Abstract
PURPOSE
Unplanned health care encounters (UHEs) such as emergency room visits can occur commonly during cancer chemotherapy treatments. Patients at an increased risk of UHEs are typically identified by clinicians using performance status (PS) assessments based on a descriptive scale, such as the Eastern Cooperative Oncology Group (ECOG) scale. Such assessments can be bias prone, resulting in PS score disagreements between assessors. We therefore propose to evaluate PS using physical activity measurements (eg, energy expenditure) from wearable activity trackers. Specifically, we examined the feasibility of using a wristband (band) and a smartphone app for PS assessments.
METHODS
We conducted an observational study on a cohort of patients with solid tumor receiving highly emetogenic chemotherapy. Patients were instructed to wear the band for a 60-day activity-tracking period. During clinic visits, we obtained ECOG scores assessed by physicians, coordinators, and patients themselves. UHEs occurring during the activity-tracking period plus a 90-day follow-up period were later compiled. We defined our primary outcome as the percentage of patients adherent to band-wear ≥ 80% of 10 am to 8 pm for ≥ 80% of the activity-tracking period. In an exploratory analysis, we computed hourly metabolic equivalent of task (MET) and counted 10 am to 8 pm hours with > 1.5 METs as nonsedentary physical activity hours.
RESULTS
Forty-one patients completed the study (56.1% female; 61.0% age 40-60 years); 68% were adherent to band-wear. ECOG score disagreement between assessors ranged from 35.3% to 50.0%. In our exploratory analysis, lower average METs and nonsedentary hours, but not higher ECOG scores, were associated with higher 150-day UHEs.
CONCLUSION
The use of a wearable activity tracker is generally feasible in a similar population of patients with cancer. A larger randomized controlled trial should be conducted to confirm the association between lower nonsedentary hours and higher UHEs.
INTRODUCTION
Recent advances in miniaturization and sensor technology have greatly improved availability and affordability of consumer wearable activity trackers. Although these devices produce measurements that are still less accurate than those obtained in a controlled environment of a clinic, they can provide real-world outpatient activity data that can be useful to inform physicians of patients’ physical activity (PA) level. Furthermore, the accuracy of these devices is expected to benefit from rapid iterative improvements by well-capitalized consumer electronics manufacturers, with validations of these devices’ measurements investigated by the research community.1,2
CONTEXT
Key Objective
Can wearable activity trackers be used to assess performance status, instead of or in tandem with the Eastern Cooperative Oncology Group (ECOG) scale, in patients with cancer with solid tumors undergoing highly emetogenic chemotherapy? How well do ECOG scores and measures derived from activity trackers correlate with unplanned health care encounters (UHEs)?
Knowledge Generated
In our study cohort, > 65% of patients were able to adhere to the activity tracker wear protocol. ECOG scores did not have a signification correlation with UHEs. In an exploratory analysis, our data suggest that higher average metabolic equivalents of task and higher nonsedentary physical activity hours are associated with lower UHEs.
Relevance
Data from activity trackers can potentially be used to evaluate a patient’s risk of UHEs.
Cancer chemotherapy treatments are commonly associated with significant toxicity, often requiring unplanned health care encounters (UHEs), such as emergency room visits for complications such as asthenia, emesis, pain, infection, and dehydration. Performance status (PS) measures, such as the Eastern Cooperative Oncology Group (ECOG) scale,3,4 have been used to identify patients at an increased risk of UHEs, enabling health care professionals to actively prescribe preventive measures to improve patients’ quality of life. However, ECOG assessments rely on qualitative descriptions of patients and therefore can be prone to biases, such as the patient’s recall bias resulting in inaccurate information available to the assessor, or the assessor’s observer bias partially due to limited interaction time. This potential disagreement between physician- and patient-assessed ECOG scores has been associated with worse outcomes in patients with cancer.5
In cancer medicine, higher PA has been associated with improved outcomes, including recurrence, overall survival, and disease-free survival, in multiple studies involving colorectal, prostate, breast, pancreatic, endometrial, ovarian, and lung cancer.6-11 A recent study12 on the feasibility of activity tracker use in patients with advanced cancer reported a correlation between step counts and ECOG scores and associated increased step counts with reduced odds for adverse events. Outside of cancer medicine, the utility of activity trackers has also been extensively evaluated,13,14 and correlations of higher PA level with better outcomes were also observed.15
To examine the feasibility of activity tracker use in cancer chemotherapy, we performed an observational study (ClinicalTrials.gov identifier: NCT03098277) with a population of patients with solid tumors undergoing highly emetogenic chemotherapy, according to the Hesketh classification.16 In this work, we report on our primary outcome, the percentage of patients adherent to activity tracker wear. We also report on the disagreements between ECOG scores assessed by physicians, coordinators, and patients and the predictive correlation between ECOG scores and UHEs. Finally, in an exploratory analysis, we report on the correlation between ECOG scores and physical activity measures and the predictive correlation between physical activity measures and UHEs.
METHODS
After approval by local institutional review boards, the study was opened at four hospital sites: University of Southern California (USC) Norris, Los Angeles County, plus USC Medical, USC Newport, and MD Anderson. Study inclusion criteria consisted of being ≥ 18 years of age with a diagnosis of solid tumor anticipated to undergo at least two future cycles of highly emetogenic palliative or adjuvant chemotherapy. Patients could have received prior chemotherapy at the time of enrollment so long as additional therapy was planned. Patients had to be willing to wear an activity tracker and provide health outcomes on a provided smartphone during a 60-day activity-tracking (band-wear) period. They also had to be able to ambulate without assistive devices (this is because this study is a part of a larger study that includes PS assessments using Microsoft Kinect). Exclusion criteria included patients with missing limbs, symptomatic brain metastasis, or known movement disorders, including essential tremor if requiring drug therapy.
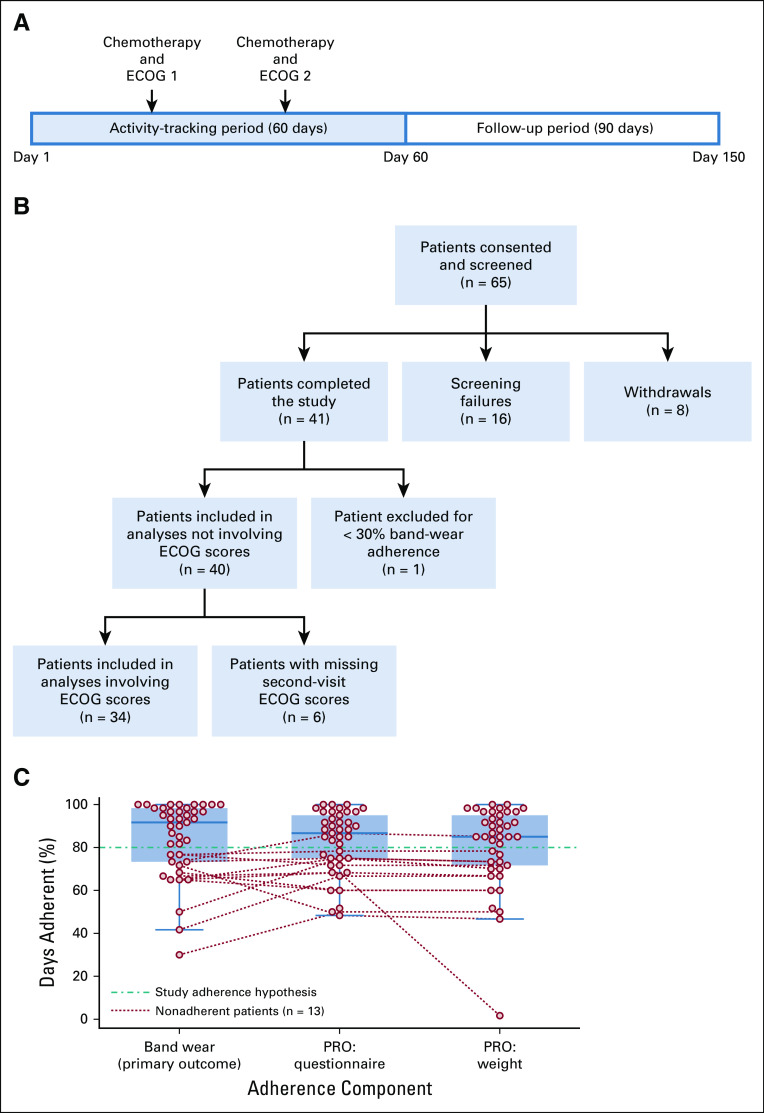
After consent and successful screening, patients received a Microsoft Band 2 (band) and a Microsoft smartphone with our patient-reported outcomes (PRO) app, available in English, Spanish, and Mandarin Chinese. Patients were instructed to always wear the band while they were awake, charge it every night, and respond to nightly PRO app prompts for the duration of the activity-tracking period. The study period is 150 days long and consists of a 60-day activity-tracking period and a 90-day follow-up period (Fig 1A). The primary outcome of this study is discussed below.
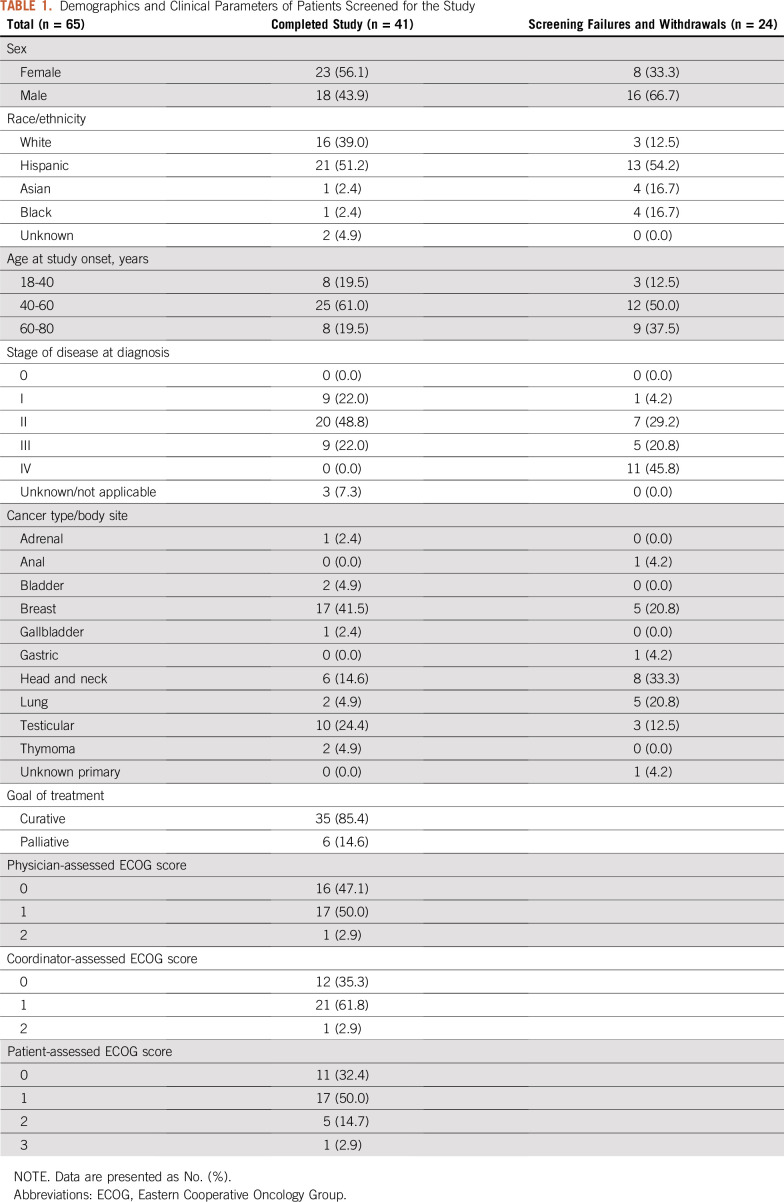
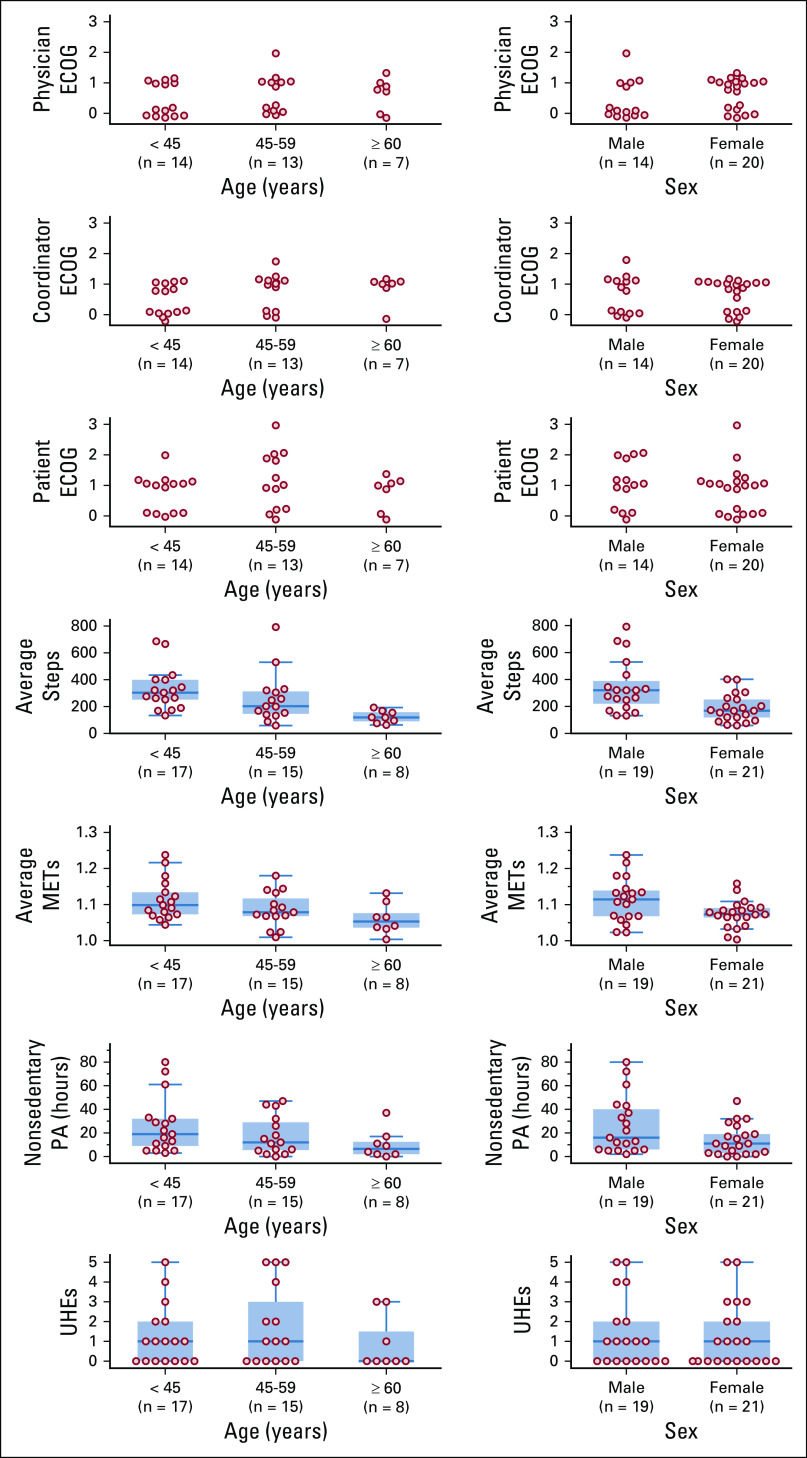
FIG 1.
(A) Study timeline. During each office visit, patients receive chemotherapy treatment and undergo Eastern Cooperative Oncology Group (ECOG) score assessment. The first and second office visits do not have a fixed day in the timeline. The study inclusion criteria only require two cycles of chemotherapy during the activity-tracking period. (B) Study cohort flowchart, showing screening failures, withdrawals, and number of patients in each part of our analysis. (C) Study adherence of patients who completed the study (n = 41). Dotted lines connect each adherence component of the same non–band-wear–adherent patient. Both patient-reported outcomes (PRO) questionnaire and PRO weight data were collected via the PRO app.
Wearable Activity Tracker
Details on Microsoft Band 2 can be found in the Appendix. After the activity-tracking period ended, patient hourly band data (energy expenditure, step counts, and heart rate) were downloaded to a secure server for further analysis. None of the data were missing, so imputation was not performed.
We defined an hour of band-wear as an o’clock hour (eg, 11 am to 12 pm) with nonzero heart rate. We have selected nonzero heart rate as the sole indicator for band-wear because Microsoft incorporates measurements from the paired phone’s sensors17 to estimate all other activity measures (eg, step counts), and hence they are not a reliable indicator for band-wear. We defined daily band-wear adherence as the participant wearing the band for ≥ 80% of the 10 am to 8 pm period; this period was selected based on this cohort’s expected common waking hours. This ensures that we can compare band data with the ECOG scores, as the ECOG scale specifies that assessments should only consider the patient’s waking hours (Appendix Table A1). We then defined overall band-wear adherence as the participant being daily band-wear adherent for ≥ 80% of days during the band-wear period. Finally, the primary outcome was defined as the percentage of band-wear–adherent patients. The details of the statistical power, along with the hypotheses, can be found in the Appendix.
For the analysis, we also considered data only for the 10 am to 8 pm period. To allow for activity level comparison across patients, we estimated the hourly metabolic equivalent of task (MET), where 1 MET corresponds to a person’s basal metabolism.18 METs were estimated by dividing hourly energy expenditure by daily patient-reported weight (see Patient-Reported Outcomes section) to account for possible weight changes. Baseline activity level was then corrected to 1 MET using line-fit to normalize METs across patients.
The Sedentary Behavior Research Network has proposed a common definition of sedentary behavior as “any waking behaviour characterized by an energy expenditure ≤ 1.5 METs while in a sitting or reclining posture.”19(p540) Accordingly, we defined and computed nonsedentary PA hours as the number of 10 am to 8 pm hours where the patient exhibited > 1.5 METs. Note that because band data were provided in an hourly interval, a 1.5-MET threshold corresponds to an energy expenditure of 1.5 METs on average for the entire hour. Finally, using an exploratory threshold of 10 hours of nonsedentary PA, patients were separated into two similar-sized groups for analysis: higher PA (HPA) and lower PA (LPA).
Patient-Reported Outcomes
During the activity-tracking period, we collected PRO data daily using our smartphone app, available in English, Spanish, and Mandarin Chinese. PRO data included daily body weight measured on a provided home scale and daily questionnaires based on Patient-Reported Outcomes Measurement Information System (PROMIS).20 Detailed PRO questionnaire information and data analysis can be found in Broderick et al.21 Missing PRO weight data were imputed using linear interpolation (and forward/backward fill for the end/beginning).
PS Assessments
The ECOG scale (Table A1)3,4 was used for PS assessments. We collected ECOG scores from the physician, coordinator, and patient during two office visits in the activity-tracking period. Patients were instructed on how to self-assess during office visits. ECOG scores were missing from the earliest enrolled patients, before additional coordinator training, so ECOG scores from the second office visit were used for analysis. Patients had good ECOG scores overall; therefore, we considered two ECOG score groups for analysis: ECOG = 0 and ECOG > 0.
UHEs
We defined UHEs to include urgent office visits, urgent hospitalizations, unplanned day hospital visits, and emergency room visits, excluding routine office visits, planned chemotherapy hospitalizations, scheduled chemotherapy, and planned surgery hospitalizations. This combined end point of UHEs aims to capture significant complications, which in other medical specialty areas might be similarly captured by the count of emergency room visits and hospitalizations.
Retrospective reviews were conducted by physicians to categorize encounters recorded on the electronic health record system as planned or unplanned; UHEs were then counted over the 150-day study period. To reduce the effect of outliers on averaged UHEs when comparing between two groups, we considered patients with more than five UHEs as having five UHEs in our analysis.
Statistical Analysis
Our data analysis was performed in Python. Normality assumptions were tested using the Shapiro-Wilk test. Homoscedasticity assumptions were tested using Levene’s test. Correlation between two variables was calculated using Spearman’s rho rank correlation. Difference between mean/median values of a variable sampled from two different groups was tested using independent two-sample t test (independent t test) if that variable exhibited normality and homoscedasticity in both groups and the Wilcoxon-Mann-Whitney rank-sum test (WMW-test) otherwise. One-sided tests were used to compare between groups where a direction assumption can be made; otherwise, two-sided tests were used. A threshold of 0.05 for P was used to establish significance.
RESULTS
Between May 2016 and May 2017, 65 patients were consented and screened for the study. There were 16 screening failures and eight withdrawals within the first 30 days. Of these 24 patients, median age was 58 years (range, 24-71 years) and 11 had stage IV disease. Reasons for withdrawal included: nonadherent with the devices (n = 4), uncomfortable using the devices (n = 1), mature cataracts preventing the use of a smartphone (n = 1), moving out of town (n = 1), and misplacing the devices (n = 1). A study cohort flowchart is provided in Figure 1B.
Patients who completed the study (n = 41) were 56% female, 51% Hispanic, and 39% white. The median age at cancer diagnosis was 48 years (range, 24-72 years). The plurality (19/41, 46%) of patients had stage II disease at diagnosis, with nine patients with stage I disease and nine patient with stage III disease. The majority (35/41, 85%) of patients were being treated with curative intent. Patients’ solid tumor site included breast (42%), testicular (24%), and head and neck (15%). More details can be found in Table 1. The most common chemotherapy regimens included bleomycin, etoposide, cisplatin; doxorubicin and cyclophosphamide; and cisplatin, doxorubicin, cyclophosphamide.
TABLE 1.
Demographics and Clinical Parameters of Patients Screened for the Study
Band-Wear Adherence
Out of the 41 patients who completed the study, 28 patients (68%) were band-wear adherent. The median percentage of days adherent was 92% (Fig 1C). The average age of the adherent and nonadherent group (50 and 44 years) was not significantly different (independent t test; two-sided P = .15); the median was 48 and 47 years. Fourteen males and 14 females comprised the adherent group; and six males and seven females comprised the nonadherent group.
In the following sections, all but one of the 41 patients were included in our exploratory analysis, irrespective of their adherence status; the only patient excluded was adherent for only 30% of days. These 40 patients were composed of 19 males and 21 females; their age distribution (< 45, 45-59, and ≥ 60 years) was 17, 15, and eight. This group is labeled with (n = 40) in the remainder of the analysis.
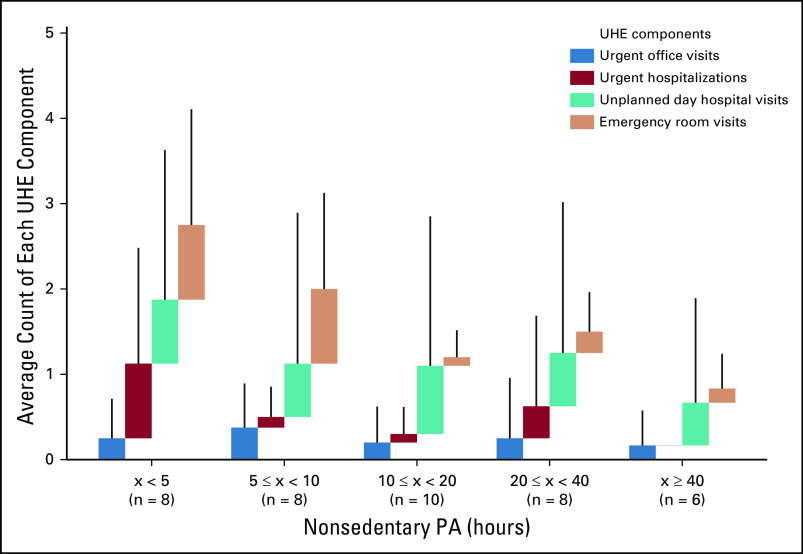
Physical Activity Data
The average steps per hour was 262; the median was 225. Steps per hour exhibited a decrease with age. The median average steps per hour of ages < 45, 45-59, and ≥ 60 years were 303, 202, and 119. The median average steps per hour was 320 for men, significantly different from 168 for women (WMW test; two-sided P = .0026).
The average hourly METs was 1.09; the median was 1.08. Hourly METs also exhibited a decrease with age. The median average hourly METs of ages < 45, 45-59, and ≥ 60 years were 1.10, 1.08, and 1.05. The median average hourly METs was 1.11 for men, significantly different from 1.07 for women (WMW test; two-sided P = .048).
The average nonsedentary PA hours was 19.9 hours; the median was 12.5. The average nonsedentary PA hours of ages < 45, 45-59, and ≥ 60 years was 26.1, 17.9, and 10.3; the median was 19.0, 12.0, and 6.5. The average nonsedentary PA hours was 26.4 for men and 14.0 for women; the median was 16.0 for men, not significantly different from 11.0 for women (WMW test; two-sided P = .073; Fig 2).
FIG 2.
Physician-, coordinator-, and patient-assessed Eastern Cooperative Oncology Group (ECOG) scores (n = 34), physical activity measures (average hourly step counts, average hourly metabolic equivalents of task [METs], and nonsedentary physical activity [PA] hours) over the 60-day activity-tracking period (n = 40), and unplanned health care encounters (UHEs) over the 150-day study period (n = 40) demographics. Patients with more than five UHEs were considered as having five UHEs to reduce the effect of outliers. Jitters were added to ECOG scores to allow individual data points to be shown.
Using an exploratory threshold of 10 nonsedentary PA hours, patients were separated into two similar-sized groups: HPA (n = 24) and LPA (n = 16). The HPA group comprised 13 males and 11 females; their age distribution for < 45, 45-59, and ≥ 60 years was 12, nine, and three. The LPA group comprised six males and 10 females; their age distribution for < 45, 45-59, and ≥ 60 years was five, six, and five.
ECOG Score Data
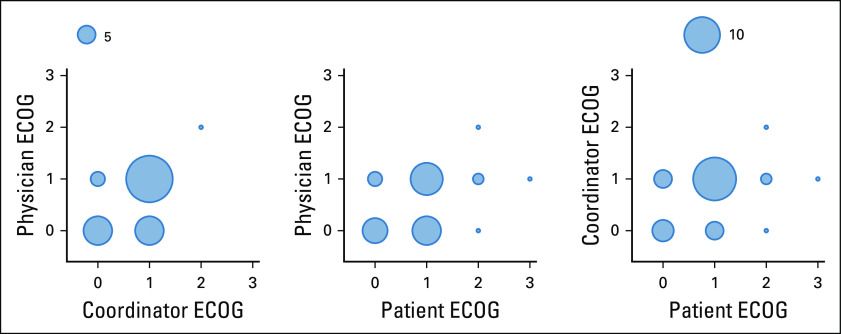
The physician-, coordinator-, and patient-assessed ECOG scores from the second office visit were successfully recorded for 34 out of 40 patients. This group is labeled with (n = 34) in the remainder of the analysis. There were disagreements in 35.3%, 50.0%, and 44.1% of the physician-coordinator, physician-patient, and coordinator-patient ECOG score pairs (Appendix Fig A1). Patients had good ECOG scores overall (only one, one, and six patients had physician-, coordinator-, and patient-assessed ECOG score > 1).
UHEs Data
Patients rarely encountered more than UHEs (three out of 40 patients had 11, 13, and 21 UHEs). To reduce the effect of outliers, we considered patients with more than five UHEs as having five UHEs in the following analysis. The average UHEs of 40 patients was 1.35. Average UHEs of ages < 45, 45-59, and ≥ 60 years was 1.23, 1.73, and 0.88; average UHEs of men and women were not significantly different at 1.42 and 1.29 (WMW test; two-sided P = .81; Fig 2).
Predictive Correlation Between ECOG Scores and UHEs
The average UHEs (n = 34) for physician-, coordinator-, and patient-assessed ECOG = 0 and ECOG > 0 score groups were 1.44 and 0.83, 0.92 and 1.23, and 1.00 and 1.17. None of the ECOG > 0 score group for physician-, coordinator-, and patient-assessed ECOG scores had significantly higher UHEs than the corresponding ECOG = 0 score group (WMW test; one-sided P = .87, .18, .28). We also did not observe significant correlations between UHEs and physician-, coordinator-, and patient-assessed (ECOG = 0, ECOG > 0) score groups. Note that the physician-assessed (ECOG = 0, ECOG > 0) score groups unexpectedly exhibited negative correlation with UHEs (Fig 3, right column).
FIG 3.
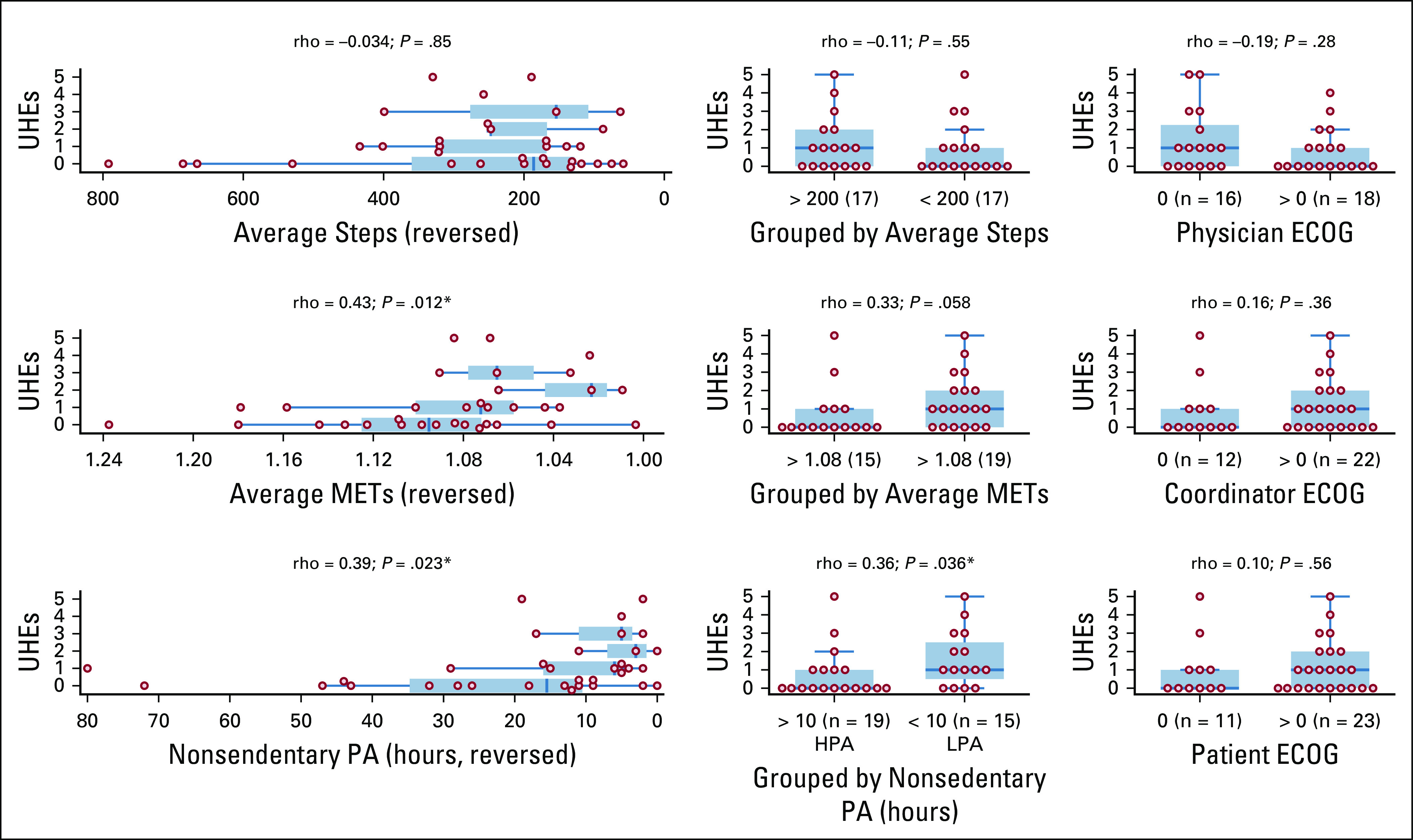
Unplanned health care encounters (UHEs) over the 150-day study period (n = 34), stratified by physical activity measures and Eastern Cooperative Oncology Group (ECOG) scores: (left) as a function of physical activity measures (average hourly steps, average hourly metabolic equivalents of task [METs], nonsedentary physical activity [PA] hours), (middle) as dichotomized into PA levels (average hourly steps groups, average hourly METs groups, nonsedentary PA hours groups), and (right) as dichotomized into ECOG = 0 and ECOG > 0 score groups (assessed by physicians, coordinators, and patients). All the subplots are arranged such that the population depicted toward the right side of each plot is expected to have higher UHEs than the left. Spearman’s rho rank correlation and the associated P value are shown above each subplot. HPA, higher PA; LPA, lower PA. (*) P < .05.
The average UHEs for patients with and without physician-patient ECOG score disagreement were 0.94 and 1.29. In contrast to findings in Schnadig et al,5 we did not observe that physician-patient ECOG score disagreement is associated with significantly higher UHEs (WMW test; one-sided P = .69).
Correlation Between Physical Activity Measures and ECOG Scores
We observed two significant correlations between the ECOG = 0/ECOG > 0 score groups and the PA measures: (1) between physician-assessed score groups and average steps (Spearman’s rho, −0.44; P = .0085), and (2) between coordinator-assessed score groups and average steps (Spearman’s rho, −0.37; P = .031; Fig 4), consistent with findings in Gresham et al.12
FIG 4.
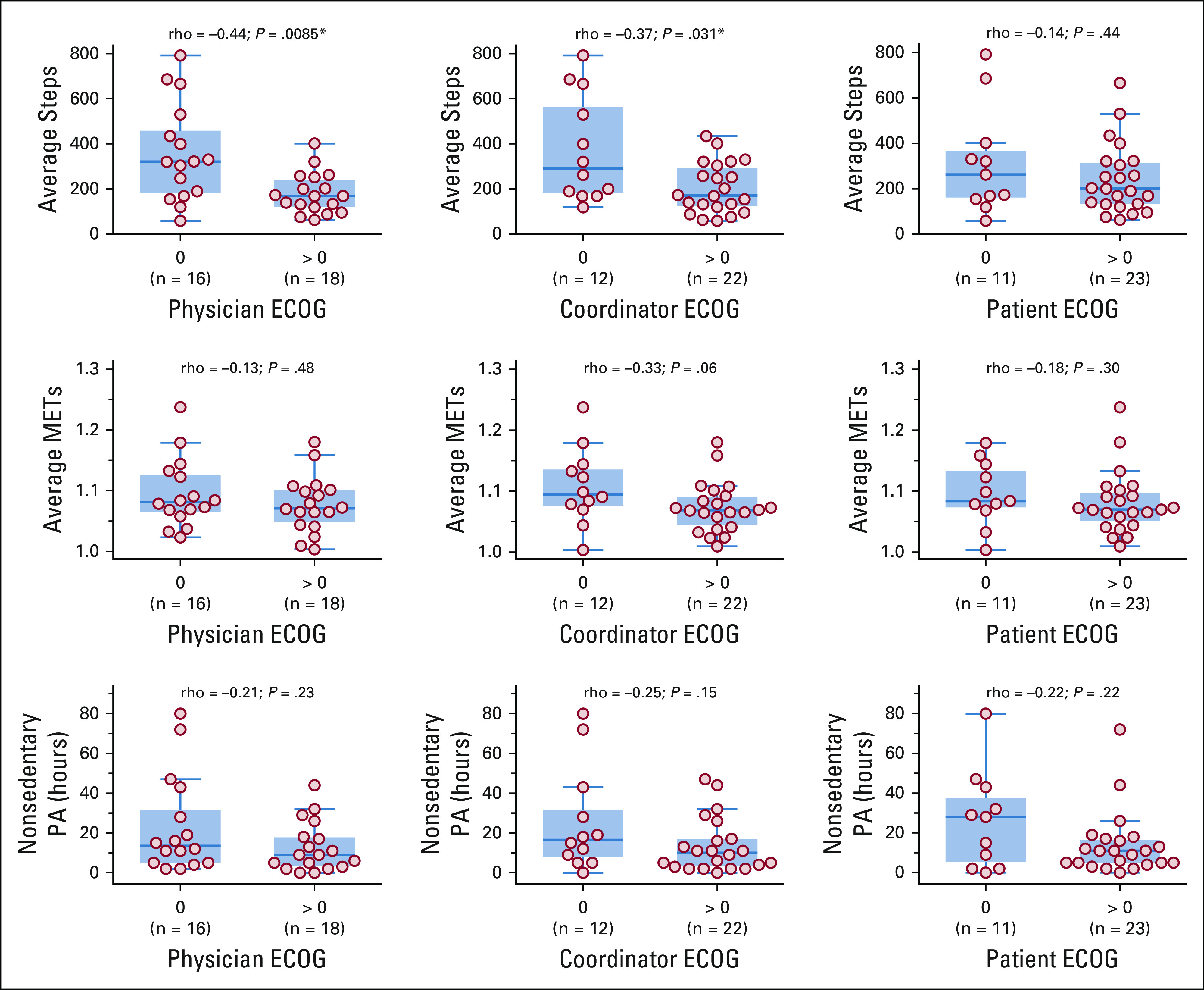
Average hourly step counts, average hourly metabolic equivalents of task (METs), and nonsedentary physical activity (PA) hours over the 60-day activity-tracking period (n = 34), stratified by Eastern Cooperative Oncology Group (ECOG) = 0 and ECOG > 0 score groups. Six patients with missing ECOG scores were excluded. Spearman’s rho rank correlation and the associated P values are shown above each subplot. (*) P < .05.
Predictive Correlation Between Physical Activity Measures and UHEs
The average UHEs (n = 34) for HPA and LPA groups were 0.74 and 1.60. The LPA group had significantly higher UHEs than the HPA group (WMW test; one-sided P = .019). We observed significant correlations between UHEs and average METs and nonsedentary PA hours (Fig 3). In contrast to findings in Gresham et al,12 we did not observe a significant correlation between step counts and UHEs (WMW test; one-sided P = .73). The correlation between UHEs and nonsedentary PA hours still holds when patients with missing ECOG scores were included (n = 40; Fig 5).
FIG 5.
Average count of unplanned health care encounter (UHE) components over the 150-day study period (n = 40), stratified by nonsedentary physical activity (PA) hours. A thin vertical line above each bar shows its standard deviation. Any UHE component with more than five encounters (nine, 10, and 13 in unplanned day hospital visits; none in other components) was considered as five encounters to reduce the effect of outliers.
A visualization of individual patient day-by-day average step counts, average METs, and nonsedentary PA hours, along with their ECOG scores and UHEs, can be found in Appendix Figures A2-A4.
DISCUSSION
Band-wear adherence may have been hindered by the learning curve of using new technology in an older population present in this study (80% age > 40 years and 20% age > 60 years). As participation in the study did not provide any direct benefit to the enrolled patients, a common reason for early dropout was that the patient had advanced disease and did not want the burden of dealing with new technology concurrently with difficult treatments. Future clinical studies of activity trackers could obtain higher adherence if enrollment is restricted to patients who already own smartphones. Recent improvements in activity tracker technology should also help improve adherence, as newer devices are smaller and require less-frequent charging (charging were required every night in this study). Attention to repetitive coaching and educating during office visits seemed to improve band-wear adherence as the study progressed and the dropout rate lowered over time.
The common waking hours selected for this study (10 am to 8 pm) possibly did not capture the entirety of waking hours of all participants. However, it was necessary to establish common hours for analysis so that the derived physical activity measures are comparable to the ECOG scores, as the ECOG scale only includes activity during waking hours. Although many activity trackers claim to be able to determine waking/sleeping hours, we prefer not to rely on proprietary algorithms that are device dependent and may not be validated for the target population. Given the relatively short consumer-market lifespan of any particular activity tracker, it is valuable to use measures that can be reliably reproduced across all devices.
There are known limitations of using METs to describe PA intensity. For example, patients with similar weight but different lean body mass are expected to have different resting metabolic rate, because resting metabolic rate is roughly proportional to lean body mass; METs do not account for this. Studies1,2 have also shown that activity trackers exhibit notable variances in estimating energy expenditure compared with gold-standard calorimetry; median errors for Microsoft Band 1 were found to be 30%-40%. We partially mitigated this issue by performing baseline activity line-fit such that the activity level at rest is equal to 1 MET across patients. Nonetheless, MET estimation remains practical, allowing for PA estimation that is comparable across patients.
Although a wearable activity tracker can capture longitudinal physical activity of a patient over an extended period, its detail of observation is limited to cumulative measures, such as step counts and energy expenditure. These measures capture the frequency and intensity aspects of physical activity but are not well-suited to capture the effortfulness and effectiveness aspects, which are also integral to performance status. This limitation of activity trackers can be addressed with a task-based clinic evaluation by physicians and/or body tracking systems. We evaluated the use of Microsoft Kinect for this purpose and reported preliminary analysis in Hasnain et al.22
This study demonstrated the feasibility of outpatient wearable activity tracker use as a method of assessing physical activity in patients with cancer. We documented a 50% disagreement in the physician-patient ECOG score pair; however, we did not observe that such disagreements are associated with significantly higher UHEs. We observed significant correlations between step counts and physician- and coordinator-assessed ECOG scores but not patient-assessed ECOG scores. We also did not observe significant predictive correlation between ECOG scores and UHEs. In our exploratory analysis, we observed significant predictive correlations between (1) average METs and UHEs, and (2) nonsedentary physical activity hours and UHEs.
There are multiple potential uses for wearable activity tracker in outpatient oncology practice, including quantifying the extent to which a cancer therapy changes PA level of patients; identifying patients at increased risk of complications who may benefit from additional office visits, engagement of home care, or increased attention to symptom management; selecting patients for clinical trial participation; and informing toxicity attribution. Clinical studies testing this last use case are presently underway.
ACKNOWLEDGMENT
We thank all the patients who participated in the study. We also thank the clinical research staff at participating study sites—University of Southern California (USC) Norris, USC-Los Angeles County, USC Newport, and MD Anderson—for their support. We thank Summer Jackson at MD Anderson for her essential assistance with the study, as well as Kien Nguyen and Minh Nguyen at USC Integrated Media Systems Center and InfoLAB for assistance with data acquisition.
Appendix
Microsoft Band 2 Information
Microsoft Band 2 is a wrist-worn activity tracker bracelet containing multiple sensors, including a three-axis accelerometer and an optical heart rate sensor. A microprocessor in the bracelet processes these sensor measurements and periodically sends them to the Microsoft Health app installed on a paired phone, which later forwards these data to Microsoft. Microsoft’s proprietary program then processes the data to estimate hourly (smallest time denomination available) activity measurements, including energy expenditure, step counts, and heart rate (see Shcherbina et al1 for accuracy validation).
Primary Outcome Hypothesis Test and Statistical Power
The following hypothesis test was designed for the primary outcome, where pct is the percentage of patients that are adherent to band-wear:
H0: pct ≥ 55%
Ha: pct < 35%
Overall, there is 93% power to reject H0, when pct = 35%, with α of .05.
FIG A1.
Interrater disagreement of Eastern Cooperative Oncology Group (ECOG) scores between physicians, coordinators, and patients (n = 34). Circle radii scale linearly with the number of ECOG scores recorded.
FIG A2.
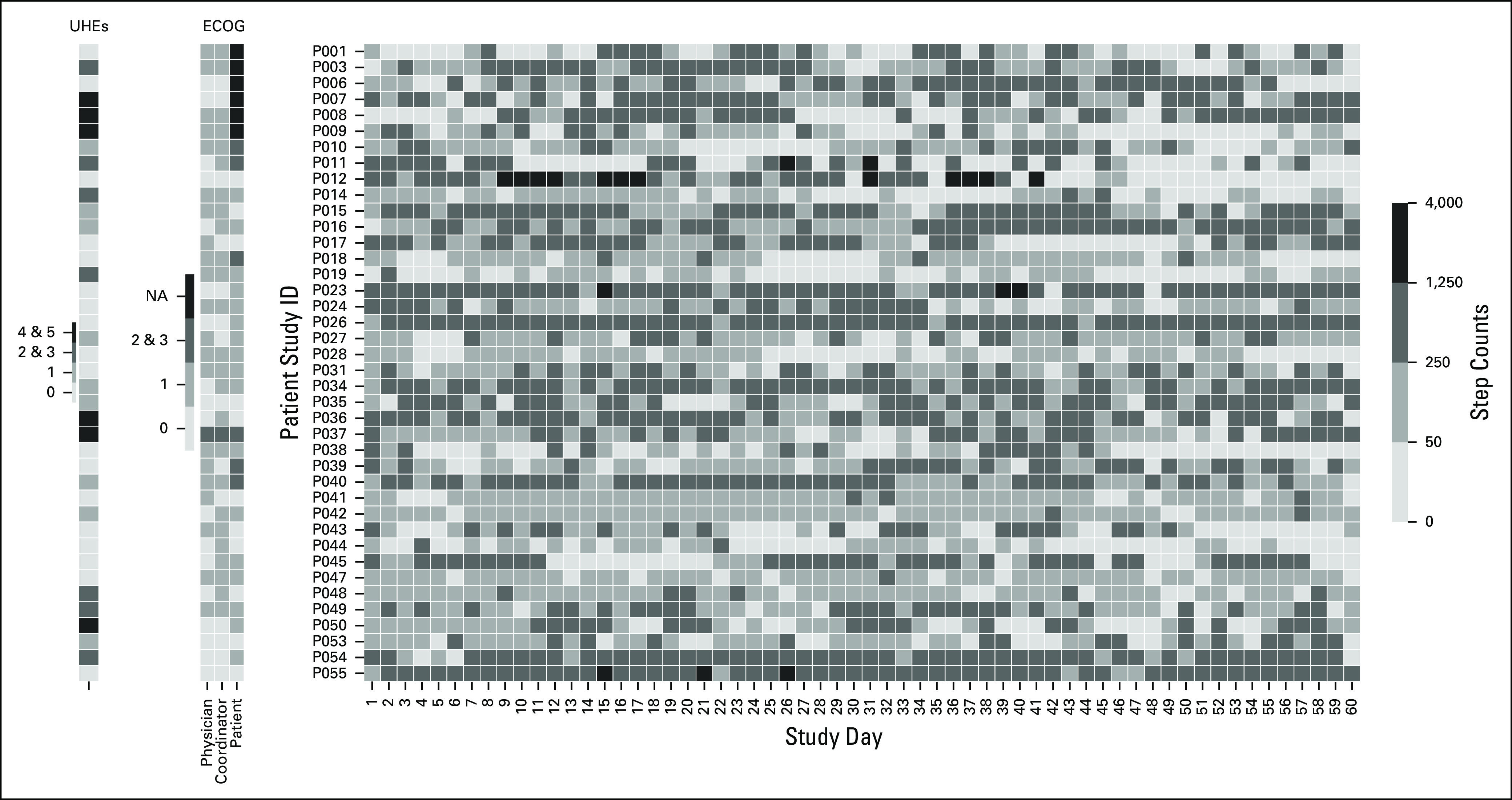
Heat map of average hourly step counts for each study day. Each row represents an individual patient. On the left side, Eastern Cooperative Oncology Group (ECOG) scores and unplanned health care encounters (UHEs) are provided for reference. NA, not available.
FIG A3.
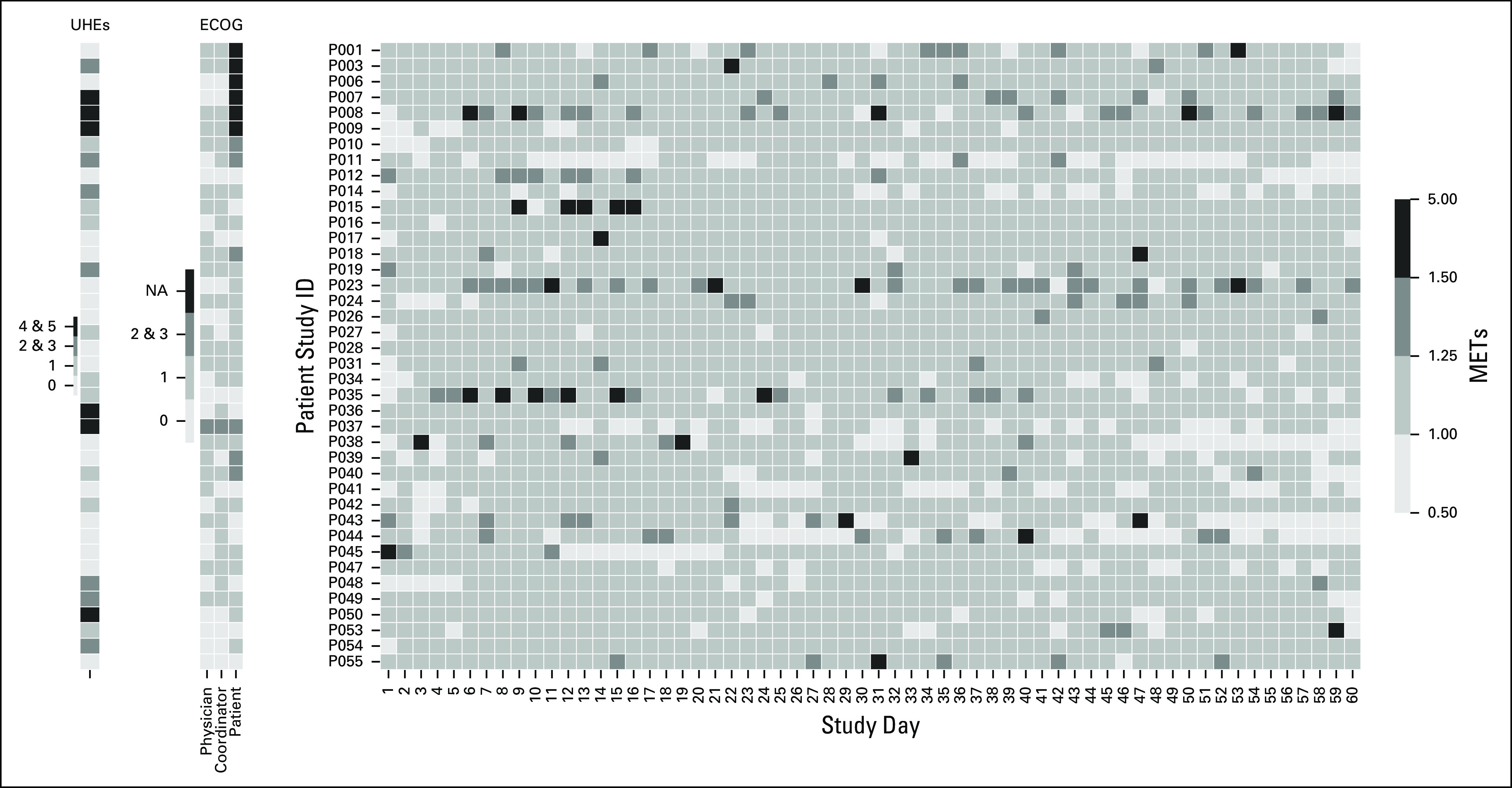
Heat map of average hourly metabolic equivalents of task (METs) for each study day. Each row represents an individual patient. On the left side, Eastern Cooperative Oncology Group (ECOG) scores and unplanned health care encounters (UHEs) are provided for reference. NA, not available.
FIG A4.
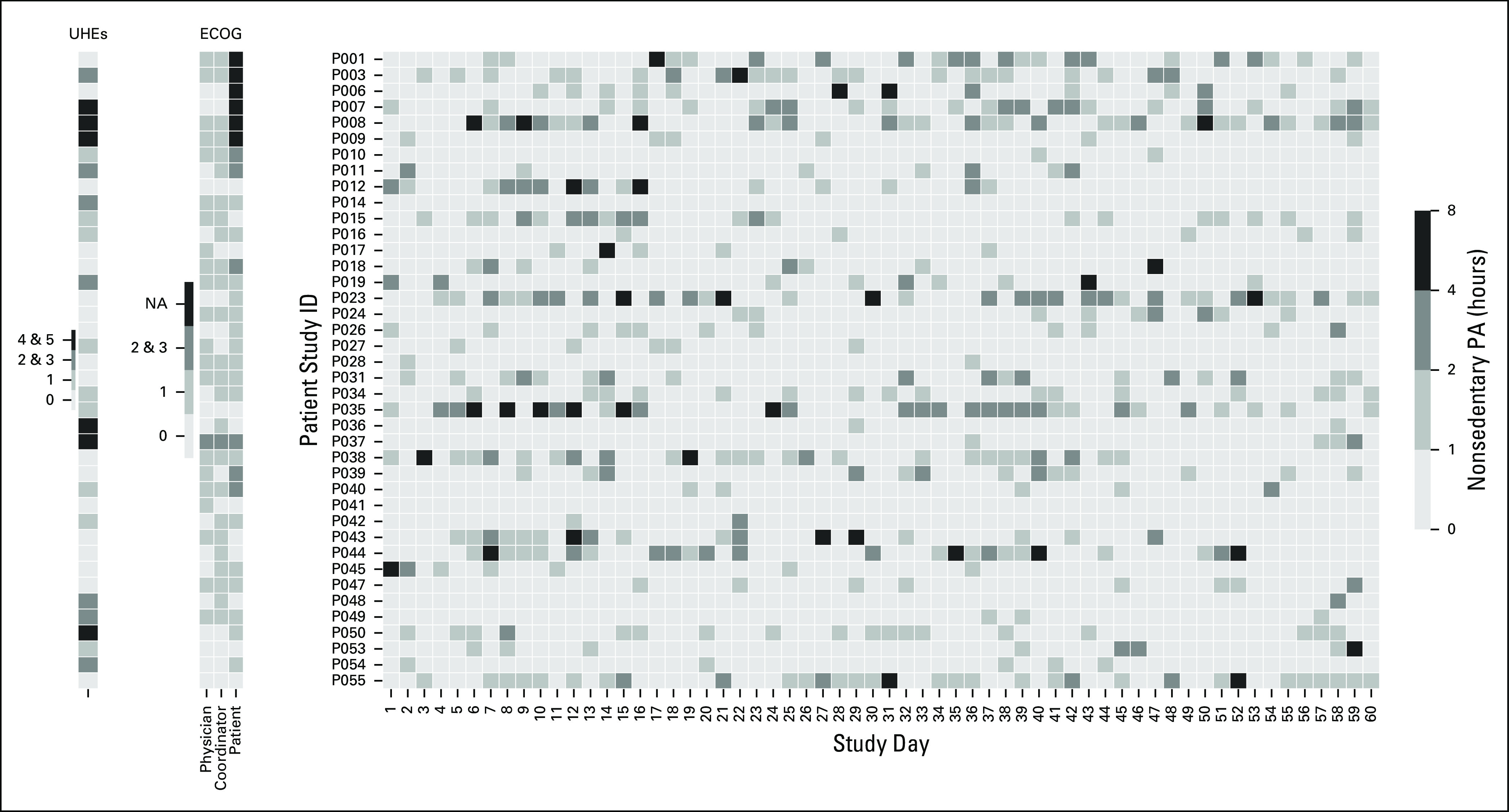
Heat map of nonsedentary physical activity (PA) hours for each study day. Each row represents an individual patient. On the left side, Eastern Cooperative Oncology Group (ECOG) scores and unplanned health care encounters (UHEs) are provided for reference. NA, not available.
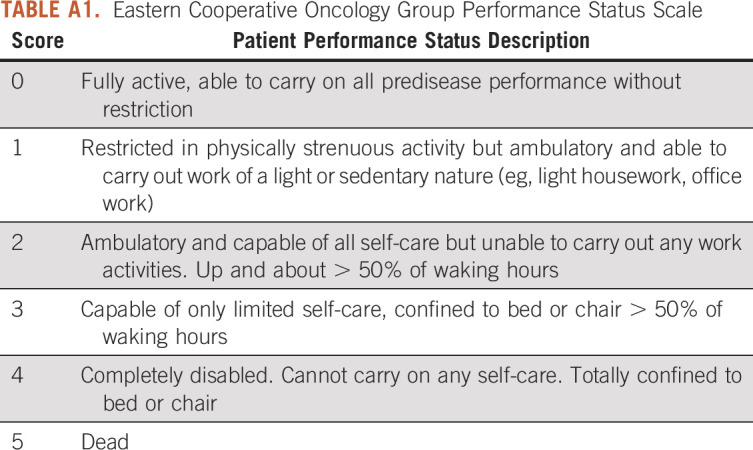
TABLE A1.
Eastern Cooperative Oncology Group Performance Status Scale
PRIOR PRESENTATION
Presented at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by Department of Defense and National Cancer Institute (NCI) Contract No. HHSN261201500003B via Noetic pass-through funds, University of Southern California (USC) Norris Cancer Center NCI Cancer Center Support Grant Award No. P30CA014089, USC Integrated Media Systems Center (IMSC), and unrestricted cash gifts from Oracle, Microsoft, and Google.
CLINICAL TRIAL INFORMATION
NCT03098277 (PROSPER)
AUTHOR CONTRIBUTIONS
Conception and design: Luciano P. Nocera, Alexander S. Martin, Zaki Hasnain, Ming Li, Jerry S. H. Lee, Sean E. Hanlon, Frankie A. Cozzens Philips, Joan Broderick, Cyrus Shahabi, Peter Kuhn, Jorge J. Nieva
Financial support: Frankie A. Cozzens Philips, Joan Broderick, Peter Kuhn, Jorge J. Nieva
Administrative support: Marcella May, Sriram Yennu, Peter Kuhn
Provision of study material or patients: Naoto T. Ueno, Sriram Yennu, David I. Quinn, Jorge J. Nieva
Collection and assembly of data: Tanachat Nilanon, Luciano P. Nocera, Anand Kolatkar, Marcella May, Zaki Hasnain, Sriram Yennu, Angela Alexander, Aaron E. Mejia, Roger Wilson Boles, Ming Li, Joan Broderick, Peter Kuhn, Jorge J. Nieva
Data analysis and interpretation: Tanachat Nilanon, Luciano P. Nocera, Zaki Hasnain, Naoto T. Ueno, Sriram Yennu, Angela Alexander, Roger Wilson Boles, Jerry S. H. Lee, David I. Quinn, Paul K. Newton, Cyrus Shahabi, Peter Kuhn, Jorge J. Nieva
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Luciano P. Nocera
Patents, Royalties, Other Intellectual Property: Application No. 62/825 965: System and method for determining quantitative health-related performance status of a patient (Inst)
Anand Kolatkar
Stock and Other Ownership Interests: Epic Sciences
Patents, Royalties, Other Intellectual Property: HD-SCA technology was developed by me and members of my team while at The Scripps Research Institute, which has subsequently licensed the technology to Epic Sciences for exclusive commercial development.
Naoto T. Ueno
Honoraria: Kyowa Hakko Kirin, Roche Taiwan, Amgen, Chugai/Roche, Henry Steward Talks, Taiho Pharmaceutical, Eisai
Consulting or Advisory Role: Samsung Bioepis, Daiichi Sankyo, Immunomedics
Research Funding: Medivation, Bayer, Amgen, Puma Biotechnology, Merck, Daiichi Sankyo, Celgene, GlaxoSmithKline, Kyowa Hakko Kirin, Bio-Path Holdings, Novartis, Sysmex
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
Sriram Yennu
Consulting or Advisory Role: Pfizer
Research Funding: Bayer (Inst), Genentech/Roche (Inst), Helsinn Therapeutics (Inst)
Angela Alexander
Research Funding: Merck, Genentech/Roche
David I. Quinn
Honoraria: Bayer, Astellas Pharma, Pfizer, Genentech/Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Exelixis, Janssen Oncology, Novartis, Mundipharma, Pharmacyclics, Clovis Oncology, Seattle Genetics
Consulting or Advisory Role: Astellas Pharma, Pfizer, Bristol Myers Squibb, Genentech/Roche, Merck Sharp & Dohme, Bayer, Exelixis, AstraZeneca, Janssen Oncology, Eisai, Novartis, US Biotest, Clovis Oncology, Seattle Genetics, AstraZeneca
Research Funding: Millennium (Inst), Genentech/Roche (Inst), Sanofi (Inst), GlaxoSmithKline (Inst), Merck (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Merck, Roche, Bayer, Bristol Myers Squibb Japan, Exelixis, Astellas Pharma
Uncompensated Relationships: Eisai, US Biotest
Peter Kuhn
Stock and Other Ownership Interests: Epic Sciences
Consulting or Advisory Role: Epic Sciences
Research Funding: Gilead Sciences, Kite Pharma, Amgen, AbbVie, Daiichi Sankyo, Eli Lilly, Novartis, Fluidigm
Patents, Royalties, Other Intellectual Property: HD-SCA technology was developed by me and members of my team while at The Scripps Research Institute, which has subsequently licensed the technology to Epic Sciences for exclusive commercial development. Patent 62/783,921: “System and Method for Determining Human Performance;” that is, the development of systems and methods in human performance to determine whether a patient with cancer will need unplanned medical care during cancer therapy.
Expert Testimony: BDMK Law
Jorge J. Nieva
Stock and Other Ownership Interests: Epic Sciences, Cansera
Consulting or Advisory Role: AstraZeneca, Western Oncolytics, Fujirebio Diagnostics, Takeda
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patent Pending – movement and unexpected health care encounters
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shcherbina A, Mattsson CM, Waggott D, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017;7:3. doi: 10.3390/jpm7020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zubrod CG, Schneiderman M, Frei E, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 4.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 5.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenreich CM, Wang Q, Neilson HK, et al. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576–585. doi: 10.1016/j.eururo.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ. 2017;189:E268–E274. doi: 10.1503/cmaj.160464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 9.Brown JC, Winters-Stone K, Lee A, et al. Cancer, physical activity, and exercise. Compr Physiol. 2012;2:2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkes AL, Pakenham KI, Chambers SK, et al. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: A randomized controlled trial. Ann Behav Med. 2014;48:359–370. doi: 10.1007/s12160-014-9610-2. [DOI] [PubMed] [Google Scholar]
- 11.Dieli-Conwright CM, Lee K, Kiwata JL. Reducing the risk of breast cancer recurrence: An evaluation of the effects and mechanisms of diet and exercise. Curr Breast Cancer Rep. 2016;8:139–150. doi: 10.1007/s12609-016-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1038/s41746-018-0032-6. Gresham G, Hendifar AE, Spiegel B, et al: Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digit Med . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1038/s41746-018-0027-3. Noah B, Keller MS, Mosadeghi S, et al: Author correction: Impact of remote patient monitoring on clinical outcomes: An updated meta-analysis of randomized controlled trials. NPJ Digit Med . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gualtieri L, Rosenbluth S, Phillips J. Can a free wearable activity tracker change behavior? The impact of trackers on adults in a physician-led wellness group. JMIR Res Protoc. 2016;5:e237. doi: 10.2196/resprot.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Kumamaru M, Jenkins S, et al. In-patient step count predicts re-hospitalization after cardiac surgery. J Cardiol. 2015;66:286–291. doi: 10.1016/j.jjcc.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: Relevance to clinical practice. Oncologist. 1999;4:191–196. [PubMed] [Google Scholar]
- 17. Microsoft: Track your calories burned. https://support.microsoft.com/en-us/help/4000329/band-health-and-exercise-calories-burned.
- 18.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(suppl 9):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 19. Sedentary Behaviour Research Network: Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours.” Appl Physiol Nutr Metab 37:540-542, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5) suppl 1:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broderick JE, May M, Schwartz JE, et al. Patient reported outcomes can improve performance status assessment: A pilot study. J Patient Rep Outcomes. 2019;3:41. doi: 10.1186/s41687-019-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasnain Z, Li M, Dorff T, et al. Low-dimensional dynamical characterization of human performance of cancer patients using motion data. Clin Biomech (Bristol, Avon) 2018;56:61–69. doi: 10.1016/j.clinbiomech.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]