Abstract
Background:
Coronavirus disease 2019 is a pandemic with no specific therapeutic agents or vaccination. Small published case series on critically ill adults suggest improvements in clinical status with minimal adverse events when patients receive coronavirus disease 2019 convalescent plasma, but data on critically ill pediatric patients are lacking. We report a series of four critically ill pediatric patients with acute respiratory failure who received coronavirus disease 2019 convalescent plasma as a treatment strategy for severe disease.
Case Summary:
Patients ranged in age from 5 to 16 years old. All patients received coronavirus disease 2019 convalescent plasma within the first 26 hours of hospitalization. Additional disease modifying agents were also used. All patients made a full recovery and were discharged home off of oxygen support. No adverse events occurred from the coronavirus disease 2019 convalescent plasma transfusions.
Conclusion:
Coronavirus disease 2019 convalescent plasma is a feasible therapy for critically ill pediatric patients infected with severe acute respiratory syndrome coronavirus 2. Well-designed clinical trials are necessary to determine overall safety and efficacy of coronavirus disease 2019 convalescent plasma and additional treatment modalities in pediatric patients.
Keywords: acute respiratory failure, convalescent plasma, convalescent plasma; pediatric intensive care unit, pediatrics
BACKGROUND
Since its origin in Wuhan, China, the epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide, leading the World Health Organization to declare coronavirus disease 2019 (COVID-19) a pandemic (1, 2). As of June 19, 2020, the virus has caused over 450,000 deaths worldwide and infected patients in more than 215 countries (2). Several published studies suggest that COVID-19 illness might have a milder course in pediatric patients (3–6), but severe disease does occur (7).
There is currently no specific treatment or vaccine for COVID-19. Multiple therapies including antivirals and immunomodulators are under investigation (8, 9). Convalescent plasma has been used to treat other high-risk viral pathogens, and its use for COVID-19 is being explored (10–13). Several small case series in critically ill adults receiving COVID-19 convalescent plasma (CCP) suggest improvement in patient clinical status with minimal adverse events (8–10, 14–16). A larger analysis of 5,000 adult patients with severe COVID-19 infection suggested safety in hospitalized patients (17). There are no published studies regarding the use of CCP in critically ill pediatric patients. In this report, we describe a single-center’s initial experience with CCP in four critically ill pediatric patients with SARS-CoV-2.
CASE SUMMARY
All four patients were admitted to the PICU with a diagnosis of SARS-CoV-2 and acute respiratory failure requiring high-flow nasal cannula (HFNC) at admission. Table 1 summarizes key demographic features of the four patients, the timing of CCP therapy, and additional therapeutic modalities employed. Three of the four patients received CCP obtained via apheresis from donors donating to the University of North Carolina Blood Donation Center. One patient received CCP from an outside supplier. Donors met Food and Drug Administration (FDA) requirements for eligibility by both standard blood donor and CCP donor criteria. Donors had documented SARS-CoV-2 infection by a diagnostic test (e.g., nasal swab polymerase chain reaction [PCR]) or a positive serologic test for SARS-CoV-2 antibodies. All donors had complete resolution of symptoms for at least 14 days prior to donation. For each case, a single-patient emergency Investigational New Drug (E-IND) application was submitted to the FDA and approved. Parental consent was obtained in each case prior to administration of CCP. Donor units obtained at the University of North Carolina Blood Donation Center were tested for the presence of anti-SARS-CoV-2 antibodies using a customized enzyme-linked immunosorbent assay binding assay targeted to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein (18).
Case 1
A 15-year-old obese, Hispanic male was transferred to the PICU from an outside hospital due to rapidly progressing acute respiratory failure secondary to SARS-CoV-2 which was diagnosed by viral PCR. The patient had presented to the hospital with 7 days of fever (temperature 40.2°C), worsening cough, shortness of breath, chest tightness, nausea, vomiting, and poor per os (PO) intake. He initially required only low-flow nasal cannula but quickly escalated to HFNC. On arrival to the PICU, he was febrile and tachycardic with normal blood pressure and perfusion. He was tachypneic and in moderate respiratory distress on 30 L HFNC, 100% Fio2. Admission chest radiograph (CXR) demonstrated hypoinflated lungs with patchy perihilar and left midlung opacities compatible with viral pneumonia and history of confirmed COVID-19. Laboratories were significant for elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin, lactate dehydrogenase (LDH), fibrinogen, and d-dimer, as well as lymphopenia. Troponin, pro-b-type natriuretic peptide (pro-BNP), and cardiac function were normal. Table 1 provides admission laboratory values.
Table 1.
Demographics and Clinical Characteristics of Four Critically Ill Pediatric Patients With Coronoavirus Disease 2019 Treated With Convalescent Plasma
| Patients | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age (yr, mo) | 15, 9 | 16, 4 | 5, 11 | 12 , 6 |
| Sex | Male | Male | Female | Female |
| Body mass index (kg/m2) | 35.97 | 37.4 | 20.32 | 44.92 |
| Ethnicity | Hispanic | Asian | Hispanic | Hispanic |
| Blood type | O positive | A positive | O positive | A positive |
| Comorbidities | Obesity | Obesity | None | Obesity, asthma |
| Maximum O2 support | HFNC (60 L, 100%) | HFNC (50 L, 100%) | Venoarterial extracorporeal membrane oxygenation | Mechanical ventilation |
| Hospital LOS (d) | 10 | 7 | 24 | 11 |
| ICU LOS (d) | 9 | 4 | 15 | 7 |
| Laboratories at admission | ||||
| C-reactive protein (mg/L) | 357.9 | 31 | 179 | 60.3 |
| Erythrocyte sedimentation rate (mm/hr) | 60 | 64 | 32 | 54 |
| Fibrinogen (mg/dL) | 926 | 504 | 547 | 486 |
| Ferritin (ng/mL) | 340 | 803 | 388 | 235 |
| Lactate dehydrogenase (U/L) | 1,163 | 1,467 | 626 | 1,244 |
| d-dimer (ng/mL) | 1,004 | 459 | 1,979 | < 150 |
| WBC (× 109/L) | 12.8 | 3.7 | 14.7 | 5.2 |
| Absolute lymphocyte count (× 109/L) | 0.8 | 1.1 | 0.7 | 0.5 |
| Absolute neutrophil count (× 109/L) | 11.6 | 2.1 | 13.5 | 4.5 |
| Platelet count (× 109/L) | 124 | 272 | 157 | 237 |
| Troponin I (ng/mL) | < 0.034 | < 0.034 | 0.599 | < 0.034 |
| Pro-b-type natriuretic peptide (pg/mL) | 256 | 65 | 10,400 | 12 |
| Creatinine (mg/dL) | 0.77 | 0.71 | 0.32 | 0.37 |
| Aspartate aminotransferase (U/L) Alanine aminotransferase (U/L) | 39 117 | 37 24 | 94 86 | 56 46 |
| Hospital day of CCP | 3 | 2 | 2 | 1 |
| CCP units transfused | 2 (same donor) | 2 | 10 mL/kg | 2 (separate donors) |
| Receptor-binding domain endpoint titer | 1:160 | Unknown | 1:1,280 | Unit 1 = 1: 2,560, |
| Unit 2 = 1:640 | ||||
| Additional therapies | Remdesivir, anakinra | Remdesivir | Remdesivir, IV immunoglobulin, steroids, anakinra | Remdesivir, steroids |
| Outcome | Discharged home 7 d after CCP | Discharged home 5 d after CCP | Discharged home 23 d after CCP | Discharged home 10 d after CCP |
CCP = coronavirus disease 2019 convalescent plasma, HFNC = high-flow nasal cannula, LOS = length of stay.
On the day of transfer from the outside hospital, he had been started on remdesivir which was continued for 7 days (hospital day [HD] 1–HD7) and ultimately stopped due to rising ALT. In the setting of increasing HFNC requirements, the patient received two units of CCP from the same donor (RBD binding titer 1:160) on HD3 (26 hr after admission). He had no adverse effects from the transfusion. Figure 1 illustrates the change in Fio2, fever curve, and CRP following CCP transfusion. Following receipt of CCP, he had improvement in some inflammatory markers (CRP), but others continued to increase (ferritin and platelets), and his respiratory support maxed at 60 L HFNC, 100% Fio2. Therefore, on HD4, he was started on IV anakinra which was continued for 6 days. Respiratory requirements plateaued at 50–60 L HFNC and 100% Fio2 until HD6 when he slowly started to wean support. He was stable on room air on HD9 and discharged home on HD10 (7 d following CCP administration). Figure 2 shows a timeline of his hospital course.
Figure 1.
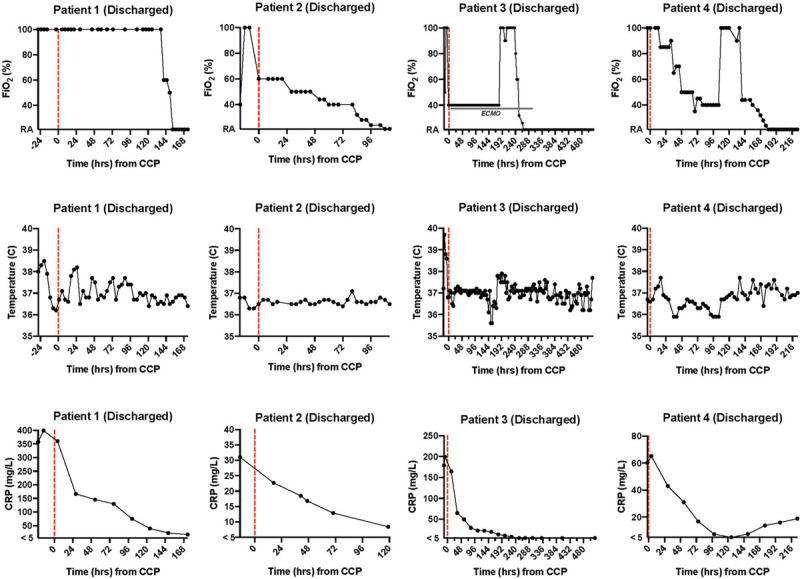
Trend over time in Fio2, temperature, and C-reactive protein (CRP) of four critically ill pediatric patients with coronavirus disease 2019 (COVID-19) treated with COVID-19 convalescent plasma (CCP). Dashed red lines indicate time of CCP administration. Patient outcomes as of end of study observation given in parentheses.
Figure 2.
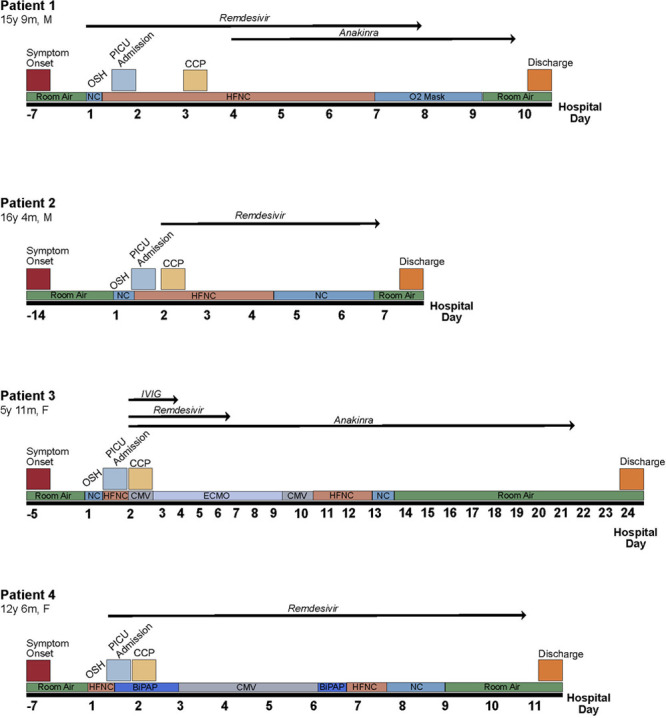
Timeline of hospital course including treatment strategies employed, respiratory support, and outcome. BiPAP = bilevel positive airway pressure, CCP = coronavirus disease 2019 convalescent plasma, CMV = conventional mechanical ventilation, ECMO = veno-arterial extracorporeal membrane oxygenation, F = female, HFNC = high-flow nasal cannula, IVIG = intravenous immunoglobulin, m = months, M = male, NC = nasal cannula, OSH = outside hospital, y = years.
Case 2
A 16-year-old obese, Asian male was transferred to the PICU from an outside hospital due to hypoxia from SARS-CoV-2 which was diagnosed by viral PCR. The patient had presented to the hospital with 14 days of symptoms including fever, worsening cough, shortness of breath, rhinorrhea, chest pain, malaise, nausea, and poor PO intake. He also complained of new onset hemoptysis. On arrival to the PICU, he was hemodynamically stable with normal heart rate, blood pressure, and perfusion. He was tachypneic with mild respiratory distress on 6 L HFNC, Fio2 100%. Admission CXR demonstrated hazy and patchy bilateral pulmonary infiltrates consistent with a viral pneumonia. Laboratories were significant for elevated CRP, ESR, ferritin, LDH, fibrinogen, and d-dimer, as well as lymphopenia. Troponin, pro-BNP, and cardiac function were normal. Table 1 provides admission laboratory values.
On the evening of transfer, the patient’s respiratory status quickly worsened, and respiratory support was escalated to a maximum of 50 L HFNC, Fio2 100%. In the setting of increasing HFNC requirements, the patient received two units of CCP (from an outside vendor) on HD2 (14 hr after admission). He had no adverse effects from the transfusion. Figure 1 illustrates the change in Fio2, fever curve, and CRP following CCP transfusion. Remdesivir was also started on HD2 and continued for a 5-day course. Following CCP and initiation of remdesivir, he had improvement in oxygen requirement and was able to wean down on his HFNC flow and Fio2. He was stable on room air on HD6 and discharged home on HD7 (5 d following CCP administration). Figure 2 shows a timeline of his hospital course.
Case 3
A previously healthy, 5-year-old Hispanic female was transferred to the PICU from an outside hospital emergency department due to acute respiratory failure, fluid-refractory hypotension, and elevated inflammatory markers in the setting of SARS-CoV-2 which was diagnosed by viral PCR. The patient presented to the outside hospital with 5 days of fever, abdominal pain, dysuria, nausea, vomiting, and new onset erythematous rash on her chest and abdomen. Laboratories were significant for elevated CRP, ESR, ferritin, fibrinogen, and d-dimer. She also had elevated troponin and pro-BNP. Table 1 provides admission laboratory values.
At admission to our PICU, she was afebrile but tachycardic and hypotensive on a high-dose epinephrine infusion (0.2 µg/kg/min). She was also tachypneic but maintaining normal oxygen saturations on 6 L HFNC, Fio2 100%. She was alert and oriented without focal neurologic deficits. Echocardiogram at admission revealed mildly decreased systolic function and mild left anterior descending coronary artery dilation. She remained hypotensive despite stress-dose steroids and the addition of a norepinephrine infusion. Additionally, she had worsening hypoxia despite escalation in HFNC support. Less than 3 hours after admission, she was intubated due to persistent hypoxemia and hypotension. Initial oxygenation index was 19, indicating severe acute respiratory distress syndrome (ARDS). Given escalating support, the patient received 10 mL/kg of CCP (RBD binding titer 1:1,280) on HD2 (19 hr after admission) and was also started on remdesivir. She had no adverse effects from the transfusion. Figure 1 illustrates the change in Fio2, fever curve, and CRP following CCP transfusion. Remdesivir was continued for a total of 5 days (HD2–HD6).
A second echocardiogram obtained nine hours after admission revealed severely diminished left ventricular systolic function. On HD2 (< 24 hr after admission), she was cannulated to venoarterial extracorporeal membrane oxygenation (VA ECMO) for cardiogenic shock and myocarditis. SARS-CoV-2 immunoglobulin G (IgG) assay (Abbott Laboratories, Abbott Park, IL) testing which was collected prior to the administration of CCP or IV immunoglobulin (IVIG), returned positive on HD2; these results did not return until after the administration of CCP. This disease process appeared more consistent with multisystem inflammatory syndrome in children (MIS-C) than with acute COVID-19 infection. Following cannulation on HD2, she also received high-dose steroids, IVIG, and was started on IV anakinra. Cardiac function and respiratory status improved while on VA ECMO, and the patient was decannulated on HD9 (extracorporeal membrane oxygenation day 7). The patient was extubated to HFNC on HD10 and weaned to room air on HD13. She was transferred out of the PICU on HD15 and was discharged home on HD24 (23 d following CCP administration). Figure 2 shows a timeline of her hospital course.
Case 4
A 12-year-old obese, Hispanic female with a history of asthma was transferred to our PICU from an outside hospital due to shortness of breath and a known diagnosis of SARS-Cov-2 diagnosed by viral PCR. She was also group A streptococcus positive by oropharyngeal swab at the outside facility. The patient presented to the hospital with seven days of fever, cough, and fatigue and three days of shortness of breath. On arrival to the PICU, she was afebrile, tachycardic and tachypneic but with normal blood pressure and perfusion. CXR showed bilateral patchy opacities without pleural effusions consistent with viral pneumonia. Laboratories were significant for elevated CRP, LDH, and ferritin as well as lymphopenia. Troponin, pro-BNP and screening electrocardiogram were normal. Table 1 provides admission laboratory values.
On arrival, she demonstrated dyspnea on 45 L HFNC, Fio2 100%, and was escalated to bilevel positive airway pressure (BiPAP) therapy. On the night of admission (HD1, 2 hr after admission), she received two units of CCP (RBD binding titer unit 1 = 1:2,560, unit 2 = 1:640). She had no adverse effects from the transfusion. Figure 1 illustrates the change in Fio2, fever curve, and CRP following CCP transfusion. Remdesivir was also started on HD1 and continued for 10 days (HD1–HD10). She received ceftriaxone for 5 days to treat strep pharyngitis. On HD3, she had worsening respiratory distress and progressed to intubation. She initially had difficulty oxygenating requiring high mean airway pressures with an initial oxygenation index of 18, indicating severe ARDS. IV methylprednisolone was started at the time of intubation. Mean airway pressures were able to be weaned, and she was extubated to BiPAP on HD6 and quickly weaned to HFNC on the same day. She was transferred out of the PICU on low-flow nasal cannula on HD7 and weaned to room air on HD9. She was discharged home on HD11 (10 d following CCP administration). Figure 2 shows a timeline of her hospital course.
DISCUSSION
The global pandemic of SARS-CoV-2 has led to substantial worldwide morbidity and mortality. Although many treatment strategies are being studied, there are currently no vaccines or specific drugs available for the treatment of severe COVID-19 (10, 11). Convalescent plasma has been successfully used for the treatment of other viral infections including SARS-CoV, Middle East respiratory syndrome, influenza A (H1N1), and Ebola (11, 12). However, definitive clinical evidence showing the efficacy of convalescent plasma is scarce and inconsistent with some studies showing benefit (11, 13, 19), whereas others have not (20). Due to the rapidly progressing pandemic, as well as no definitive treatment strategies or vaccination, the FDA allowed the use of convalescent plasma with an E-IND to treat critically ill patients with life-threatening diseases including respiratory failure and shock (21).
A few small case series, ranging from one to 10 critically ill adult patients, have been published that suggest improvement in patient clinical status with minimal adverse events when patients receive convalescent plasma (8–10, 14–16). A larger randomized control trial demonstrated trends toward more favorable outcomes but did not reach statistically significant differences when comparing CCP administration with standard supportive care in adult hospitalized patients with benefit limited to those with severe but not life-threatening COVID-19 (22). However, this trial terminated early due to inability to enroll enough patients and may have not been powered to demonstrate statistical significance (22, 23). Shen et al (9) showed improvement in the Sequential Organ Failure Assessment score, Pao2/Fio2 ratio, and viral loads within 12 days of administration of CCP in five critically ill adults. Duan et al (15) demonstrated feasibility of CCP in 10 critically ill adults and showed improvement in clinical symptoms within 3 days of transfusion.
To our knowledge, our case series is the first series to describe the use of CCP in critically ill pediatric patients. Figlerowicz et al (24) published a case report describing a pediatric patient with pancytopenia related to COVID-19 who received CCP. Unlike our series, the patient described in this report was hemodynamically stable and not in respiratory distress (24). Although adverse reactions can be seen with plasma transfusions, including transfusion-related lung injury, transfusion-associated circulatory overload (TACO), and anaphylaxis (17), no reactions or complications were seen in our patients. In a pediatric population, TACO can be a specific concern due to large volume transfusions in a small weight patient. Joyner et al (17) analyzed key safety metrics after CCP transfusion in 5,000 hospitalized adults with severe COVID 19 and found the incidence of serious adverse events to be less than 1%.
Data from other respiratory infections suggest that convalescent plasma is most effective when given early in the disease process (19). All four of our patients received CCP early in their hospitalization (all within the first 26 hr). Patients 1 and 2 both plateaued or improved in their respiratory support need following transfusion of CCP. They were able to be managed without intubation and were discharged home off of all respiratory support and oxygen on HD10 and HD7, respectively (Table 1). Discharge occurred 5–7 days after the administration of CCP. Patient 4 did develop severe ARDS and escalated to needing intubation on HD3, but she was able to be extubated on HD6 (5 d after CCP) and weaned off of oxygen support on HD9. She was discharged home off of all respiratory support on HD11, 10 days after the administration of CCP (Table 1).
Patient 3 presented a challenging case. Initially, she was believed to have acute COVID-19 infection due to respiratory failure and positive PCR and thus was given CCP as respiratory support quickly escalated. However, as her case progressed and she was found to have IgG antibodies to COVID-19 in a sample collected prior to receiving CCP, our diagnosis changed to MIS-C. Data are lacking on the benefit of CCP in critically ill patients with MIS-C. Patient 3 did improve throughout hospitalization. She was decannulated from VA ECMO on HD9 (7 d post CCP) and extubated the following day. She decreased on her respiratory support and was discharged home off of all respiratory support on HD24, 23 days after the administration of CCP (Table 1).
Whether the stability and eventual improvements in the patients would have occurred without CCP cannot be answered from this case series. As is common in many hospitalized and critically ill patients, all patients received other therapies in addition to CCP. Perhaps, the improvement in patients was related to combination therapy as all patients also received immune-modulating drugs. Although these confounding other therapies and a small sample size limit our ability to assign efficacy to CCP, our case series demonstrates that CCP can feasibly be given to critically ill pediatric patients without adverse events. Additionally, all of our patients tolerated all therapies aimed at modulating COVID-19, including remdesivir and anakinra. A systematic review published in May of 2020 found that there are 47 ongoing studies evaluating convalescent plasma (10), including at our own institution. The Randomized Evaluation of COVID-19 therapy trial in Europe is a randomized control trial that is enrolling hospitalized children (25). Additionally, the National Institutes of Health lists another phase 1 study looking at the safety and pharmacokinetics of CCP in high risk children exposed or infected with COVID-19 which has not yet started enrolling (26). Our case series suggests that broader inclusion of pediatric patients should occur in additional studies as children can have severe disease with COVID-19 and appear to tolerate similar therapeutic modalities being tested in adults. Well-designed and controlled trials that include pediatric patients are critical to assess the safety and efficacy of CCP to this unique patient population.
CONCLUSIONS
We present the first case series of pediatric patients receiving CCP. Our results indicate that CCP is a feasible therapy for critically ill pediatric patients infected with SARS-CoV-2. Well-designed clinical trials are necessary to determine overall safety and efficacy of CCP and other disease modifying agents in pediatric patients.
ACKNOWLEDGMENTS
We would like to thank all of the individuals who donated their plasma to the UNC Blood Donation Center, the UNC Health Foundation, the UNC School of Medicine, Aravinda de Silva’s support of the coronavirus disease 2019 convalescent plasma antibody assays and D.M. Margolis for support and UM1-AI126619 to D.M. Margolis. This project was also supported by the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus Relief Fund established and approved by the North Carolina General Assembly.
Footnotes
This work was performed at the University of North Carolina at Chapel Hill, Chapel Hill, NC.
Dr. Rimland receives training support from the UNC Medical Scientist Training Program and the National Institutes of Health MD/PhD Partnership Program. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) Pandemic. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed June 10, 2020
- 3.CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr. 2020; 174:882–889 [DOI] [PubMed] [Google Scholar]
- 5.Kanthimathinathan HK, Dhesi A, Hartshorn S, et al. COVID-19: A UK children’s hospital experience. Hosp Pediatr. 2020; 10:802–805 [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020; 382:1663–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBiasi RL, Song X, Delaney M, et al. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020; 233:199–203.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020; 158:e9–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020; 323:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review. Cochrane Database Syst Rev. 2020; 5:CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown BL, McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci. 2020; 59:102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan HC, Roback JD. Convalescent plasma: Therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev. 2020; 34:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Lancet Haematology. The resurgence of convalescent plasma therapy. Lancet Haematol. 2020; 7:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020; 92:1890–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020; 117:9490–9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020; 35:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020; 130:4791–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020; 5:eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. ; Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis. 2015; 211:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey RT, Jr, Fernández-Cruz E, Markowitz N, et al. ; INSIGHT FLU-IVIG Study Group. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): A double-blind, randomised, placebo-controlled trial. Lancet Respir Med. 2019; 7:951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020; 368:m1256. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020; 324:460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for COVID-19-potentially hopeful signals. JAMA. 2020; 324:455–457 [DOI] [PubMed] [Google Scholar]
- 24.Figlerowicz M, Mania A, Lubarski K, et al. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia. Transfus Apher Sci. 2020. July 1102866. [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NHS Blood and Transport: Randomised Evaluation of COVID-19 Therapy (RECOVERY). 2020 Available at: https://www.recoverytrial.net/files/recovery-protocol-v8-0-2020-07-08.pdf. Accessed August 18, 2020.
- 26.Deville JG. COVID-19 Convalescent Plasma as Prevention and Treatment for Children with Underlying Medical Conditions. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04462848. Accessed August 18, 2020


