially causes tissue injury resembling BP, adult hNC16A mice were reconstituted intravenously with human eosinophils and injected i.p. with anti-NC16A IgE. The hFcεRI/hNC16A with systemic reconstitution of human eosinophils and injection of pathogenic IgE exhibited deposition of human IgE at the BMZ (Figure 6d), severe BP disease (Figure 6e) and significantly increased eosinophil infiltration (Figure 6f). Taken together, these results demonstrate that hFcεRI-expressing eosinophils are necessary and sufficient for anti-hNC16A IgE induced BP in hNC16A mice.
Figure 6. Reconstitution of hFcεRI-expressing eosinophils restores BP in hNC16A mice.
Adult hNC16A mice were injected i.v. with 5x106 Eos from hNC16A or hFcεRI/hNC16A mice and then injected at ear with anti-hNC16A IgE or control IgE (100 ng/g body weight). Neonatal hNC16A mice were injected i.d. at dorsal back with anti-hNC16A IgE or anti-hNC16A IgE (100 ng/g body weight) plus 0-2.5x106 Eos from hNC16A or hFcεRI/hNC16A mice. The mice were examined 48 h post IgE injection. Only mice reconstituted with hFcεRI-expressing Eos and injected with anti-hNC16A IgE developed BP (a, bar 4; b) and their disease activity correlated with numbers of reconstituted hFcεRI-expressing Eos (c, bars 2-7). Adult hNC16A mice were injected i.v. with 5x106 human Eos and then injected i.p. with anti-hNC16A IgE or control IgE (250 ng/g body weight). The mice were examined at ear site 48 h post IgE injection. Mice reconstituted with human eosinophils plus anti-NC16A IgE showed human IgE deposition at the BMZ by direct IF (d), dermal-epidermal separation (e) and significantly increased eosinophil infiltration (f). Scale bars = 100 μm for panel d. *p<0.01 (bar 4 vs. bar 3 in panel f). Arrow, blister site.
DISCUSSION
Our findings show that anti-hNC16A IgE purified from BP patients are pathogenic in mice expressing human hNC16A and human FcεRI as evidenced by a subepidermal split accompanied by IgE deposition at the BMZ and eosinophil infiltration. Disease severity of anti-hNC16A IgE induced BP is dose dependent and correlates with the degree of eosinophil infiltration. In this animal model, BP anti-hNC16A IgE induced blister formation requires eosinophils and occurs independently of neutrophils. Thus, this study establishes that our mouse model of IgE induced BP requires both hNC16A expression and infiltrating eosinophils that express hFcεRI.
Eosinophil infiltration has long been a hallmark of BP histologically and eosinophils have been speculated to be pathogenically relevant for more than half a century (Charles, 1960; Dvorak et al., 1982; Schaumburg-Lever et al., 1972). However, the role of eosinophils in disease pathogenesis has not been established primarily due to lack of an appropriate model system. While animal models of disease may not reproduce all mechanisms of human disease, they do provide insight into pathogenesis and allow for the dissection of multiple mechanisms that may contribute to complex diseases such as BP. This in vivo study clearly links eosinophils to BP disease pathophysiology using double humanized mice that express both human BP180 region hNC16A and the human FcεRI. The presence of hFcεRI on eosinophils from BP patients has recently been described (Messingham et al., 2014; Tanaka et al., 1995) and activated eosinophils are present in BP lesional skin (Engmann et al., 2017). Our results corroborate the importance of hFcεRI in eosinophil mediated tissue pathology. These findings also confirm that IgE autoantibodies are pathogenic in BP. Binding of anti-hNC16A IgE autoantibodies to basal keratinocytes leads to eosinophil infiltration, and molecular interactions between IgE autoantibodies and hFcεRI on infiltrating eosinophils appears to be pivotal in BP blister formation. This study provides a long awaited mechanism by which eosinophils may be recruited to the BMZ and explain the development of the histopathology characteristic of BP (i.e., eosinophil infiltration and BMZ separation). It is possible that the pathogenic activity of anti-NC16A IgE-eosinophils may involve other cells and secreted factors. Currently, we are dissecting the exact functional interplay between anti-NC16A IgE, high affinity IgE receptor, eosinophils and mast cells, and the relative contributions of eosinophils vs. mast cells in this model setting.
In our animal model, infiltrating eosinophils are located in the dermis. In human BP, the presence of eosinophils along the dermal epidermal junction is considered to be a diagnostic clue for bullous pemphigoid. Such an observation, however, is neither a diagnostic requirement, nor a consistent finding. Eosinophils at the BMZ are seen in a minority of cases. More frequently, the eosinophils are located around capillaries and dispersed in the interstitial papillary and reticular dermis. It is well documented that the number and distribution of eosinophils are variable. Variations in the number and types of inflammatory cells in skin biopsies of lesional BP may correlate with the target antigen and type of autoantibodies. The lack of eosinophils abutting the BMZ in our animal model could be the result of sample selection, the short time course (acute nature) of the disease in our model system, or possibly the fact that eosinophil mediated tissue destruction does not require direct contact with the BMZ and instead occurs through a mechanism not yet identified.
There are no or very low levels of expression of FcεRI on naïve/resting eosinophils under normal/physiological conditions (de Andres et al., 1997). However, expression of FcεRI on eosinophils is upregulated under certain pathological conditions (e.g. inflammation) (Kayaba et al., 2001). In this study, eosinophils of hFcεRI/NC16A mice with human FCER1A driven under its own promoter express hFcεRI, providing a potential molecular mechanism underlying subepidermal blistering caused by anti-NC16A IgE and hFcεRI-expressing eosinophils.
We clearly demonstrate that anti-NC16A IgE and eosinophils in concert are sufficient to induce subepidermal blister formation in our murine model system. It has recently been shown that BP antibodies may also induce blister formation in the presence of activated eosinophils in an alternative model system (de Graauw et al., 2017). Though the individual contributions of BP IgG and IgE were not separated in this study, blister formation was inhibited by blocking FcγR raising the possibility that BP IgG may also play a role in eosinophil mediated blister formation. Our data demonstrate that anti-NC16A IgG does not significantly induce eosinophil recruitment or activation compared to anti-NC16A IgE. However, how anti-NC16A IgE and eosinophils intersect with anti-NC16A IgG and infiltrating neutrophils in human BP is unclear. Sophisticated animal models such as ours are necessary to dissect the multifactorial and complicated disease machinery involved in BP pathogenesis.
The potential role of eosinophils in human disease has expanded in recent years (Jacobsen et al., 2012; Lee et al., 2010) and while eosinophils have historically been associated with allergic inflammation and parasitic infections, these granulocytes may play an important role in immune regulation and the tissue remodeling and repair associated with both health and disease (Jacobsen et al., 2012). These roles for eosinophils are manifested in a diverse group of diseases such as eosinophilic esophagitis (Blanchard et al., 2011; Mishra et al., 2008), inflammatory bowel disease (Forbes et al., 2004; Lampinen et al., 2008; Takedatsu et al., 2004; Vieira et al., 2009), and even cancer (Lotfi et al., 2007; Samoszuk, 1997). Here we report the description of eosinophils as key players in human autoimmune disease, thus supporting another role for eosinophils outside of the classic allergy and parasitic infection.
There are numerous clinical implications that result from the understanding that eosinophils may be directly involved in mediating tissue destruction in BP. It has previously been described that eosinophilia correlates with disease severity (Bushkell and Jordon, 1983; Yu et al., 2014). This information coupled with evidence of direct contribution to tissue injury suggests that eosinophils may be useful as a biomarker for disease activity and/or treatment success or failure. In addition, eosinophils may be a target for new therapeutics in BP. Omalizumab shows efficacy in the treatment of BP and is associated with a decrease in tissue eosinophilia, supporting the use of this therapeutic strategy (Fairley et al., 2009; Yu et al., 2014). New therapies that specifically target eosinophils are likely to be beneficial as well (Radonjic-Hoesli et al., 2015). Furthermore, the double humanized hFcεRI/hNC16A mouse model will allow for direct manipulation, drug development and testing in a clinically relevant in vivo model.
In summary, these data provide direct evidence for eosinophils as a pathogenic mediator of human autoimmune disease using the autoimmune blistering skin disease BP as a model. In addition to expanding the current understanding of eosinophil biology, our study highlights eosinophils as a target for the treatment of BP and establishes a model to systematically dissect the role of eosinophils in the immunopathogenesis of BP.
MATERIALS AND METHODS
Patients, sera, and antibody purification
Serum samples were collected from three patients with active BP (BP1, BP2, and BP3). These patients presented with generalized tense blisters and dermal-epidermal separation with inflammatory cell infiltration by routine histology. Direct IF showed deposition of IgG at the BMZ of perilesional skin. Indirect IF showed “roof staining” of salt-split human skin cryosections with IgG titer of 1:640 (BP1) and 1:320 (BP2, BP3). NC16A-specific IgE levels were 292 (BP1), 127 (BP2) and 631 (BP3) index units determined by ELISA as described (Messingham et al., 2009). hNC16A-specific total IgG were purified from BP patient sera using a protein G column, followed by an hNC16A-specific glutathione sepharose column as described (Liu et al., 2008). hNC16A-specific IgE were purified from BP patients’ sera using a protocol described previously with modification (Fairley et al., 2007). Briefly, IgG-depleted fractions of BP sera (by a protein G column) were loaded onto an anti-human IgE antibody (ATCC, cat#HB-235)-coupled Affigel-10 affinity column (Bio-Rad, Hercules, CA). The eluted IgE fractions were then loaded onto an NC16A-specific glutathione sepharose column. The concentrations of purified IgG and IgE were quantified by human IgG- and IgE-specific ELISA (Southern Biotechnology). The purity of hNC16A-specific IgG and hNC16A-specific IgE were determined by amount of hNC16A-specific IgG or IgE in total amount of protein in the antibody preparations. The purity of hNC16A-specific IgG and IgE were 94% and 92%, respectively. Purified anti-hNC16A IgG and IgE fractions were concentrated by ultrafiltration (Millipore) and used for in vitro and in vivo experiments.
Mice and antibody passive transfer
The humanized hNC16A, humanized FcεRI, hFcεRI/hNC16A, eosinophil-deficient hNC16A mice were generated as described (Liu et al., 2008) and in Supplemental Section. For antibody passive transfer in neonatal mice (24-48 h old), hNC16A-specific IgG (100 μg/g body weight) or IgE (100-500 ng/g body weight) in 50μl of PBS was injected intradermally into the dorsal back (Liu et al., 2008). For adult mice (8 weeks old), hNC16A-specific IgG (100 μg/g body weight) or IgE (0-200 ng/g body weight) in 25μl of PBS was injected into the ear (Chen et al., 2001). The antibody-injected skin was examined 0-48h post injection. The disease activity was scored as “- “ to “3+” as described in Supplemental Section. After clinical examination, the animals were sacrificed, and skin and serum specimens were obtained. The skin sections were used for H/E staining to determine histologic evidence of subepidermal separation. Deposition of anti-hNC16A IgG and IgE at the BMZ was detected by direct immunofluorescence (IF) using FITCconjugated anti-human IgG (Thermo Fisher Scientific cat# 62-8411) and IgE antibodies (Thermo Fisher Scientific cat# H15801). Skin infiltrating eosinophils was detected by indirect IF using anti-mouse major basic protein (MBP) monoclonal antibody (provided by Dr. J. Lee, Mayo Clinic Arizona), followed by Alexa Fluor 488-conjugated goat anti-rat antibody (Life Technologies, cat# a11006).
Quantification of infiltrating neutrophils and eosinophils
Infiltrating neutrophils in the antibody-injected skin were quantified by measuring tissue MPO activity as described (Bradley et al., 1982; Liu et al., 1997; Liu et al., 2008) using purified MPO as standard. MPO content was expressed as relative MPO activity (OD460nm reading/mg protein of the mouse skin injected with pathogenic antibodies minus OD460nm reading/mg protein of the mouse skin injected with control antibodies). Similarly, infiltrating eosinophils in the skin were quantified using the eosinophil peroxidase (EPO) activity assay (Schneider and Issekutz, 1996). Briefly, serial dilutions of skin protein extract were incubated with the substrate OPD at room temperature. The reactions were stopped by adding 4 N H2SO4 and read at 490 nm. EPO content was expressed as relative EPO activity (OD490nm reading/mg protein of the mouse skin injected with pathogenic antibodies minus OD490nm reading/mg protein of the mouse skin injected with control antibodies). Protein concentrations were determined by the Bio-Rad dye-binding assay using BSA as a standard.
Purification of mouse eosinophils and human eosinophil culture
Eosinophils were purified from the peripheral blood of hNC16A and hFcεRI/hNC16A mice using the MACS cell separation system (Miltenyi Biotec, Auburn, CA) (Li et al., 2009). Eosinophils were also isolated from peritoneal cavity of hNC16A and hFcεRI/hNC16A mice injected i,p. with 1ml of 4% thioglycollate broth for 5 days using Chemicon’s Eosinophil Isolation Kit (EMD Millipore, Temecula, CA). Purity of eosinophils by MACS system and Eosinophil Isolation Kit were >96% (median=94) and >89% (median=87), respectively. Expression of hFcεRI on the surface of purified eosinophils was confirmed by flow cytometry (Beckman Coulter Cyan ADP). Briefly, the cells were incubated with human IgE (BD Bioscience, cat# Ab65866), followed by staining with APC-conjugated anti-human IgE antibody (BioLegend, cat# 325508, clone MHE-18). The flow data was displayed by APC against APC-Cy7 (nothing labeled in this color, just for visualization purpose). Human eosinophil cell line HL60 (ATCC, HL-60 clone 15) was derived from a leukemia cell line and has been used as a cell culture for human eosinophil research (Fischkoff et al., 1984). HL60 cells were maintained in RPMI 1640 media (Gibco), supplemented with 10% FBS (Sigma), and eosinophilic differentiation was induced by treating HC15 cells with 0.5 μM butyric acid (Sigma) for 5 days (Fischkoff et al., 1984).
In vitro eosinophil activation
Eosinophil activation in vitro was determined by eosinophil superoxide production (oxidative burst) and degranulation (EPO release) as described in Supplemental Section.
Neutrophil depletion studies
To deplete neutrophils, adult hFcεRI/hNC16A mice were pretreated with i.p. injection of rat anti-mouse Ly6G antibody or match control antibody (BioLegend, cat#127601) at a dose of 150μg per mouse and 12 h later injected at ear with anti-hNC16A IgE or control IgE (Liu et al., 2008). Neutrophil levels in circulation were monitored by direct cell counting of blood smears stained with Wright dye (Baxter Diagnostics Inc., McGaw Park, IL).
Reconstitution of hFcεRI-expressing eosinophils in hNC16A mice
Adult hNC16A mice were injected i.v. with 5x106 Eos from hNC16A or hFcεRI/hNC16A mice and 30min later injected at ear with anti-hNC16A IgE or control IgE (100ng/g body weight). Adult hNC16A mice were injected i.v. with 5x106 human Eos and 30min later injected i.p. with anti-hNC16A IgE or control IgE (250ng/g body weight). Neonatal hNC16A mice were injected i.d. at dorsal back with anti-hNC16A IgE (100ng/g body weight) or anti-hNC16A IgG (100μg/g body weight) plus 2.5x106 Eos from hNC16A or hFcεRI/hNC16A mice and examined at 48h post IgE injection.
Statistics
The data are expressed as mean ± SEM and were analyzed using the Student's t-test or 2-Way ANOVA. A p value less than 0.05 was considered significant.
Study approval
Animal care and animal experiments were in accordance with the Animal Care Committee at the University of North Carolina-Chapel Hill. Written informed consent was received from participants prior to inclusion in the study. This study was approved by the University of North Carolina Institutional Review Board.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Daniel Zedek for his assistance with histology. This study was supported by NIH grants AI07924 and AI40768 (ZL), AR06372 (NL), R21AI88628 (BHK), AR32599 (LAD), RAR061567A (JJL), National Natural Science Foundation of Science 81301370 (LL) and the Dermatology Foundation (DAC). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. The authors have no conflicting financial interests.
We dedicate this work to our co-author, Dr. James J. Lee, who passed away March 25, 2017. As an expert in eosinophil biology, Dr. Lee’s contribution and collaboration in this body of work, and countless others, was substantial.
Abbreviations:
- BP
bullous pemphigoid
- BMZ
basement membrane zone
- hNC16A
human BP180 noncollagenous 16A domain
- MPO
myeloperoxidase
- EPO
eosinophil peroxidase
Footnotes
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernard P, Venot J, Constant F, Bonnetblanc JM. Blood eosinophilia as a severity marker for bullous pemphigoid. J Am Acad Dermatol 1987;16:879–81. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol 2011;127:208–17, 17 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982;78:206–9. [DOI] [PubMed] [Google Scholar]
- Bushkell LL, Jordon RE. Bullous pemphigoid: a cause of peripheral blood eosinophilia. J Am Acad Dermatol 1983;8:648–51. [DOI] [PubMed] [Google Scholar]
- Charles A Electron microscopic observations on pemphigoid. Br J Dermatol 1960;72:439–47. [DOI] [PubMed] [Google Scholar]
- Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest 2001;108:1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, et al. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood 1997;89:3826–36. [PubMed] [Google Scholar]
- de Graauw E, Sitaru C, Horn M, Borradori L, Yousefi S, Simon HU, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy 2017;72:1105–13. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Ratrie H 3rd, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol 2009;160:429–32. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Mihm MC Jr., Osage JE, Kwan TH, Austen KF, Wintroub BU. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J Invest Dermatol 1982;78:91–101. [DOI] [PubMed] [Google Scholar]
- Engmann J, Rudrich U, Behrens G, Papakonstantinou E, Gehring M, Kapp A, et al. Increased Activity and Apoptosis of Eosinophils in Blister Fluids, Skin and Peripheral Blood of Patients with Bullous Pemphigoid. Acta Derm Venereol 2017;97:464–71. [DOI] [PubMed] [Google Scholar]
- Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009;123:704–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol 2007;127:2605–11. [DOI] [PubMed] [Google Scholar]
- Fischkoff SA, Pollak A, Gleich GJ, Testa JR, Misawa S, Reber TJ. Eosinophilic differentiation of the human promyelocytic leukemia cell line, HL-60. J Exp Med 1984;160:179–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol 2004;172:5664–75. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243–50. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol 1993;151:5742–50. [PubMed] [Google Scholar]
- Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood 2012;120:3882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordon RE, Nordby JM, Milstein H. The complement system in bullous pemphigoid. III. Fixation of C1q and C4 by pemphigoid antibody. J Lab Clin Med 1975a;86:733–40. [PubMed] [Google Scholar]
- Jordon RE, Schroeter AL, Good RA, Day NK. The complement system in bullous pemphigoid. II. Immunofluorescent evidence for both classical and alternate-pathway activation. Clin Immunol Immunopathol 1975b;3:307–14. [DOI] [PubMed] [Google Scholar]
- Kayaba H, Dombrowicz D, Woerly G, Papin JP, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J Immunol 2001;167:995–1003. [DOI] [PubMed] [Google Scholar]
- Lampinen M, Backman M, Winqvist O, Rorsman F, Ronnblom A, Sangfelt P, et al. Different regulation of eosinophil activity in Crohn's disease compared with ulcerative colitis. J Leukoc Biol 2008;84:1392–9. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 2010;40:563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever W (1965) Pemphigus and Pemphigoid. Thomas CC: Springfield, Illinois. [Google Scholar]
- Li Z, Garantziotis S, Jia W, Potts EN, Lalani S, Liu Z, et al. The extracellular matrix protein mindin regulates trafficking of murine eosinophils into the airspace. J Leukoc Biol 2009;85:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Liao W, Long H, Chang CC, Lu Q. The Eosinophil in Health and Disease: from Bench to Bedside and Back. Clin Rev Allergy Immunol 2016;50:125–39. [DOI] [PubMed] [Google Scholar]
- Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organspecific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest 1993;92:2480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest 1997;100:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun 2008;31:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Zhang G, Wang L, Lu Q. Eosinophilic Skin Diseases: A Comprehensive Review. Clin Rev Allergy Immunol 2016;50:189–213. [DOI] [PubMed] [Google Scholar]
- Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother 2007;30:16–28. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods 2009;346:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messingham KN, Holahan HM, Frydman AS, Fullenkamp C, Srikantha R, Fairley JA. Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLoS One 2014;9:e107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology 2008;134:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med 2007;13:378–83. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y, Uematsu J, Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem 1993;113:493–501. [DOI] [PubMed] [Google Scholar]
- Parodi A, Rebora A. Serum IgE antibodies bind to the epidermal side of the basement membrane zone splits in bullous pemphigoid. Br J Dermatol 1992;126:526–7. [DOI] [PubMed] [Google Scholar]
- Provost TT, Tomasi TB Jr. Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol 1974;18:193–200. [PMC free article] [PubMed] [Google Scholar]
- Radonjic-Hoesli S, Valent P, Klion AD, Wechsler ME, Simon HU. Novel targeted therapies for eosinophilassociated diseases and allergy. Annu Rev Pharmacol Toxicol 2015;55:633–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013;13:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoszuk M Eosinophils and human cancer. Histol Histopathol 1997;12:807–12. [PubMed] [Google Scholar]
- Schaumburg-Lever G, Orfanos CE, Lever WP. Electron microscopic study of bullous pemphigoid. Arch Dermatol 1972;106:662–7. [PubMed] [Google Scholar]
- Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods 1996;198:1–14. [DOI] [PubMed] [Google Scholar]
- Soh H, Hosokawa H, Asada Y. IgE and its related phenomena in bullous pemphigoid. Br J Dermatol 1993;128:371–7. [DOI] [PubMed] [Google Scholar]
- Takedatsu H, Mitsuyama K, Matsumoto S, Handa K, Suzuki A, Funabashi H, et al. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol 2004;34:1561–9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Takenaka M, Matsunaga Y, Okada S, Anan S, Yoshida H, et al. High affinity IgE receptor (Fc epsilon RI) expression on eosinophils infiltrating the lesions and mite patch tested sites in atopic dermatitis. Arch Dermatol Res 1995;287:712–7. [DOI] [PubMed] [Google Scholar]
- van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol 2016;12:267–77. [DOI] [PubMed] [Google Scholar]
- Vieira AT, Fagundes CT, Alessandri AL, Castor MG, Guabiraba R, Borges VO, et al. Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Pathol 2009;175:2382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayli S, Pelivani N, Beltraminelli H, Wirthmuller U, Beleznay Z, Horn M, et al. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol 2011;165:1133–7. [DOI] [PubMed] [Google Scholar]
- Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol 2014;71:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol 1997;109:573–9. [DOI] [PubMed] [Google Scholar]
- Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol 2007;127:1167–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.