Abstract
As SARS-CoV-2 and its related clinical syndrome (COVID-19) became a pandemic worldwide, questions regarding its clinical presentation, infectivity, and immune response have been the subject of investigation. We present a case of a patient previously considered recovered from nosocomially transmitted asymptomatic COVID-19 illness, who presented with new respiratory, radiological, and RT-PCR findings consistent with COVID-19, while on high-dose prednisolone due to a suspected secondary demyelinating disease. Importantly, it led to three subsequent cases within patient’s household after discharge from the hospital. After reviewing this case in light of current evidence and debates surrounding SARS-CoV-2 RT-PCR results, we hypothesize that patients on corticosteroids may have particular viral shedding dynamics and should prompt a more conservative approach in regard to isolation discontinuation and monitoring.
Keywords: COVID-19, RT-PCR, Viral shedding, Corticosteroids, Case report
Introduction
Since the identification of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as the cause of the disease which was later named COVID-19, and as it progressed to the current worldwide pandemic, much investigation has been made regarding its clinical presentation, transmission route, and immunity. Here, we present an atypical case regarding clinical evolution and SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) dynamics and discuss it in light of the current published evidence.
Case Report
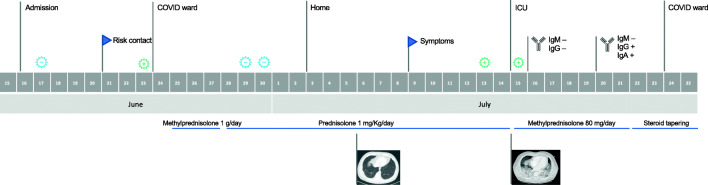
We report the case of a 41-year-old with no relevant underlying medical conditions who was admitted to our hospital on June 15 due to a 10-day history of gait ataxia, dizziness, headache, and vomiting. On admission, he was screened for COVID-19 as a standard procedure for hospitalized patients (BD MAX™ System: negative SARS-CoV-2 RT-PCR). Thorough diagnostic workup raised the suspicion of a secondary demyelinating disease, and the patient was started on high-dose steroids (methylprednisolone 1 g/day for 3 days followed by prednisolone 1 mg/Kg/day) (Fig. 1).
Fig. 1.
Timeline of events regarding location, SARS-CoV-2 test results, symptoms, serology, and steroid usage. Legend:  Positive SARS-CoV-2;
Positive SARS-CoV-2;  Negative SARS-CoV-2
Negative SARS-CoV-2
On June 21, a patient who had been admitted for a bilateral pneumonia (with two negative tests for SARS-CoV-2) and was sharing the same room was intubated as an emergency in the context of a cardiopulmonary arrest, with a subsequent post-mortem nasopharyngeal and oropharyngeal swab revealing SARS-CoV-2 RNA. Hence, our patient collected a swab on the 23rd of June as screening, which proved to be positive (QuantStudio™ 7 Flex RT PCR system: N1 Ct 35.8, N2 Ct 37.3, RP Ct 29). He was moved to a COVID-19 isolation area, and during his hospital stay reported no fever, respiratory, or gastrointestinal symptoms. He subsequently presented two negative SARS-CoV-2 tests on June 29 and 30 (Aptima® SARS-CoV-2 assay, which uses target capture and transcription-mediated amplification), so he was considered cured from asymptomatic COVID-19. He was discharged on July 3 on prednisolone 80 mg/day. While at home (July 6), he had chest computed tomography (CT), which had been ordered to exclude signs of sarcoidosis as part of the secondary demyelinating disease workup and which revealed a peripheral ground-glass opacity compatible with early COVID-19 (Fig. 2).
Fig. 2.
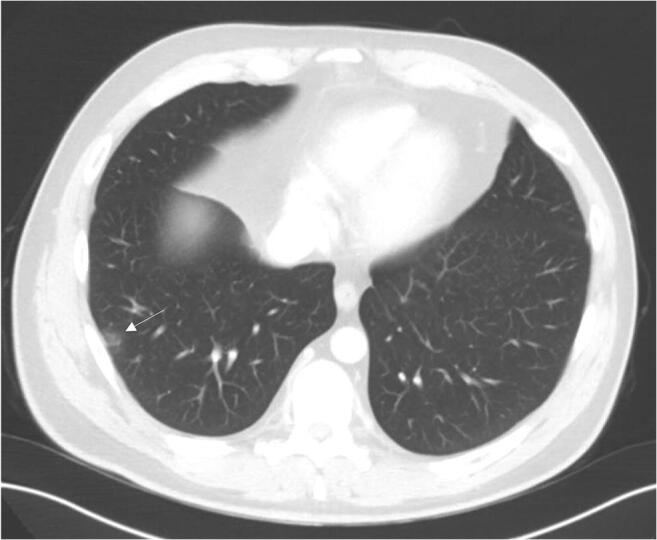
Chest computed tomography from July 6 showing a subpleural focal ground-glass densification area in the right lateral basal segment (arrow)
On July 9, he represented with fever, headache, myalgias, and cough, and on July 13, he was tested as an outpatient for SARS-CoV-2 with a positive result. His family (mother, father, and wife) also developed respiratory symptoms and all tested positive for SARS-CoV-2. Importantly, they were shielding and reported no other risk contact besides the one with our patient.
On July 15, he was admitted to our hospital, reporting worsening of the symptoms, with dyspnea and thoracalgia. On admission, he was tachypneic and presented with severe respiratory failure, rapidly requiring intubation and transfer to our intensive care unit (ICU). Chest CT showed diffuse bilateral ground-glass opacification and extensive subpleural consolidation of the lower lobes (Fig. 3), and SARS-CoV-2 swab was positive (Xpert® Xpress SARS-CoV-2: E Ct 15.9; N2 Ct 17.8; SPC Ct 0.0). Serologic tests were performed on July 16 (negative IgM and IgG) and on July 20 (positive IgG (8.00 UA/mL); positive IgA (9.18 ICO); negative IgM (4.5 UA/mL)). The patient clinically improved on methylprednisolone 80 mg/day (for 7 days followed by tapering), piperacillin/tazobactam, and remdesivir and was extubated after 7 days. At the time of the writing of this report, he has been discharged from the ICU and is progressing favorably in the COVID ward.
Fig. 3.
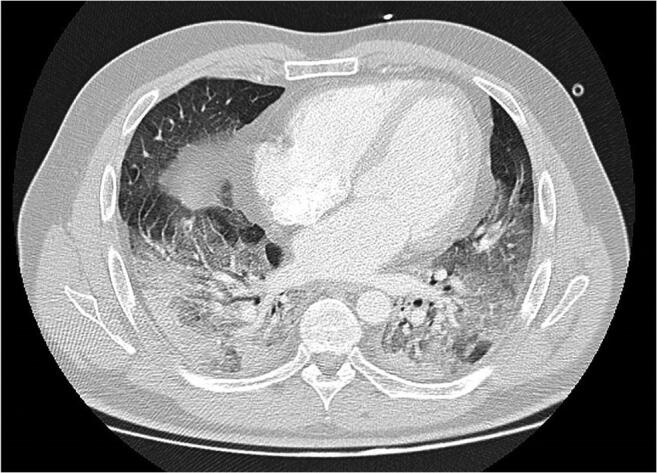
Chest computed tomography from July 15 showing diffuse bilateral ground-glass opacities with some areas of “crazy-paving” pattern and extensive subpleural consolidation of the lower lobes
Conclusions
We found this case remarkable for its clinical evolution, infectivity, and immune response. Firstly, this patient had an initial positive SARS-CoV-2 RT-PCR test 48 h after a risk contact, remaining asymptomatic for 10 days after this test, which is a criteria for releasing asymptomatic patients from isolation according to the WHO guidance [1]. Additionally, the two negative SARS-CoV-2 tests further reinforced the criteria for cure of the (asymptomatic) disease.
After 16 days of the positive swab, he presented with his first symptoms. This could point either to a reactivation of the disease in a patient who first presented as asymptomatic or to a long incubation period (18 days from risk contact until developing symptoms, with a CT performed 3 days prior to the onset of symptoms showing an evolving disease, which is consistent with previous studies reporting typical radiological findings of COVID-19 in asymptomatic or presymptomatic patients [2]). Some outbreak studies have followed up patients who were asymptomatic at the time of diagnosis and concluded that some patients developed symptoms and were actually presymptomatic [2–4]. In one study, the median time for developing symptoms after the initial positive RT-PCR test was 4 days (range from 3 to 5) [3].
Interestingly, this patient seems to have transmitted the disease to his family during the presymptomatic period, since he was at home for the 6 days prior to symptom onset and his household members reported symptoms 2 to 3 days after him, having no other risk contacts. There are numerous reports on transmission of SARS-CoV-2 from asymptomatic individuals or individuals who were within the incubation period [5–7]. Current data suggests that infectiousness peaks in the earlier stages of infection [8, 9], and one modeling study suggested individuals could be infectious 2 to 3 days prior to symptom onset [9].
Finally, analyzing serology markers for this patient, immune response seems to be delayed considering his first exposure to the virus (IgG and IgA detected 27 days after the first positive test). However, most published seroconversion studies evaluated immunoglobulins levels according to symptom onset. Our patient presented with detectable IgG and IgA 11 days after symptom onset. A recent study which compared IgA, IgM, and IgG responses in COVID-19 patients [10] has showed that medium seroconversion time was 4 to 6 days for IgA and IgM and 5 to 10 days for IgG. Notably, this patient’s IgA levels were not available on the first serology sample collected on the seventh day after symptoms onset, which is of importance since IgA detection seems to show the highest sensitivity at the beginning of disease (highest positive diagnostic rate at 4 to 10 days after symptom onset) [10] and to contribute to a greater extent to virus neutralization [11]. In the same study [10], IgM showed a lower positive diagnostic rate, which is in line with our patient’s results (whose IgM was negative).
Fluctuating shedding of SARS-CoV-2 has been described on COVID-19 patients [12, 13] and not only has asymptomatic infection been associated with a higher likelihood of SARS-CoV-2 RNA clearance within the first week of diagnosis when compared with symptomatic infection [14] but there have also been reports of COVID-19 reactivation [15, 16]. Hence, a possible explanation is that this case represents a reactivation of COVID-19 in a patient which first presented as an asymptomatic carrier. Another is that the two negative SARS-CoV-2 could simply reflect the fluctuant presence of the virus.
Examining the timeline (Fig. 1), it could also be speculated that the fact that the patient was on high-dose steroids in the early course of disease might have delayed the clinical presentation at first. Since the beginning of the pandemic, the effect of steroids on viral clearance and their role in the management of COVID-19 have been the subject of much speculation. Some small retrospective studies early in the pandemic suggested that low-dose corticosteroid therapy did not delay viral clearance in patients with COVID-19 [17, 18]. There were also concerns raised around steroids being associated with higher mortality, longer length of stay, and higher rate of bacterial infection, extrapolating data from retrospective studies among patients with coronaviruses infections (including Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus) [19]. Recently, the RECOVERY trial indicated that dexamethasone reduced 28-day mortality among COVID-19 pneumonia patients requiring invasive mechanical ventilation or oxygen [20]. However, there was no benefit in patients not receiving respiratory support [20]. This is in line with the currently known pathophysiology of COVID-19 illness which appears to progress from an early infection stage related with a viral response phase, followed by a pulmonary phase and finally a hyperinflammation phase during which steroids are thought to have its greatest value [21]. Notably, 15% of the patients recruited for RECOVERY were not considered suitable for randomization to dexamethasone, although it was not clarified the amount of them who was considered by the managing physician to have a definite contraindication and which one was it [20]. Moreover, it is unclear whether that, even in severe disease, steroids may be less beneficial for some subsets of patients, such as the elderly and those immunosuppressed. Since the administration of steroids comes with risks, the risk-benefit balance should always be assessed. To the best of our knowledge, no data on the effect of steroids in asymptomatic (or presymptomatic) patients has been reported, nor on SARS-CoV-2 dynamics in patients who are chronically on steroids.
In conclusion, this case highlights that the ongoing pandemic urges for a better understanding of SARS-CoV-2 replication, infectivity, and immunity. We hypothesize that patients on corticosteroids or other immunosuppressive medication may have particular viral shedding dynamics and should prompt a more conservative approach in regard to isolation discontinuation and monitoring, even with a negative nucleic acid amplification test. Importantly, after having been considered cured for asymptomatic COVID-19 illness, this case led to three subsequent cases. Studies including determination of SARS-CoV-2 infectivity through viral culture, immunity assessment, and genomic comparison of viral strains can prove to be paramount in further understanding these factors and, ultimately, in improving management of cases. Lastly, this case underlines the potential for nosocomial transmission of COVID-19 and the importance of infection control measures.
Authors’ Contributions
All authors meet the ICMJE authorship criteria. RPJ, RS, LL, MP, LA, PD, CC, ACA, SC, JJM, SG, IS, and PF were involved in the treatment, decision-making, and clinical management of the patient. RPJ wrote the manuscript. EA assisted in the review of the case report and pathological findings. All the authors critically revised the manuscript for intellectual content and approved the final version of the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent for Publication
The patient has consented this publication.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Criteria for releasing COVID-19 patients from isolation. 2020. https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation. Accessed 23 Jul 2020.
- 2.Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, Wang J, Hu Z, Yi Y, Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai A, Sasaki T, Kato S, Hayashi M, Tsuzuki S, Ishihara T, Iwata M, Morise Z, Doi Y. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med. 2020;383:885–886. doi: 10.1056/NEJMc2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spice K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhmer MM, Buchholz U, Corman VM, Hoch M, Katz K, Marosevic DV, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8). 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed]
- 6.Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian G, Yang N, Hoi A, Ma Y, Wang L, Li G, Chen X. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis. 2020;71(15):861–862. doi: 10.1093/cid/ciaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–655. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 10.Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, Cheng L, Li Y, Ma X, Jin T. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. medRxiv. 2020. 10.1101/2020.06.10.20126532. [DOI] [PMC free article] [PubMed]
- 12.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau NVV, Lam VT, Dung NT, Yen LM, Minh NNQ, Hung LM, et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin Infect Dis. 2002:ciaa711. 10.1093/cid/ciaa711.
- 15.Loconsole D, Passerini F, Palmieri VO, Centrone F, Sallustio A, Pugliese S, et al. Recurrence of COVID-19 after recovery: a case report from Italy. 2020. 10.1007/s15010-020-01444-1. [DOI] [PMC free article] [PubMed]
- 16.Chen D, Xu W, Lei Z, Huang Z, Liu J, Gao Z, Peng L. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, Geng S, Pan A. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Inf Secur. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Lv Y, Xu C, Sun W, Chen W, Peng Z, Chen C, Cui X, Jiao D, Cheng C, Chi Y, Wei H, Hu C, Zeng Y, Zhang X, Yi Y. Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front Public Health. 2020;8:355. doi: 10.3389/fpubh.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Inf Secur. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone for COVID-19-preliminary report effect of dexamethasone in hospitalized patients with COVID-19-preliminary report RECOVERY Collaborative Group*. N Engl J Med. 2020:NEJMoa2021436. 10.1101/2020.06.22.20137273.
- 21.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Hear Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]