Atrial fibrillation (AF) is a major cause of morbidity and mortality in the United States (US). With an increasingly diverse US population, ensuring minority participation in clinical trials is important for the generalizability of AF therapies and practice guidelines across the country. We sought to evaluate current gaps in racial/ethnic minority participation in major AF clinical trials by examining trials cited in the 2019 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Focused Update of the 2014 AHA/ACC/HRS Guideline for Atrial Fibrillation (1).
We included all papers cited in the 2019 guidelines that we identified as randomized clinical trials – studies that described initial participant randomization with post-randomization outcomes. We identified a total of 34 trials from 1996 to 2019 that addressed varying topics including anticoagulation, procedural interventions, and antiarrhythmic therapies. We examined manuscripts and supplementary data for participant-level racial/ethnic data. We classified studies by reported proportion of non-Hispanic white (NHW), African American (AA), Hispanic, and/or Asian participants. If NHW participation was not explicitly described, participants reported as white were categorized as NHW. For each race/ethnicity, we pooled participant-level data to determine overall percentage of participation across trials. We also classified studies by reporting of geographic areas (for example, North America, Europe, Latin America, or Asia-Pacific) without participant-level data.
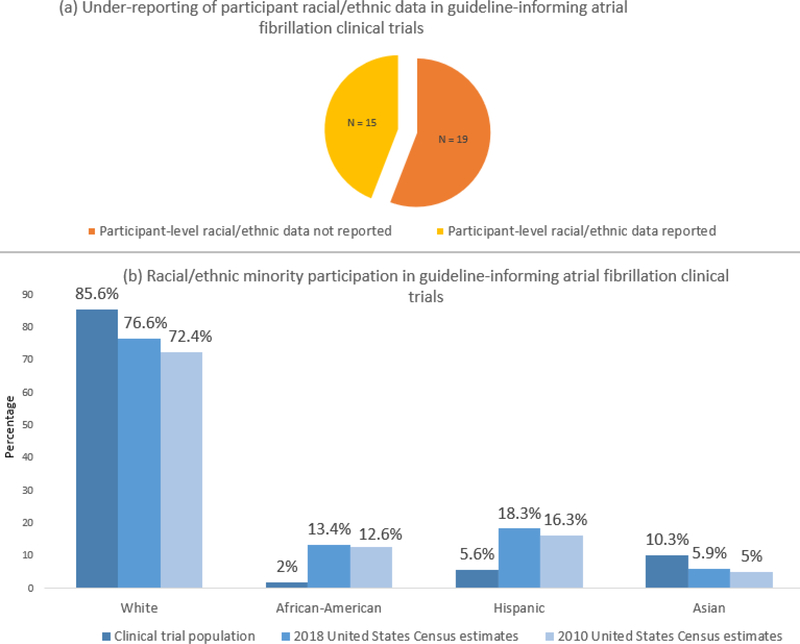
We found that 15 out of 34 trials (44%) reported participant-level racial/ethnic data. An additional 8 trials reported geographic areas without participant-level data. All trials reporting participant-level data were published between 2009 and 2019. Trials that did not report participant-level data addressed a variety of topics including anticoagulation/antithrombotic therapy, antiarrhythmic therapy, procedures, ambulatory monitoring, or lifestyle interventions. A total of fourteen trials reported NHW participation rates. Only eight total studies reported AA participants. Six trials reported Hispanic participation rates, and seven trials reported Asian participation rates. No studies provided disaggregated Hispanic or Asian subgroup data. One trial reported a combined proportion of European and Arab participants.
Pooled NHW participation among the fourteen NHW-reporting trials was 85.6% of total participants. Pooled AA participation was 2% in the trials reporting participant-level AA data. Hispanic participation was 5.6% and Asian participation was 10.3% among trials that reported Hispanic or Asian data, respectively. When participation rates were compared with 2010 and 2018 US census data, AA and Hispanic populations were under-represented in clinical trials (Figure)(2,3).
Figure -.
Reporting (a) and representation (b) of racial/ethnic minority participation in 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society guideline-informing atrial fibrillation trials.
Under-reporting of racial/ethnic minority participation limits our ability to assess generalizability of trial results across diverse populations. Importantly, we also found heterogeneity in reporting across major AF trials, with inconsistently reported rates of AA, Hispanic, and/or Asian participation. Hispanic and Asian groups were not disaggregated likely due to low representation, which is notable given documented health differences within disaggregated subgroups(4). There is a need for uniform reporting standards for participant-level racial/ethnic data in clinical trials to allow assessment of generalizability across diverse groups and identification of minority participation gaps.
The Food and Drug Administration has recognized the importance of minority trial participation with various initiatives including designating 2016 the “Year of Clinical Trial Diversity”(5). Our results demonstrating AA and Hispanic under-representation underscore the need to intensify outreach and recruitment of diverse participants.
Our analysis has certain limitations. We explored 2019 guidelines to ensure that our analysis reflected contemporary literature; we may have excluded newer trials. We were unable to address disaggregated Hispanic and Asian subgroups given lack of data. Due to inconsistent reporting, we were unable to comprehensively differentiate White Hispanic from NHW participation which may lead to NHW percentage overestimation.
In conclusion, in contemporary guideline-informing AF clinical trials, participant-level racial/ethnic minority data are reported inconsistently and heterogeneously. Based on their proportion in the US population, African American and Hispanic populations are under-represented in AF clinical trials. Future efforts should focus on standardized reporting and improved participation of racial/ethnic minorities in clinical trials.
Footnotes
Disclosures: The authors report no relevant disclosures.
Sample Tweet: #JACCEP @StanfordMed study suggests that African-Americans and Hispanics are under-reported and under-represented in major atrial fibrillation trials. More work to be done for diversity in trial recruitment. #Afib @StanCVFellows @AshishSarraju, @FaRodriguezMD
References
- 1.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 2.United States Census Bureau QuickFacts. 2018. Available at https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed December 15, 2019
- 3.United States Census Bureau. US Population - 1940 to 2010. 2012. Available at https://www.census.gov/newsroom/cspan/1940census/CSPAN_1940slides.pdf. Accessed December 15, 2019.
- 4.Rodriguez F, Hastings KG, Boothroyd DB et al. Disaggregation of Cause-Specific Cardiovascular Disease Mortality Among Hispanic Subgroups. JAMA Cardiol 2017;2:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration Office of Minority Health. Clinical Trial Diversity Stakeholder Communications Toolkit. 2016. Available at https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed December 15, 2019.