Abstract
Lesbian, gay, bisexual, and transgender (LGBT) older adults comprise a unique and growing subset of the aging population. The historical context in which they came of age was imbued with victimization and discrimination. These experiences are subjectively stressful and collectively known as minority stress. Older LGBT adults continue to face stressors related to their gender and sexual identities in their daily lives. Importantly, chronic minority stress (CMS), like other forms of chronic stress, is harmful to health and well-being. CMS contributes to LGBT health disparities, including cardiovascular disease and depression, conditions that in turn increase risk for premature cognitive decline. Furthermore, long-term exposure to stress hormones is associated with accelerated brain aging. Yet, the cognitive functioning of LGBT elders and the influence of CMS on their cognition is all but unexplored. In this review, we examine the influences of CMS in LGBT elders and connect those influences to existing research on stress and cognitive aging. We propose a testable model describing how CMS in LGBT elders heightens risk for premature cognitive aging and how ameliorating factors may help protect from CMS risk. Research is desperately needed to calibrate this model toward improving LGBT quality of life and mental health practices.
Keywords: LGBT, disparities, Healthcare Needs, Cognition
Social, economic, and environmental factors impact health (“Healthy People 2020,” 2012), and the differences in health among disadvantaged groups compared to most of the population are defined as health disparities (Carter-Pokras & Baquet, 2002). Racial minorities have health disparities that are predicated by race-related stressors, including discrimination and internalized racism (Merritt, Bennett, Williams, Edwards, & Sollers, 2006; Utsey & Hook, 2007). Over the lifespan, chronic stress stemming from social inequality leads to physiological deterioration and accelerated aging (Geronimus, 1992; McEwen & Gianaros, 2010). This mechanism applies to other forms of social inequality such as ethnic discrimination (Lauderdale, 2006) and ageism (Allen, 2016).
Lesbian, gay, bisexual, and transgender (LGBT) older adults1 are another health disparate population. They are at an elevated risk for developing cardiovascular diseases and other metabolic disorders (Fredriksen-Goldsen, Emlet, et al., 2013; Fredriksen-Goldsen, Kim, Barkan, Muraco, & Hoy-Ellis, 2013; Lick, Durso, & Johnson, 2013). These diseases can be attributed to chronic exposure to minority stress, which is the specific set of stressors conferred based on one’s actual or perceived social identities (Hendricks & Testa, 2012; Meyer, 2003, 2015). Protracted exposure to stress hormones (e.g., cortisol) damages the structures and functions of numerous body systems. Also, social inequality can impede access to adequate and affirmative healthcare opportunities (Ferraro & Shippee, 2009).
Elevated levels of stress hormones are associated with accelerated brain aging and cognitive decline (Lupien, McEwen, Gunnar, & Heim, 2009). Thus, LGBT elders experiencing chronic minority stress (CMS) may be at elevated risk of cognitive decline (Fredriksen-Goldsen et al., 2017; Hatzenbuehler, 2016). Yet, little is known about cognitive aging for LGBT elders as compared to heterosexual older adults, much less about LGBT subgroups and the mechanisms underlying such developmental changes. Geriatric health care and mental health care cannot be comprehensively informed without greater knowledge of minority groups and subgroups, including LGBT elders. Therefore, the objectives of this article are twofold: 1) detail what is directly and indirectly known about the risks for cognitive decline that LGBT elders may incur and 2) propose an introductory model to guide future research and clinical practice.
Studies of LGBT Cognition
Typically, older adulthood is associated with declines in cognitive abilities. The experiences of cognitive aging for LGBT elders specifically have only recently been documented. Older adults who either self-identified as LGBT or reported engaging in same-sex sexual behavior, having romantic relationship(s) with someone of the same sex or gender, or having an attraction to someone with the same sex or gender endorse having mild to moderate cognitive difficulties (Fredriksen-Goldsen, Jen, Bryan, & Goldsen, 2016). In a separate sample, 23% of adults age 50 and older who identify as LGBT reported significant cognitive declines in memory and at least one other cognitive function (Flatt et al., 2018). Of the 1.1 million LGBT adults age 65 and older, general population estimates (Alzheimer’s Association, 2016; Hebert, Weuve, Scherr, & Evans, 2013) suggest that roughly 121,000 (11%) would currently be living with Alzheimer’s disease, or AD, (Fredriksen-Goldsen, 2016; Fredriksen-Goldsen et al., 2016). Another estimate puts the prevalence of all-cause dementia for self-identifying LGBT older adults at 23% (McGovern, 2014). One recent examination of mild cognitive impairment and all-cause dementia diagnoses indicates no differences between adults age 55 and older in same-sex versus opposite-sex relationships (Perales-Puchalt et al., 2019).Yet, these estimates are not based on objective assessment; there are currently no studies directly examining dementia prevalence or cognitive functioning in self-identified LGBT older adults regardless of relationship status.
Neurocognitive studies of sexual orientation, as measured by participants’ self-identification and sexual attractions, have thus far focused on early adulthood (Rahman, Abrahams, & Wilson, 2003; Rahman, Andersson, & Govier, 2005; Rahman, Sharp, McVeigh, & Ho, 2017; Rahman & Wilson, 2003; Rahman, Wilson, & Abrahams, 2003, 2004a, 2004b; Xu, Norton, & Rahman, 2017), concluding that cognition is generally intact in non-heterosexual adults, but with sex-atypicality reflecting a “cross-sex shift” (Willmott & Brierley, 1984; Xu et al., 2017), wherein cognitive abilities in gay men are comparable to heterosexual women, and lesbian women’s abilities are commensurate with heterosexual men.
To date only one cross-sectional study has investigated cognitive abilities and sexual orientation in adults of more advanced age (Maylor et al., 2007). Yet, it examined age only from 20 to 65, precluding evaluation of later adulthood. Overall, women outperformed men on category fluency and spatial memory, while men outperformed women on visuospatial tasks. Moreover, cognitive performance was poorer with greater age, as is typically found. When sexual orientation was also considered, the cross-sex shift was apparent but without a meaningful pattern of interaction with age, suggesting little impact of sexual orientation on cognition through middle adulthood. No studies of LGBT older adults in such a context yet exist. Thus, the cognitive abilities of LGBT elders are unknown.
Minority Stress and Its Mechanisms
LGBT older adults came of age during an era of repression when non-heterosexuality was pathologized and criminalized (Fredriksen-Goldsen & Muraco, 2010). For instance, for decades, LGBT adults have been barred from marrying or adopting children. Homosexuality has been pathologized and was included in the American Psychiatric Association’s Diagnostic and Statistical Manual for Mental Disorders (DSM) until 1973. Still today, actual or threatened violence, discrimination, and hate speech are frequent (D’Augelli & Grossman, 2001; Huebner, Rebchook, & Kegeles, 2004; Woodford, Howell, Silverschanz, & Yu, 2012). Moreover, policies in public and private sectors can intentionally or unintentionally burden LGBT individuals’ resources and well-being and place them at social disadvantages (Hatzenbuehler, 2014). For example, in many U.S. states, sexual identity is not included as a protected identity, and employment may be terminated because of individuals’ sexual orientation (e.g., “Zarda & Moore, Jr. v. Altitude Express, Inc.,” 2017). Furthermore, LGBT people have been discriminated from obtaining housing or appropriate healthcare. Due to their age and the era in which they came of age, older LGBT adults have been chronically exposed to an array of identity-related stressors (Fredriksen-Goldsen & Muraco, 2010; Kertzner, Meyer, Frost, & Stirratt, 2009). These stressors may negatively impact the way in which LGBT adults age.
The minority stress model (Meyer, 1995, 2003) postulates that a unique set of distal and proximal stressors experienced by LGBT individuals increases their risk for physical and mental health disorders. Distal stressors are objectively stressful, external events that happen to an individual or other people that the individual learns about. On the other hand, proximal stressors are personal and psychological; they are the appraisals and internalization of distal stressors (i.e., internalized stigma2). Both distal and proximal stressors contribute to negative views of the self and maladaptive psychological phenomena that can trigger physical and mental health disorders (Cox, Dewaele, van Houtte, & Vincke, 2011; Hatzenbuehler, 2009; Newcomb & Mustanski, 2010). For example, LGBT individuals frequently internalize society’s negative attitudes against non-heterosexuality (Meyer & Dean, 1998; Walch, Ngamake, Bovornusvakool, & Walker, 2016), which can be experienced as shame, self-hatred, and poor self-esteem (Brubaker, Garrett, & Dew, 2009; Herek, Gillis, & Cogan, 2009). Internalized stigma also contributes to discomfort in disclosing one’s sexual or gender identity, feeling disconnected from other LGBT individuals, and being uncomfortable with same-sex romantic relationships and sexual activities (Newcomb & Mustanski, 2010). Through these mechanisms, sexual stigma negatively impacts the mental health of LGBT people.
Anticipating confrontation or discrimination places an individual in a state of hypervigilance and arousal, thereby activating stress responses (Brosschot, Pieper, & Thayer, 2005). While stress responses are adaptive in the short-term, protracted stress activation can lead to “allostatic load,” which is the set of physiological changes causing functional and structural damage throughout the body (Cohen, Janicki-Deverts, & Miller, 2007; Epel et al., 2004; McEwen & Stellar, 1993), including excessive circulation of stress hormones that trigger immune responses and chronic inflammation (Segerstrom & Miller, 2004). Moreover, protracted stress can cause hypertension, place strain on the cardiovascular system, and increase risk for myocardial infarctions (i.e., heart attacks; McEwen, 1998a; Muller, Tofler, & Stone, 1989). Lifetime exposure to identity-related stigma alters cortisol levels, cortisol regulation, and hypothalamic-pituitary-adrenal (HPA) axis function, and these mechanisms are thought to underlie many stress-related sexual and gender minority health disparities (Fredriksen-Goldsen & Kim, 2017; Hatzenbuehler & McLaughlin, 2014; Hoy-Ellis & Fredriksen-Goldsen, 2016; Huebner & Davis, 2005; Juster, Smith, Ouellet, Sindi, & Lupien, 2013; Meyer, 2003; Pascoe & Smart Richman, 2009) through heightened allostatic load (Cohen et al., 2007; McEwen & Stellar, 1993).
LGBT Health Disparities and Cognitive Aging
Social inequality is stressful, and throughout one’s lifespan, inequality confers significant health risks that alter aging trajectories and increase mortality (Ferraro & Shippee, 2009). Heterosexism creates a stressful social environment that LGBT individuals navigate across a lifetime, and chronic minority stress (CMS) has significant consequences on LGBT elders’ physical and mental health. Late adulthood is typified by gradual declines in cognitive abilities and based on a lifetime of social inequality in conjunction with known health disparities, LGBT individuals may be at risk of having accelerated cognitive declines (cf. Ferraro & Shippee, 2009). Cognitive aging in the LGBT population has been infrequently examined, and the specific impact of minority stress on cognition for LGBT individuals is currently unknown.
Historical oppression has shaped the healthcare utilization of individuals from victimized backgrounds, including LGBT people (Brotman, Ryan, Jalbert, & Rowe, 2002; Fredriksen-Goldsen et al., 2016; Fredriksen-Goldsen, Kim, et al., 2013). Many LGBT adults are concerned about being treated poorly by their healthcare providers (Espinoza, 2014), and those who fear disclosing their sexual/gender minority status to healthcare providers are more likely to delay routine care and examinations, especially if they have previously encountered homophobia or transphobia from providers (Brotman et al., 2002). Clearly, stigma impacts the way LGBT people access healthcare, and as such, sexual orientation and gender identity/variance are critical social variables that affect health outcomes (“Healthy People 2020,” 2012; Institute of Medicine, 2011; Logie, 2012).
In addition to barriers to accessing healthcare, minority stress specifically impacts health outcomes for LGBT adults aged 50 years or greater. Lifetime victimization and internalized stigma predict poor general health for LGBT elders, and internalized heterosexism increases the number of chronic health conditions (e.g., angina, arthritis, congestive heart failure, diabetes, heart attack, high cholesterol, hypertension, osteoporosis, and stroke) that LGBT adults aged 50 and older endorse (Fredriksen-Goldsen, Emlet, et al., 2013; see also, Lick et al., 2013). Moreover, negative experiences of minority stress are associated with greater “disability” (i.e., functional impairment stemming from physical, mental, or emotional conditions). Ultimately, lifetime victimization and discrimination are negatively associated with elders’ reports of physical health quality of life, which suggests that CMS impedes successful aging (Fredriksen-Goldsen, Kim, Shiu, Goldsen, & Emlet, 2015).
The negative consequences of sexual minority stress are not limited to physical health; they also extend to mental health (King & Richardson, 2016). Internalized homophobia increases symptoms of anxiety and depression, and age exacerbates the relationship between internalized heterosexism and mental health problems (Newcomb & Mustanski, 2010). Recent studies of participants 50 and older showed that those who reported greater sexual minority stress also reported more symptoms of major depressive disorder (Fredriksen-Goldsen, Emlet, et al., 2013; Hoy-Ellis & Fredriksen-Goldsen, 2016). Older adults who identify as lesbian, gay, or bisexual are more likely to smoke cigarettes and engage in excessive drinking compared to heterosexual elders (Fredriksen-Goldsen, Kim, et al., 2013). Through the minority stress model, mental health disparities among LGBT individuals are the consequence of identity-related prejudice, discrimination, and stigma (Meyer, 2003).
Psychological disorders and chronic stress are known to increase risk for dementia (Diniz, Butters, Albert, Dew, & Reynolds, 2013; Lathe, Sapronova, & Kotelevtsev, 2014; Muela et al., 2017; Ownby, Crocco, Acevedo, John, & Loewenstein, 2006), likely via HPA axis impairments and chronic exposure to cortisol (Simard, Hudon, & van Reekum, 2009; Sotiropoulos et al., 2008). Indeed, major depressive disorders, which LGBT people have to a greater degree than heterosexuals, place older adults in the general population at heightened risk for cognitive impairment, vascular dementia, and AD (Caraci, Copani, Nicoletti, & Drago, 2010; Diniz et al., 2013; Houde, Bergman, Whitehead, & Chertkow, 2008; Modrego & Ferrandez, 2004; Ownby et al., 2006; Sotiropoulos et al., 2008). Specifically, prolonged exposure to cortisol during adulthood is associated with damage to age- and dementia-vulnerable neural networks, including reductions in hippocampal function and volume via cell death (Lupien et al., 1998; Lupien, Maheu, Tu, Fiocco, & Schramek, 2007; Lupien et al., 2009; McEwen, 1998b), hippocampal dendritic arbor retraction (Ulrich-Lai & Herman, 2009), disrupted dendritic connections between the hippocampus and the prefrontal cortex (Arnsten, 2009; Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007), and suppressed neurogenesis (McEwen, 2007). Elevated cortisol levels may be one pathophysiological cause of Alzheimer’s disease (Landfield, Blalock, Chen, & Porter, 2007). In fact, animals exposed to frequent stress have more neuropathology that is comparable to the plaques and tangles found in the brains of humans with AD (Green, Billings, Roozendaal, McGaugh, & LaFerla, 2006). Further, allostatic load increases risk for cardiovascular diseases, chronic inflammation, and vulnerability to pathogenic infection (McEwen & Stellar, 1993), which can be further exacerbated by poor health behaviors such as lack of exercise and substance use (Conron, Mimiaga, & Landers, 2010). These health consequences exacerbate typical brain aging processes, specifically within the hippocampus, amygdala, and prefrontal cortex, and contribute to all-cause cognitive decline (Garrido, 2011; Lupien et al., 2009). Although cognitive functioning in LGBT elders is as yet unexplored, their exposure to CMS suggests that their cognitive functions, most specifically memory and executive functioning, may be particularly vulnerable to accelerated aging and dementia (Arnsten, 2009; Roozendaal, 2002; Sapolsky, Krey, & McEwen, 1986).
Conceptual Model of Cognitive Aging for LGBT Older Adults
The size of the LGBT population is underappreciated and growing. By 2060, there will be an estimated 2.2 million LGBT older adults in the U.S. alone (Fredriksen-Goldsen et al., 2016; Fredriksen-Goldsen & Kim, 2017). Minority stress will continue to impact their mental health and healthcare utilization, which may alter their aging trajectory (Ferraro & Shippee, 2009). Aging trajectories are shaped by environmental and social systems, and these systems are imbued with advantages and disadvantages based on one’s position within society. Successful aging is impeded in individuals whose identities like race, gender, and sexual orientation are dissimilar from the group(s) with the most social power. Specifically, inequality confers disadvantages via exposure to risk and the absence of opportunity. The consequences of inequality accumulate across the lifespan and thereby burden typical aging processes (Ferraro & Shippee, 2009). Thus, stress mechanisms underlying health disparities in minority populations may contribute to premature cognitive aging because chronic stress is a risk factor for accelerated aging and Alzheimer’s disease (Sotiropoulos et al., 2008). Coping mechanisms, specifically social support and LGBT identity disclosure, are expected to mitigate the effects of CMS by modulating stress hormone exposure and improving quality of life.
Specific studies examining the neuropsychological abilities of LGBT adults through late-life have not been conducted. Although LGBT aging research is increasing, cognitive outcomes have not been reported. Nevertheless, LGBT older adults may be at an increased risk for cognitive impairment and dementia because of their long-term exposure to minority stress, which elevates their rate of health conditions, including substance use, and all-cause dementia risks (Center for Disease Control and Prevention, 2011; Fredriksen-Goldsen et al., 2016; Fredriksen-Goldsen, Kim, et al., 2013; McGovern, 2014). Furthermore, lack of attention to and underreporting of sexual identity in longitudinal research prevents discernment of typical and pathological LGBT cognitive aging trajectories relative to their heterosexual counterparts. Even if such studies ultimately reveal comparable risk profiles (cf. Perales-Puchalt et al., 2019), history of discrimination for LGBT elders may hinder their pursuit of early interventions, contributing to poorer cognitive outcomes (Gardner, de Vries, & Mockus, 2014; McGovern, 2014). Thus, specific studies examining the cognitive functioning of LGBT adults through late-life and their patterns and rates of decline are critically needed.
LGBT Minority Stress and Cognition
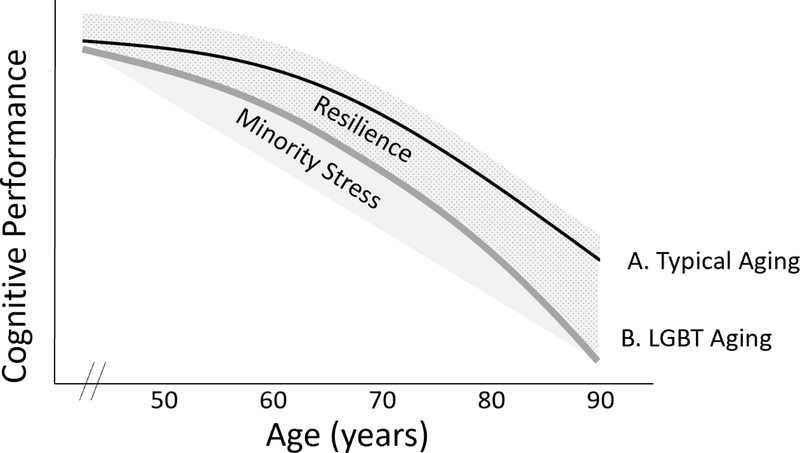
We propose an introductory conceptual model of minority stress-related cognitive decline in LGB elders for the purpose of compelling and guiding critically important future research (see Figure 1). Line A represents the typical cognitive aging trajectory whereby all-cause brain and cognitive functioning gradually decline across older adulthood. Line B is the theoretical average all-cause decline for LGBT older adults. This cognitive trajectory is theoretically modifiable by minority stress (i.e., shaded region below) and resilience (i.e., shaded and stippled region above). Line B has a steeper slope than Line A because lifelong exposure to stress hormones contributes to deficits in neurocognitive functioning (Lupien et al., 1998; Lupien et al., 2009; Sapolsky et al., 1986). The mechanisms by which stress accelerates decline include the neurotoxic effects of stress hormones on the hippocampus and prefrontal cortex as well as metabolic disruptions that harm general brain functioning (Arnsten, 2009; de la Torre, 2012; Lupien et al., 1998; Lupien et al., 2009; McEwen, Nasca, & Gray, 2016; Muela et al., 2017; Raz & Rodrigue, 2006; Stefanidis, Askew, Greaves, & Summers, 2017). Cognitive consequences would therefore be particularly evident in learning, memory, and executive functions.
Figure 1. Theoretical trajectory of cognitive decline for LGBT older adults.
Cognitive decline is accelerated in LGBT aging (Line B) relative to typical aging (Line A) due to the degree of chronic minority stress, which increases physical and mental health risk factors (de la Torre, 2012; Diniz et al., 2013; Fredriksen-Goldsen, Emlet, et al., 2013; Fredriksen-Goldsen et al., 2016; Ownby et al., 2006; Simard et al., 2009; Sotiropoulos et al., 2008; Stefanidis et al., 2017) and neurotoxic effects of stress hormones on brain structure and function (Lupien et al., 2009). Neuropsychological consequences include learning, memory, and executive functioning. Factors such as discrimination and social inequality accumulate to modify the trajectory (Ferraro & Shippee, 2009) in an additive manner (Minority Stress shaded area). Protective factors (e.g., social support) theoretically promote resilience to cognitive aging (Resilience shaded and stippled area) (Fredriksen-Goldsen, Emlet, et al., 2013; Fredriksen-Goldsen et al., 2015; Meyer, 2015). Protective factors also include those beneficial to typical older adults, including education, socioeconomic status, and physical activity (Alzheimer’s Association, 2016). Genetics can be additive or subtractive but are excluded from the model for simplicity.
Importantly, social systems, one’s position in society, and location apportion exposure to risks and access to resources (Brotman et al., 2002; Ferraro & Shippee, 2009). Health disparities are a manifestation of social inequality as people from various minority backgrounds are at elevated risks for health complications. As such, many LGBT individuals may ultimately face other forms of oppression in addition to heterosexism (Bowleg, Huang, Brooks, Black, & Burkholder, 2003; Kertzner et al., 2009; Kim & Fredriksen-Goldsen, 2017; Kum, 2017; Veenstra, 2011). Stigma-related stress is typically multi-faceted, likely accumulating to compound risks for detrimental aging (Ferraro & Shippee, 2009; Meyer, 2015). This is accounted for by the gray area below Line B. Notably, LGBT health disparities research samples tend to include mostly White, healthy, educated, and “out” participants. It is therefore imperative that future research specifically examine discrete identities as well as the interacting effects of oppression/privilege associated with individuals’ unique identities(Kertzner et al., 2009). This will necessitate multilevel predictive modeling and examination of mediating and moderating factors. For example, transgender older adults have poorer health outcomes than non-transgender sexual minorities, which is indirectly mediated by the impact of a transgender identity on healthcare access, physical activity, minority stress, and social support, in addition to other mediators such as obesity and disability (Fredriksen-Goldsen et al., 2014).
Protective Factors
Resource mobilization, human agency, environmental enrichment (e.g., social support), and early intervention are factors that mitigate the negative effects of early disadvantage (Ferraro & Shippee, 2009; Lupien et al., 2009). Thus, cognitive decline for LGBT elders is likely modifiable by protective factors such as: social support, LGBT community engagement, and disclosure of one’s identity (Burton, Bonanno, & Hatzenbuehler, 2014; Butler, 2004; Caceres & Frank, 2016; Fredriksen-Goldsen, Emlet, et al., 2013; Fredriksen-Goldsen et al., 2015; Hoy-Ellis & Fredriksen-Goldsen, 2016; Hsieh, 2014; Kim, Fredriksen-Goldsen, Bryan, & Muraco, 2017; Lyons & Pepping, 2017; Masini & Barrett, 2008; Walch et al., 2016). These variables likely moderate the stress-related aging trajectories in Figure 1 by modulating cortisol responses (Burton et al., 2014) in an additive manner (Kremen, Lachman, Pruessner, Sliwinski, & Wilson, 2012). For example, in adults3 24–75 years of age, protective factors had an additive effect toward protecting cognition over the next decade (Agrigoroaei & Lachman, 2011).
Implications and Recommendations
The U.S. economy and healthcare system may be impacted by the ability of current scientists and healthcare providers to discover and eliminate health disparities experienced by LGBT older adults (“Healthy People 2020,” 2012). Currently, there are over 3 million LGBT people age 55 and older (Espinoza, 2014; Fredriksen-Goldsen et al., 2016), and the LGBT population is projected to double by 2040 (Espinoza, 2014). With discrepant prevalence rates of physical and mental health conditions compared to their heterosexual counterparts, LGBT elders’ care will require disparately more resources (Fredriksen-Goldsen et al., 2016; Fredriksen-Goldsen & Kim, 2017). Given their particularly high risk for exposure to stress, pathological aging risk might be exacerbated in LGBT elders. As such, understanding of their risks for cognitive decline and dementia and the potential avenues to prevent their particular risks is critically needed.
Sexual and gender identities are important considerations in clinical and cognitive evaluations of older adults, both to better address potential risks and to better target and afford access to LGBT-affirmative services (Gardner et al., 2014). Healthcare providers and aging researchers are uniquely positioned to provide recommendations to enhance health and well-being. Examples include offering or providing referrals to culturally sensitive interventions, recommending interactions with LGBT community organizations and social support groups, and encouraging engagement in supportive social networks. Finally, research on the cognitive impact and mechanisms of lifelong minority stress is essential to the mitigation and prevention of disease and healthcare costs and to maintain quality of life for LGBT elders (Meyer, 2015).
Acknowledgements
An earlier version of this manuscript fulfilled a portion of the requirements for the Doctor of Philosophy degree at Marquette University. This work was supported by a National Science Foundation Graduate Research Fellowship (#1452781), a Summer Fellowship from Marquette University’s Graduate School and Vice President of Research and Innovation, a Milwaukee Gay Sports Network & Windhover Foundation LGBTQ+ Scholarship from the Cream City Foundation, and grants from the National Center for Advancing Translational Sciences and the National Institutes of Health (UL1TR001436 and TL1TR001437). The authors thank Drs. Nicholas Heck and Anthony Porcelli for consultation on earlier versions of this project and Dr. Allison Jahn and Ms. Elizabeth Paitel for consultation and editing assistance. The authors have no conflicts of interest to report.
Footnotes
Definitions of older adulthood vary across the literature and in popular culture. Although research on LGBT aging tends to focus on those age 50+ (cf. Fredriksen-Goldsen & Muraco, 2010), studies vary, with some focusing on age 65+ and others on ages 45–75. Herein “older adult” and “elder” are synonymous and defined as age 65 and older.
Internalized stigma has also been described as “self-stigma,” “internalized homophobia,” “internalized heterosexism,” and “internalized homonegativity.”
Sexual identity was not reported.
References
- Agrigoroaei S, & Lachman ME (2011). Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J Gerontol B Psychol Sci Soc Sci, 66 Suppl 1, i130–140. doi: 10.1093/geronb/gbr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JO (2016). Ageism as a Risk Factor for Chronic Disease. Gerontologist, 56(4), 610–614. doi: 10.1093/geront/gnu158 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2016). 2016 Alzheimer’s disease facts and figures. Alzheimers Dement, 12(4), 459–509. doi:j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci, 10(6), 410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L, Huang J, Brooks K, Black A, & Burkholder G (2003). Triple jeopardy and beyond: multiple minority stress and resilience among black lesbians. J Lesbian Stud, 7(4), 87–108. doi: 10.1300/J155v07n04_06 [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, & Thayer JF (2005). Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30(10), 1043–1049. doi: 10.1016/j.psyneuen.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Brotman S, Ryan B, Jalbert Y, & Rowe B (2002). The impact of coming out on health and health care access: the experiences of gay, lesbian, bisexual and two-spirit people. J Health Soc Policy, 15(1), 1–29. doi: 10.1300/J045v15n01_01 [DOI] [PubMed] [Google Scholar]
- Brubaker MD, Garrett MT, & Dew BJ (2009). Examining the relationship between internalized heterosexism and substance abuse among lesbian, gay, and bisexual individuals: A critical review. Journal of LGBT Issues in Counseling, 3(1), 62–89. doi: 10.1080/15538600902754494 [DOI] [Google Scholar]
- Burton CL, Bonanno GA, & Hatzenbuehler ML (2014). Familial social support predicts a reduced cortisol response to stress in sexual minority young adults. Psychoneuroendocrinology, 47, 241–245. doi: 10.1016/j.psyneuen.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SS (2004). Gay, Lesbian, Bisexual, and Transgender (GLBT) Elders: The Challenges and Resilience of this Marginalized Group. Journal of Human Behavior in the Social Environment, 9(4), 25–44. doi: 10.1300/J137v09n04_02 [DOI] [Google Scholar]
- Caceres BA, & Frank MO (2016). Successful ageing in lesbian, gay and bisexual older people: a concept analysis. Int J Older People Nurs, 11(3), 184–193. doi: 10.1111/opn.12108 [DOI] [PubMed] [Google Scholar]
- Caraci F, Copani A, Nicoletti F, & Drago F (2010). Depression and Alzheimer’s disease: neurobiological links and common pharmacological targets. Eur J Pharmacol, 626(1), 64–71. doi: 10.1016/j.ejphar.2009.10.022 [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O, & Baquet C (2002). What is a “health disparity”? Public Health Rep, 117(5), 426–434. doi: 10.1093/phr/117.5.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2011). Cognitive impairment: A call for action now! Retrieved from https://www.cdc.gov/aging/pdf/cognitive_impairment/cogimp_poilicy_final.pdf
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, & Sousa N (2007). The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci, 27(11), 2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, & Miller GE (2007). Psychological stress and disease. JAMA, 298(14), 1685–1687. doi: 10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Conron KJ, Mimiaga MJ, & Landers SJ (2010). A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health, 100(10), 1953–1960. doi: 10.2105/AJPH.2009.174169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N, Dewaele A, van Houtte M, & Vincke J (2011). Stress-related growth, coming out, and internalized homonegativity in lesbian, gay, and bisexual youth. An examination of stress-related growth within the minority stress model. J Homosex, 58(1), 117–137. doi: 10.1080/00918369.2011.533631 [DOI] [PubMed] [Google Scholar]
- D’Augelli AR, & Grossman AH (2001). Disclosure of sexual orientation, victimization, and mental health among lesbian, gay, and bisexual older adults. J Interpers Violence, 16(10), 1008–1027. doi: 10.1177/088626001016010003 [DOI] [Google Scholar]
- de la Torre JC (2012). Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol, 2012, 367516. doi: 10.1155/2012/367516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, & Reynolds CF 3rd. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry, 202(5), 329–335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A, 101(49), 17312–17315. doi: 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza R (2014). Out and visible: The experiences and attitudes of lesbian, gay, bisexual and transgender older adults, ages 45–75. Retrieved from SAGE (Services and Advocacy for GLBT Elders) website: http://www.sageusa.org/resources/outandvisible.cfm [Google Scholar]
- Ferraro KF, & Shippee TP (2009). Aging and cumulative inequality: how does inequality get under the skin? Gerontologist, 49(3), 333–343. doi: 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, & Brennan-Ing M (2018). Correlates of Subjective Cognitive Decline in Lesbian, Gay, Bisexual, and Transgender Older Adults. J Alzheimers Dis, 64(1), 91–102. doi: 10.3233/JAD-171061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI (2016). The Future of LGBT+ Aging: A Blueprint for Action in Services, Policies, and Research. Generations, 40(2), 6–15. [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Bryan AE, Jen S, Goldsen J, Kim HJ, & Muraco A (2017). The Unfolding of LGBT Lives: Key Events Associated With Health and Well-being in Later Life. Gerontologist, 57(suppl 1), S15–S29. doi: 10.1093/geront/gnw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Cook-Daniels L, Kim HJ, Erosheva EA, Emlet CA, Hoy-Ellis CP, . . . Muraco A (2014). Physical and mental health of transgender older adults: an at-risk and underserved population. Gerontologist, 54(3), 488–500. doi: 10.1093/geront/gnt021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Emlet CA, Kim HJ, Muraco A, Erosheva EA, Goldsen J, & Hoy-Ellis CP (2013). The physical and mental health of lesbian, gay male, and bisexual (LGB) older adults: the role of key health indicators and risk and protective factors. Gerontologist, 53(4), 664–675. doi: 10.1093/geront/gns123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Jen S, Bryan AE, & Goldsen J (2016). Cognitive Impairment, Alzheimer’s Disease, and Other Dementias in the Lives of Lesbian, Gay, Bisexual and Transgender (LGBT) Older Adults and Their Caregivers. J Appl Gerontol, 733464816672047. doi: 10.1177/0733464816672047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, & Kim HJ (2017). The Science of Conducting Research With LGBT Older Adults- An Introduction to Aging with Pride: National Health, Aging, and Sexuality/Gender Study (NHAS). Gerontologist, 57(suppl 1), S1–S14. doi: 10.1093/geront/gnw212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Kim HJ, Barkan SE, Muraco A, & Hoy-Ellis CP (2013). Health disparities among lesbian, gay, and bisexual older adults: results from a population-based study. Am J Public Health, 103(10), 1802–1809. doi: 10.2105/AJPH.2012.301110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Kim HJ, Shiu C, Goldsen J, & Emlet CA (2015). Successful Aging Among LGBT Older Adults: Physical and Mental Health-Related Quality of Life by Age Group. Gerontologist, 55(1), 154–168. doi: 10.1093/geront/gnu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, & Muraco A (2010). Aging and Sexual Orientation: A 25-Year Review of the Literature. Res Aging, 32(3), 372–413. doi: 10.1177/0164027509360355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AT, de Vries B, & Mockus DS (2014). Aging out in the desert: disclosure, acceptance, and service use among midlife and older lesbians and gay men. J Homosex, 61(1), 129–144. doi: 10.1080/00918369.2013.835240 [DOI] [PubMed] [Google Scholar]
- Garrido P (2011). Aging and stress: past hypotheses, present approaches and perspectives. Aging Dis, 2(1), 80–99. [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis, 2(3), 207–221. [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, & LaFerla FM (2006). Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci, 26(35), 9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML (2009). How does sexual minority stigma “get under the skin”? A psychological mediation framework. Psychol Bull, 135(5), 707–730. doi: 10.1037/a0016441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML (2014). Structural stigma and the health of lesbian, gay, and bisexual populations. Curr Dir Psychol Sci, 23(2), 127–132. doi: 10.1177/0963721414523775 [DOI] [Google Scholar]
- Hatzenbuehler ML (2016). Structural stigma: Research evidence and implications for psychological science. Am Psychol, 71(8), 742–751. doi: 10.1037/amp0000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, & McLaughlin KA (2014). Structural stigma and hypothalamic-pituitary-adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Ann Behav Med, 47(1), 39–47. doi: 10.1007/s12160-013-9556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthy People 2020. (2012). Topics and objectives index. Retrieved from http://www.healthypeople.gov/2020/topicsobjectives2020/default.aspx
- Hebert LE, Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks ML, & Testa RJ (2012). A conceptual framework for clinical work with transgender and gender nonconforming clients: An adaptation of the minority stress model. Prof Psychol-Res Pr, 43(5), 460–467. doi: 10.1037/a0029597 [DOI] [Google Scholar]
- Herek GM, Gillis JR, & Cogan JG (2009). Internalized stigma among sexual minority adults: Insights from a social psychological perspective. J Couns Psychol, 56(1), 32–43. doi: 10.1037/a0014672 [DOI] [Google Scholar]
- Houde M, Bergman H, Whitehead V, & Chertkow H (2008). A predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry, 23(10), 1028–1033. doi: 10.1002/gps.2028 [DOI] [PubMed] [Google Scholar]
- Hoy-Ellis CP, & Fredriksen-Goldsen KI (2016). Lesbian, gay, & bisexual older adults: linking internal minority stressors, chronic health conditions, and depression. Aging Ment Health, 20(11), 1119–1130. doi: 10.1080/13607863.2016.1168362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh N (2014). Explaining the mental health disparity by sexual orientation: The importance of social resources. Soc Ment Health, 4(2), 129–146. doi: 10.1177/2156869314524959 [DOI] [Google Scholar]
- Huebner DM, & Davis MC (2005). Gay and bisexual men who disclose their sexual orientations in the workplace have higher workday levels of salivary cortisol and negative affect. Ann Behav Med, 30(3), 260–267. doi: 10.1207/s15324796abm3003_10 [DOI] [PubMed] [Google Scholar]
- Huebner DM, Rebchook GM, & Kegeles SM (2004). Experiences of harassment, discrimination, and physical violence among young gay and bisexual men. Am J Public Health, 94(7), 1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (2011). The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academics Press; Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22013611. [PubMed] [Google Scholar]
- Juster RP, Smith NG, Ouellet E, Sindi S, & Lupien SJ (2013). Sexual orientation and disclosure in relation to psychiatric symptoms, diurnal cortisol, and allostatic load. Psychosom Med, 75(2), 103–116. doi: 10.1097/PSY.0b013e3182826881 [DOI] [PubMed] [Google Scholar]
- Kertzner RM, Meyer IH, Frost DM, & Stirratt MJ (2009). Social and psychological well-being in lesbians, gay men, and bisexuals: the effects of race, gender, age, and sexual identity. Am J Orthopsychiatry, 79(4), 500–510. doi: 10.1037/a0016848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, & Fredriksen-Goldsen KI (2017). Disparities in Mental Health Quality of Life Between Hispanic and Non-Hispanic White LGB Midlife and Older Adults and the Influence of Lifetime Discrimination, Social Connectedness, Socioeconomic Status, and Perceived Stress. Res Aging, 39(9), 991–1012. doi: 10.1177/0164027516650003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fredriksen-Goldsen KI, Bryan AE, & Muraco A (2017). Social Network Types and Mental Health Among LGBT Older Adults. Gerontologist, 57(suppl 1), S84–S94. doi: 10.1093/geront/gnw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SD, & Richardson VE (2016). Influence of income, being partnered/married, resilience, and discrimination on mental health distress for midlife and older gay men. Journal of Gay & Lesbian Mental Health, 20(2), 127–151. doi: 10.1080/19359705.2015.1127191 [DOI] [Google Scholar]
- Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, & Wilson RS (2012). Mechanisms of age-related cognitive change and targets for intervention: social interactions and stress. J Gerontol A Biol Sci Med Sci, 67(7), 760–765. doi: 10.1093/gerona/gls125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kum S (2017). Gay, gray, black, and blue: An examination of some of the challenges faced by older LGBTQ people of color. Journal of Gay & Lesbian Mental Health, 21(3), 228–239. doi: 10.1080/19359705.2017.1320742 [DOI] [Google Scholar]
- Landfield PW, Blalock EM, Chen KC, & Porter NM (2007). A new glucocorticoid hypothesis of brain aging: implications for Alzheimer’s disease. Curr Alzheimer Res, 4(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R, Sapronova A, & Kotelevtsev Y (2014). Atherosclerosis and Alzheimer--diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr, 14, 36. doi: 10.1186/1471-2318-14-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS (2006). Birth outcomes for Arabic-named women in California before and after September 11. Demography, 43(1), 185–201. [DOI] [PubMed] [Google Scholar]
- Lick DJ, Durso LE, & Johnson KL (2013). Minority Stress and Physical Health Among Sexual Minorities. Perspect Psychol Sci, 8(5), 521–548. doi: 10.1177/1745691613497965 [DOI] [PubMed] [Google Scholar]
- Logie C (2012). The case for the World Health Organization’s Commission on the Social Determinants of Health to address sexual orientation. Am J Public Health, 102(7), 1243–1246. doi: 10.2105/AJPH.2011.300599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, . . . Meaney MJ (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci, 1(1), 69–73. doi: 10.1038/271 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, & Schramek TE (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn, 65(3), 209–237. doi: 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Lyons A, & Pepping CA (2017). Prospective effects of social support on internalized homonegativity and sexual identity concealment among middle-aged and older gay men: a longitudinal cohort study. Anxiety Stress and Coping, 30(5), 585–597. doi: 10.1080/10615806.2017.1330465 [DOI] [PubMed] [Google Scholar]
- Masini BE, & Barrett HA (2008). Social support as a predictor of psychological and physical well-being and lifestyle in lesbian, gay, and bisexual adults aged 50 and over. Journal of Gay and Lesbian Social Services, 20(1–2), 91–110. doi: 10.1080/10538720802179013 [DOI] [Google Scholar]
- Maylor EA, Reimers S, Choi J, Collaer ML, Peters M, & Silverman I (2007). Gender and sexual orientation differences in cognition across adulthood: age is kinder to women than to men regardless of sexual orientation. Arch Sex Behav, 36(2), 235–249. doi: 10.1007/s10508-006-9155-y [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998a). Protective and damaging effects of stress mediators. N Engl J Med, 338(3), 171–179. doi: 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998b). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev, 87(3), 873–904. doi: 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci, 1186, 190–222. doi: 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 41(1), 3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual. Mechanisms leading to disease. Arch Intern Med, 153(18), 2093–2101. [PubMed] [Google Scholar]
- McGovern J (2014). The forgotten: dementia and the aging LGBT community. J Gerontol Soc Work, 57(8), 845–857. doi: 10.1080/01634372.2014.900161 [DOI] [PubMed] [Google Scholar]
- Merritt MM, Bennett GG Jr., Williams RB, Edwards CL, & Sollers JJ 3rd. (2006). Perceived racism and cardiovascular reactivity and recovery to personally relevant stress. Health Psychol, 25(3), 364–369. doi: 10.1037/0278-6133.25.3.364 [DOI] [PubMed] [Google Scholar]
- Meyer IH (1995). Minority stress and mental health in gay men. J Health Soc Behav, 36(1), 38–56. [PubMed] [Google Scholar]
- Meyer IH (2003). Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull, 129(5), 674–697. doi: 10.1037/0033-2909.129.5.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer IH (2015). Resilience in the study of minority stress and health of sexual and gender minorities. Psychology of Sexual Orientation and Gender Diversity, 2(3), 209–213. doi: 10.1037/sgd0000132 [DOI] [Google Scholar]
- Meyer IH, & Dean L (1998). Internalized homophobia, intimacy and sexual behaviour among gay and bisexual men In Herek G (Ed.), Stigma and sexual orientation (pp. 160–186). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Modrego PJ, & Ferrandez J (2004). Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol, 61(8), 1290–1293. doi: 10.1001/archneur.61.8.1290 [DOI] [PubMed] [Google Scholar]
- Muela HC, Costa-Hong VA, Yassuda MS, Moraes NC, Memoria CM, Machado MF, . . . Bortolotto LA (2017). Hypertension Severity Is Associated With Impaired Cognitive Performance. J Am Heart Assoc, 6(1). doi: 10.1161/JAHA.116.004579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE, Tofler GH, & Stone PH (1989). Circadian variation and triggers of onset of acute cardiovascular disease. Circulation, 79(4), 733–743. [DOI] [PubMed] [Google Scholar]
- Newcomb ME, & Mustanski B (2010). Internalized homophobia and internalizing mental health problems: a meta-analytic review. Clin Psychol Rev, 30(8), 1019–1029. doi: 10.1016/j.cpr.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, & Loewenstein D (2006). Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry, 63(5), 530–538. doi: 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: a meta-analytic review. Psychol Bull, 135(4), 531–554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales-Puchalt J, Gauthreaux K, Flatt J, Teylan MA, Resendez J, Kukull WA, . . . Vidoni ED (2019). Risk of dementia and mild cognitive impairment among older adults in same-sex relationships. Int J Geriatr Psychiatry, 34(6), 828–835. doi: 10.1002/gps.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Q, Abrahams S, & Wilson GD (2003). Sexual-orientation-related differences in verbal fluency. Neuropsychology, 17(2), 240–246. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Andersson D, & Govier E (2005). A specific sexual orientation-related difference in navigation strategy. Behav Neurosci, 119(1), 311–316. doi: 10.1037/0735-7044.119.1.311 [DOI] [PubMed] [Google Scholar]
- Rahman Q, Sharp J, McVeigh M, & Ho ML (2017). Sexual Orientation-Related Differences in Virtual Spatial Navigation and Spatial Search Strategies. Arch Sex Behav, 46(5), 1279–1294. doi: 10.1007/s10508-017-0986-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Q, & Wilson GD (2003). Large sexual-orientation-related differences in performance on mental rotation and judgment of line orientation tasks. Neuropsychology, 17(1), 25–31. [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, & Abrahams S (2003). Sexual orientation related differences in spatial memory. J Int Neuropsychol Soc, 9(3), 376–383. doi: 10.1017/S1355617703930037 [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, & Abrahams S (2004a). Biosocial factors, sexual orientation and neurocognitive functioning. Psychoneuroendocrinology, 29(7), 867–881. doi: 10.1016/S0306-4530(03)00154-9 [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, & Abrahams S (2004b). Performance differences between adult heterosexual and homosexual men on the Digit-Symbol Substitution subtest of the WAIS-R. J Clin Exp Neuropsychol, 26(1), 141–148. doi: 10.1076/jcen.26.1.141.23934 [DOI] [PubMed] [Google Scholar]
- Raz N, & Rodrigue KM (2006). Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev, 30(6), 730–748. doi: 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem, 78(3), 578–595. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, & McEwen BS (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev, 7(3), 284–301. doi: 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull, 130(4), 601–630. doi: 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Hudon C, & van Reekum R (2009). Psychological distress and risk for dementia. Curr Psychiatry Rep, 11(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, & Almeida OF (2008). Stress and glucocorticoid footprints in the brain-the path from depression to Alzheimer’s disease. Neurosci Biobehav Rev, 32(6), 1161–1173. doi: 10.1016/j.neubiorev.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Stefanidis KB, Askew CD, Greaves K, & Summers MJ (2017). The Effect of Non-Stroke Cardiovascular Disease States on Risk for Cognitive Decline and Dementia: A Systematic and Meta-Analytic Review. Neuropsychol Rev. doi: 10.1007/s11065-017-9359-z [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci, 10(6), 397–409. doi: 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsey SO, & Hook JN (2007). Heart rate variability as a physiological moderator of the relationship between race-related stress and psychological distress in African Americans. Cultur Divers Ethnic Minor Psychol, 13(3), 250–253. doi: 10.1037/1099-9809.13.3.250 [DOI] [PubMed] [Google Scholar]
- Veenstra G (2011). Race, gender, class, and sexual orientation: intersecting axes of inequality and self-rated health in Canada. International Journal for Equity in Health, 10. doi: 10.1186/1475-9276-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch SE, Ngamake ST, Bovornusvakool W, & Walker SV (2016). Discrimination, internalized homophobia, and concealment in sexual minority physical and mental health. Psychology of Sexual Orientation and Gender Diversity, 3(1), 37–48. doi: 10.1037/sgd0000146 [DOI] [Google Scholar]
- Willmott M, & Brierley H (1984). Cognitive characteristics and homosexuality. Arch Sex Behav, 13(4), 311–319. [DOI] [PubMed] [Google Scholar]
- Woodford MR, Howell ML, Silverschanz P, & Yu L (2012). “That’s so gay!”: Examining the covariates of hearing this expression among gay, lesbian, and bisexual college students. J Am Coll Health, 60(6), 429–434. doi: 10.1080/07448481.2012.673519 [DOI] [PubMed] [Google Scholar]
- Xu Y, Norton S, & Rahman Q (2017). Sexual orientation and neurocognitive ability: A meta-analysis in men and women. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Zarda & Moore v. Jr. Altitude Express, Inc., No. 15–3775 (U.S. Court of Appeals for the Second Circuit 2017). [Google Scholar]