Abstract
Objective:
We performed these studies to learn how iodine in the form of free iodide behaves during stress.
Design:
Prospective observational trial using samples obtained from human trauma patients and retrospective observational study using remnant samples from human sepsis patients and arctic ground squirrels. Preclinical interventional study using hind-limb ischemia and reperfusion injury in mice.
Setting:
Level I trauma center emergency room and ICU and animal research laboratories.
Subjects:
Adult human sepsis and trauma patients, wild-caught adult arctic ground squirrels, and sexually mature laboratory mice.
Interventions:
Ischemia and reperfusion injury was induced in mice by temporary application of tourniquet to one hind-limb. Iodide was administered IV just prior to reperfusion.
Measurements and Main Results:
Free iodide was measured using ion chromatography. Relative to iodide in plasma from normal donors, iodide was increased 17-fold in plasma from trauma patients and 26-fold in plasma from sepsis patients. In arctic ground squirrels, iodide increases over three-fold during hibernation. And during ischemia/reperfusion injury in mice, iodide accumulates in ischemic tissue and reduces both local and systemic tissue damage.
Conclusions:
Iodide redistributes during stress and improves outcome after injury. Essential functions of iodide may have contributed to its evolutionary selection and be useful as a therapeutic intervention for human patients.
Keywords: hibernation, iodine, sepsis, stress, trauma
Animals use a variety of means to respond to life-threatening conditions (1). One of the most acute threats is low oxygen. If compensation for low oxygen is not sufficient, tissue can be damaged, and animals can die. However, damage can be variable in severity, and death is not inevitable. Human survival of stressful conditions involving local and/or systemic hypoxia has been documented since ancient times (2, 3). Some animals, such as the Arctic ground squirrel (AGS), have evolved innate resistance to hypoxia (4). What enables some people and animals to better survive the stress of low oxygen? We have learned that much of the loss of cell and tissue viability is caused not by low oxygen itself but rather by its return and the increase in reactive oxygen containing molecules such as hydrogen peroxide which can lead to cell and tissue damage (5). Reperfusion injury is well known in myocardial infarction where it is characterized by excessive innate and adaptive immune responses (6). Indeed, excessive inflammation is a phenomenon that leads to increased morbidity and mortality in many injuries and diseases (7).
Immune responses associated with inflammation are affected by thyroid hormone (8). The most active thyroid hormone, T3, which contains three iodine atoms, is known to stimulate oxygen consumption in neutrophils, macrophages, and dentritic cells during inflammation (9). This effect of thyroid hormone on leukocytes is apparent in hypo- and hyperthyroid patients that show reduced and excessive oxygen consumption, respectively (10–13). Stimulation of oxygen consumption, sometimes referred to as oxidative burst, results in the production of superoxide by nicotinamide adenine dinucleotide phosphate oxidases which rapidly is converted into hydrogen peroxide, required to mount an inflammatory response and produce inflammatory cytokines by nuclear factor kappa-light-chain-enhancer of activated B cells (14–16).
Whereas T3 plays a role in establishing an inflammatory response, its turnover may play a role in diminishing it as deiodination is strongly associated with proper leukocyte function (17–19). Deiodination of thyroid hormones releases free iodide which is able to suppress oxidative burst in neutrophils (20). Consistent with this, we found that increasing blood levels of iodide suppresses inflammation in animal models of ischemia and reperfusion injury and functions as a catalytic anti-inflammatory insofar as it is able to catalytically destroy hydrogen peroxide (21, 22). This capacity to improve outcome in models of ischemia and reperfusion injury is shared by other small fully reduced anions which we call elemental reducing agents (ERAs) that include bromide, selenide, and sulfide (21, 23, 24). These findings suggest that iodine and perhaps other ERAs, have common intrinsic properties used in biology that cannot be replaced by other elements.
Because the ERA selenide rapidly redistributes during injury in animals (24) and because selenium rapidly disappears from human blood upon injury (25), we tested whether iodine is also redistributed in response to physiologic challenges. To do this, we developed a quantitative assay using ion chromatography (IC) that is sensitive enough to measure iodide in mammalian blood. Using this method, we measured iodide levels in both healthy and disease states as well as different states of animation.
MATERIALS AND METHODS
Blood Iodide analysis
Blood iodide was quantitated using IC with amperometric detection (Metrohm, Herisau, Switzerland). Plasma was deproteinated using 75% acetonitrile. The limit of detection of this method is less than 1 ng/mL (ppb).
Healthy Plasma Samples
Plasma samples from 208 healthy nonmedical volunteers were obtained from Bloodworks Northwest remnant donor sample pool. Equal numbers of samples from males and females representing four ethnic groups were assayed: Caucasian (North American/European), Asian (Chinese, Southeast Asian, South Asian, Korean, Japanese), Mexican (Mexican/Chicano), and African American (African-American/Black, African/Black).
Trauma Plasma Samples
The prospective sample collection protocol for this study was approved by the Institutional Review Boards at Fred Hutchinson Cancer Research Center, University of Washington/Harborview Medical Center, and Department of Defense. One-hundred eleven patients age greater than or equal to 18 admitted to Harborview Medical Center (Seattle, WA) within 6 hours of sustaining severe blunt trauma injuries were enrolled into the study. Due to need for rapid patient capture, inclusion required clinical evidence of severe blunt injury and presence of hemorrhagic shock defined by either hypotension (blood pressure [BP] ≤ 90 systolic) or a base deficit of greater than or equal to six, and high likelihood of requiring blood transfusion within 6 hours of injury. Average injury severity score was 32 (range 5–75). Exclusion criteria included severe, preexisting organ failure, severe head injury, pregnancy, severe burns, prisoners, chronic steroid use, and those who were not expected to survive 48 hours, due to the need for serial sampling over several days, similar to other investigations such as the Glue Grant study of whole genome response to trauma (26). A 4-mL venous sample and 4 mL of discarded urine were obtained from all subjects within 12 hours of their injuries prior to the first CT with iodinated contrast. The dates and times of all CTs were obtained as well as the amount and type of contrast. All study samples were centrifuged to obtain plasma which was stored at –70°C. Iodine containing antiseptic solution was not used. Amiodarone (iodinated antiarrhythmic drug) was not administered nor was its use documented in patient history. Samples with high iodide measured by IC were subsequently assayed by inductively coupled plasma mass spectrometry (ICPMS) to measure total iodine; if it was greater than combined endogenous plus IC measured iodide, that sample was excluded from analysis.
Sepsis Plasma Samples
Plasma samples from sepsis patients were obtained as remnants from a previous prospective observational study performed at two emergency departments (EDs) in Seattle (Harborview Medical Center and University of Washington) with ~80,000 combined annual visits, between June 2016 and December 2017 (27, 28). The local Institutional Review Board approved this study with waiver of informed consent. The initial study included adult (> 17 yr old) admitted patients meeting at least one of the following signs of life-threatening organ dysfunction: 1) two systemic inflammatory response syndrome (SIRS) criteria and organ dysfunction (29), 2) systolic BP less than 90 mm Hg, or 3) lactate greater than 4.0 mmol/L during the ED stay. The parent study recruited patients regardless of ED diagnosis, excluding patients with trauma, patients transferred for management of intracranial hemorrhage, and those without remnant blood samples available for collection. Presence of infection in the ED was adjudicated independently by two of three attending emergency physicians, by review of the entire hospital course. The third physician adjudicated ties if necessary. Adjudications occurred at least 1 month after hospital discharge. The first two reviewers for each subject demonstrated high diagnostic agreement (kappa 0.82; 95% CI 0.75–0.88), with 28 of 301 cases (9.3%) requiring tie-breaking adjudication by the third attending physician. Patients adjudicated to not have infection in the ED were excluded from the current study. We obtained remnant EDTA blood samples, drawn during the ED stay, from the clinical laboratory for enrolled patients. The clinical laboratory kept samples refrigerated at 4°C and released remnant samples for research after 48 hours from the time of blood draw. All study samples were obtained between 48 and 72 hours from the blood draw, centrifuged to obtain plasma, and stored at –70°C. Patients for the current analysis were limited to those patients who had sufficient remaining plasma for iodide testing.
AGSs
All procedures using squirrels were performed and approved in accordance with University of Alaska Fairbanks Institutional Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals. AGSs (Urocitellus parryii) were trapped in early July in the northern foothills of the Brooks Range, Alaska, 40 miles south of Toolik Field Station (68°38 N, 149°38 W; elevation 809 m) and were transported to Fairbanks, Alaska, under Alaska Department of Fish and Game permit. AGS were housed in ambient temperature (Ta) between 16°C and 18°C with a 16:8 light/dark cycle until August 15, when they were moved to an environmental chamber maintained at Ta of 2°C and 4L:20D light cycle. During euthermic phases, water was provided ad libitum, and food was restricted to approximately 47 g per day of rodent chow (no 5663, Mazuri, PMI Nutrition International, Richmond, IN or 9GU5, formulated with Laboratory Diet, St. Louis, MO). Animals were housed individually in 12” × 19” × 12” stainless steel wire mesh hanging cages over ammonia absorbing corn cob litter. Once animals exhibited hibernation behavior (respiration under 5 breaths/min, curled posture, inactivity), food was withdrawn, and animals were placed in polycarbonate cages (8.5” ×1 7” × 8.5”) with shavings, cotton bedding, and gel hydration packets. Care was taken to minimize all disturbances in the hibernation chamber during the hibernation season, and access was restricted. Animals were implanted with iButton temperature loggers (Maxim Integrated, San Jose, CA) using sterile technique in the abdominal cavity as previously described (30). Euthermic animals were anesthetized with 5% isoflurane and maintained at 3% mixed with 100% medical grade oxygen delivered at a flow rate of 1.5 L/min; torpid animals did not require anesthesia. Blood was collected via cardiac puncture using heparinized syringes just prior to euthanasia. Plasma was separated by centrifugation, frozen and stored at –60°C.
Mouse Hindlimb Injury
All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center. Buprenorphine (0.05 mg/kg) was administered at the beginning of the experiment. Mice were preanesthetized using Ketamine (100 mg/kg) and Xylazine (10 mg/kg) and maintained under 1–3% isoflurane in 100% oxygen for 2 hours during tourniquet induced ischemia. A Mcgivney hemorrhoidal ligator (Integra, York, PA) was used to apply a small o-ring on one hind leg. After 2 hours, the o-ring was removed, and the animal was placed at 30°C for 3 hours of reperfusion. Iodide or saline was administered by retro-orbital injection at the end of the ischemic period before removal of the o-ring. After 3 hours of reperfusion, animals were euthanized, and blood and tissues were collected for analysis. All animals, treated and control, received identical anesthetic, injury, and recovery procedures. Plasma iodide levels were measured before and after injury in control and treated groups. Although the iodide-treated groups had increased plasma iodide compared with untreated groups, there was no difference in either treated or control groups before and after injury.
Muscle was homogenized in a small Kontes glass homogenizer and deproteinated using 75% acetonitrile. Lung tissue was collected and weighed before and after lyophilization. Blood concentrations of muscle creatine kinase and cardiac troponin I were assayed using enzyme-linked imunosorbent assay kits from Abcam (Cambridge, MA) and Life Diagnostics (West Chester, PA), respectively.
Data Collection and Analysis
We collated, graphed, and analyzed data using Prism software (GraphPad Software LLC, San Diego, CA).
Data and Materials Availability
All data collected are available herein, and each measurement is represented as an individual data point in the dot plots in the figures. All materials are commercially available, and protocols are described in relevant subsections of Materials and Methods.
RESULTS
We measured and compared blood iodide levels in healthy blood donors and patients suffering from severe blunt force trauma and sepsis. The trauma patients had experienced sufficient injury to display signs of hemorrhagic shock and require surgery followed by at least 2 days of intensive care; the sepsis patients had confirmed infection with signs of organ dysfunction and sepsis in the ED. Although the average iodide level in healthy donors was 5.7 ng/mL, we found that the average blood iodide in trauma patients was 99 ng/mL which is a 17-fold increase (p = 0.0063) (Fig. 1). Similarly, the average blood iodide in sepsis patients was 146 ng/mL, a 26-fold increase relative to healthy blood donors (p < 0.0001) (Fig. 1). All blood samples were taken prior to CT and administration of iodinated contrast media, which could increase blood iodide. We also used ICPMS to quantitate total iodine in the patient samples to ensure they were contrast-free.
Figure 1.
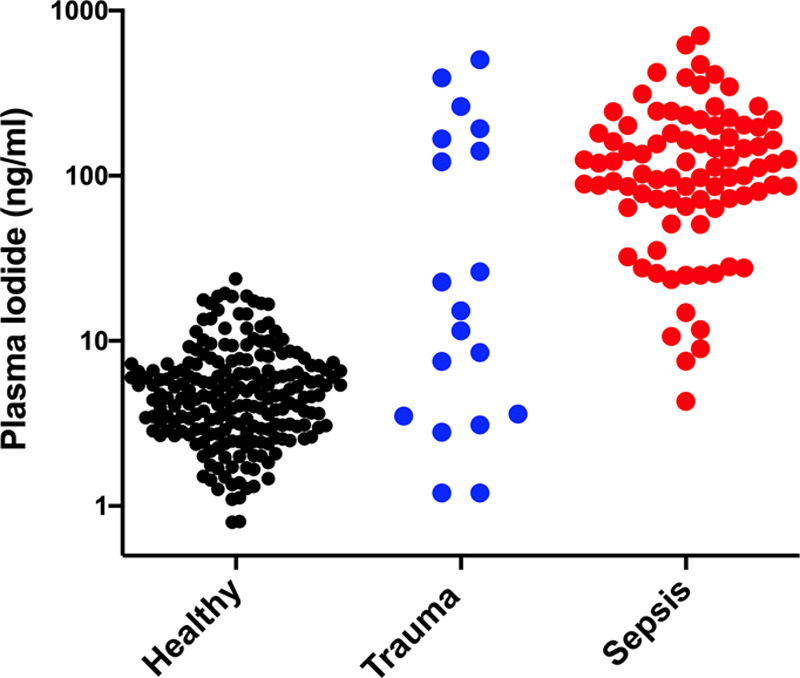
Blood iodide increases in sepsis and trauma patients. Black dots represent plasma iodide values from healthy nonmedical volunteers, n = 208, mean = 5.7 ng/mL, sd = 4.1, sem = 0.28, range = 0.80–23 ng/mL; blue dots are trauma plasma iodide values, n = 19, mean = 99 ng/mL, sd = 147, sem = 34, range = 1.2–504 ng/mL; red dots are sepsis, n = 86, mean = 146 ng/mL, sd = 130, sem = 14, range = 4.3–706 ng/mL. t test comparing Healthy and Trauma using Welch’s correction for unequal variance, p = 0.0063, and t test comparing Healthy and Sepsis using Welch’s correction for unequal variance, p < 0.0001
To learn if blood iodide increases in a natural state of reduced metabolism, we measured blood iodide in hibernating AGSs (Urocitellus parryii). While hibernating, these animals depress metabolism to survive prolonged cold exposure and food deprivation during winter in Alaska. Hibernation is composed of a series of multiday bouts of torpor (low metabolism) punctuated by brief (multihour) periods of interbout arousal during which metabolism and temperature increase. Blood iodide remains relatively unchanged between the summer active state and early torpor (53–76 ng/mL; p = 0.09). However, during late torpor and interbout arousal, blood iodide increases to 177 and 168 ng/mL, respectively (p = 0.018 and p = 0.005) (Fig. 2).
Figure 2.
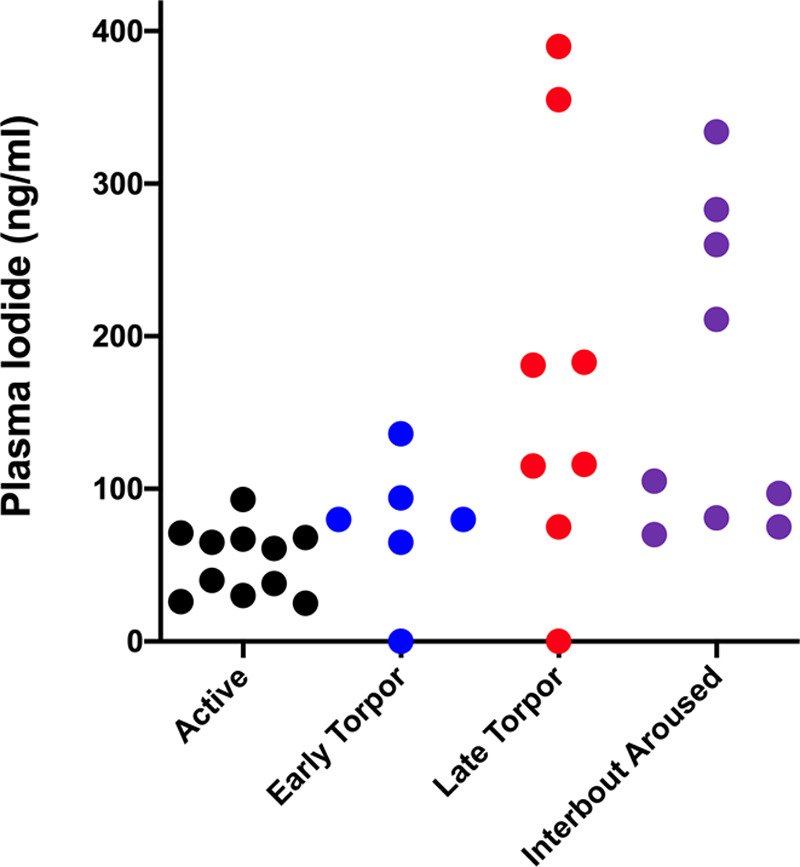
Blood iodide increases in hibernating Arctic ground squirrels. Black dots represent plasma iodide values in summer active squirrels, n = 11, mean = 53 ng/mL, sd = 22, sem = 6.7, range = 25–93 ng/mL. Blue dots are early torpor, n = 6, mean = 76 ng/mL, sd = 44, sem = 18, range = 0–136 ng/mL. Red dots are late torpor, n = 8, mean = 177 ng/mL, sd = 134, sem = 48, range = 0–390 ng/mL. Purple dots are interbout aroused, n = 9, mean = 168 ng/mL, sd = 104, sem = 35, range = 70–334. t test using Welch’s correction for unequal variance comparing: active versus early torpor, p = 0.09; active versus late torpor, p = 0.018; active versus interbout aroused, p = 0.005; early versus late torpor, p = 0.04; early torpor versus interbout aroused, p = 0.031; late torpor versus interbout aroused, p = 0.4.
To determine if an increase in blood iodide was beneficial, we designed a controlled experiment using a mouse model of localized ischemia and reperfusion injury that was less severe than whole body shock and hibernation, in which blood flow to one back leg was temporarily stopped by applying a tourniquet high up on the leg then allowed to reperfuse. This unilateral hindlimb injury is not severe enough to stimulate increased blood iodide. When iodide was administered after injury but before reperfusion, injury-dependent muscle damage assayed by blood concentration of muscle creatine kinase was significantly reduced (saline treated = 18,760 U/L vs iodide treated = 10,556 U/L; p = 0.00073) (Fig. 3A). We also found that iodide administration reduced both heart damage as measured by blood cardiac troponin I (saline = 2.6 vs iodide = 1.4 ng/mL; p = 0.022) (Fig. 3B) and lung edema measured by comparing the ratios of wet and dry lung tissue weights (saline = 4.4 vs iodide = 3.6; p = 0.014) (Fig. 3C). This result suggests that iodide administration may reduce systemic inflammation. In addition, iodide localizes in muscle from injured legs relative to uninjured legs (Fig. 4, A and B). In untreated animals, endogenous muscle iodide was 20.5 ng/mg in the injured leg and 8.4 ng/mg in the uninjured leg (p < 0.0001) (Fig. 4A). In iodide animals treated with therapeutic iodide, muscle from the injured leg contained 232 ng/mg and muscle from the uninjured leg contained 52 ng/mg (p < 0.0007) (Fig. 4B).
Figure 3.
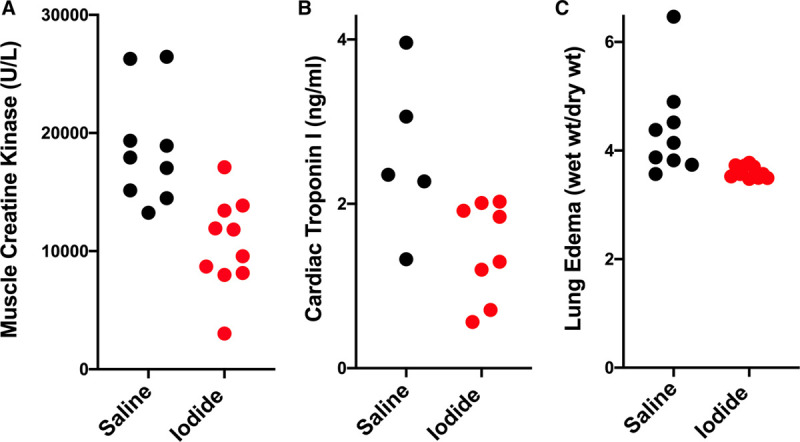
Iodide treatment reduces damage after mouse hindlimb ischemia and reperfusion injury. In both panels, black dots represent values from saline treated animals, and red dots represent values from iodide treated animals. A, Blood concentration of skeletal muscle creatine kinase in animals treated with saline (n = 9, mean = 18,760 U/L, sd = 4,765, sem = 1,588, range 13,246–26,458 U/L) and iodide (n = 10, mean = 10,556 U/L, sd = 3,932, sem = 1,243, range = 3,019–17,097 U/L), p = 0.00073. B, Blood concentration of cardiac troponin I in saline (n = 5, mean = 2.6 ng/mL, sd = 0.98, sem = 0.44, range = 1.3–4.0 ng/mL) and iodide treated animals (n = 8, mean = 1.45 ng/mL, sd= 0.59, sem = 0.21, range = 0.56–2.0 ng/mL), p = 0.022. C, Lung edema (wet weight/dry weight) in saline (n = 9, mean = 4.4, sd = 0.89, sem = 0.30, range = 3.6–6.5) and iodide treated animals (n = 10, mean = 3.6, sd = 0.11, sem = 0.04, range = 3.5–3.8), p = 0.014.
Figure 4.
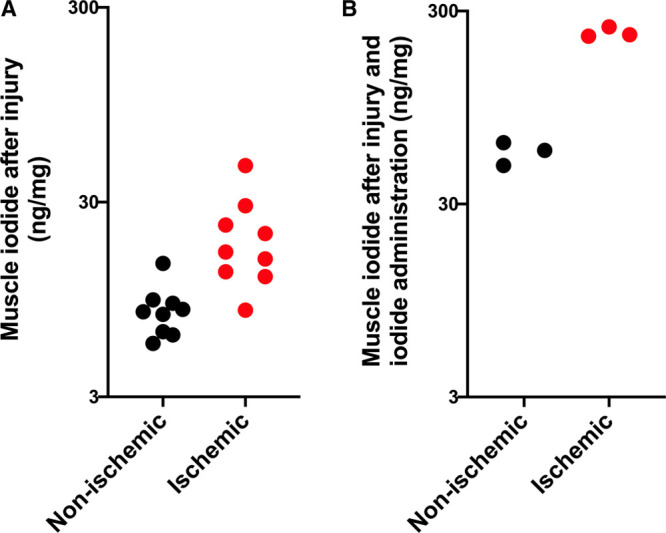
Iodide increases in ischemic muscle tissue after injury. In both panels, black dots represent iodide values from nonischemic muscle tissue, and red dots represent iodide values from ischemic muscle tissue. A, Iodide in nonischemic (n = 9, mean = 8.4 ng/mg, sd = 2.6, sem = 0.88, range = 5.6–14.5 ng/mg) and ischemic leg muscle (n = 9, mean = 20.5 ng/mg, sd = 11.4, sem = 3.8, range = 8.4–46 ng/mg) after ischemia reperfusion injury, p < 0.0001. B, Muscle iodide after ischemia reperfusion injury and 1 mg/kg iodide administration in nonischemic (n = 3, mean = 56 ng/mg, sd = 7.5, sem = 4.4, range 48–62 ng/mg) and ischemic (n = 3, mean = 232 ng/mg, sd = 14, sem = 8.2, range 222–249 ng/mg), p = 0.0007.
DISCUSSION
We present data showing that blood iodide increases in patients suffering from sepsis and traumatic shock and in squirrels during hibernation. Although these conditions differ in their specific stimuli (injury and infection in the cases of traumatic shock and sepsis and extreme environmental conditions in hibernation), they are similar in that they represent significant physiologic challenges of their respective organismal constancies. Many early investigators recognized the conserved nature of stress responses and hypothesized that fundamental biological details are probably shared in divergent species in response to seemingly unrelated stimuli (1, 31, 32). This idea set the stage for basic and clinical research on stress responses in health and disease. The increase in blood iodide in divergent species in unrelated circumstances is consistent with the idea that it plays a role in a conserved stress response.
We also showed that iodide administered at supra-normal levels improved outcome after injury and localized to the site of injured muscle tissue in mice. It is likely the increased iodide in the injured tissue came from deiodinated thyroid hormone. This idea is consistent with the fact that blood levels of both T3 and T4 decrease in many different diseases (33). This phenomenon is referred to by several different names over the last 70 years including nonthyroidal illness (NTIS), low T3, and euthyroid sick syndrome and has been associated with sepsis, trauma, heart attack, diabetes, renal insufficiency, and burns, and its extent of change correlates directly with severity of illness (33). Although the underlying mechanism of this phenomenon remains unclear, we demonstrated in a previous study that iodide administration sustains normal thyroid hormone levels during injury (22). This suggests that increasing blood iodide counteracts NTIS. Given that iodide is the product of thyroid hormone degradation by deiodinases which are upregulated in disease (34), it is logical to consider the degradation a natural stress response in which thyroid hormone is deiodinated to create iodide.
If increased blood iodide is part of a conserved stress response then what is the mechanism by which iodide improves outcome? Hydrogen peroxide plays a critical role in the establishment of the inflamed state (35) and, when over expressed, is likely to cause cell and tissue damage and disease resulting from excessive inflammation (36–40). The facts that iodide can catalytically convert hydrogen peroxide to oxygen and water (41) and administration of exogenous iodide increases antiperoxidant activity in the blood of animals after injury (22) suggest that this catalytic antiperoxidant activity is a mechanism by which iodide may improve outcome. The catalytic nature of iodide’s antioxidant activity distinguishes it from all other antioxidants. Vitamin C, Vitamin E, and other small molecule antioxidants are sacrificial reducing agents, becoming oxidized during the reactions with hydrogen peroxide and/or other reactive oxygen species and rapidly depleted during inflammatory states. Spent antioxidant products of these reactions must be rereduced before they are able to detoxify more reactive oxygen species (42). In conditions such as sepsis and trauma when the reducing power of many cells is exhausted, the accumulation of the oxidized forms of antioxidant molecules in tissues and blood hastens the accumulation of irreversible oxidative damage (43). During hibernation, the increase in blood iodide we observe could complement existing antioxidant defense pathways that minimize oxidative damage during torpor and emergence from torpor (44–46). As such, iodide redistribution to the blood could serve as a safe and synergistic compensatory response to limit damage caused by hydrogen peroxide.
The first two ERAs we found that reduce ischemia and reperfusion damage were sulfide and selenide (23, 24). Both are able to effectively reduce oxygen demand in small mammals and improve survival in extreme conditions. However, they are quite toxic, and their physiologic profiles do not scale to large mammals. We then found that bromide, previously known as one of the first sedatives (47), and iodide also had similar properties (21). In contrast to sulfide and selenide, iodide is not toxic, and its ability to improve survival in life-threatening conditions did scale to large animals in preclinical studies (22). These preclinical demonstrations provided the foundation for phase I and phase II clinical safety trials of iodide in healthy human volunteers and patients suffering from ischemia and reperfusion injury to the heart (i.e., during acute myocardial infarction). In these clinical studies, exogenously administered 1 mg/kg iodide safely increased blood iodide to an average of 8,290 ng/mL, which is more than 1,400-fold greater than healthy volunteers and 84-fold greater than the blood iodide in trauma patients (48). Furthermore, although these clinical safety trials were not designed or provisioned to test efficacy, which will be tested in a phase III study beginning in 2020, trends in clinical measures suggested that iodide administration improved outcome in humans suffering from myocardial infarction. Although clinical evidence to support iodide administration during critical illness does not yet exist, clinicians show a willingness to use exogenous micronutrients as part of metabolic resuscitation (49). In the future, iodide administration may be integrated into the routine care of critically ill patients as well. Episodes of severe stress such as blunt trauma and sepsis, known to induce an intense inflammatory response fueled in large part by excessive oxidant generation and bystander cell injury and organ dysfunction, may well benefit from elevated levels of exogenous iodide during the early stages and/or ongoing activation of these destructive processes.
We hypothesize that iodine redistribution is an evolutionarily conserved life process used by diverse organisms to respond to stress. Although we studied iodide in mammals, others have shown that brown algae (e.g., kelp) release iodide in response to hydrogen peroxide created by exposure to air and sunlight when the tide goes out (50). Likewise, moon jellyfish also concentrate iodine and use it to degrade hydrogen peroxide (51). The results presented here demonstrate that in response to significant physiologic stress, mammals increase blood iodide and that iodide supplementation improves outcome after injury. We conclude that iodide redistribution enables organisms to better tolerate stress, leading to its evolutionary selection as an essential stress response mechanism in many organisms. For these reasons, iodide may be useful as a clinical therapeutic.
ACKNOWLEDGMENTS
Laura Hennessey coordinated trauma patient sample collection with assistance of Hikmatullah Arif, Elizabeth Barnes, Mahrukh Kadri, and Keegan Stromberg. Sakshi Sehgal help procure sepsis patient samples.
Footnotes
Supported, in part by grants from Army Research Office (to Dr. Roth) and National Institutes of Health (to Dr. Drew).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Canon WB. The Wisdom of the Body. 1932. New York, NY, W. W. Norton and Co. [Google Scholar]
- 2.Freilich H. Ancient accounts of mouth-to-mouth resuscitation. JAMA. 1964; 189:383 [Google Scholar]
- 3.Ekmektzoglou KA, Johnson EO, Syros P, et al. Cardiopulmonary resuscitation: A historical perspective leading up to the end of the 19th century. Acta Med Hist Adriat. 2012; 10:83–100 [PubMed] [Google Scholar]
- 4.Bhowmick S, Drew KL. Mechanisms of innate preconditioning towards ischemia/anoxia tolerance: Lessons from mammalian hibernators. Cond Med. 2019; 2:134–141 [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen PR. Myocardial reperfusion injury: Experimental evidence and clinical relevance. Eur Heart J. 1995; 16:734–740 [DOI] [PubMed] [Google Scholar]
- 6.Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol. 2010; 2:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013; 123:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montesinos MDM, Pellizas CG. Thyroid hormone action on innate immunity. Front Endocrinol (Lausanne). 2019; 10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Spek AH, Jim KK, Karaczyn A, et al. The thyroid hormone inactivating type 3 deiodinase is essential for optimal neutrophil function: Observations from three species. Endocrinology. 2018; 159:826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández V, Videla LA. On the mechanism of thyroid hormone-induced respiratory burst activity in rat polymorphonuclear leukocytes. Free Radic Biol Med. 1995; 19:359–363 [DOI] [PubMed] [Google Scholar]
- 11.Nagy JT, Szabó T, Komáromi I, et al. A possible competition between 5’-monodeiodination of thyroxine and the respiratory burst in human granulocytes. Horm Metab Res. 1986; 18:415–417 [DOI] [PubMed] [Google Scholar]
- 12.Russo-Carbolante EM, Polizzelo AC, Azzolini AE, et al. Neutrophils from Brazilian patients with Graves’ disease: Some biochemical and functional aspects. Cell Biochem Funct. 2005; 23:297–306 [DOI] [PubMed] [Google Scholar]
- 13.Marino F, Guasti L, Cosentino M, et al. Thyroid hormone regulation of cell migration and oxidative metabolism in polymorphonuclear leukocytes: Clinical evidence in thyroidectomized subjects on thyroxine replacement therapy. Life Sci. 2006; 78:1071–1077 [DOI] [PubMed] [Google Scholar]
- 14.Sacco KA, Smith MJ, Bahna SL, et al. NAPDH oxidase-specific flow cytometry allows for rapid genetic triage and classification of novel variants in chronic granulomatous disease. J Clin Immunol. 2020; 40:191–202 [DOI] [PubMed] [Google Scholar]
- 15.Park HS, Jung HY, Park EY, et al. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004; 173:3589–3593 [DOI] [PubMed] [Google Scholar]
- 16.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991; 10:2247–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff SJ, Durack DT, Rosen H, et al. Functional studies on human peritoneal eosinophils. Infect Immun. 1977; 17:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woeber KA, Doherty GF, Ingbar SH. Stimulation by phagocytosis of the deiodination of L-thyroxine in human leukocytes. Science. 1972; 176:1039–1041 [DOI] [PubMed] [Google Scholar]
- 19.Woeber KA, Ingbar SH. Metabolism of L-thyroxine by phagocytosing human leukocytes. J Clin Invest. 1973; 52:1796–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyachi Y, Niwa Y. Effects of potassium iodide, colchicine and dapsone on the generation of polymorphonuclear leukocyte-derived oxygen intermediates. Br J Dermatol. 1982; 107:209–214 [DOI] [PubMed] [Google Scholar]
- 21.Iwata A, Morrison ML, Roth MB. Iodide protects heart tissue from reperfusion injury. PLoS One. 2014; 9:e112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison ML, Iwata A, Keyes CC, et al. Iodide improves outcome after acute myocardial infarction in rats and pigs. Crit Care Med. 2018; 46:e1063–e1069 [DOI] [PubMed] [Google Scholar]
- 23.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005; 308:518. [DOI] [PubMed] [Google Scholar]
- 24.Iwata A, Morrison ML, Blackwood JE, et al. Selenide targets to reperfusing tissue and protects it from injury. Crit Care Med. 2015; 43:1361–1367 [DOI] [PubMed] [Google Scholar]
- 25.Hawker FH, Stewart PM, Snitch PJ. Effects of acute illness on selenium homeostasis. Crit Care Med. 1990; 18:442–446 [DOI] [PubMed] [Google Scholar]
- 26.Raymond SL, Hawkins RB, Wang Z, et al. Prospective validation of a transcriptomic metric in severe trauma. Ann Surg. 2020; 271:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henning DJ, Hall MK, Watsjold BK, et al. Interleukin-6 improves infection identification when added to physician judgment during evaluation of potentially septic patients. Am J Emerg Med. 2020; 38:947–952 [DOI] [PubMed] [Google Scholar]
- 28.Henning DJ, Bhatraju PK, Johnson NJ, et al. Physician judgment and circulating biomarkers predict 28-day mortality in emergency department patients. Crit Care Med. 2019; 47:1513–1521 [DOI] [PubMed] [Google Scholar]
- 29.Dellinger RP, Carlet JM, Masur H, et al. ; Surviving Sepsis Campaign Management Guidelines Committee. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004; 32:858–873 [DOI] [PubMed] [Google Scholar]
- 30.Frare C, Jenkins ME, McClure KM, et al. Seasonal decrease in thermogenesis and increase in vasoconstriction explain seasonal response to N6 -cyclohexyladenosine-induced hibernation in the Arctic ground squirrel (Urocitellus parryii). J Neurochem. 2019; 151:316–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard C. An Introduction to the Study of Experimental Medicine. 1949. Reprinted New York, NY, Henry Schuman Inc; originally published in 1865 [Google Scholar]
- 32.Selye H. Stress and the general adaptation syndrome. Br Med J. 1950; 1:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braverman LE. Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text. 2012. Philadelphia, PA, Lippincott Williams & Wilkins [Google Scholar]
- 34.Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 2008; 4:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Navarro FJ, Martínez-Morcillo FJ, de Oliveira S, et al. Hydrogen peroxide in neutrophil inflammation: Lesson from the zebrafish. Dev Comp Immunol. 2020; 105:103583. [DOI] [PubMed] [Google Scholar]
- 36.Eskici G, Axelsen PH. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry. 2012; 51:6289–6311 [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Atwood CS, Hartshorn MA, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999; 38:7609–7616 [DOI] [PubMed] [Google Scholar]
- 38.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006; 440:944–948 [DOI] [PubMed] [Google Scholar]
- 39.Burgoyne JR, Oka S, Ale-Agha N, et al. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal. 2013; 18:1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunawardena D, Raju R, Münch G. Hydrogen peroxide mediates pro-inflammatory cell-to-cell signaling: A new therapeutic target for inflammation? Neural Regen Res. 2019; 14:1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebhafsky HA. The catalytic decomposition of hydrogen peroxide by the iodine-iodide couple at 25°. J Am Chem Soc. 1932; 54:1792–1806 [Google Scholar]
- 42.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999; 13:1007–1024 [DOI] [PubMed] [Google Scholar]
- 43.van der Crabben SN, Stegenga ME, Blümer RM, et al. Erythrocyte glutathione concentration and production during hyperinsulinemia, hyperglycemia, and endotoxemia in healthy humans. Metabolism. 2011; 60:99–106 [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Zhang J, Xu S, et al. Controllable oxidative stress and tissue specificity in major tissues during the torpor–arousal cycle in hibernating Daurian ground squirrels. Open Biol. 2018; 8:180068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma YL, Zhu X, Rivera PM, et al. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R1297–R1306 [DOI] [PubMed] [Google Scholar]
- 46.Orr AL, Lohse LA, Drew KL, et al. Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol. 2009; 153:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macleod N. The bromide sleep: A new departure in the treatment of acute Mania. Br Med J. 1900; 1:134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adlam D, Uren N, Oldroyd K, et al. A randomized double-blind, dose ranging clinical trial of intravenous FDY-5301 in acute STEMI patients undergoing primary PCI.. Seattle, WA, Faraday Pharmaceuticals; Available at: https://static1.squarespace.com/static/55664ddde4b0349f890f744a/t/5e192962896438638101c294/1578707322480/FDY-5301-AHA+2019.pdf. [DOI] [PubMed] [Google Scholar]
- 49.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest. 2017; 151:1229–1238 [DOI] [PubMed] [Google Scholar]
- 50.Küpper FC, Carpenter LJ, McFiggans GB, et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci U S A. 2008; 105:6954–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berking S, Czech N, Gerharz M, et al. A newly discovered oxidant defence system and its involvement in the development of Aurelia aurita (Scyphozoa, Cnidaria): Reactive oxygen species and elemental iodine control medusa formation. Int J Dev Biol. 2005; 49:969–976 [DOI] [PubMed] [Google Scholar]


