Abstract
Background
Interleukin-6 signal blockade showed preliminary beneficial effects in treating inflammatory response against SARS-CoV-2 leading to severe respiratory distress. Herein we describe the outcomes of off-label intravenous use of Sarilumab in severe SARS-CoV-2-related pneumonia.
Methods
53 patients with SARS-CoV-2 severe pneumonia received intravenous Sarilumab; pulmonary function improvement or Intensive Care Unit (ICU) admission rate in medical wards, live discharge rate in ICU treated patients and safety profile were recorded. Sarilumab 400 mg was administered intravenously on day 1, with eventual additional infusion based on clinical judgement, and patients were followed for at least 14 days, unless previously discharged or dead.
Findings
Of the 53 SARS-CoV-2pos patients receiving Sarilumab, 39(73·6%) were treated in medical wards [66·7% with a single infusion; median PaO2/FiO2:146(IQR:120–212)] while 14(26·4%) in ICU [92·6% with a second infusion; median PaO2/FiO2: 112(IQR:100–141.5)].
Within the medical wards, 7(17·9%) required ICU admission, 4 of whom were re-admitted to the ward within 5–8 days. At 19 days median follow-up, 89·7% of medical inpatients significantly improved (46·1% after 24 h, 61·5% after 3 days), 70·6% were discharged from the hospital and 85·7% no longer needed oxygen therapy. Within patients receiving Sarilumab in ICU, 64·2% were discharged from ICU to the ward and 35·8% were still alive at the last follow-up. Overall mortality rate was 5·7%.
Interpretation
IL-6R inhibition appears to be a potential treatment strategy for severe SARS-CoV-2 pneumonia and intravenous Sarilumab seems a promising treatment approach showing, in the short term, an important clinical outcome and good safety.
Keywords: Severe sars-cov-2 pneumonia, Sarilumab, Inflammation
Research in context.
Evidence before this study
SARS-CoV-2 infection remains a disease with many unknown aspects for which there are no therapies with proven efficacy. To date, it is recognized that COVID-19 disease may lead to the development of a cytokine storm for which drugs as IL-6R inhibitors may have beneficial effect.
Added value of this study
In this observational clinical study, we report the efficacy and safety of intravenous Sarilumab use in SARS-CoV-2 severe pneumonia with a global resolution rate of 83·0% (89·7% in medical wards and 64·3% in ICU) and an overall mortality rate of 5·7%.
Implications of all the available evidence
IL-6R-inhibition is an effective approach for severe SARS-CoV-2 pneumonia and intravenous Sarilumab is a promising treatment approach leading to an important clinical benefit and good safety in the short term.
Alt-text: Unlabelled box
1. Introduction
On December 31, 2019, an outbreak of pneumonia of unknown etiology was reported in the Chinese city of Wuhan and 10 days later the Chinese Center Disease Control (CDC) identified the causative agent of this infection, a new coronavirus called SARS-CoV-2 [1]. On February 21, 2020, the first Italian patient with SARS-CoV-2 disease was admitted to the Codogno Hospital [2] and over the next weeks there has been an exponential growth of cases in our country. To date, Italy is globally one of the most affected countries, with 205.463 confirmed cases, 27.967 deaths and 75.945 people declared recovered at April 30th 2020 [3].
Although 81% of SARS-CoV-2pos patients develop a mild disease, severe pneumonias requiring hospitalization are documented in about 14% of cases and the remaining 5% needs admission to Intensive Care Unit (ICU) [4], [5], [6], [7]. Severe clinical scenario mimics a real "cytokines storm" similar to that occurring in Car-T leukemia treatment [8,9]. In this context, Interleukin-6 (IL-6) is considered a key cytokine mediating the aberrant activation of immune cells arising as a putative mediator of SARS-CoV-2 induced cytokine storm despite being lower than levels detected in sepsis [10]. Therefore, multiple studies tested IL6 pathway inhibition efficacy in SARS-CoV-2 pneumonia [11], [12], [13]. In particular, IL-6 direct inhibition (i.e. Siltuximab) or its receptor blockade (i.e. Tocilizumab or Sarilumab) have been or are currently tested in the repression of excessive pro-inflammatory effects in the lung of SARS-CoV-2 pneumonia [14,15]. Due to its increased use and limited supply, Tocilizumab became rapidly unavaiable in Italy and Sarilumab, an alternative IL-6R inhibitor approved for Rheumatoid Arthritis (RA) [16], has been chosen by the Covid-19 Gemelli Task Force to treat patients with severe SARS-CoV-2 pneumonia. Intravenous use of Sarilumab for the treatment of severe SARS-CoV-2 pneumonia was firstly chosen by analogy with Tocilizumab in the treatment of the Car-T induced hyperinflammatory syndrome [9], in which a quick start of the effect is also required.
In the present study, we report the outcomes of the off-label intravenous use of Sarilumab in patients with severe SARS-CoV-2-related pneumonia. In particular, the study endpoints were to assess (i) the impact of Sarilumab for the treatment of severe SARS-CoV-2 pneumonia in terms of pulmonary function improvement and prevention of ICU admission in a ward setting as of live discharge rate in patients treated in ICU care; (ii) safety and (iii) putative biological and clinical parameters related to improvement or ICU transfer after Sarilumab.
2. Methods
2.1. Patients enrollment
From March 14th 2020, Fondazione Policlinico Universitario A. Gemelli-IRCCS became a referral hospital for SARS-CoV-2 infected people. Hospitalized patients with severe SARS-CoV-2 pneumonia, defined as SARS-CoV2 infection confirmed by RT-PCR assay, interstitial pneumonia at imaging (chest X-Ray or CT scan), impairment of respiratory function (PaO2/FiO2 ratio<300), rapid worsening of the respiratory condition or need for ICU admission received anti-IL6R therapy, unless contraindicated (i.e. septic state, total neutrophil count <1500/mm3, serum levels of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) more than five times the ULN, diverticulitis/diverticulosis or pregnancy). Based on these issues, no control group was included as comparison in the present study. Moreover, the shortage of Tocilizumab together with the increasingly number of patients with progressive respiratory symptoms led the multidisciplinary team made of Immuno-rheumatologists, Infectious Disease specialists, Pulmonologists and Covid wards clinicians to use Sarilumab, according to a shared clinical-pharmacological protocol (Prot.n.926 approved by the Ethics Comittee of the Fondazione Policlinico Universitario A. Gemelli-IRCCS, Università Cattolica del Sacro Cuore). Patients enrollment started on March 23rd till April 4th, 2020, and data collection continued until April 18th, 2020, to ensure at least 14 days follow-up for each patient. Sarilumab treatment was scheduled as follows: 400 mg intravenously on day 1, according to the published Italian Medicines Agency (AIFA) protocol (final injectable solution was obtained combining 2 Sarilumab 200 mg prefilled syringes mixed in 100 ml 0·9% sodium chloride solution for intravenous use). In case of clinical worsening or unchanged status, a repeated dose of Sarilumab 400 mg i.v. was administered. Each patient or next of kin provided informed consent.
Concomitant therapies were set for all patients at the time of confirmed positive naso-pharyngeal swab for SARS-CoV-2 upon entering the Hospital, unless differently required by clinical situation, as follow: lopinavir/ritonavir 400/100 mg BID or darunavir/ritonavir 800/100 mg once daily, orally); Hydroxychloroquine (400 mg BID for the first day, followed by 200 mg BID); Azithromycin combined at the dose of 500 mg orally or intravenously. Moreover, prophylactic dose heparin subcutaneously (4000–6000 UI QD) was added. In case of ICU admission, the Intensivist may use glucocorticoids (GC) as suggested by the Society of Critical Care Medicine [17].
2.2. Clinical, inflammatory and immunological parameters collection
For each patient, demographic, clinical and immunological data were collected at study entry and after 24 h, 3, 5, 7 and 14 days of follow-up and clinical outcome was reported at last follow-up (ongoing hospitalization, hospital discharge or death, respectively). In particular, data on oxygen saturation, PaO2/FiO2 ratio, SpO2 at rest, oxygen-support requirement (room air, Ventimask, CPAP, high flow nasal cannula-HFNC, with high-FiO2 ≥40 mmHg or low-flow oxygen, noninvasive ventilation-NIV, invasive mechanical ventilation-MV), fever, laboratory values were recorded daily from enrolment through day 28 of hospitalization, ICU admission, discharge, or death, whichever came first. Immediately before or at least within 24 h before Sarilumab administration and at the same timepoints, peripheral blood was collected for the assessment of IL6 plasma levels using ELISA assay (Multi-cytokine test for ELLA-Bio-Techne, Minneapolis).
2.3. Clinical improvement and outcomes assessment
Clinical improvement was defined as the reduction of oxygen-support requirement or improvement of at least 20% of the PaO2/FiO2 ratio from baseline, as the discharge rate and/or the no longer need of oxygen supplementation. In addition, the need of ICU admission for patients treated in medical wards (and the subsequent discharge/death) and the discharge towards medical wards for ICU-treated patients were evaluated. Moreover, the clinical outcome for each patient (in the medical and ICU setting respectively) was assessed using the WHO Clinical progression scale [18].
2.4. Statistical analysis
Statistical analysis was performed using SPSS V.20.0 (SPSS. Chicago, Illinois, USA) and Prism software (GrapAHad, San Diego, California, USA). Categorical and quantitative variables were described as frequencies, percentage and Median (25–75 interquartile range, IQR). Data on demographic and clinical parameters were compared between patients by the non-parametric Mann-Whitney U test or χ2 test, as appropriate. Univariate analysis was conducted to assess adequate event frequency between the outcomes and the candidate predictors. Predictors with a p < 0·05 entered within the multivariable logistic regression analysis.
A Receiver Operating Characteristic (ROC) curve analysis of clinical and laboratory parameters differentially distributed among SARS-CoV-2pos patients based on the considered outcome was performed to obtain relevant threshold for the prediction of “clinical improvement at 3 days after Sarilumab treatment” and of “transfer to ICU”, respectively. The optimal cut-off points were determined to yield the maximum corresponding sensitivity and specificity. Moreover, Kaplan Meyer analysis was performed to estimate the probability of “transfer to ICU” in SARS-CoV-2pos patients based on the previously identified cut-off values of clinical and laboratory parameters.
Finally, a nomogram was built to distinguish patients with the outcome (transfer to ICU) and those without. The performance of the nomogram was assessed by discrimination and calibration. The discriminative ability of the model was determined by the area under the ROC curve, which ranged from 0·5 (no discrimination) to 1 (perfect discrimination). The model was developed and validated. The statistical analyses and graphics were performed using the rms statistical packages of R 3.5,3 (The R Foundation for Statistical Computing, Vienna, Austria). For all the analyses, a p < 0·05 was considered statistically significant.
3. Results
3.1. Baseline demographic, clinical and inflammatory characteristics of SARS-CoV-2pos patients treated with Sarilumab
Fifty-three patients with severe SARS-CoV-2-related pneumonia were enrolled, of whom 47(88·7%) were male with an age range of 40–95 years (median 66 years), 33(62·3%) were overweight/obese and 34(64·2%) had at least one comorbidity (Supplementary Tables 1–2).
Considering concomitant therapies, 37(69·8%) were treated with darunavir/ritonavir, 13(24·5%) with lopinavir/ritonavir while 3(5·7%) received no antiviral treatment. Moreover, 50(94·3%) were treated with hydroxychloroquine, 45(74·9%) were receiving prophylactic dose heparin and 29(54·7%) received azythromicin. No patients were taking GC at baseline. Among the whole cohort, 39(73·6%) patients were treated in medical wards while 14(26·4%) received their first Sarilumab dose in ICU or within the 24 h of ICU admission (Supplementary Tables 1–3).
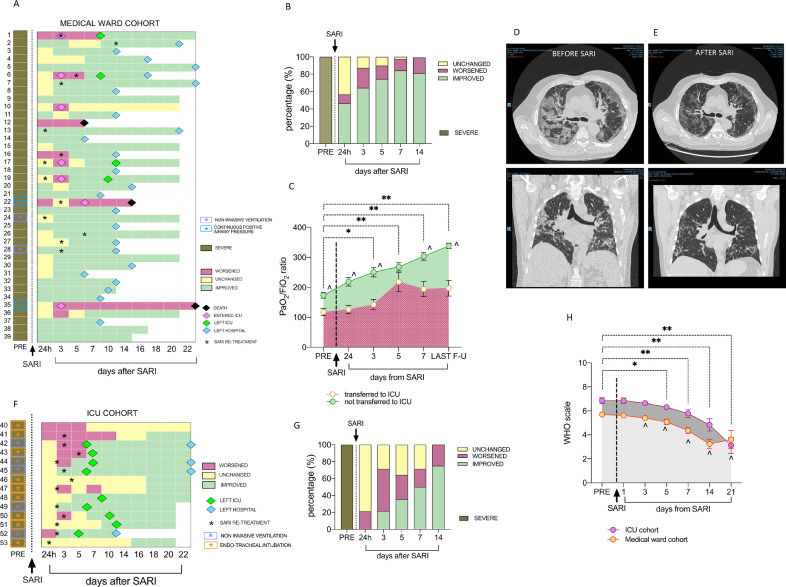
Fig. 1A and 2A show individual clinical course of SARS-CoV-2pos patients in medical wards and ICU, respectively. Twenty-six (66·7%) patients belonging to medical wards received a single course of Sarilumab, while 13(33·3%) received a second dose at a median interval of 3 days (range 1–11 days), based on the clinical course (Fig. 1A). Conversely, 13(92·9%) ICU-treated patients received two Sarilumab infusions [median interval: 2·5 days (range 1–5 days)] (Fig. 1F). The dose of Sarilumab was reduced in two patients: one HIVpos and HBVpos patient under chronic specific treatment received two courses of Sarilumab (200 mg each, 3 days apart), experiencing clinical improvement at day 3 and discharged from the hospital at day 14; another 95 years-old patient received a single infusion of Sarilumab 200 mg, showing PaO2/FiO2 ratio improvement from 134 to 270 within 24 h and oxygen support reduction from 50% to 24% in 7 days. Two patients received a reduced dose of Sarilumab at re-treatment, due to mild neutropenia development after the first dose.
Fig. 1.
A-H. Clinical course of SARS-CoV-2pos patients treated with Sarilumab in medical wards and in the ICU. A) Individual disease course of SARS-CoV-2pos patients in medical wards. Each individual disease course is depicted in a line from pre-treatment to maximum 22 days after Sariumab administration; Pink diamonds refer to transfer towards ICU; Green diamonds refer to transfer back from ICU to medical wards; Light blue diamonds refer to discharge from the hospital and black diamond refers to death. Black stars indicate repetition of infusion of Sarilumab during the follow-up. Respiratory support is highlighted for each patient as follows: light blue box for non invasive ventilation and light violet box for continuous positive airway pressure; B) Rate of clinical response to Sarilumab in SARS-CoV-2pos patients within 14 days follow-up (24 h, 3,5,7 and 14 days) in medical wards. C) PaO2/FiO2 ratio in SARS-CoV-2pos patients treated with Sarilumab based on their need to be transferred to ICU during the follow-up; comparison between baseline vs 3 days *p < 0.001; **p < 0.0001 refer to comparison between baseline vs 5, 7 days and last follow-up respectively; ^p < 0.01 for each time-point comparing patients trasferred to ICU vs patients not transferred to ICU after Sarilumab treatment. d-E) Example photos of pulmonary CT-scan of SARS-Cov-2pos patients before (D) and 10 days after (E) Sarilumab treatment. Among the whole cohort, 12 (30.8%) and 2(14.3%) patients in the clinical ward or in the ICU underwent serial CT scan during hospitalization respectively. CT scan was not routinely performed in the initial phases of the pandemic. F) Individual disease course of SARS-CoV-2pos patients in ICU. Each individual disease course is depicted in a line from pre-treatment to maximum 22 days after Sarilumab administration; Green diamonds refer to transfer towards medical wards and light blue diamond refers to discharge from the hospital. Black stars indicate repetition of infusion of Sarilumab during the follow-up. Respiratory support is highlighted for each patient as follows: light violet box for continuous positive airway pressure and orange box for endo-tracheal intubation; G) Rate of clinical response to Sarilumab in SARS-CoV-2pos patients within 14 days follow-up (24 h, 3,5,7 and 14 days) in ICU. Severe and critical SARS-CoV-2 pneumonia was defined based on PaO2/FiO2 ratio <300 and/or multiorgan, respiratory failure or shock. H) WHO clinical progression scale across disease course in SARS-CoV-2 positive patients treated with Sarilumab in Medical Ward and ICU. * p < 0.05 and **p < 0.01 Wilcoxon test comparing pre-treatment WHO scale and 5, 7, 14 and 21 days after Sarilumab administration in ICU patients; ^ p < 0.05 Wilcoxon test comparing pre-treatment WHO scale and 5, 7, 14 and 21 days after Sarilumab administration in ICU patients.
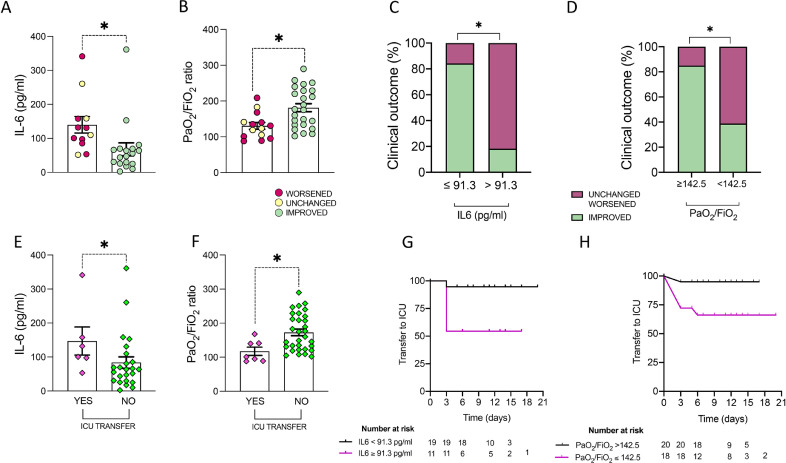
Fig. 2.
A-H. Clinical and biological baseline predictors of clinical outcome in SARS-Cov-2pos patients treated with Sarilumab. A) Baseline IL-6 plasma levels in SARS-Cov-2pos patients treated with Sarilumab based on clinical improvement at 3 days after Sarilumab treatment, *p = 0.001. B) Baseline PaO2/FiO2 in SARS-Cov-2pos patients treated with Sarilumab based on clinical improvement at 3 days after Sarilumab treatment, *p = 0.004. C) Rate of clinical improvement at 3 days in SARS-Cov-2pos patients treated with Sarilumab based on baseline IL-6 plasma levels, *p < 0.0001. D) Rate of clinical improvement at 3 days in SARS-Cov-2pos patients treated with Sarilumab based on baseline PaO2/FiO2, p = 0.003. E) Baseline IL-6 plasma levels in SARS-Cov-2pos patients based on transfer towards ICU within 14 days after Sarilumab treatment, *p = 0.05. F) Baseline PaO2/FiO2 in SARS-Cov-2pos patients based on transfer towards ICU within 14 days after Sarilumab treatment, *p = 0.008. G) Kaplan-Meier analysis of transfer to ICU rate in SARS-Cov-2pos patients treated with Sarilumab based on pre-treatment IL-6 plasma levels, p = 0.01. H) Kaplan-Meier analysis of transfer to ICU rate in SARS-Cov-2pos patients treated with Sarilumab based on pre-treatment PaO2/FiO2, p = 0.03.
3.2. Clinical course and safety profile in SARS-CoV-2pos patients treated with Sarilumab
3.2.1. Medical wards
among 39 treated patients, 7(17·9%) required ICU admission, of whom 4(57·1%) were readmitted to the ward after 5–8 days and, at the time of manuscript submission, 1 patient was still hospitalized in ICU, whereas 2 patients died in ICU (one with chemical peritonitis due to duodenal perforation, not attributable to Sarilumab) (Fig. 1A).
As shown in Fig. 1B, the clinical improvement occurred in 18(46·2%) patients after 24 h after Sarilumab infusion, while response rate at 3 days was 64·1%, reaching 89·7% at the last observation (median follow-up time of 16 days, range14–24). Twenty-four (70·6%) were discharged after a median of 12 days (range 5–22) after the first Sarilumab infusion. In addition, 30(85·7%) were no longer needing oxygen supplementation, 3(8·8%) were still at FiO2 24%, 1(2·8%) at FiO2 35% and 1(2·8%) in HFNC (FiO2 35%), respectively. After Sarilumab infusion there was a progressive increase of PaO2/FiO2 ratio in SARS-CoV-2pos patients (Fig. 1C) mirroring the improvement of pulmonary inflammation at CT-scan (Fig. 1D-E). A patient died due to pulmonary embolism 5 days after Sarilumab administration.
3.2.2. ICU
Among 14 patients in ICU, 9(64·3%)(4 assisted with endotracheal intubation) were discharged to the medical wards within 5–12 days after Sarilumab administration (median 6·5 days). At the last observation, 8(88·9%) patients discharged to medical wards were no longer needing oxygen supplementation (4 of them discharged at home) and 1(11·1%) was still in HFNC (FiO2 26%), while 5(35·7%) patients were still alive in ICU (4 significantly improved and one stable)(Fig. 1F-–G).
Overall mortality rate was 5·7% after Sarilumab administration: 1(2·5%) patient died in the Medical Ward whilst 2(14·2%) patients died in ICU, respectively.
In addition, using the newly released WHO clinical progression scale for COVID-19, we observed a significant improvement both in medical ward and ICU patient cohorts receiving Sarilumab (Fig. 1H).
Supplementary Table 4 summarizes the adverse events in SARS-CoV-2pos patients treated with Sarilumab.
3.3. Predictors of response to Sarilumab treatment in SARS-CoV-2pos patients
Among patients treated in medical wards, 25(64·1%) had clinical improvement 3 days after Sarilumab infusion. SARS-CoV-2pos patients who improved were younger [63(53–73) years], with better respiratory parameters [PaO2/FiO2:175·9(133·4–229·2)] and with lower neutrophil/lymphocyte ratio [5·1(4·2–10·0)] than patients who remained unchanged or worsened at the same time point [age:74·0(67·5–77·3) years, p = 0·01; PaO2/FiO2:127·3(99·5–147·6), p = 0·004; N/L ratio: 8·2(6·1–11·9), p = 0·03]. Moreover, patients who improved 3 days after Sarilumab administration had lower pre-treatment IL6 plasma levels [51·6(25·6–69·5) pg/ml] than patients who remained unchanged or worsened [120·4(88·2–158·4) pg/ml, p = 0·001] (Fig. 2A-B, Supplementary Figure 1).
ROC curve analysis revealed that PaO2/FiO2 ratio≥142·5 and IL6 plasma levels≤91·3 pg/ml identifies SARS-CoV2pos patients more likely to achieve clinical improvement at 3 days from Sarilumab administration (Fig. 2C-D, Supplementary Figure 2).
Table 1 shows the baseline characteristics of SARS-CoV-2pos patients treated with Sarilumab in medical wards according to the need of ICU transfer, revealing that patients transferred to ICU had higher baseline IL-6 plasma levels (p = 0·05) and lower PaO2/FiO2 ratio (p = 0·007) than patients who did not need it (Fig. 2E-F).
Table 1.
Baseline demographic, clinical and inflammatory parameters of patients with SARS-Cov-2 pneumonia in medical ward setting, according to the need of ICU admission.
| Variables | Ward setting-treated patients (n = 39) | p | |
|---|---|---|---|
| No ICU care n = 32 | ICU care n = 7 | ||
| Age, years [median(IQR)] | 64·5 (54·3–73·8) | 75·0 (71·0–79·0) | 0·01 |
| Male, n(%) | 30 (93·8) | 6 (85·7) | 0·47 |
| Weight, Kg [median(IQR)] | 80 (70·0–83·0) | 84 (75·0–100·0) | 0·21 |
| Overweight/obese, n(%) | 17 (53·1) | 6 (85·7) | 0·11 |
| SIGNS AND SYMPTOMS | |||
| Fever, n(%) | 39 (100·0) | 14 (100·0) | 1·00 |
| cough, n(%) | 20 (51·3) | 9 (64·3) | 0·40 |
| dyspnea, n(%) | 23 (59·0) | 8 (57·1) | 0·91 |
| Days from symptoms to SARI infusion, [median(IQR)] | 11 (9·0–15·0) | 8 (3·0–11·0) | 0·06 |
| FiO2 | 40 (35·0–50·0) | 60 (40·0–60·0) | 0·04 |
| PaO2/FiO2 | 167·5 (125·4–226·5) | 101·0 (89·0–141·0) | 0·007 |
| LABORATORY FINDINGS | |||
| White blood cells count, x103/ml [median(IQR)] | 6·1 (4·7–6·8) | 7·1 (5·7–10·1) | 0·22 |
| Lymphocytes count, x103/ml [median(IQR)] | 0·8 (0·6–0·9) | 0·7 (0·5–0·9) | 0·38 |
| neutrophils/lymphocytes ratio, [median(iqr)] | 5·7 (4·2–11·3) | 8·0 (6·3–12·5) | 0·22 |
| hemoglobin, g/l [median(iqr)] | 13·0 (11·8–13·9) | 13·3 (12·3–13·5) | 0·91 |
| Platelet, x103/ml [median(IQR)] | 304 (247–420) | 271 (246·3–303·8) | 0·30 |
| Albumin, g/L [median(IQR)] | 2·9 (2·7–3·1) | 3·1 (2·7 −3·8) | 0·28 |
| D-dimers, mg/L [median(IQR)] | 1345 (1056·5–2702) | 1955 (1108·3–3828·5) | 0·64 |
| GPT, mg/L [median(IQR)] | 32·5 (22·5–48·0) | 34·0 (17·0–48·0) | 0·82 |
| Creatinine, mg/L [median(IQR)] | 0·9 (0·7–1·2) | 1·2 (0·7–1·5) | 0·33 |
| C-Reactive Protein, mg/L [median(IQR)] | 148·5 (59·2–200·1) | 132·0 (102·8–161·5) | 0·78 |
| Lactate Dehydrogenase, [median(IQR)] | 315 (245·5–398·3) | 350 (306·5–469·0) | 0·24 |
| SOLUBLE BIOMARKERS | |||
| IL-6 plasma levels, pg/ml [median(IQR)] | 63·9 (30·0–103·7)*24 | 116·3 (86·3–203·7)*6 | 0·05 |
| COMORBIDITY | |||
| Diabetes, n(%) | 6 (18·8) | 1 (14·3) | 0·78 |
| Hypertension, n(%) | 15 (46·9) | 6 (85·7) | 0·06 |
| Cardiovascular disease, n(%) | 5/28 (17·9) | 2/6 (33·3) | 0·40 |
| Chronic Obstructive pulmonary disease, n(%) | 3/28 (10·7) | 1/6 (16·7) | 0·55 |
| Malignancy, n(%) | 0/28 (0·0) | 0/6 (0·0) | 1·00 |
| Dyslipidemia, n(%) | 6/29 (20·7) | 2/6 (33·3) | 0·50 |
| Chronic Infection, n(%) | 3/28 (10·7) | 0/6 (0·0) | 0·40 |
ICU: Intensive Care Unit; IQR: interquartile range; SARI: sarilumab; FiO2: fractional inspired oxygen; PaO2: arterial oxygen partial pressure; GPT: glutamine-pyruvate transaminase; IL: interleukin; g/L: grams/liter. *number of patients with available IL-6 value.
ROC curve analysis revealed that PaO2/FiO2 ratio≤142·5 and IL6 plasma levels ≥91·3 pg/ml significantly identify SARS-CoV2pos patients more likely to need to be transferred towards ICU within 14 days after Sarilumab treatment (Fig. 2G-H, Supplementary Figure 2).
3.4. Development of nomogram for the prediction of transfer to ICU in SARS-CoV-2pos patients treated with Sarilumab in medical wards
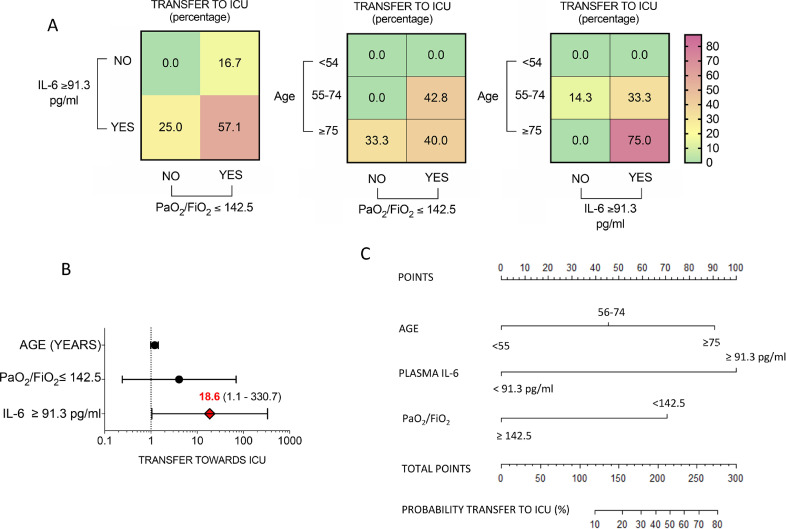
Assessing the combined fulfillment of pre-treatment demographic (age), clinical (PaO2/FiO2) and laboratory parameters (IL6 plasma levels) in SARS-CoV2pos patients treated with Sarilumab in medical wards, patients older than 75 years and having PaO2/FiO2≤142·5 had 40% incidence rate to be transferred to ICU. Moreover, patients with IL6 plasma levels≥91·3 pg/ml and PaO2/FiO2≤142·5 had 57·1% incidence to be transfer to ICU and that rate increases to 75% if older than 75 years (Fig. 3A-B).
Fig. 3.
A-CC. Development of multi-parametric nomogram predictive of transfer towards ICU in SARS-CoV-2pos patients treated with Sarilumab in medical ward setting. A) Rate of transfer to ICU in SARS-CoV-2pos patients treated with Sarilumab in medical wards based on pre-treatment IL-6 plasma levels (91.3 pg/ml), PaO2/FiO2 ratio value (142.5) and age category (<54 years, 55–74 years and >75 years respectively); Values are expressed as percentages. B) Odd ratios of transfer to ICU in SARS-CoV-2pos patients treated with Sarilumab in medical wards based on the fulfillment of pre-treatment definite parameters; Values are expressed as Odd Ratio (95%CI); C) Nomogram for the computation of probability of transfer to ICU in SARS-CoV-2pos patients treated with Sarilumab in medical wards. IL: Interleukin; ICU: Intensive Care Unit.
A nomogram was created (Fig. 3C) incorporating the three significant risk factors predicting the “transfer to ICU”. The value of each variable was given a score on the “points” scale axis. A total score was calculated by adding each single point score and by projecting the value of the “total points” score to the lower “Probability” line.
4. Discussion
Reports of different cohorts stated that nearly 80% of SARS-CoV-2 infected subjects have a spontaneous recovery, while 15% experience symptoms needing hospitalization and up to 5% may require care within ICU. The most aggressive phase is thought to have an aggressive inflammatory background (cytokine release syndrome-CRS) that leads to the respiratory distress [4]. At this stage, IL-6 is a key player in regulating such cascade [11] and SARS-CoV-2pos patients show increased circulating IL-6 levels associated with subsequent development of cytokines release syndrome [19]. Therefore, IL-6R inhibitors (i.e. Tocilizumab or Sarilumab) may help to prevent disease progression and promote faster resolution of SARS-CoV-2 pneumonia [11,[19], [20], [21]]. Thus, in China, nearly 200 patients with severe SARS-CoV-2 pneumonia were treated with Tocilizumab (source: Roche Ltd, China) and, although the results of this study have not been made public to date, the Chinese CDC, approved Tocilizumab use in patients with SARS-CoV-2 severe pneumonia needing to be admitted to ICU [22]. These indications were also implemented in the SIMIT Lombardy guidelines released on March 20th, 2020. Given the emerging evidence on the efficacy of Tocilizumab in patients with severe pneumonia, currently this drug is used even in a medium-severe phase.
The ensuing shortage of Tocilizumab led COVID-19 Task Force of our Hospital to adopt the option of using Sarilumab as an alternative anti-IL-6R. At the time of manuscript submission, there were multiple registered controlled trials testing Sarilumab as possible effective alternative to treat SARS-CoV-2 severe pneumonia [23]. Recently, Della Torre and colleagues described the first experience with intravenous sarilumab in Italian patients with severe SARS-CoV-2 pneumonia with hyperinflammatory phenotype [13]. Herein, we report, the clinical outcome and safety profile of Sarilumab in SARS-CoV-2 severe pneumonia both in medical wards and ICU setting. Sarilumab is available in subcutaneous formulation which is administered every 2 weeks in patients with RA [24] showing 20 times higher affinity for IL6R (both soluble and membrane-bound IL6 receptors) than Tocilizumab [25] and it was chosen to be used intravenously for the faster rapidity of action in analogy to Tocilizumab's use.
Our data show that Sarilumab has a good global rate of clinical outcome to treat SARS-CoV-2 severe pneumonia in terms of clinical improvement and mortality. Overall, 83·0% of 53 SARS-CoV2pos patients (89·7% in medical wards and 64·3% in ICU) safely resolved their severe respiratory syndrome, with an overall mortality rate of 5·7%. Moreover, even considering patients with a critical lung involvement, Sarilumab treatment led up to 92·8% clinical improvement with an ICU discharge rate of 64·3% in a mean recovery time of 7 days. These findings were supported by the significant decrease of WHO clinical progression scale after Sarilumab administration both in medical and ICU wards, which well reflects patient trajectory across the disease and seems to be independent by study design [18]. Interestingly, only 2 patients were treated with a short course of GC. Avoiding secondary infections due to GC is clinically relevant, also in light of preliminary results of corticosteroid use in the treatment of Covid-19 patients whose mortality varied considerably according to the level of respiratory support that the patients were receiving at the time of randomization [26,27].
Moreover, Sarilumab appears to be safe and we did not register drug-related serious adverse events and no secondary infections up to the last follow-up.
Initial reports revealed that baseline demographic and clinical parameters may be useful to assess SARS-CoV-2 infection prognosis [28]. In this contest, we found that young SARS-CoV-2pos patients with severe pneumonia with PaO2/FiO2 ratio>142·7 and not extreme systemic inflammation (IL-6 plasma levels<91·3 pg/ml) have the highest chance of Sarilumab efficacy in terms of early clinical response and less need to be transferred to ICU than patients not fulfilling these criteria. Whether a higher intensity drug exposure (more i.v. infusions) would better control the more aggressive underlying inflammation, will be highligthed only by a targeted randomized trial using a biomarker-oriented approach. Conversely, a multiparametric nomogram including such variables allows to predict the need of being transferred to ICU up to 80% of patients with severe pneumonia. Interestingly, analysing the dynamics of clinical response of patients treated with Sarilumab, it arose that the sooner therapy is started, the sooner the positive result in terms of clinical improvement is observed (“window of opportunity”). In this study neither d-Dimers levels, nor lymphopenia arose as predictors of a critical outcome, while the pre-treatment PaO/FiO2 value had the greatest role along with age.
The limitations of this report are its open-label nature with no comparison group, the single center setting, the limited number of critical-ICU patients and adoption of concomitant treatments of unproven efficacy (possibly increasing the safety results). Moreover, a longer follow-up may be useful to increase the strength of our findings in terms of long-term efficacy and safety of Sarilumab in SARS-CoV-2 severe pneumonia. Yet, an outpatient clinic for these patients has already been set-up to collect prospective clinical and biological informations.
In conclusion, IL-6R inhibition leads to good clinical outcome in patients with severe SARS-CoV-2 pneumonia and Sarilumab is a valid and safe alternative in the therapeutic armamentarium of this disease without defined standardized treatment algorythms.
Declaration of Competing Interest
All authors declare no conflict of interest with the submitted manuscript.
Acknowledgments
Aknowledgements
We would like to thank all the patients who sadly were infected by SARS-CoV-2 during this pandemic and participated to the study. We would like to thank Prof. Rocco Bellantone, Dr. Andrea Cambieri, Prof. Marco Elefanti and Prof. Giovanni Scambia of the Fondazione Policlinico Universitario A. Gemelli IRCCS for continuous support to all the activities of the FPG-IRCCS COVID-19 multidisciplinary Task-Force.
Authors contribution
EG, AC, MA and AG conceived the study; EG, AC, SLB, SA and BT analysed the data; SLB, SA, BT, SP, FL, MP, RM, AS, MG, MS, GDP, MG, FB, MM, AT, GG, GLR, AI, LZDV, LP, ALF, MML, ET, GN, LG, DB, LV, ET, AC, FL, RB, RC, FF, RL, LR, MS and MF collected data; EG, AC, SLB, SA, BT, GG, FL, RM, MA and AG wrote the first draft manuscript; All authors edited the manuscript and agreed on the last submitted version.
Funding
No funding was used for the conduction of the study.
Data sharing statement
The dataset is not subject to restrictions or embargo and is accessible after reasonable request to the Corresponding Author (EG).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100553.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carinci F. Covid 19: preparedness, decentralization and dehunt for patient zero. BMJ. 2020;368 doi: 10.1136/bmj.m799. bmjm799. [DOI] [PubMed] [Google Scholar]
- 3.Italian Ministry of Health (www.salute.gov.it)
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wuang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus –infected pneumonia in Wuhan China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A. COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 Apr 6 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown M., Sanchez E., Tattersall R.S., Manson J.J. on behalf of the HLH Across Speciality Collaboration, UK: COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le R.Q., Li L., Yuan W. FDA Approval Summary: tocilizumab for treatment of Chimeric antigen receptor T Cell-Induced severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damas P., Ledoux D., Nys M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage activation syndrome-like disease. Autoimmun Rev. 2020 Apr 3 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab PNAS May 19, 2020;117:10970–5. [DOI] [PMC free article] [PubMed]
- 13.Della-Torre E., Campochiaro C., Cavalli G. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020 Jul 3 doi: 10.1136/annrheumdis-2020-218122. annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gremese E., Ferraccioli E.S., Alivernini S., Tolusso B. Ferraccioli G Basic Immunology may lead to translational therapeutic rationale: sARS-CoV-2 and Rheumatic diseases. G.Eur J Clin Invest. 2020 Jul 9:e13342. doi: 10.1111/eci.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alivernini S., Cingolani A., Gessi M. Comparative analysis of synovial inflammation after SARS-CoV-2 infection. Ann Rheum Dis. 2020 Jul 6 doi: 10.1136/annrheumdis-2020-218315. annrheumdis-2020-218315. [DOI] [PubMed] [Google Scholar]
- 16.Burmester G.R., Lin Y., Patel R. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76:840–847. doi: 10.1136/annrheumdis-2016-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surviving Sepsis campaign Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Critical Care Medicine. 2020 doi: 10.1097/CCM.0000000000004363. www.ccmjournal.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;12 doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2 viral load is closely associated with drastically elevated Interleukin-6 (IL-6) levels in critically ill COVID 19 patients. Clin Infect Dis. 2020 Apr 17 doi: 10.1093/cid/ciaa449. pii: ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Thoracic Society-Diagnosis and Management of COVID-19 Disease. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.2020C1. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Alhazzani W., Møller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 Mar 28 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.www.chinacdc.cn and personal communication, on March 6, 2020 by Prof. Feng Li, State Key Laboratory of Virology, School of Basic Medical Findings, Wuhan University, Wuhan 4300071, China. Fli222@whu.edu.cn
- 23.https://clinicaltrials.gov/
- 24.https://www.ema.europa.eu/en/documents/product-information/kevzara-epar-product-information_en.pdf
- 25.Rafique A., Martin J., Blome M., Huang T., Ouyang A., Papadopoulos N. Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human IL-6 receptor alpha. Ann Rheum Dis. 2013;72(Suppl 3):A797. doi: 10.1136/annrheumdis-2013-eular.1. [DOI] [Google Scholar]
- 26.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Jul, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.