The prevalence of dental caries is still high around the world. Dental caries is initiated when the teeth are exposed to acid, such as lactic acid, produced via carbohydrate metabolism by acidogenic microorganisms. Veillonella species, which are among the major oral microorganisms, are considered to be beneficial bacteria due to their ability to convert lactic acid to weaker acids and to produce NO2– from NO3–, which is thought to be good for both oral and general health. Therefore, it is clear that there is a need to elucidate the biochemical characteristics of NO2– production in Veillonella species. The significance of our research is that we have found that lactate metabolism is linked to NO2– production by Veillonella species in the environment found in the oral cavity. This study suggests that Veillonella species are potential candidates for maintaining oral and general health.
KEYWORDS: Veillonella, diet, lactate, metabolism, nitrate, nitrite
ABSTRACT
Veillonella species are among the major anaerobes in the oral cavity and are frequently detected in both caries lesions and healthy oral microbiomes. They possess the ability to utilize lactate and convert nitrate (NO3–) into nitrite (NO2–). Recently, interest in NO2– has increased rapidly because of its beneficial effects on oral and general health; i.e., it inhibits the growth and metabolism of oral pathogenic bacteria, such as Streptococcus mutans, and lowers systemic blood pressure. However, there is only limited information about the biochemical characteristics of NO2– production by Veillonella species. We found that NO3– did not inhibit the growth of Veillonella atypica or Veillonella parvula, and it inhibited the growth of Streptococcus mutans only at a high concentration (100 mM). However, NO2– inhibited the growth of Streptococcus mutans at a low concentration (0.5 mM), while a higher concentration of NO2– (20 mM) was needed to inhibit the growth of Veillonella species. NO2– production by Veillonella species was increased by environmental factors (lactate, acidic pH, and anaerobic conditions) and growth conditions (the presence of NO3– or NO2–) and was linked to anaerobic lactate metabolism. A stoichiometric evaluation revealed that NO3– is reduced to NO2– by accepting reducing power derived from the oxidization of lactate. These findings suggest that the biochemical characteristics of NO2– production from NO3– and its linkage with lactate metabolism in oral Veillonella species may play a key role in maintaining good oral and general health.
IMPORTANCE The prevalence of dental caries is still high around the world. Dental caries is initiated when the teeth are exposed to acid, such as lactic acid, produced via carbohydrate metabolism by acidogenic microorganisms. Veillonella species, which are among the major oral microorganisms, are considered to be beneficial bacteria due to their ability to convert lactic acid to weaker acids and to produce NO2– from NO3–, which is thought to be good for both oral and general health. Therefore, it is clear that there is a need to elucidate the biochemical characteristics of NO2– production in Veillonella species. The significance of our research is that we have found that lactate metabolism is linked to NO2– production by Veillonella species in the environment found in the oral cavity. This study suggests that Veillonella species are potential candidates for maintaining oral and general health.
INTRODUCTION
The oral cavity is an important part of the human body, acting as the gateway for every substrate used in the body. It also plays important roles in mastication, esthetics, and phonetics. Hence, maintaining the health of the oral cavity is essential for general health and quality of life. However, the prevalence of oral diseases, notably dental caries, in children is still relatively high in some less developed countries and even in developed countries (1). In general, dental caries is a multifactorial disease, but acid production by the oral biofilm microbiota is widely known to be a direct cause of this disease (2), since dental caries is initiated through the demineralization of tooth surfaces by bacterial acid production from dietary carbohydrates. Thus, controlling bacterial acid production from carbohydrates is an effective way of preventing dental caries and maintaining oral health.
Among the oral biofilm microbiota, Veillonella species are known to be particularly abundant; they are frequently detected, especially on the tongue surface, buccal mucosa, and dental surfaces, and have also been found in severe dental caries in children (early childhood caries [ECC]) (3, 4). Recently, several Veillonella species, including Veillonella atypica, V. dispar, V. rogosae, V. tobetsuensis, V. parvula, and V. denticariosi, have been detected in the oral cavities of children and healthy young adults (4, 5). Veillonella species utilize lactic acid as an essential carbon and energy source, converting it into weaker acids, such as acetic, propionic, and formic acid (6). As mentioned above, dental caries is initiated by acids produced by acidogenic bacteria, such as Streptococcus mutans, while acid neutralization, such as the conversion of lactic acid to weaker acids, can contribute to countering the demineralization of tooth surfaces and promote their remineralization (6). Therefore, Veillonella species are assumed to be beneficial bacterial species for preventing dental caries.
In addition to utilizing lactic acid, Veillonella species possess the ability to produce nitrite (NO2–) by reducing nitrate (NO3–) (7). NO3– can be obtained from leafy green vegetables, such as spinach, lettuce, and cabbage (8). After it is consumed and absorbed through the gastrointestinal tract, approximately 25% of ingested NO3– is secreted in saliva (9, 10). Therefore, NO3– is always available in the oral cavity, and some of it is reduced to NO2– by Veillonella species.
NO2– exhibits antimicrobial activity and therefore is widely used for food preservation. In the dental field, NO2– is reported to inhibit the acid production of dental plaque (11), as well as the growth of oral pathogenic bacteria, such as Streptococcus mutans and Porphyromonas gingivalis (9, 12). Hence, the NO2– produced by Veillonella species might contribute to preventing oral diseases, such as dental caries. In addition, NO2– is known to contribute to general health by normalizing blood pressure (13, 14). The NO2– produced in the oral cavity enters the circulation and is converted into nitric oxide (NO) by mammalian nitrite reductase or nonenzymatically by the acidic environment found in the stomach, resulting in vasodilatation and a significant reduction in blood pressure (15, 16).
However, there is only limited information about the regulation of NO2– production by Veillonella species in the oral cavity. Therefore, the aim of this study was to elucidate the environmental conditions that allow Veillonella species to produce NO2– and the biochemical mechanism by which their NO2– production is regulated.
RESULTS
Effects of NO3– and NO2– on bacterial growth.
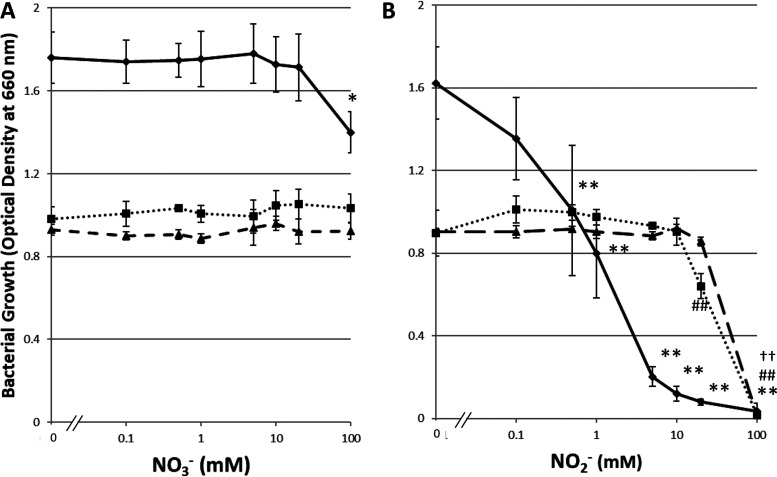
NO3– had no effect on the growth of Streptococcus mutans or Veillonella species, except at a high concentration (100 mM), at which it inhibited the growth of Streptococcus mutans (Fig. 1A) (P < 0.05). The growth of Streptococcus mutans was reduced as the NO2– concentration in the growth medium increased (Fig. 1B), and it was even inhibited at a low concentration of 0.5 mM NO2– (P < 0.01). On the other hand, a higher concentration of NO2– (20 mM) was required to inhibit the growth of Veillonella species (P < 0.01).
FIG 1.
Effects of NO3– (A) and NO2– (B) on bacterial growth. The bacterial growth (OD values) observed over 24 h is shown. Data are shown as means ± standard deviations. Symbols indicate significant differences from the control (without NO3– or NO2–) in the numbers of Streptococcus mutans (*, P < 0.05; **, P < 0.01), Veillonella atypica (##, P < 0.01), or Veillonella parvula (††, P < 0.01) bacteria (by Dunnett’s test). Circles and solid lines, Streptococcus mutans; squares and dotted lines, Veillonella atypica; triangles and dashed lines, Veillonella parvula.
NO2– production by Veillonella species.
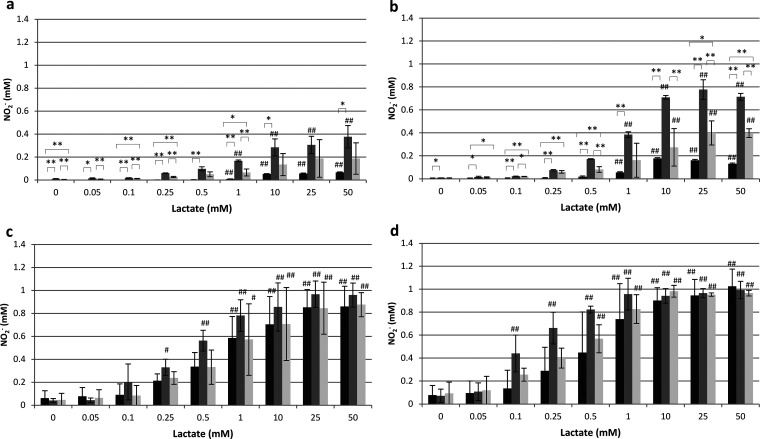
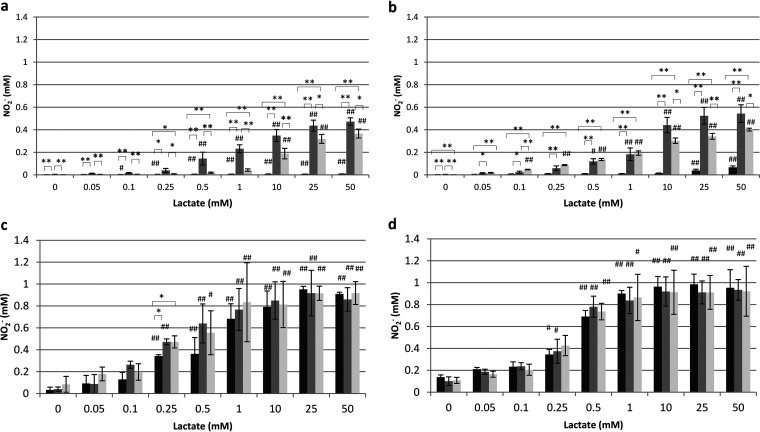
The effects of environmental factors (lactate, pH, and atmospheric conditions) and growth conditions (the presence of NO3– or NO2–) on NO2– production by Veillonella species were investigated (Fig. 2 and 3). Under aerobic conditions, both Veillonella atypica and Veillonella parvula required lactate to produce NO2–, and the NO2– production of these species was increased under acidic conditions (pH 5). When they were grown with NO3– or NO2–, both bacterial strains exhibited increased NO2– production. Furthermore, the NO2– production of Veillonella atypica tended to be higher than that of Veillonella parvula. Under anaerobic conditions, the NO2– production of Veillonella atypica and Veillonella parvula was generally higher at pH 5 than at pH 7, and NO2– production increased with the lactate concentration. NO2– production was higher under anaerobic conditions than under aerobic conditions under all experimental conditions for both Veillonella strains, and NO2– production was detected in the absence of exogenous lactate, although the amounts of NO2– produced were small.
FIG 2.
NO2– production of Veillonella atypica under aerobic conditions at pH 7 (a) and pH 5 (b) and under anaerobic conditions at pH 7 (c) and pH 5 (d). Data are shown as means ± standard deviations. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) in NO2– production among bacterial cells grown in TYL, KNO3-containing TYL, and KNO2-containing TYL (by Tukey’s test). Hashtags indicate significant differences (#, P < 0.05; ##, P < 0.01) in NO2– production from that with 0 mM lactate under the same growth conditions (by Dunnett’s test). Black bars, no addition; dark gray bars, KNO3; light gray bars, KNO2.
FIG 3.
NO2– production by Veillonella parvula under aerobic conditions at pH 7 (a) and pH 5 (b) and under anaerobic conditions at pH 7 (c) and pH 5 (d). Data are shown as means ± standard deviations. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) in NO2– production among bacterial cells grown in TYL, KNO3-containing TYL, and KNO2-containing TYL (by Tukey’s test). Hashtags indicate significant differences (#, P < 0.05; ##, P < 0.01) in NO2– production from that seen in the presence of 0 mM lactate under the same growth conditions (by Dunnett’s test). Black bars, no addition; dark gray bars, KNO3; light gray bars, KNO2.
Metabolic end products of lactate metabolism during NO2– production by Veillonella species.
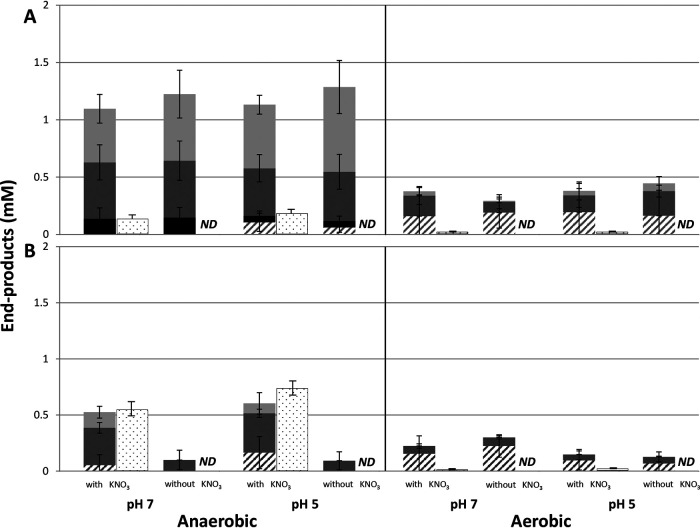
Under anaerobic conditions, Veillonella atypica produced mainly propionate and acetate, followed by formate and pyruvate in the presence and absence of KNO3 (Fig. 4A). Larger amounts of pyruvate were detected at pH 5. Under aerobic conditions, the main end products were pyruvate and acetate with a small amount of propionate. There were no clear differences in the levels of the metabolic end products examined between pH 7 and pH 5. The total amount of end products derived from lactate metabolism was higher under anaerobic conditions than under aerobic conditions. NO2– production was observed only in the presence of KNO3.
FIG 4.
Metabolic end products produced from lactate during NO2– production by Veillonella atypica (A) or Veillonella parvula (B). Data are shown as means ± standard deviations. ND, not detected. Each parameter has two bars. For those on the left, light gray shading indicates propionate, dark gray shading indicates acetate, black shading indicates formate, and a hatched pattern indicates pyruvate. For those on the right, the dotted pattern indicates nitrite.
Under anaerobic conditions, Veillonella parvula produced mainly acetate, with small amounts of pyruvate and propionate in the presence of KNO3 (Fig. 4B). Only a small amount of acetate was detected in the absence of KNO3. Under aerobic conditions, the main end products seen during NO2– production were pyruvate and acetate. A significant amount of end products was also detected in the absence of KNO3. In the presence of KNO3, the total amount of end products from lactate was higher under anaerobic conditions than under aerobic conditions, and there was no clear difference between pH 7 and pH 5. NO2– production was observed only in the presence of KNO3.
DISCUSSION
In the present study, we showed the effects of NO3– and NO2– on the growth of bacterial strains (Fig. 1). NO3– did not affect the growth of Streptococcus mutans, except at a high concentration (100 mM), while NO2– (0.5 mM) inhibited its growth even at a low concentration, as reported previously (12). It has been reported that NO2– has multiple inhibitory effects; e.g., it interferes with energy metabolism, oxidative phosphorylation, and proton-dependent active transport (17), causes the collapse of the proton gradient, inhibits metabolic enzymes (18), and damages the cell membrane, iron-sulfur proteins, and DNA (13). However, neither NO3– nor NO2– had inhibitory effects on the growth of Veillonella species, except for a high concentration of NO2– (20 mM). These results suggest that Veillonella species have a system that allows them to tolerate the toxic effects of NO2–, although this system has not been elucidated yet. In the oral cavity, the concentrations of NO3– range from 0.8 mM (unstimulated saliva) to 4 mM (stimulated saliva), and that of NO2– is around 0.3 mM (19). Another study showed that the concentration of NO2– in the oral cavity normally ranges from 0.2 to 2 mM (12) and that it varies according to several factors, such as dietary NO3– intake, bacterial nitrate production activity, the salivary flow rate, and endogenous nitrate production (20). Hence, our results suggest the possibility that in the oral cavity, NO3– itself cannot inhibit the growth of Streptococcus mutans and Veillonella species; however, NO2– that is produced in the oral cavity can inhibit the growth of cariogenic bacteria, such as Streptococcus mutans.
The present study clearly showed that Veillonella species can produce NO2– from NO3– under both aerobic and anaerobic conditions and that such production requires lactate (Fig. 2 and 3). Higher levels of NO2– production were seen under anaerobic conditions, suggesting that this metabolic activity is oxygen sensitive, although the enzymes responsible for the reduction of NO3– to NO2– have not been identified. In addition, higher NO2−-producing activity was seen under acidic conditions, as has been reported for the production of hydrogen sulfide from cysteine by Veillonella species (3). In most cases, Veillonella species grown with NO3– exhibited the highest NO2– production, indicating that NO3– induces a system that utilizes NO3–, as well as a system that protects Veillonella species from the toxic effects of NO2–. NO2– also demonstrated a similar effect in some cases, suggesting that NO2– is involved in the induction system.
Analyses of the amounts of metabolic end products produced from lactate and NO3– revealed a link between lactate metabolism and NO3– reduction. Veillonella parvula required NO2– production from NO3– to occur in order to metabolize lactate under anaerobic conditions, indicating that lactate metabolism and NO2– production are closely linked under these conditions (Fig. 4B). Under aerobic conditions, however, Veillonella parvula was capable of metabolizing lactate without NO3–, indicating that the link between lactate metabolism and NO2– production is weak under these conditions. Conversely, there were no clear differences in the amounts of metabolic end products produced from lactate by Veillonella atypica in the presence or absence of NO3– under anaerobic and aerobic conditions (Fig. 4A), indicating that the link between lactate metabolism and NO2– production is weak in this species. The differences in the closeness of the relationship between lactate metabolism and NO3– reduction between the Veillonella species were probably due to variations in the characteristics of each bacterial species.
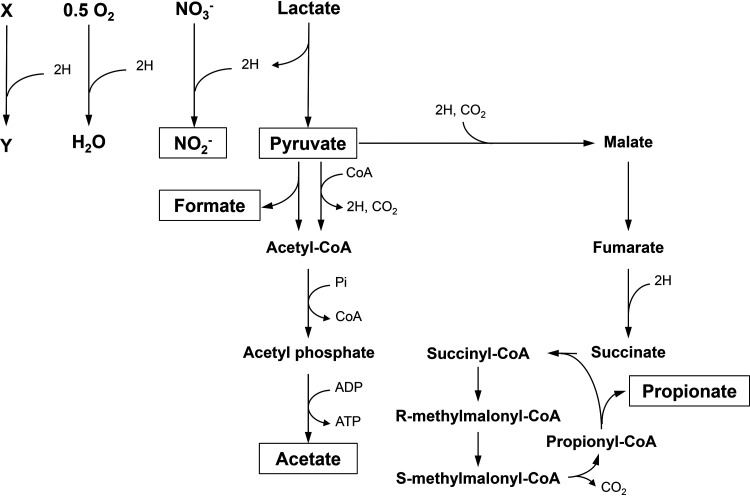
An assessment of the reduction-oxidation balance based on stoichiometric considerations (Table 1) of metabolic end products further supported the idea that there is a metabolic link between lactate metabolism and NO2– production. Under anaerobic conditions, Veillonella species produce propionate, acetate, formate, and pyruvate from lactate and produce NO2– from NO3– (Fig. 4). Specifically, lactate is oxidized to pyruvate; NO3– is reduced to NO2–; and pyruvate is further metabolized to formate, acetate, and propionate through the formate-acetate pathway and the propionate pathway, depending on the reduction-oxidation balance (Fig. 5). The amount of reducing power generated and the amount of oxidation that occurred were calculated based on the levels of metabolic end products (Table 1), and the results clearly indicated that a reduction-oxidation balance was achieved in Veillonella parvula under anaerobic conditions, supporting the suggestion of a link between lactate and NO3– metabolism. However, in the case of Veillonella atypica, the amount of reducing power produced under anaerobic conditions was estimated to be higher than the required amount (Table 1). This suggests that Veillonella atypica is able to metabolize lactate by using an unknown electron acceptor instead of NO3– under anaerobic conditions, possibly hydrogen ions (2H+ + 2e– → H2), which were previously reported to be involved in hydrogen metabolism in Veillonella species (21).
TABLE 1.
Reduction-oxidation balance during lactate metabolism with NO3– based on stoichiometric calculations
| Process or balance | Parameters at pH 7/pH 5 under the indicated conditions |
|||
|---|---|---|---|---|
|
Veillonella atypica |
Veillonella parvula |
|||
| Anaerobic | Aerobic | Anaerobic | Aerobic | |
| Oxidation (2H production [mM]) | ||||
| Lactate utilization (Pyr + Ace + Pro)a | 0.959/1.075 | 0.377/0.380 | 0.525/0.603 | 0.224/0.148 |
| Acetate production via oxidation (Ace – For)b | 0.353/0.359 | 0.180/0.146 | 0.332/0.350 | 0.070/0.053 |
| Reduction (2H consumption [mM]) | ||||
| Propionate production (Pro × 2)c | 0.935/1.110 | 0.075/0.079 | 0.279/0.177 | 0.000/0.000 |
| NO3– reduction (NO2–)d | 0.149/0.214 | 0.020/0.021 | 0.544/0.687 | 0.012/0.029 |
| Reduction-oxidation balance ([a + b]/[c + d]) | 1.21/1.08 | 5.89/5.28 | 1.04/1.10 | 24.47/6.99 |
The amount of lactate utilized was estimated from the total amounts of pyruvate (Pyr), acetate (Ace), and propionate (Pro). One mole of 2H (reducing power) can be produced by oxidizing 1 mol of lactate to 1 mol of Pyr (Fig. 5).
The amount of acetate produced via oxidation was estimated by subtracting the amount of formate (For) produced from the amount of Ace produced. One mole of 2H can be produced by oxidizing 1 mol of Pyr to 1 mol of Ace (Fig. 5).
Two moles of 2H can be consumed by reducing 1 mol of Pyr to 1 mol of Pro (Fig. 5).
One mole of 2H can be consumed by reducing 1 mol of NO3– to 1 mol of NO2– (Fig. 5).
FIG 5.
Proposed NO3– and lactate metabolic pathways of Veillonella species. CoA, coenzyme A.
Under aerobic conditions, acetate and pyruvate were mainly produced together with trace amounts of NO2– from NO3– (Fig. 4), and it was not obvious that a reduction-oxidation balance was achieved, suggesting that most of the reducing power was oxidized by atmospheric oxygen (22) and only a limited amount of reducing power was used for NO3– reduction, and/or that NO3– reduction is labile to oxygen, as discussed above (Fig. 5).
The present study clearly showed that Veillonella species produce NO2– efficiently in the presence of lactate at a wide range of pHs (neutral to acidic pHs) under anaerobic conditions. It is well known that the environment in the oral biofilm and some areas of the oral cavity is anaerobic and becomes acidic and lactate dominant after carbohydrate intake (23). The constant supply of NO3– from saliva and its intermittent supply from food, such as leafy green vegetables, might help Veillonella species to produce NO2– and subsequently suppress other oral bacteria that are associated with oral diseases, such as caries. Some studies have suggested that consuming vegetables could reduce the severity of caries (24), indicating that consuming leafy green vegetables containing NO3– induces and enhances NO2– production by oral NO2−-producing bacteria, such as Veillonella. In this context, in addition to the classical view of Veillonella species, which states that they metabolically convert cariogenic lactate to weaker acids, these bacteria might play a key role in maintaining a health-promoting oral microbiome by controlling excessive metabolic activity by, and the growth of, oral bacteria.
Furthermore, after NO2– produced in the oral cavity is swallowed, it enters the acidic stomach, where it either is nonenzymatically metabolized or comes into contact with mammalian nitrite reductases, which enzymatically metabolize it, forming bioactive nitrogen oxides, such as nitric oxide (NO). Orally ingested NO3– clearly has marked systemic NO-like effects; e.g., it induces vasodilation and lowers blood pressure (15, 16). The presence of nitrate-reducing bacteria, such as Veillonella species, in the oral cavity has a crucial effect on general health, and it has been reported that the elimination of oral nitrate-reducing bacteria by antiseptic reagents reduced the plasma nitrite level, resulting in a concomitant increase in blood pressure (16).
This study reveals Veillonella species as potential candidates for maintaining oral and general health. Further experiments are necessary to extend the biochemical properties of the lactate/NO3– metabolism of Veillonella species to the in vivo situation and to confirm and generalize the inhibitory effect of nitrite production by Veillonella species on S. mutans to other oral bacteria, such as non-mutans streptococci, Actinomyces species, and Lactobacillus species.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Veillonella atypica ATCC 17744, Veillonella parvula ATCC 10740, and Streptococcus mutans NCTC 10449 were used in this study. These bacteria were maintained on CDC anaerobe blood agar (Nippon BD, Tokyo, Japan) at 37°C in an anaerobic glove box (N2, 80%; CO2, 10%; H2, 10%; type NHC; Hirasawa Works, Tokyo, Japan). Single colonies of the Veillonella strains were inoculated in a complex medium containing 0.5% tryptone (Difco Laboratories, Detroit, MI, USA), 0.3% yeast extract (Difco Laboratories), and 1.26% sodium lactate (Wako, Tokyo, Japan) in 50 mM potassium phosphate buffer (PPB; pH 7) (TYL), while single colonies of Streptococcus mutans were inoculated in a complex medium containing 1.7% tryptone, 0.3% yeast extract, 0.5% NaCl (Wako, Tokyo, Japan), and 0.5% glucose (Wako, Tokyo, Japan) in 50 mM PPB (pH 7) (TYG). All bacteria were grown under anaerobic conditions in an NHC-type glove box, and all media were kept under anaerobic conditions for at least 3 days before use. The purity of the cultures was confirmed by macroscopic observation of colony morphogenesis, including color on agar plates, and further Gram staining was used to verify purity.
Effects of NO3– and NO2– on bacterial growth.
The Veillonella strains and Streptococcus mutans were grown in TYL or TYG, with various concentrations (0 to 100 mM) of potassium nitrate (KNO3) or potassium nitrite (KNO2), at 37°C for 24 h under anaerobic conditions. Bacterial growth was estimated by monitoring the optical density (OD) of the culture medium at 660 nm using a spectrophotometer (WPA, Cambridge, UK).
NO2– production by Veillonella species.
Bacterial cells of Veillonella species were harvested in the late-logarithmic phase (OD at 660 nm, 0.8 to 0.9) using centrifugation (10,000 rpm for 7 min at 4°C) and were then washed twice before being resuspended in washing buffer containing 75 mM potassium chloride, 75 mM sodium chloride, and 2 mM magnesium chloride in 2 mM PPB (pH 7). These bacterial-cell suspensions were stored at 4°C until use. The bacterial cells were harvested using double-sealed centrifuge tubes to maintain anaerobic conditions. The washing, resuspending, and storage of the cells were carried out under anaerobic conditions in another anaerobic glove box (N2, 90%; H2, 10%; type NH; Hirasawa Works, Tokyo, Japan).
Reaction mixtures, containing bacterial-cell suspensions (OD at 660 nm, 1.0), various concentrations of sodium lactate (0 to 50 mM), and 1 mM KNO3 in 40 mM PPB (pH 7 or 5), were incubated at 37°C under aerobic or anaerobic conditions for 30 min. After being incubated, the reaction mixtures were centrifuged (10,000 rpm for 3 min at 4°C) to obtain the supernatant. The amounts of NO2– in the supernatant were measured using a Griess reagent kit (Dojindo, Kumamoto, Japan) (25, 26) and a microplate reader (Thermo Scientific Varioskan Flash, Vantaa, Finland) at 540 nm.
Metabolic end products from lactate metabolism during NO2– production by Veillonella species.
Veillonella strains were grown in TYL medium, and bacterial-cell suspensions were prepared as described above. Reaction mixtures (1 ml), containing bacterial-cell suspensions (OD at 660 nm, 1.0) with or without 1 mM KNO3 and 10 mM sodium lactate in 40 mM PPB (pH 7 or 5), were incubated for 15 min at 37°C under aerobic or anaerobic conditions. Subsequently, 0.45 ml of the reaction mixture was mixed with 0.05 ml of 6 N perchloric acid for the organic acid analysis. The samples were filtered through a polypropylene membrane (pore size, 0.20 μm; Toyo Roshi Ltd., Tokyo, Japan). Then the filtrates were analyzed by high-performance liquid chromatography (Shimadzu Prominence LC-20AD; Shimadzu Co., Ltd., Kyoto, Japan) (27) to determine the concentrations of various organic acids (pyruvate, malate, succinate, lactate, fumarate, formate, acetate, and propionate). The amount of NO2– in the sample was also measured as described above.
Statistical analysis.
The significance of differences among multiple groups was analyzed using Tukey’s test and Dunnett’s test. P values of <0.05 were considered statistically significant (StatFlex, version 6).
ACKNOWLEDGMENTS
We express our gratitude and appreciation to all staff and members of the Division of Oral Ecology and Biochemistry, Graduate School of Dentistry, Tohoku University, for providing facilities and constant support.
The experimental research conducted in the present study was supported partly by KAKENHI grants (17K12033, 17H04420, 18K19629) and a FUTOKUKAI Foundation grant from Lion Co.
REFERENCES
- 1.Anil S, Anand PS. 2017. Early childhood caries: prevalence, risk factors, and prevention. Front Pediatr 5:157. doi: 10.3389/fped.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strużycka I. 2014. The oral microbiome in dental caries. Pol J Microbiol 63:127–135. doi: 10.33073/pjm-2014-018. [DOI] [PubMed] [Google Scholar]
- 3.Washio J, Sato T, Koseki T, Takahashi N. 2005. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol 54:889–895. doi: 10.1099/jmm.0.46118-0. [DOI] [PubMed] [Google Scholar]
- 4.Mashima I, Kamaguchi A, Nakazawa F. 2011. The distribution and frequency of oral Veillonella spp. in the tongue biofilm of healthy young adults. Curr Microbiol 63:403–407. doi: 10.1007/s00284-011-9993-2. [DOI] [PubMed] [Google Scholar]
- 5.Mashima I, Nakazawa F. 2014. The influence of oral Veillonella species on biofilms formed by Streptococcus species. Anaerobe 28:54–61. doi: 10.1016/j.anaerobe.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N. 2015. Oral microbiome metabolism. J Dent Res 94:1628–1637. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 7.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. 2005. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 8.Brkić D, Bošnir J, Bevardi M, Bošković AG, Miloš S, Lasić D, Krivohlavek A, Racz A, Mojsović–Ćuić A, Trstenjak NU. 2017. Nitrate in leafy green vegetables and estimated intake. Afr J Tradit Complement Altern Med 14:31–41. doi: 10.21010/ajtcam.v14i3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allaker RP, Silva-Mendez LS, Hardie JM, Benjamin N. 2001. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol Immunol 16:253–256. doi: 10.1034/j.1399-302x.2001.160410.x. [DOI] [PubMed] [Google Scholar]
- 10.Fejerskov O, Nyvad B, Kidd E. 2015. Dental caries: the disease and its clinical management, 3rd ed Wiley Blackwell, Oxford, United Kingdom. [Google Scholar]
- 11.Yamamoto Y, Washio J, Shimizu K, Igarashi K, Takahashi N. 2017. Inhibitory effects of nitrite on acid production in dental plaque in children. Oral Health Prev Dent 15:153–156. doi: 10.3290/j.ohpd.a37926. [DOI] [PubMed] [Google Scholar]
- 12.Silva-Mendez LS, Allaker RP, Hardie JM, Benjamin N. 1999. Antimicrobial effect of acidified nitrite on cariogenic bacteria. Oral Microbiol Immunol 14:391–392. doi: 10.1034/j.1399-302x.1999.140612.x. [DOI] [PubMed] [Google Scholar]
- 13.Cammack R, Joannou CL, Cui XY, Torres MC, Maraj SR, Hughes MN. 1999. Nitrite and nitrosyl compounds in food preservation. Biochim Biophys Acta 1411:475–488. doi: 10.1016/S0005-2728(99)00033-X. [DOI] [PubMed] [Google Scholar]
- 14.Gilchrist M, Shore AC, Benjamin N. 2011. Inorganic nitrate and nitrite and control of blood pressure. Cardiovasc Res 89:492–498. doi: 10.1093/cvr/cvq309. [DOI] [PubMed] [Google Scholar]
- 15.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. 2015. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montenegro MF, Sundqvist ML, Larsen FJ, Zhuge Z, Carlström M, Weitzberg E, Lundberg JO. 2017. Blood pressure-lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension 69:23–31. doi: 10.1161/HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]
- 17.Rowe JJ, Yarbrough JM, Rake JB, Eagon RG. 1979. Nitrite inhibition of aerobic bacteria. Curr Microbiol 2:51–54. doi: 10.1007/BF02601735. [DOI] [Google Scholar]
- 18.Yarbrough JM, Rake JB, Eagon RG. 1980. Bacterial inhibitory effects of nitrite: inhibition of active transport, but not of group translocation, and of intracellular enzymes. Appl Environ Microbiol 39:831–834. doi: 10.1128/AEM.39.4.831-834.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez GA, Miozza VA, Delgado A, Busch L. 2014. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 36:31–35. doi: 10.1016/j.niox.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Dykhuizen RS, Frazer R, Duncan C, Smith CC, Golden M, Benjamin N, Leifert C. 1996. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother 40:1422–1425. doi: 10.1128/AAC.40.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentine RC, Wolfe RS. 1963. Role of ferredoxin in the metabolism of molecular hydrogen. J Bacteriol 85:1114–1120. doi: 10.1128/JB.85.5.1114-1120.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino E, Karino H, Yamada T. 1981. Lactate metabolism by human dental plaque and Veillonella under aerobic and anaerobic conditions. Arch Oral Biol 26:17–22. doi: 10.1016/0003-9969(81)90066-2. [DOI] [PubMed] [Google Scholar]
- 23.Huang R, Li M, Gregory RL. 2011. Bacterial interactions in dental biofilm. Virulence 2:435–444. doi: 10.4161/viru.2.5.16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punitha VC, Amudhan A, Sivaprakasam P, Rathanaprabu V. 2015. Role of dietary habits and diet in caries occurrence and severity among urban adolescent school children. J Pharm Bioallied Sci 7(Suppl 1):S296–S300. doi: 10.4103/0975-7406.155963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyungerel B, Lim H, Lee CH, Choi EH, Li GH, Choi KD. 2014. Anti-inflammatory effects of Magnolia sieboldii extract in lipopolysaccharide-stimulated RAW264.7 macrophages. Trop J Pharm Res 12:913–918. doi: 10.4314/tjpr.v12i6.8. [DOI] [Google Scholar]
- 26.Sasaki Y, Oguchi H, Kobayashi T, Kusama S, Sugiura R, Moriya K, Hirata T, Yukioka Y, Takaya N, Yajima S, Ito S, Okada K, Ohsawa K, Ikeda H, Takano H, Ueda K, Shoun H. 2016. Nitrogen oxide cycle regulates nitric oxide levels and bacterial cell signaling. Sci Rep 6:22038–22011. doi: 10.1038/srep22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manome A, Abiko Y, Kawashima J, Washio J, Fukumoto S, Takahashi N. 2019. Acidogenic potential of oral Bifidobacterium and its high fluoride tolerance. Front Microbiol 10:1099. doi: 10.3389/fmicb.2019.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]