Abstract
Estimated heritability of coffee intake ranges from 0.36 to 0.58, however, these point estimates assume that inherited effects are the same throughout the distribution of coffee intake, i.e., whether consumption is high or low relative to intake in the population. Quantile regression of 4788 child-parent pairs and 2380 siblings showed that offspring-parent and sibling concordance became progressively greater with increasing quantiles of coffee intake. Each cup/d increase in the parents’ coffee intake was associated with an offspring increase of 0.020±0.013 cup/d at the 10th percentile of the offsprings’ coffee intake (slope±SE, NS), 0.137±0.034 cup/d at their 25th percentile (P=5.2×10−5), 0.159±0.029 cup/d at the 50th percentile (P=5.8×10−8), 0.233±0.049 cup/d at the 75th percentile (P=1.8 ×10−6), and 0.284±0.054 cup/d at the 90th percentile (P=1.2×10−7). This quantile-specific heritability suggests that factors that distinguish heavier vs. lighter drinkers (smoking, male sex) will likely manifest differences in estimated heritability, as reported.
Keywords: coffee, heritability, quantile-specific heritability, quantile-dependent penetrance, smoking, sex differences, gene-environment interactions
Coffee consumption is higher in men, smokers, daily alcohol consumers, and older subjects (Loftfield et al. 2016). Benefits of coffee consumption include wakefulness, mental alertness, and improved ability to process information (Harland 2000, Soroko et al. 1996). There are also health benefits that may promote its use, including 10% reduction in total mortality risk in men and 15% reduction in women (Freedman et al. 2012), and reductions in risks for Parkinson’s disease, liver disease, and type 2 diabetes (Cornelis 2014). Its sustained use may be facilitated by headaches, diminished alertness, depression, and irritability from caffeine withdrawal (Juliano et al. 2004). Alternatively, caffeinated coffee may be contraindicated in persons with anxiety, elevated heart rate, hypertension (Farag et al. 2010), difficulty sleeping (Soroko et al. 1996), or low bone mineral density (Hallström et al. 2010). Most individual are able to assess the physiologic effects of caffeine and consume it accordingly (Smith 2002).
Genetic factors appear to play a prominent role in determining coffee intake. Concordance for coffee consumption was reported to be greater in monozygotic than dyzygotic twins in Italians in 1962 (Conterio et al. 1962). Subsequent twin studies reported heritabilities for coffee or caffeine intake of 0.36 in Caucasian American World War II male veterans (Swan et al. 1996), 0.41 in female twins living in the United Kingdom (Teucher et al. 2007), 0.42 in the Swedes (Reynolds et al. 2006), 0.43 in Caucasian women (Kendler et al. 1999), 0.45 in Finnish women (Laitala et al. 2008), 0.51 in Australians (Luciano et al. 2005), 0.56 in Finnish men (Laitala et al. 2008), and 0.58 in Virginians (Hettema et al. 1999).
The reason for different heritabilities across populations is not known, but may relate to the fact that coffee consumption patterns differ geographically (Heckman et al. 2010). “Quantile-dependent penetrance” (or expressivity) hypothesizes that the influence of genes may depend upon whether the phenotype (e.g., coffee consumption) is high or low relative to its population distribution (Williams 2012). In this regard, it is hypothesized that the environment (e.g., culture) affects coffee consumption, which in turn affects the phenotypic expression of genetic traits (quantile-specific expressivity: environment→phenotype→genetic expression) rather than the more traditional interpretation of gene-environment interaction (environment→genetic expression→phenotype). Quantile-specific effects have been shown to play a fundamental role in the genetics of body weight and lipoprotein concentrations while not affecting other traits such as height (Williams 2012). The differences in heritability between populations could relate in part to mean differences in consumption.
A predicted consequence of quantile-dependent expressivity is that the selection of subjects by environmental factors or behaviors that distinguish high vs. low coffee consumers may produce different genetic estimates, suggestive of gene-environment interactions. Multiple studies have reported greater caffeine consumption in smokers than nonsmokers (Loftfield et al. 2016, Swan et al. 1996, Hettema et al. 1999, Treur et al. 2017, Fredholm et al. 1999). In addition, Sachse et al. (1999) reported that the influence of genetic variation on caffeine metabolism is larger in smokers. It is not known whether the heritability of coffee consumption is affected by smoking, i.e., greater heritability for coffee intake in smokers due to their higher intake levels.
We therefore applied quantile regression to reported coffee intake from the Original and Offspring Cohorts of the Framingham Study to assess whether quantile-specifc expressivity affects the inheritance of coffee consumption. Quantile regression is a robust nonparametric method for estimating the regression slope at each quantile of the dependent variable. Bootstrap resampling provides nonparametric significance levels for the quantile-specific regression slopes and their differences. Their robust, nonparametric properties are important given coffee intake is discrete and not normally distributed.
Methods
The data were obtained from the National Institutes of Health FRAMCOHORT, GEN3, FRAMOFFSPRING Research Materials obtained from the National Heart Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center. The Original (generation 1) Framingham cohort consisted of 5,209 men and women between the ages of 30 and 62 from the town of Framingham, Massachusetts who were recruited and examined between 1948 and 1953 and re-examined biannually thereafter (Kannel et al. 1979). The Offspring (generation 2) cohort consisted of 5,124 adult children of the original participants and their spouses who were first examined between 1971 and 1975, re-examined eight years later, and then every three to four years thereafter (Kannel et al. 1979). Subjects were at least 16 years of age and not self-identified as nonwhite or Hispanic in the Offspring cohort (race and ethnicity were not requested in the Original cohort, but reported to be overwhelmingly white). Number of cups of regular (caffeinated) coffee consumed per day was requested at 12 examinations of the Framingham Original Cohort (Exams 4, 12, 13, 14, 15, 17–23) and 5 examinations of the Offspring Cohort (Exams 2–6). Cups of coffee consumed per day were not requested in the Framingham Third Generation Cohort (they reported categories of coffee intake).
Statistics
Age and sex adjustment was performed separately in the Original and Offspring Cohorts using standard least-squares regression with the following independent variables: female (0,1), age, age2, female x age, and female x age2. Individual subject values were taken as the average of the residuals over all available examinations. Although coffee intake was reported in cup or half-cup increments for the individual surveys, the age and sex-adjusted intakes averaged over multiple surveys had a continuous distribution. Offspring-father and offspring-mother correlations and regression slopes were computed using the age and sex-adjusted offspring and parent intakes from the Offspring and Original Cohorts, respectively. Offspring-parent correlations and regression slopes were computed by assigning a weight of one-half to the child-father and one-half to the child-mother pair (if both parents available), and assigning a weight of one to the child-parent pair if only one parent was available. Offspring-midparental correlations and regression slopes were computed by comparing each child’s age and sex-adjusted consumption to the average of the age and sex-adjusted parental values in those families having both parents. Full-sibling correlations were obtained by constructing all possible pairs using double entry (Karlin et al. 1981). The number of degrees of freedom for the standard error was Σki-2 for offspring-parent and midparental regression slopes and correlations, and Σ(ki-1) for sibship correlations and regression slopes, where ki is the number of offspring in family i and the summation is taken over all i, i=1,…, N nuclear families. Slopes are presented ±SE.
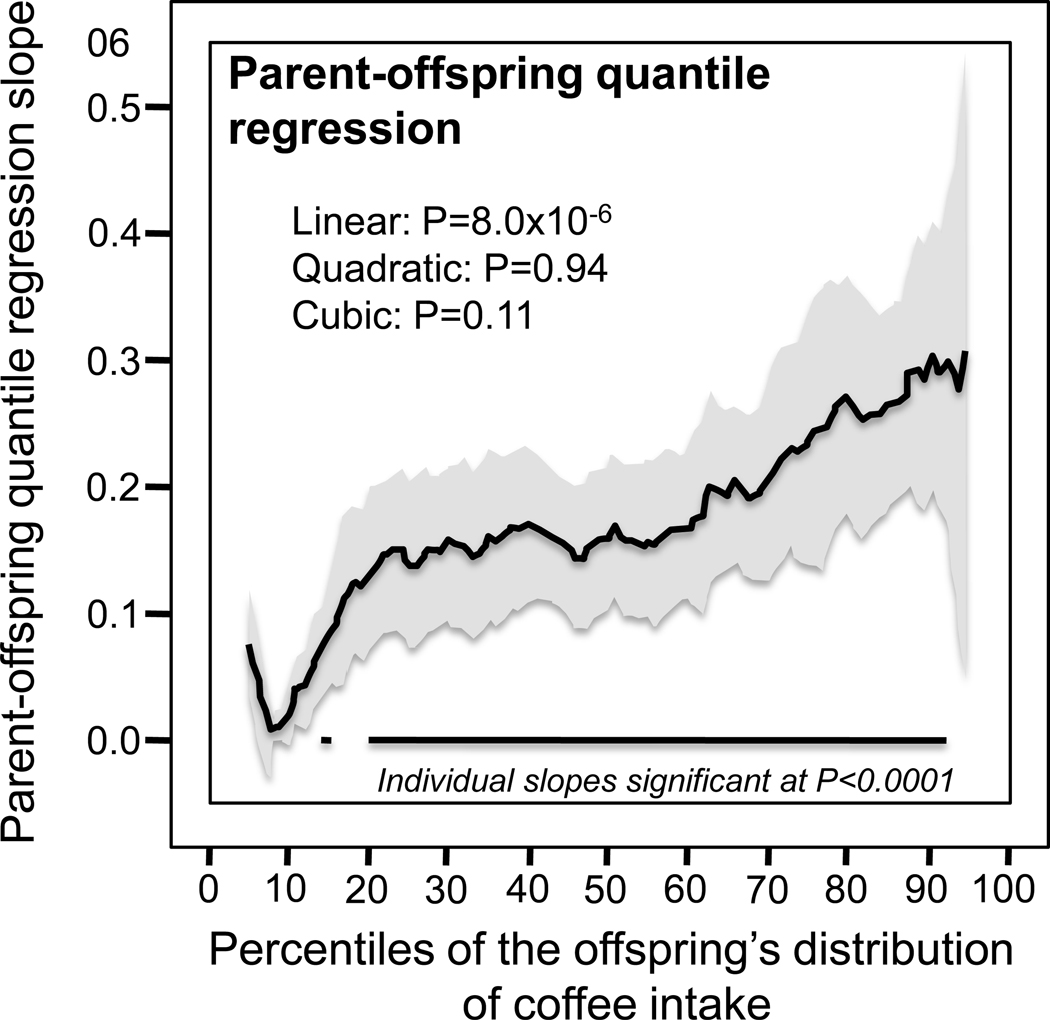
Simultaneous quantile regression is a well-developed statistical procedure (Koenker et al. 2001, Gould 1992) that estimates the regression coefficients for multiple quantiles using linear programming to minimize the sum of asymmetrically weighted absolute residuals, and bootstrap resampling to estimate their corresponding variances and covariances (Gould 1992). Quantile regression uses all the data for estimating the slope at each quantile by minimizing the absolute deviations. Simultaneous quantile regression was performed using the “sqreg” command of Stata (version. 11, StataCorp, College Station, TX) with one thousand bootstrap samples drawn to estimate the variance-covariance matrix for the 91 quantile regression coefficients between the 5th and 95th percentiles, and the post-estimation procedures (test and lincom) to test linear combinations of the slopes after estimation with Σki-2 degrees of freedom for offspring-parent regression slopes and Σ(ki-1) degrees of freedom for sibship regression slopes. Quantile-specific penetrance was assessed by: 1) estimating quantile-specific β-coefficient for the 5th, 6th, 95th percentiles of the sample distribution using simultaneous quantile regression (Figure 1, the <5th and >95th percentiles ignored because they were thought to be less stable); 2) plotting the quantile-specific β coefficients vs. the percentile of the trait distribution; and 3) testing whether the resulting graph is constant, or changes as a linear, quadratic, or cubic function of the percentile of the trait distribution using orthogonal polynomials (Winer et al. 1991). Heritability in the narrow sense (h2) was estimated as 2βOP/(1+r) from offspring-parent regression slopes (βOP) and [(1+8rβFS)0.5-1]/2r from full-sibs regression slopes (βFS) where r is the spouse correlation (Falconer and Mackay, 1996).
Figure 1.
(upper panel) presents the offspring-parent regression slopes (βOP) for selected quantiles of the offspring’s coffee intake (cups/d) and estimated heritability (h2). The slopes became progressively greater (i.e., steeper) with increasing quantiles of coffee intake. These quantile-specific regression slopes were included with those of other quantiles to create the quantile-specific heritability function in the lower panel. Shaded region designates the 95% confidence interval for the quantile-specific slopes. Parents and offspring data were adjusted for sex, age, age2, sex x age, and sex x age2. Heritability in the narrow sense (h2) was estimated as 2βOP/(1+r) from offspring-parent regression slopes (βOP) where r is the spouse correlation (Falconer and Mackay, 1996).
Gene-environment interactions were tested using standard regression analysis of the age- and sex-adjusted parent and offspring values, where the offspring consumption was predicted by the main effects of offspring’s smoking status and parents’ coffee consumption, and the interaction term between offspring’s smoking status and parents’ coffee consumption.
Results
Coffee intakes were reported by 1834 spouse-pairs, 4788 parent-child pairs in 1456 families with one or more parents, 3668 parent-child pairs in 904 families with both parents, and 2380 siblings in 906 families with two or more children per family. Their characteristics are displayed in table 1. Eliminating non-drinkers left 1703 spouse-pairs, 4183 parent-child pairs in 1346 families, and 2029 siblings in 795 families with two or more siblings. 61.3% of the offspring reported their coffee intake (including non-intake) at all five surveys, 14.8% at four surveys, 9.2% at three surveys, 7.1% at two surveys, and 7.6% at one survey. 10.1% of male offspring reported not drinking coffee, 13.3% consumed ≤1 cup/d, 22.2% consumed 1.1 to 2 cups/d, 20.0% consumed 2.1–3 cups/d, 14.9% consumed 3.1–4 cups/d, 8.5% consumed 4.1 to 5 cups/d, and 10.9% consumed >5 cups/d. Consumption in female offspring was less, 14.9% non-drinkers, 19.8% ≤1 cup/d, 24.6% 1.1 to 2 cups/d, 19.3% 2.1–3 cups/d, 10.2% 3.1–4 cups/d, 5.2% 4.1 to 5 cups/d, and 6.1% >5 cups/d.
Table 1.
Sample characteristics averaged over all visits
| Offspring-Parent | Sibships | |||||
|---|---|---|---|---|---|---|
| Fathers | Mothers | Sons | Daughter | Brothers | Sisters | |
| Sample size (N) | 1122 | 1208 | 1413 | 1541 | 1151 | 1229 |
| Age (years) | 63.37 ±8.99 |
65.96 ±8.32 |
49.61 ±10.09 |
50.23 ±10.23 |
49.45 ±10.26 |
49.86 ±10.27 |
| BMI (kg/m2) | 26.67 ±3.47 |
26.57 ±4.64 |
27.50 ±3.83 |
26.21 ±5.49 |
27.52 ±3.89 |
26.24 ±5.61 |
| Smokers (%) | 59.83 | 41.49 | 57.96 | 48.09 | 58.99 | 48.17 |
| Coffee intake | ||||||
| Nonconsumers (%) | 3.57 | 4.64 | 10.12 | 14.86 | 9.21 | 13.18 |
| Cups/d among drinkers | 2.37 ±1.56 |
2.12 ±1.40 |
3.00 ±2.10 |
2.43 ±1.82 |
2.98 ±2.12 |
2.40 ±1.78 |
Spouse and offspring-parent regression analyses
Reported intake was correlated r=0.18 between all spouses (r=0.20 excluding non-coffee drinkers). As traditionally estimated by least squares regression, the offspring-parent and offspring-midparental regression slopes (Table 2) for coffee correspond to estimates of heritability of 0.36 (0.31 adjusted for assortative mating) and 0.27, respectively (Falconer and Mackay, 1996).
Table 2.
Classical and quantile regression analyses of coffee intake
| Traditional regression analysis | Quantile regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Classical regression slope | Increase in slope per 1% increase in the percentile of the dependent variable’s distribution | Difference in slope between the 90th and 10th percentiles | |||||||
| Correlation | Linear effect | Nonlinear effects | |||||||
| Slope±SE | Significance | Slope±SE | Linear P | Quadratic P | Cubic P | Difference±SE | Signifcance | ||
| All | |||||||||
| Spouse | 0.18 | 0.1621±0.0209 | 7.8×10−15 | 0.0025±0.0005 | 2.7×10−6 | 0.003 | 0.50 | 0.1405±0.0435 | 0.001 |
| Offspring-parent | 0.13 | 0.1818±0.0262 | 3.8×10−12 | 0.0025±0.0006 | 8.0×10−5 | 0.94 | 0.11 | 0.2646±0.0538 | 8.6×10−7 |
| Son-parent | 0.15 | 0.2218±0.0397 | 2.4×10−8 | 0.0025±0.0010 | 0.009 | 0.54 | 0.63 | 0.2193±0.0834 | 0.009 |
| Daughter-parent | 0.11 | 0.1431±0.0344 | 3.2×10−5 | 0.0022±0.0009 | 0.01 | 0.76 | 0.09 | 0.2192±0.0809 | 0.007 |
| Offspring-midparent | 0.14 | 0.2659±0.0441 | 1.6×10−9 | 0.0031±0.0011 | 0.003 | 0.34 | 0.12 | 0.4110±0.0827 | 6.7×10−7 |
| Full siblings | 0.13 | 0.1257±0.0205 | 8.9×10−10 | 0.0024±0.0006 | 3.9×10−5 | 0.45 | 0.62 | 0.2128±0.0462 | 4.0×10−6 |
| Excluding non-coffee drinkers | |||||||||
| Spouse | 0.20 | 0.1836±0.0218 | <10−15 | 0.0027±0.0006 | 4.6×10−6 | 0.004 | 0.26 | 0.1451±0.0690 | 0.04 |
| Offspring-parent | 0.13 | 0.1859±0.0281 | 3.8×10−11 | 0.0030±0.0008 | 0.0001 | 0.63 | 0.66 | 0.2621±0.0697 | 0.0002 |
| Son-parent | 0.17 | 0.2448±0.0415 | 3.7×10−9 | 0.0033±0.0011 | 0.002 | 0.53 | 0.94 | 0.2574±0.1152 | 0.03 |
| Daughter-parent | 0.09 | 0.1234±0.0378 | 0.001 | 0.0025±0.0011 | 0.02 | 0.92 | 0.38 | 0.2498±0.0948 | 0.009 |
| Offspring-midparent | 0.16 | 0.2814±0.0465 | 1.4×10−9 | 0.0036±0.0011 | 0.001 | 0.48 | 0.43 | 0.3442±0.0975 | 0.0004 |
| Full siblings | 0.16 | 0.1257±0.0265 | 2.0×10−6 | 0.0024±0.0008 | 0.001 | 0.56 | 0.70 | 0.2128±0.0596 | 0.0004 |
Quantile-regression analyses
Figure 1 (upper panel) presents the offspring-parent regression slopes (βOP) for selected quantiles of the offsprings’ coffee intake (cups/d) and their corresponding heritabilities (h2). The slopes became progressively greater (i.e., steeper) with increasing quantiles of intake. These quantile-specific regression slopes were included with those of other quantiles to create the quantile-specific heritability function in the lower panel, i.e., where the parent-offspring slope (Y-axis) is plotted as a function of the quantile of the offsprings’ sample distribution (X-axis). Specifically, the Y-axis represents the slope at the 5th quantile of consumption, the 6th quantile of consumption…, and the 95th quantiles of the offsprings’ coffee consumption. The shaded area presents the 95% confidence intervals for the individual slopes at each quantile. The figure shows that each cup/d increase in the parents’ coffee intake was associated with an offspring’s increase of 0.020±0.013 cup/d at the 10th percentile of the offspring’s coffee intake (NS), 0.137±0.034 cup/d at their 25th percentile (P=5.2×10−5), 0.159±0.029 cup/d at the 50th percentile (P=5.8×10−8), 0.233±0.049 cup/d at the 75th percentile (P=1.8 ×10−6), and 0.284±0.054 cup/d at the 90th percentile (P=1.2×10−7). If the offspring-parent slope for coffee consumption was the same for all offspring quantiles as traditionally assumed, then the upper panel would display parallel regression lines, and the lower graph would present a simple horizontal line. In fact, the graph shows that the slopes became progressively greater with increasing quantiles of its offspring’s distribution, such that on average each 1-percent increase in the offspring distribution was associated with a 0.0025±0.0006 linear increase in the βOP slope (P=8.0×10−5).
The offspring-parent regression slope was over 14-fold greater at the 90th than at the 10th percentile of offspring reported consumption. The difference in slope between heavy and light drinkers (i.e., 90th −10th percentile±SE: 0.265±0.054, P=8.6×10−7) was over 50% greater than the traditional slope for the entire offspring sample (0.182±0.026, Table 2). Figure 2 shows that the offspring-parent slope was statistically significant (P<0.0001) at every percentile between the 18th and the 93rd percentiles of the offspring’s coffee intake. Table 2 and Figure 2 show that the increase in the offspring-parents regression slopes with increasing percentiles of the offspring distribution was further supported by the midparental-offspring, parent-son, parent-daughter, and full-sib analyses.
Figure 2.
Full-sib regression slopes (vertical axis) by quantiles of the sib’s coffee intake (horizontal axis). Shaded region designates the 95% confidence interval for the quantile-specific slopes. Siblings’ reported coffee intake (cups/d) were adjusted for sex, age, age2, sex x age, and sex x age2. Heritability in the narrow sense (h2) was estimated as [(1+8rβFS)0.5-1]/2r from full-sibs regression slopes (βFS) where r is the spouse correlation (Falconer and Mackay, 1996).
The quantile-specific effects were not weakened when non-coffee drinkers were eliminated from the analyses (Table 2). The effects became, in fact, stronger as measured by the average linear increase in the offspring-parent slope per 1% increase in the percentile of the offspring’s distribution, albeit significance was somewhat diminished due to smaller sample size.
Gene-environment interaction.
Standard regression analysis was used to test whether the higher coffee intake for smokers than nonsmokers (mean±SE: 3.12±0.06 vs. 2.20±0.04 cups/d) would result in greater heritability of coffee intake in smokers. Excluding abstainers, the parent-offspring regression slope for smokers was significantly greater than the slope for nonsmokers (slope±SE: 0.224±0.040 vs. 0.106±0.040, P=0.02 for difference) when the data were adjusted for age and sex (P=0.07 including nondrinkers).
Discussion
Prior studies tacitly assume that the genetic and non-genetic factors affecting coffee consumption in parents, offspring, and other related individuals apply to all quantiles of the phenotype (Conterio et al. 1962, Swan et al. 1996, Teucher et al. 2007, Reynolds et al. 2006, Kendler et al. 1999, Laitala et al. 2008, Luciano et al. 2005, Hettema et al. 1999, Kendler et al. 1999; Coffee and Caffeine Genetics Consortium et al. 2015). We have shown that the offspring-parent slope at the 90tth percentile of the population distribution was over 14-fold greater than the offspring-parent slope at the 10th percentile, and their difference was over 50% greater than the traditional offspring-parent slope for the entire sample. If the offspring-parent regression slope (representing approximately one-half the additive heritability, Falconer and Mackay 1996) is a fundamental characteristic of coffee inheritance, then surely a difference in the parent-offspring regression slopes between the 90th and 10th percentiles is at least as important, given it is 50% greater. Quantile specific effects were also significant for full-sib (including dominance and shared environmental effects) and spouse associations (reflecting phenotypic assortment, cohabitation, and social homogamy). These quantile-specific effects represent fundamental departures from the underlying statistical models used in the analyses of twin pairs and pedigrees.
Implications regarding gene-environment interactions
Figures 1 and 2 suggests that estimates of genetic heritability, cultural transmission, or shared environment are likely to increase with consumption levels. Correspondingly, environmental factors that tend to distinguish heavier vs. lighter coffee drinkers such as smoking will likely manifest differences in genetics, cultural transmission, or shared environment. For example, caffeine consumption is greater in smokers than smokers, possibly due to the co-adoption of these behaviors (Loftfield et al. 2016, Swan et al. 1996, Hettema et al. 1999, Treur et al. 2017, Fredholm et al. 1999). Smokers in the Framingham Offspring Study tended to drink more, and correspondingly, the offspring-parent regression slope for the smokers was significantly greater than the regression slope for the nonsmoker. Others have also suggested that the influence of genetic variation on caffeine metabolism is larger in smokers (Sachse et al. 1999). However, it is also true that genetic factors are reported to explain most of the association between smoking and caffeine consumption (Swan et al. 1996, Hettema et al. 1999, Treur et al. 2017) and it is also possible that the weaker offspring-parent relationship in coffee intake in nonsmoking than smoking twins is because the analyses exclude the inheritance of genotypes affecting both coffee and smoking use.
In our analyses, higher coffee intake in male than female offspring was associated with greater heritability for male than female offspring (i.e., greater offspring-parent slopes in Table 2), as predicted by their quantile-specific effects. Correspondingly, Rodenburg et al. (2012) reported a significantly greater decline per G variant allele of rs2472299 (a proxy for the GA allele of rs762551) in men than women. CYP1A2 enzyme activity is higher in men than women and is induced by polycyclic aromatic aromatic hydrocarbons in cigarette smoke (Gunes et al. 2008, Backman et al. 2008).
Coffee intake varies substantially between populations of European ancestry and throughout the world, with average consumption being nearly twice as high in Europe than America (Cornelis 2015). The quantile-specific effects we observed could contribute study differences in reported heritability of coffee consumption. Unfortunately, almost none of the reported twin studies describe the coffee intakes of their subjects, making it difficult to determine how the quantile-specific effects of Figures 1 and 2 may contribute to these differences.
Limitations.
Heritability as estimated from familial phenotypes lacks the specificity of genetic effects provided by associations with directly measured genotypes, however, they are important given that the proportion of the variance they explain, between 36% and 58%, is substantially greater than the 7.1% explained by the additive effects of single nucleotide polymorphisms (Coffee and Caffeine Genetics Consortium et al., 2015). Heritability estimates are not invariant to transformations and are dependent upon the variance of the phenotype, which may vary from population to population. The simple estimates of h2 from offspring-parent regression slopes provided by Falconer and Mackay (1966) does not include shared environmental effects, and those from full siblings does not include dominance and shared environment as well as restrictions on the effects of assortative mating. Another important limitation of these analyses is that the gene-environment interactions between smoking and coffee consumption may not be necessarily generalizable to the current population given the reductions in smoking rates since the 1970s.
In conclusion, these analyses suggest that that the heritability of coffee consumption is significantly dependent upon whether intake is high or low relative to its distribution in the population. Quantile-specific effects may also affect non-genetic determinants of coffee consumption. The quantile-specific effects are quite large relative to the effects individual specific loci identified from genomewide association studies which rely heavily on pooling across cohorts to achieve genomewide statistical significance (Coffee and Caffeine Genetics Consortium et al. 2015). Our results suggest that reliance on average effects may ignore important differences throughout the distribution of consumption, and that existing statistical model for disentangling environmental and genetic influences may ignore important quantile-dependent effects.
Acknowledgement
The data were obtained from the National Institutes of Health FRAMCOHORT, GEN3, FRAMOFFSPRING Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center. The author (PTW) was responsible for the project conception, development of overall research plan, analyzing data including performing the statistical analysis, and wrote the paper. The sole author had responsibility for all parts of the manuscript. There are no conflicts of interest to report. The Framingham Heart Study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195 and HHSN268201500001I). This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, FRAMCOHORT, GEN3, FRAMOFFSPRING, Boston University, or NHLBI.
This research was supported by grant R21ES020700 from the National Institute of Environmental Health Sciences, and an unrestricted gift from HOKA ONE ONE.
Footnotes
Conflicts of interest: None to report.
Literature cited
- Backman JT, Schroder MT, Neuvonen PJ (2008) Effects of gender and moderate smoking on the pharmacokinetics and effects of the CYP1A2 substrate tizanidine. Eur J Clin Pharmacol 64:17–24. [DOI] [PubMed] [Google Scholar]
- Coffee and Caffeine Genetics Consortium, Cornelis MC, Byrne EM, Esko T, Nalls MA, Ganna A, Paynter N, Monda KL, Amin N, Fischer K, Renstrom F, Ngwa JS, Huikari V, Cavadino A, Nolte IM, Teumer A, Yu K, Marques-Vidal P, Rawal R, Manichaikul A, Wojczynski MK, Vink JM, Zhao JH, Burlutsky G, Lahti J, Mikkilä V, Lemaitre RN, Eriksson J, Musani SK, Tanaka T, Geller F, Luan J, Hui J, Mägi R, Dimitriou M, Garcia ME, Ho WK, Wright MJ, Rose LM, Magnusson PK, Pedersen NL, Couper D, Oostra BA, Hofman A, Ikram MA, Tiemeier HW, Uitterlinden AG, van Rooij FJ, Barroso I, Johansson I, Xue L, Kaakinen M, Milani L, Power C, Snieder H, Stolk RP, Baumeister SE, Biffar R, Gu F, Bastardot F, Kutalik Z, Jacobs DR Jr, Forouhi NG, Mihailov E, Lind L, Lindgren C, Michaëlsson K, Morris A, Jensen M, Khaw KT, Luben RN, Wang JJ, Männistö S, Perälä MM, Kähönen M, Lehtimäki T, Viikari J, Mozaffarian D, Mukamal K, Psaty BM, Döring A, Heath AC, Montgomery GW, Dahmen N, Carithers T, Tucker KL, Ferrucci L, Boyd HA, Melbye M, Treur JL, Mellström D, Hottenga JJ, Prokopenko I, Tönjes A, Deloukas P, Kanoni S, Lorentzon M, Houston DK, Liu Y, Danesh J, Rasheed A, Mason MA, Zonderman AB, Franke L, Kristal BS; International Parkinson’s Disease Genomics Consortium (IPDGC).; North American Brain Expression Consortium (NABEC).; UK Brain Expression Consortium (UKBEC)., Karjalainen J, Reed DR, Westra HJ, Evans MK, Saleheen D, Harris TB, Dedoussis G, Curhan G, Stumvoll M, Beilby J, Pasquale LR, Feenstra B, Bandinelli S, Ordovas JM, Chan AT, Peters U, Ohlsson C, Gieger C, Martin NG, Waldenberger M, Siscovick DS, Raitakari O, Eriksson JG, Mitchell P, Hunter DJ, Kraft P, Rimm EB, Boomsma DI, Borecki IB, Loos RJ, Wareham NJ, Vollenweider P, Caporaso N, Grabe HJ, Neuhouser ML, Wolffenbuttel BH, Hu FB, Hyppönen E, Järvelin MR, Cupples LA, Franks PW, Ridker PM, van Duijn CM, Heiss G, Metspalu A, North KE, Ingelsson E, Nettleton JA, van Dam RM, Chasman DI (2015) Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 20:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conterio F, Chiarelli B (1962) Study of the inheritance of some daily life habits. Heredity 17:347–59. [DOI] [PubMed] [Google Scholar]
- Cornelis MC (2014) Gene-coffee interactions and health. Curr Nutr Rep 3:178–195. [Google Scholar]
- Cornelis MC (2015) Toward systems epidemiology of coffee and health. Curr Opin Lipidol. 26:20–9. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics (fourth ed.) Longmans Green, Harlow, Essex, UK: 1996 [Google Scholar]
- Farag NH, Whitsett TL, McKey BS, Wilson MF, Vincent AS, Everson-Rose SA, Lovallo WR (2010) Caffeine and blood pressure response: sex, age, and hormonal status. J Womens Health (Larchmt) 19:1171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83–133. [PubMed] [Google Scholar]
- Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R (2012) Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 366:1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould WW (1992) Quantile regression with bootstrapped standard errors. Stata Technical Bulletin. 9:19–21. [Google Scholar]
- Gunes A, Dahl ML (2008) Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 9:625–37. [DOI] [PubMed] [Google Scholar]
- Hallström H, Melhus H, Glynn A, Lind L, Syvanen AC, Michaelsson K (2010) Coffee consumption and CYP1A2 genotype in relation to bone mineral density of the proximal femur in elderly men and women: a cohort study. Nutr Metab (Lond) 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland BF (2000) Caffeine and nutrition. Nutrition 16:522–6. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Weil J, Gonzalez de Mejia E (2010) Caffeine (1, 3, 7-trime-thylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75:R77–87. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS (1999) A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend 57: 69–78. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Griffiths RR (2004) A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 176:1–29. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP (1979) An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 110:281–90. [DOI] [PubMed] [Google Scholar]
- Karlin S, Cameron EC, Williams PT (1981) Sibling and parent-offspring correlation estimation with variable family size. Proc Natl Acad Sci USA. 78:2664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA (1999) Caffeine intake, tolerance, and withdrawal in women: a population-based twin study. Am J Psychiatry 156:223–8. [DOI] [PubMed] [Google Scholar]
- Koenker R, Hallock KF (2001) Quantile regression. J Economic Perspectives. 15:143–56. [Google Scholar]
- Laitala VS, Kaprio J, Silventoinen K (2008) Genetics of coffee consumption and its stability. Addiction. 2008;103:2054–61. [DOI] [PubMed] [Google Scholar]
- Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, Graubard BI (2016) Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J Nutr. 146:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Kirk KM, Heath AC, Martin NG (2005) The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction 100:1510–7. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Barlow T, Pedersen NL. (2006) Alcohol, tobacco and caffeine use: spouse similarity processes. Behav Genet 36:201–15. [DOI] [PubMed] [Google Scholar]
- Rodenburg EM, Eijgelsheim M, Geleijnse JM, Amin N, van Duijn CM, Hofman A, Uitterlinden AG, Stricker BH, Visser LE (2012) CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking. Am J Clin Nutr. 96:182–7. [DOI] [PubMed] [Google Scholar]
- Sachse C, Brockmoller J, Bauer S, Roots I (1999) Functional significance of a C/A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 47:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A (2002) Effects of caffeine on human behavior. Food Chem Toxicol 40:1243–55. [DOI] [PubMed] [Google Scholar]
- Soroko S, Chang J, Barrett-Connor E (1996) Reasons for changing caffeinated coffee consumption: the Rancho Bernardo Study. J Am Coll Nutr. 15:97–101. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR (1996) The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. J Subst Abuse 8:19–31. [DOI] [PubMed] [Google Scholar]
- Teucher B, Skinner J, Skidmore PM, Cassidy A, Fairweather-Tait SJ, Hooper L, Roe MA, Foxall R, Oyston SL, Cherkas LF, Perks UC, Spector TD, MacGregor AJ (2007) Dietary patterns and heritability of food choice in a UK female twin cohort. Twin Res Hum Genet. 10:734–48. [DOI] [PubMed] [Google Scholar]
- Treur JL, Taylor AE, Ware JJ, Nivard MG, Neale MC, McMahon G, Hottenga JJ, Baselmans BM, Boomsma DI, Munafò MR, Vink JM (2017) Smoking and caffeine consumption: a genetic analysis of their association. Addict Biol. 22:1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PT (2012) Quantile-specific penetrance of genes affecting lipoproteins, adiposity and height. PLoS One. 7(1):e28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM (1991) Statistical principles in experimental design. Third edition McGraw-Hill; New York. [Google Scholar]