Abstract
High blood pressure is the leading cause of preventable morbidity and mortality globally. Many patients remain on single-drug treatment with poor control, although guidelines recognize that most require combination therapy for blood pressure control. Our hypothesis is that a single-pill combination of 4 blood pressure–lowering agents each at a quarter dose may provide a simple, safe, and effective blood pressure–lowering solution which may also improve long-term adherence. The Quadruple UltrA-low-dose tReaTment for hypErTension (QUARTET) double-blind, active-controlled, randomized clinical trial will examine whether ultra-low-dose quadruple combination therapy is more effective than guideline-recommended standard care in lowering blood pressure. QUARTET will enroll 650 participants with high blood pressure either on no treatment or on monotherapy. Participants will be randomized 1:1 and allocated to intervention therapy of a single pill (quadpill) containing irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, and bisoprolol 2.5 mg or to control therapy of a single identical-appearing pill containing irbesartan 150 mg. In both arms, step-up therapy of open-label amlodipine 5 mg will be provided if blood pressure is >140/90 at 6 weeks. The primary outcome is the difference between groups in the change from baseline in mean unattended automated office systolic blood pressure at 12-week follow-up. The primary outcome and some secondary outcomes will be assessed at 12 weeks; there is an optional 12-month extension phase to assess longer-term efficacy and tolerability. Our secondary aims are to assess if this approach is safe, has fewer adverse effects, and has better tolerability compared to standard care control. QUARTET will therefore provide evidence for the effectiveness and safety of a new paradigm in the management of high blood pressure.
Strengths and limitations of this study
Strengths
-
•
Large, multisite randomized trial with up to 12 months of follow-up
-
•
Double-blind design
-
•
Comparison with current guideline-based blood pressure management
-
•
Objective measurement of the primary outcome
-
•
Embedded economic and acceptability evaluations
Limitations
-
•
Single-country study. A sister trial, QUARTET USA, will provide further information on generalizability.
-
•
Trial not powered for cardiovascular events
Burden of high blood pressure and treatment gaps
High blood pressure (BP) is the leading cause of preventable morbidity and mortality globally.1 The benefits of BP lowering in reducing cardiovascular events are unequivocal,2 and there is clear evidence of greater benefits for combination-based therapy compared to monotherapy.3 Furthermore, numerous studies have indicated the benefits of more rapid control of BP and have shown that this is more likely to occur with use of combination therapy.4 Yet, control of high BP is poor, with only 1 in 3 on treatment achieving BP targets.5., 6., 7., 8.
Previous guidelines typically recommended initiating monotherapy, up-titration of dose, switching drugs if not tolerated, and adding other agents if needed.7 This often takes multiple visits to achieve target BP, and studies show that most individuals remain on monotherapy and with inadequate BP control.5 The largest global survey of hypertension practice showed that only 34% of those treated for high BP were controlled (systolic BP [SBP] <140 and diastolic BP [DBP] <90 mm Hg), and 31% of treated patients were receiving combination therapy.5 The 2017 May Measurement Month BP screening campaign included a convenience sample of 1.2 million across 34 countries and found that 54% of those treated had adequate BP control. A 2013 survey of 31 international hypertension guidelines showed that 27 (87%) now recommend use of combination for initial treatment but typically only as an option for patients at >20/10 mm Hg from goal.9 As 50% to 75% of patients require combination treatment for BP control, there has been increasing interest in the initial use of combination therapy.10 Most recently the European Society of Cardiology/European Society of Hypertension guidelines recommended initial combination therapy for most people, except those with low cardiovascular risk and SBP <150 mm Hg and frail older adults.11
There are multiple barriers to BP control that are patient, health care system, and physician related. Patient adherence is a major factor and is worsened by increased number of medications, complexity of dosing regimens, and medication adverse effects.12 , 13 “Therapeutic inertia,” the reluctance of physicians to treat mild hypertension and uptitrate medications, is also a barrier to BP control. A large study conducted in Western Europe and the United States of more than 20,000 people with hypertension found that BP control rates ranged from 31% to 63%, and only 15% to 38% of instances of elevated BP had uptitration during the visit.14 There is a clear need for improved strategies that will (a) make the treatment of high BP more effective and easier to implement for physicians and patients, (b) quickly and safely bring BP under control, and (c) increase long-term adherence with therapy. We hypothesize that a single-pill combination of 4 BP-lowering agents at quarter dose may achieve these goals.
Rationale for very low-dose combination therapy
Dose response data on BP reduction
Pharmacological dose-response curves for BP-lowering drugs indicate that a quarter dose has at least half the BP-lowering effect of a standard dose (usual maintenance dose) but with much fewer adverse effects.3
A systematic review of all randomized trials of quarter-dose BP-lowering identified a total of 42 trials, 38 of single quarter-dose comparisons, 7 of dual quarter-dose comparisons, and 2 of a quadruple quarter-dose combination.15 Compared to placebo, single quarter-dose therapy reduced BP by 5/2 mm Hg (P < .0001) with no increase in adverse events. Dual quarter-dose therapy was similarly efficacious as standard-dose monotherapy. Two studies of quadruple quarter-dose therapy have been published. One unblinded pilot with 4 control groups of standard-dose monotherapies of the components showed a reduction of 13/8 mm Hg compared to the average reduction of all 4 controls after 4 weeks.16 The other, our pilot, a double-blinded placebo-controlled crossover trial in people with newly diagnosed hypertension, showed a reduction of 22/13 mm Hg after 4 weeks of active treatment versus placebo.17
There is strong evidence that the BP-lowering effects of different classes of drugs are independent and fully additive.15 , 18 The effects of adding a second BP-lowering agent are closely concordant with those predicted by independent effects, occur across all pairs of medication classes, and are about 5 times more effective than doubling the dose of the first agent.18 The additive effects across 3 classes of low-dose drugs were also demonstrated in a placebo-controlled, crossover trial of 3 half-dose BP drugs in 86 participants aged >50 years without a history of cardiovascular disease.19 Overall, a 17.4/9.4–mm Hg BP reduction was observed compared to the anticipated 17.9/9.5–mm Hg decline expected from the cumulative effects of the 3 separate agents.
Dose response data on adverse effects
Avoiding or minimizing adverse effects is critical to long-term adherence for BP lowering given that high BP is typically symptomless. BP-lowering medications rarely cause adverse effects when used at low dose, but each doubling in dose typically leads to a steep increase in adverse effect rates.20 This contrasts to the dose response for BP reduction whereby significant effects are seen at quarter dose, with only a moderate dose response thereafter. For thiazide or thiazide-like diuretics (TZs), β-blockers (BBs), and calcium channel blockers (CCBs), there is a relatively steep increase in adverse effects across all dose ranges.20 Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II receptor blockers (ARBs) are usually well tolerated at low and standard doses but are associated with more adverse effects at higher doses, with the exception of ACE-I cough which is not dose dependent.21
Few direct data have been published on the adverse effects of ultra-low doses of antihypertensive medications. Bennett et al reviewed 15 studies that had data on adverse effects.15 Comparisons with placebo showed no difference in total adverse events for single quarter-dose (14 trials, n = 1838), dual quarter-dose (6 trials, n = 312), and quadruple quarter-dose (1 trial, n = 19) therapy. Comparisons with standard-dose monotherapy showed significantly fewer adverse events overall for single quarter-dose (15 trials, n = 1978) and dual quarter-dose (2 trials, n = 290) therapy. Biochemical changes appear minimal with quarter-dose therapy compared to standard-dose monotherapy.15 These data suggest that dose-dependent adverse effects will be minimal with this intervention, and idiosyncratic reactions are uncommon with these component medications.
Objective
The primary objective of the Quadruple UltrA-low-dose tReatment for hypErTension (QUARTET) trial is to examine whether ultra-low-dose quadruple combination therapy (quadpill) is more effective than guideline-recommended therapy with an ARB plus a CCB if required in lowering BP. Our secondary aim is to assess if this approach is safe and has fewer adverse effects compared to standard care.
Methods
Trial design
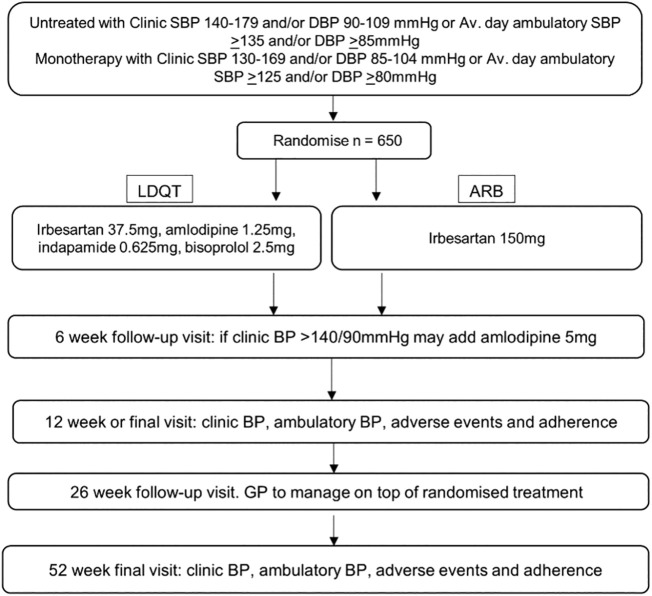
This is a 12-week double-blind randomized controlled trial of 650 patients with high BP. Participants are randomized in a 1:1 allocation ratio using a central computer-based service to initial therapy with quadpill or to a standard dose of an ARB, with a CCB added as required, as per current guideline recommendations (Figure 1 ). The primary outcome is reduction in mean office SBP measured using Omron HEM-907 at 12 weeks. Secondary outcomes include the proportion of participants with controlled BP (SBP < 140 mm Hg and DBP < 90 mm Hg) at 6 weeks and 12 weeks, ambulatory BP measures at 12 weeks, tolerability, and the occurrence of adverse events. Learnings from the quadpill pilot informed the design and conduct of the present trial.17
Figure 1.
Trial schema.
Extension study
An extension study to 12 months of follow-up involves 2 more visits at 26 and 52 weeks after randomization to examine longer-term efficacy and tolerability.
Participants
Eligibility criteria
The study enrolled the first participant on 8 June 2017, and the last participants are expected to complete follow-up by 30 November 2020. Currently, 575 (88%) participants have been randomized, and 421 have agreed to continue in the extension study. At the time of submission, COVID-19 has impacted study recruitment, as health services have paused nonessential activities (from mid-March 2020) to minimize infection risk. This may increase the likelihood of stopping the trial before the recruitment target is reached.
Inclusion criteria
-
•
Adults (≥18 years)
-
•
Previous documentation of hypertension or high BP (SBP 140-179 mm Hg and/or DBP 90-109 mm Hg) from general practitioner (GP), pharmacist, or other health care professional
-
•And either:
-
oA measure of office SBP 140-179 mm Hg and/or DBP 90-109 mm Hg documented by study staff in the last 12 weeks with a study automatic BP device or
-
oA recorded measure of daytime average SBP ≥ 135 mm Hg and/or DBP ≥ 85 mm Hg on a 24-hour ambulatory BP monitoring device in the last 12 weeks.
-
o
-
•And 1 of the following:
-
oTreatment naive (ie, never treated)
-
oCurrently not on treatment (not taken in last 4 weeks)
-
oCurrently taking 1 BP-lowering drug (that is any of the following drug classes: ACE-I, ARB, CCB, BB, aldosterone antagonist, α-blocker) at any dose.
-
o
Exclusion criteria
-
•
Contraindication to irbesartan, amlodipine, indapamide, or bisoprolol
-
•
Evidence of secondary cause of hypertension
-
•
Estimated glomerular filtration rate < 50 mL/min/1.73 m2
-
•
Raised serum potassium (greater than local laboratory normal limit)
-
•
Women who are pregnant, breast feeding, and/ or of childbearing potential and not using medically acceptable form of contraception throughout the study
-
•
Concomitant illness, physical impairment, or mental condition which in the opinion of the study team/primary care physician could interfere with the conduct of the study including outcome assessments
-
•
Participation in a concurrent interventional medical investigation or clinical trial. Patients in observational, natural history, and/or epidemiological studies not involving an intervention are eligible.
-
•
Participant's primary care physician or other responsible physician believes it is not appropriate for participant to switch current monotherapy or initiate study drug.
-
•
Inability or unwillingness to provide written informed consent
-
•
Unable to complete study procedures including 24-hour ambulatory BP monitoring (ABPM)
-
•
Definite indication for 1 or more components of the quadpill
We amended exclusion criteria in November 2017 to ensure participants in an influenza vaccination study were not precluded from participation in the trial.
-
•
Participation in a concurrent clinical trial of an investigational medical product. Patients in trials of approved medical products or in observational, natural history, and/or epidemiological studies not involving an intervention are eligible.
Changes to inclusion and exclusion criteria to facilitate recruitment
In June 2018, we further amended inclusion criteria to allow lower BP entry for those on monotherapy, as these participants are considered not at target within Australian guidelines.22
-
•
Adults (≥18 years)
-
•
Previous documentation of hypertension or high BP (SBP 140-179 mm Hg and/or DBP 90-109 mm Hg) from GP, pharmacist, or other health care professional
-
•And meeting either criterion A or B:
-
oCriterion A: In treatment naive (ie, never treated) or in patients currently not on treatment (not taken in last 4 weeks), either:
-
▪A measure of clinic SBP 140-179 mm Hg and/or DBP 90-109 mm Hg documented by study staff in the last 12 weeks with a study automatic BP device or
-
▪A recorded measure of daytime average SBP ≥135 mm Hg and/or DBP ≥85 mm Hg on a 24-hour ABPM device in the last 12 weeks
-
▪
-
oCriterion B: In patients currently taking 1 BP-lowering drug “monotherapy,” either:
-
▪A measure of clinic SBP 130-179 mm Hg and/or DBP 85-109 mm Hg documented by study staff in the last 12 weeks with a study automatic BP device or
-
▪A recorded measure of daytime average SBP ≥125 mm Hg and/or DBP ≥80 mm Hg on a 24-hour ABPM device in the last 12 weeks
-
▪
-
o
Setting, locations, and recruitment
Participants are recruited from community general practices and outpatient clinics. Current active sites are listed in Appendix 1. There are a total of 10 sites in 4 of the 8 states and territories of Australia (New South Wales, Victoria, Tasmania, and Western Australia), with 3 of these based in primary care and the rest in hospital or university locations. We use several methods to identify potentially eligible participants. This includes community advertising and awareness campaigns (using print and electronic media advertisements and radio), referral by clinicians aware of the study (advertising through clinical trial sites and communication media to health professionals), and screening of relevant patient lists by clinical investigators for potentially eligible patients. Participants are not paid for their participation. Participants may be reimbursed for travel.
Study treatment
Patients are randomized to (a) an encapsulated single pill (quadpill) containing irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, and bisoprolol 2.5 mg or to (b) an identical capsule containing irbesartan 150 mg. At 6 weeks, if the BP is greater than 140/90 mm Hg in either arm, open-label amlodipine 5 mg is added: this is provided as an additional pill.
We selected quarter standard doses of irbesartan, amlodipine, indapamide, and bisoprolol. The first 3 were chosen as the most commonly prescribed ARB, CCB, and TZ in Australia (PBS Information Management Section Pharmaceutical Policy Branch, 2013). Standard dose was determined following the method of Bennett et al.15 Although hydrochlorothiazide is included in a number of fixed-dose combinations, some recent guidelines23 and literature recommend indapamide or chlorthalidone, principally on the basis that some data suggest more cardiovascular event reduction with these agents,24 , 25 although a recent article suggests no difference.26 The additional BP reduction expected from including a quarter dose of a different class of drug is about 3 times as great as would be achieved by doubling the dose of any other component.15 We chose the fourth agent to be a BB because of its long duration of action and relatively minimal adverse effects at a quarter dose. The choice of a BB as a fourth agent of choice is also consistent with a number of international hypertension guidelines which specify BB use after renin angiotensin system blockers, CCBs, and TZs.22 , 27 , 28 We chose bisoprolol over atenolol because of its longer duration of action. The other major consideration was use of off-patent components to minimize costs.
The control group follows the recommendations of the current Australian guidelines,22 , 29 that is, initiating with an ACE-I or ARB and, if BP is not controlled, adding a CCB. This approach is also consistent with the 2011 NICE Hypertension Guidelines and among the preferred treatment options in the 2013 JNC-8 Guidelines and the 2013 European Society of Cardiology/European Society of Hypertension guidelines, which were current at the inception of this study.23 , 30 , 31 We chose irbesartan because it is the most commonly prescribed ARB in Australia and amlodipine because it is the most commonly prescribed CCB.
Patients who are on monotherapy at time of recruitment will be asked to stop their treatment while they are taking the study treatment. The drug is provided to both intervention and control arms at no cost to the participant. Medications are provided in quantities of 99 tablets at 3 monthly intervals. That is a medication kit is given to patients at baseline, week 12, and 6 months and 9 months in participants participating in the extension study. Each kit consists of 3 bottles comprising 33 tablets in each bottle. Most sites provide in-person pickup of medications, and in selected sites in New South Wales (Westmead Hospital, Royal North Shore Hospital) and Western Australia (Sir Charles Gardiner Hospital, Royal Perth Hospital), medication is mailed to participants.
Preparation of study treatment
The study drug has been made up by PCI Clinical Services (formerly Pharmaceutical Packaging Professionals), a Therapeutic Goods Administration Code of Good Manufacturing Practice audited facility approved for all stages of finished product manufacture for clinical trials. This company encapsulated the drugs listed for intervention and control arms into a single capsule, with additional placebos in the control capsule. Thus, both the intervention and control participants receive a single capsule that appears identical, inside and out, to all participants, their health care providers, and trial personnel.
Study procedures
Patients are assessed for eligibility and randomized if criteria are met. Follow-up clinical assessments are conducted at 6 and 12 weeks. The 6-week visit includes a clinic BP measurement (3 unattended automated office measures) and recording of any changes in concomitant medications, adverse events, and health service use. At 6 weeks, if clinic BP is >140/90 mm Hg, the researcher will alert the study physician who will assess the participant (BP and symptoms) and consider adding open-label amlodipine 5 mg (consistent with current guidelines). The week 12 visit includes the above plus 24-hour ABPM, quality of life, and additional laboratory assessments of sodium, potassium, chloride, bicarbonate, serum creatinine, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration formula), uric acid, liver function tests, and urine albumin to creatinine ratio. No central laboratory is used. Participants have an option to extend their involvement in the study to 12 months after randomization, involving extra visits at weeks 26 and 52 (with comparable follow-up procedures to weeks 6 and week 12 visits, respectively). Extension involves continuing to receive the randomly allocated treatment but with management through their GP or site physician. They may add additional drugs if clinically necessary with open-label treatment added without the need to unblind randomized therapy. Adherence to medications is assessed by self-report and a pill count of returned study medications at end of study time points, that is, week 12 final visit and at 12 months the final visit of the extension study. Participants are asked how many days in the last 30 days they have missed taking any of their regular medications and similarly about missed medications in the last 7 days.
During the study, we will obtain information on self-reported health service utilization and specifically ask if patients have seen and how frequently they have seen the following health providers: practice nurse, GP, physician in public hospital emergency department (not admitted), physician in public outpatients clinic for any reason, and physician in private specialist clinic for any reason. We also request consent to link data to Medical Benefits Schedule (listing of Medicare services subsidized by the Australian Government) and Pharmaceutical Benefits Scheme (listing of medicines subsidized by the Australian Government).
Information is collected on serious adverse events and adverse events of special interest (see list in Appendix 1). We specifically query participants about adverse events of special interest at each visit (6 week, 12 weeks, and additional visits for extension participants at 6 and 12 months). Adverse events of special interest include dizziness, hypotension, pedal edema, muscle cramps, bradycardia, heart failure, hypersensitivity reactions (skin rashes, itching), gastrointestinal complaints (nausea, vomiting, diarrhea), musculoskeletal complaints, and headaches. Adverse events are not adjudicated. The EuroQol Group Quality of Life questionnaire is completed by participants at their baseline, 12-week, and final visits.
Outcome measures and outcome assessment
The primary and secondary outcomes are listed in Table I .
Table I.
Primary and secondary outcomes for the QUARTET Trial
| Primary outcome | |
|---|---|
| Difference between groups in change in mean office SBP from baseline to 12 wk | |
| Secondary outcomes | |
| 24-h ambulatory BP | (a) Difference between groups in mean 24-h SBP and DBP at 12 and 52 wk (b) Difference between groups in mean change in 24-h SBP and DBP from 0 to 12 w, 0 to 52 wk, and 12 to 52 wk (c) Difference between groups in mean daytime SBP and DBP at 12 and 52 wk (d) Difference between groups in mean nighttime SBP and DBP at 12 and 52 wk (e) Difference between groups in daytime, nighttime, and 24-h BP load (percentage area under the BP curve above normal day, night, and 24-h values as per National Heart Foundation guidelines (f) Difference between groups in the proportion of nondippers (nighttime BP is not more than 10% lower than average daytime BP as per National Heart Foundation guidelines) and coefficient of variability of BP33 |
| Other BP measures | (a) Difference between groups in mean automated office SBP (52 wk) and DBP (12 and 52 wk) (b) Difference between groups in standard clinic SBP/DBP at 12 and 52 wk (c) Hypertension control (% with SBP < 140 mm Hg and DBP < 90 mm Hg) at 6, 12, 26, and 52 wk (d) Percentage requiring step-up treatment at 6 wk (e) Percentage requiring step-up BP-lowering treatment over 52 wk (f) Percentage with both BP control (as defined above) and no adverse events (g) Difference between groups in SBP and DBP variability |
| Tolerability | (a) Difference between groups in potentially related adverse effects (dizziness, blurred vision, syncope/ collapse/ fall, chest pain/ angina, shortness of breath, cough, wheeze, ankle edema, skin rash, itching, gout, hyperkalemia, hypokalemia, hyponatremia, other) (b) Difference between groups in mean potassium, uric acid, blood glucose, cholesterol and fractions, ALT, AST, urine albumin to creatinine ratio, and creatinine levels (c) Difference between groups in participant withdrawals from treatment |
| Safety | Percentage with any severe adverse event |
| Medication adherence | Self-reported measures and pill counts |
| Cost-effectiveness | The ratio of the difference in costs and outcomes between treatment arms |
| Patient and prescriber acceptability | End-of-study feedback questionnaires |
Key secondary outcomes have been put in bold. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The BP measurements are recorded using an Omron HEM907. An appropriate cuff size is selected for all BP measurements. First, a measure of clinic BP is observed and recorded by research staff. Then, automated office BP is measured following the recommendations of the European Society of Hypertension/European Society of Cardiology and Australian National Heart Foundation.22 , 30 This requires the research staff to set the automated device to take 3 separate BP measurements while the researcher steps out of the room (unattended BP measurement). The Omron HEM907 is programmed to start the first measurement after 5 minutes of rest and then at 1-minute intervals. The primary outcome “mean SBP” will be calculated using the average of these 3 unattended measures. In addition, 24-hour ABPM is conducted at baseline and at 12- and 52-week follow-up visits using a Suntech Oscar-2 programmed to measure every 30 minutes while participant is awake and hourly during sleep.32 , 33
Sample size
A sample size of 650 patients provides 90% power at P = .05 to detect a difference between randomized groups of 4 mm Hg in the primary outcome, assuming an SD of 15 mm Hg.34 A sample of 650 also has 85% power to detect a 3–mm Hg difference in average 24-hour SBP (SD 12 mm Hg)34 and 85% power to detect a 25% increase in the proportion with controlled BP assuming 50% are controlled in the comparator group. All calculations allow for a 10% dropout or data loss rate. It is assumed that irbesartan 150 mg and uptitration with the addition of amlodipine in 75% of participants in the control group will give an average reduction of 12 mm Hg from an average baseline SBP of 150 mm Hg.35 Based on the information presented in the background, quadruple combination therapy will reduce SBP by at least 16 mm Hg.16 , 20
The rate of all adverse events is predicted to be around 15% in the control group,35 and this study will have 90% power to rule out an increase of 5 percentage points (ie, a noninferiority margin of 20%) assuming the true incidence of adverse events in the quadpill group is 10% and a 1-sided test with α = 2.5%. The 10% incidence of adverse events is a conservative estimate from adding up the incidence of adverse effects from each treatment class at ½ standard dose described in a previous systematic review: BB 5.5%, TZ 2.0%, CCB 1.6%, and ARB 0%.3
Interim analyses, monitoring, and stopping guidelines
The trial data safety and monitoring committee (DSMC) monitors safety data on an ongoing basis, with the analyses performed by an independent statistician from the George Institute for Global Health. The DSMC can recommend the Steering Committee of the QUARTET Study should continue the study unchanged, adjust the duration of follow-up, or terminate the study early if there is clear and substantial evidence of benefit, if the data suggest the risk of adverse events substantially outweighs the potential benefits, or for futility. The first DSMC meeting was held after 25% of participants completed 12 weeks of follow up and recommended continuation of the study without modification.
Randomization
The unblinded statistician prepared a computer-generated randomization schedule stratified by site and using permuted blocks of variable size. This was loaded into the Web-based data management system (IBM Clinical Development, Morrisville, NC). Allocation concealment is maintained because only the unblinded statistician and unblinded data manager have access to the randomization list and allocation within the database.
Participants are enrolled at sites by blinded staff, with participant randomization and study drug allocation conducted through the database with blinding maintained. The study drug kit numbering is separate to the randomization sequence to prevent the kit allocation potentially unblinding site staff. The investigators, project management, site staff, and participants are blinded to the randomization sequence and treatment allocation.
Statistical methods
The main analyses of study outcomes will be conducted according to the principle of intention to treat. The primary analysis of change in SBP at 12 weeks will be performed using an analysis of covariance including the treatment arm and baseline SBP as a covariate. Continuous secondary outcomes will be analyzed similarly. Additional analyses will include all follow-up measurements in a longitudinal model including treatment arm, visit, and a treatment by visit interaction term as well as the baseline measurement. Within-patient correlations will be modeled using generalized estimating equations or random effects. A similar approach will be applied to binary end points (eg, BP control) with log-binomial regression used in place of linear regression. A per-protocol analysis will be performed to provide information on the difference in efficacy between the 2 study treatments. There will also be predefined subgroup analyses, including by baseline BP, gender, age, diabetes, education, and BP-lowering treatment at baseline (no treatment vs monotherapy). A detailed analysis plan will be finalized prior to unblinding.
Economic evaluation
Cost-effectiveness and cost-utility analysis
An incremental cost-effectiveness analysis will be used to compare the costs and outcomes of the treatment arms from a health system perspective. This will consider the cost per mm Hg reduction in systolic BP and the cost per quality-adjusted life-year gained for quadpill versus monotherapy to facilitate comparison with other interventions. Costs will be determined through the collection of resource use during the study period and estimates of commercial costs for the quadpill. Information on hospital admissions, physicians' visits, and medications is collected at follow-up visits.
Acceptability evaluation
A semiquantitative survey and in-depth interviews will be conducted to assess the acceptability of quadpill and to identify which factors are important to participants and health providers in BP reduction. Patient acceptability is a critical component of health care innovation. Patients and health providers in the study will be invited to answer questions assessing their perceptions, experience, and the degree of engagement with the intervention at the completion of the trial. Patients and health providers will be invited to participate in semistructured interviews on perceptions of the utility and acceptability of the intervention program. Examples of questions are included in Appendix 1. Interviews will be recorded, transcribed, and then coded using NVivo. From the coded data, key themes will be identified.
Trial management, funding, and sponsorship
The trial conduct is overseen by a steering committee (list in Appendix 1). The central coordinating center ensures implementation of the study according to the protocol, timelines, and recruitment targets. We use an electronic data management system incorporating study checks and omissions. An independent DSMC meets regularly to assess emerging evidence on safety and efficacy. The QUARTET trial received primary funding from the National Health and Medical Research Council (NHMRC) Australia (APP1100377). Investigators also received support from the NHMRC program and investigator fellowships to enable the study (see Funding statement). The University of Sydney is the current study sponsor.
Trial registration, human research ethics, and dissemination plan
The QUARTET trial is registered on the Australian New Zealand Clinical Trial Registry (ACTRN12616001144404). The Western Sydney Local Health District Human Research Ethics Committee provides lead ethics approval (HREC/15/WMEAD/422).
The main trial results will be published in the name of the QUARTET Investigators with credit assigned to the collaborating investigators and other research staff. Publication authors must meet the International Committee of Medical Journal Editors guidelines for authorship. Presentations of the study findings will be made at national and international meetings concerned with the management of cardiovascular disease and high BP. Trial data will be made available through data access agreements established following approval through the QUARTET Steering Committee. Trial data will not be publicly released or placed into an open-access repository. Trial data will be held by the University of Sydney for a minimum period of 15 years (or longer if required by applicable regulatory authorities).
Discussion
High BP is the leading risk factor for lost healthy life-years globally.1 For women, it is the leading risk factor, with 90 million disability-adjusted life-years (DALYs), and it is the second leading risk factor in men, with 124 million disability-adjusted life-yearss.36 Although the global age-standardized death rate attributable to high SBP declined by 1.35% over the last 30 years, the number of deaths attributable to high SBP has increased globally over this time, with 10.4 million deaths in 2016.37 Achieving sustainable and affordable reductions in SBP is key to addressing this leading risk factor for lost healthy life.
The QUARTET trial is the first large-scale trial to examine a quadruple, quarter-dose regimen. This approach has many theoretical benefits, including greater efficacy and fewer adverse effects, as well as pragmatic benefits that should improve adherence and decrease costs. If this new intervention achieves its conservative additional 4 mm Hg of BP reduction compared to that conferred by optimal guideline-recommended care, such a difference could translate into an additional 15% to 20% reduction in cardiovascular events.3
There has been increasing acceptance of the role of dual antihypertensive combinations in BP management due to both the observation that most patients require more than 1 agent to achieve BP control and trials showing that early use of combination is beneficial.38
Benefits of combination therapy
It is apparent that people respond differently to different BP classes23 , 39; however, it is difficult to determine which drug is most effective for each individual.40 A trial and error approach to finding an effective monotherapy regimen may contribute to low adherence. Combination therapy is more likely to provide a genuine good response more quickly and with less variability.
Fewer medications and single-pill combination therapy improve adherence. A recent meta-analysis of trials comparing combination pills containing 2 antihypertensive agents to separate pills demonstrated a significant improvement in adherence with combination therapy.41 Triple combinations are commercially available42 , 43; however, they have not included an entirely low-dose option. These products are targeted to the relatively small subpopulation of patients with severe or resistant hypertension not controlled on full doses of dual combination therapy or those already on the 3 medications.43
Some recent trials of low-dose combination therapy have demonstrated the potential of this strategy in other settings. The TRIUMPH trial evaluated a half-strength triple pill but with several points of difference, most importantly the comparison against a variety of usual-care options in Sri Lankan outpatient hospital care, with a focus on improving the access and affordability of BP-lowering medications in this setting.44 This study found that 70% of participants in the triple-pill group achieved their target BP versus 55% in the usual-care group,44 and the triple pill was cost-effective compared to usual care.45 The Quadpill Pilot trial was a placebo-controlled pilot study conducted in treatment-naive people with newly diagnosed high BP in primary care.17 The ultra-low-dose quadruple combination was very effective at lowering BP in the short-term single-center pilot study; hence, the current study is needed. A sister trial, QUARTET USA (Clinicaltrials.gov NCT03640312), is currently under way in Chicago, IL, and an individual patient data meta-analysis is planned once both trials are completed.
Conclusions
If the intervention tested here is proven to be safe and effective, the trial results could be rapidly implemented, with immediate benefits in routine clinical practice. Similar therapy could be provided to patients using available medications, including existing dual combinations and the use of dose administration aids. Ultimately, most advantage will be gained from single-pill formulations. The results of the current trial would stimulate the development of such products if the results were favorable.
In summary, ultra-low-dose combination therapy has the potential to have a major impact on current poor rates of BP control globally. The critical next step is direct evidence on effectiveness and safety in a large-scale randomized controlled trial, which the QUARTET trial aims to provide.
Authors' contributions
C. K. C. wrote the first draft of the QUARTET protocol that was subsequently funded by NHMRC with critical review from A. R. as the senior author and all CIs of the NHMRC protocol including G. H., M. S., T. U., R. W., L. B. E. R. A. has been the postdoctoral fellow on QUARTET, prepared the first draft of the manuscript, and revised the manuscript. All authors have reviewed the final manuscript. We also acknowledge Henry Krum (deceased) who provided critical review of the QUARTET protocol submitted to NHMRC.
Funding statement
The QUARTET trial received principal funding through a project grant from NHMRC Australia (APP1100377). The trail was also supported by funding from NHMRC program grants (APP1052555 and APP1092642). Individual investigators also received support to enable their time allocation to the trial: C. C. was supported by NHMRC Career Development Fellowship Level 2 (APP1105447), E. R. A. was supported by a National Heart Foundation Australia Postdoctoral Fellowship 2018-2019 (101884), C. M. R. was supported through an NHMRC Principal Research Fellowship (APP1136372), M. P. S. was supported through an NHMRC Senior Research Fellowship (APP1080404), R. W. was supported by an NHMRC Early Career Fellowship (APP1125044), and A. P. was supported by an NHMRC Principal Research Fellowship (APP1136898).
Declaration of competing interests
George Health Enterprises, the social enterprise arm of The George Institute for Global Health, has applied for patents in this research area, on which C. K. C. and A. R. are named as inventors; George Health Enterprises has also received investment to develop fixed-dose combinations containing aspirin, statins, and BP-lowering drugs.
Appendix 1. QUARTET Trial Steering Committee
-
•
Prof Clara Chow (Chair)
-
•
Dr Emily Atkins
-
•
Prof Laurent Billot
-
•
Prof John Chalmers
-
•
Prof Graham Hillis
-
•
Prof Bruce Neal
-
•
Prof Mark Nelson
-
•
Prof Anushka Patel
-
•
Prof Chris Reid
-
•
Prof Anthony Rodgers
-
•
Prof Markus Schlaich
-
•
Prof Tim Usherwood
-
•
A/Prof Ruth Webster
Site investigators (alphabetical order, excluding above): Michael Bloch, Michael Burke, Gemma Figtree, Peter Hay, Shirley Jansen.
Appendix 2. Current sites
New South Wales
-
•
Westmead Hospital, Westmead
-
•
Royal North Shore Hospital, St Leonards
-
•
Holdsworth House Medical Centre, Darlinghurst
-
•
Castle Hill Medical Centre, Castle Hill
-
•
Kildare Road Medical Centre, Blacktown
Tasmania
-
•
University of Tasmania, Hobart
Victoria
-
•
Monash University, Caulfield
Western Australia
-
•
Curtin University, Bentley
-
•
Royal Perth Hospital, Perth
-
•
Sir Charles Gairdner Hospital, Nedlands
Harms
All serious adverse events (SAEs) and adverse events of special interest (AESIs) experienced by a participant after the informed consent document is signed and until the end of the study at week 12 or 52 will be collected and reported to the Chief Coordinating Centre as per applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) and applicable regulatory guidelines. If an SAE is unresolved at the conclusion of the study, a clinical assessment will be made by the medical monitor as to whether continued follow-up of the SAE is warranted. SAE criteria, definitions, and guidance for reporting are outlined in Sections 1 to 4.
1. Adverse event
An adverse event (AE) is defined as any untoward medical occurrence in a subject or clinical investigation subject administered a pharmaceutical product at any dose that does not necessarily have to have a causal relationship with this treatment. Therefore, an AE can be any unfavorable and unintended sign (including an abnormal laboratory finding, for example), symptom, or disease temporally associated with the use of an investigational product, whether or not considered related to the investigational product. This definition includes intercurrent illnesses or injuries and exacerbation of pre-existing conditions.
2. Adverse events of special interest
The expected adverse reactions to the BP-lowering medications that will be used in QUARTET are well known (Appendix 2). To better assess participants' tolerability to the study medications, the following AESIs and whether they are new or ongoing from baseline will be reported to the Chief Coordinating Centre regardless of severity and seriousness:
-
•
Dizziness
-
•
Hypotension
-
•
Pedal edema
-
•
Headache
-
•
Muscle cramps
-
•
Bradycardia
-
•
Worsening of heart failure
-
•
Hypersensitivity reactions (skin rashes, itching)
-
•
Gastrointestinal complaints
-
•
Musculoskeletal trauma
3. Serious adverse event
An SAE is defined as any untoward medical occurrence that at any dose:
-
•
results in death
-
•
is life threatening in the opinion of the attending clinician (ie, the patient was at risk of death at the time of the event; it does not refer to an event that might hypothetically have caused death had it been more severe)
-
•
requires inpatient hospitalization or prolongation of existing hospitalization (Any hospitalization that was planned prior to randomization will not meet SAE criteria. Any hospitalization that is planned post randomization will meet the SAE criteria.)
-
•
results in persistent or significant disability or incapacity
-
•
results in congenital anomaly or birth defect (Note that the women in the study population are likely to be postmenopausal.)
-
•
is an important medical event in the opinion of the attending clinician that is not immediately life threatening and does not result in death or hospitalization but which may jeopardize the patient or may require intervention to prevent one of the other outcomes listed above
An AE that meets the above categories between when the informed consent form is signed, the end of study visit at week 12 or at 26 and 52 weeks if patient is participating in the study extension, and until the 28 days after the study drug is discontinued will be reported as an SAE. All SAEs are required to be reported to the sponsor team within 24 hours of the study team first becoming aware of the event. The SAE will also be required to be reported to the relevant Human Research Ethics Committee (HREC) - Institutional Review Board (IRB) within the time frame specified in the relevant committee guidelines. If irbesartan or the Low-dose quadruple combination therapy (LDQT) is discontinued as a result of an AE, the study team will document all events leading to the discontinuation of treatment. AEs which do not fall into these categories are defined as nonserious.
4. Suspected unexpected serious adverse reaction
An unexpected adverse reaction is an adverse reaction, the nature or severity of which is not consistent with the applicable product information. Refer to QUARTET protocol (Appendix 2) for a list of expected adverse reactions for the interventions used in this protocol.
A suspected unexpected serious adverse reaction is any unexpected adverse reaction that at any dose meets the definition of an SAE (refer to Section 3). Any event that meets the definition of a suspected unexpected serious adverse reaction between when the informed consent form is signed and the end of study visit at week 12 or week 52 will be reported to the local Human Research Ethics Committee - Institutional Review Board and the relevant regulatory authorities as per local requirements and ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting.
Examples of questions asked of providers and participants to assess acceptability of the quadpill intervention
Examples of questions asked of participants include the following:
-
-
During the trial, how easy did the participant find it to take the trial medications?
-
-
If the LDQT is available to be prescribed by participant's usual physician, how likely would the participant be to request it?
-
-
Are there any other comments the participant had about the LDQT?
Examples of questions addressed to health care providers about the quad pill include the following:
-
-
What do you think are the potential benefits of LDQT or your concerns about LDQT?
-
-
If LDQT was available, in what circumstances would you prescribe it or what evidence would you require to start prescribing LDQT?
-
-
What do you consider to be important factors in patients' decisions to take BP-lowering medications?
References
- 1.Lim S.S., Vos T., Flaxman A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 3.Law M.R., Morris J.K., Wald N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ (Clinical research ed) 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julius S., Kjeldsen S.E., Weber M. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the value randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 5.Chow C.K., Teo K.K., Rangarajan S. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 6.Kotseva K., Wood D., De Backer G. Cardiovascular prevention guidelines in daily practice: a comparison of euroaspire i, ii, and iii surveys in eight european countries. Lancet. 2009;373:929–940. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 7.Peiris D.P., Aa Patel, Cass A. Cardiovascular disease risk management for aboriginal and Torres Strait islander peoples in primary health care settings: findings from the Kanyini audit. Med J Aust. 2009;191:304–309. doi: 10.5694/j.1326-5377.2009.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 8.Webster R.J., Heeley E.L., Peiris D.P. Gaps in cardiovascular disease risk management in Australian general practice. Med J Aust. 2009;191:324–329. doi: 10.5694/j.1326-5377.2009.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers J., Arima H., Harrap S. Global survey of current practice in management of hypertension as reported by societies affiliated with the International Society of Hypertension. J Hypertens. 2013;31:1043–1048. doi: 10.1097/HJH.0b013e32835f7eef. [DOI] [PubMed] [Google Scholar]
- 10.Byrd J.B., Zeng C., Tavel H.M. Combination therapy as initial treatment for newly diagnosed hypertension. Am Heart J. 2011;162:340–346. doi: 10.1016/j.ahj.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018:ehy339. doi: 10.1093/eurheartj/ehz219. [DOI] [PubMed] [Google Scholar]
- 12.Vrijens B., Vincze G., Kristanto P. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ (Clinical research ed) 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osterberg L., Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Alexander G., Stafford R. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167:141–147. doi: 10.1001/archinte.167.2.141. [DOI] [PubMed] [Google Scholar]
- 15.Bennett A., Chow C.K., Chou M. Efficacy and safety of quarter-dose blood pressure–lowering agents. Hypertension. 2017;70:85–93. doi: 10.1161/HYPERTENSIONAHA.117.09202. [DOI] [PubMed] [Google Scholar]
- 16.Mahmud A., Feely J. Low-dose quadruple antihypertensive combination: more efficacious than individual agents—a preliminary report. Hypertension. 2007;49:272–275. doi: 10.1161/01.HYP.0000254479.66645.a3. [DOI] [PubMed] [Google Scholar]
- 17.Chow C.K., Thakkar J., Bennett A. Quarter-dose quadruple combination therapy for initial treatment of hypertension: placebo-controlled, crossover, randomised trial and systematic review. Lancet. 2017;389:1035–1042. doi: 10.1016/S0140-6736(17)30260-X. [DOI] [PubMed] [Google Scholar]
- 18.Wald D.S., Law M., Morris J.K. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Wald D.S., Morris J.K., Wald N.J. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law M.R., Wald N.J., Morris J.K. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ (Clinical Research Ed) 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Konstam, Neaton J.D., Dickstein K. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 22.National Heart Foundation Australia . 2016. Guideline for the diagnosis and management of hypertension in adults. [Google Scholar]
- 23.National Institute for Health and Clinical Excellence The clinical management of primary hypertension in adults. Clinical guideline. 2011;127 [Google Scholar]
- 24.Chalmers J., Arima H., Woodward M. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial. Hypertension. 2014;63:259–264. doi: 10.1161/HYPERTENSIONAHA.113.02252. [DOI] [PubMed] [Google Scholar]
- 25.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure–lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 26.Hripcsak G., Suchard M.A., Shea S. Comparison of cardiovascular and safety outcomes of chlorthalidone vs hydrochlorothiazide to treat hypertension. JAMA Intern Med. 2020;180:542–551. doi: 10.1001/jamainternmed.2019.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams B, Mancia G. 2018 European Society of Cardiology (ESC) and European Society of Hypertension (ESH) joint guidelines for the management of arterial hypertension. J Hypertens. 2018;in press.
- 28.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 29.National Heart Foundation Australia (National Blood Pressure and Vascular Disease Advisory Committee) 2010. Guide to management of hypertension 2008. Updated december 2010. [Google Scholar]
- 30.Mancia G., Fagard R., Narkiewicz K. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 31.James P.A., Oparil S., Carter B.L. 2014 Evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 32.Head G.A., McGrath B.P., Mihailidou A.S. Stowasser M, Mangoni Aa, Cowley D, Brown Ma, Ruta L-A, Wilson A. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J Hypertens. 2012;30:253–266. doi: 10.1097/HJH.0b013e32834de621. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien E., Parati G., Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62:988–994. doi: 10.1161/HYPERTENSIONAHA.113.02148. [DOI] [PubMed] [Google Scholar]
- 34.Farsang C. Efficacy and tolerability of fixed-dose combination of perindopril/indapamide in type 2 diabetes mellitus: Picasso trial. Adv Ther. 2014;31:333–344. doi: 10.1007/s12325-014-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobrie G., Investigators I.-A.S. I-add study: assessment of efficacy and safety profile of irbesartan/amlodipine fixed-dose combination therapy compared with irbesartan monotherapy in hypertensive patients uncontrolled with irbesartan 150 mg monotherapy: a multicenter, phase III, prospective, randomized, open-label with blinded-end point evaluation study. Clin Ther. 2012;34:1720–1734. doi: 10.1016/j.clinthera.2012.07.001. e1723. [DOI] [PubMed] [Google Scholar]
- 36.Gakidou E., Afshin A., Abajobir A.A. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marczak L., Williams J., Loeffler M. for the For the Institute for Health M, Evaluation. Global deaths attributable to high systolic blood pressure, 1990-2016. JAMA. 2018;319:2163. doi: 10.1001/jama.2018.5119. [DOI] [PubMed] [Google Scholar]
- 38.Feldman R.D., Zou G.Y., Vandervoort M.K. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. doi: 10.1161/HYPERTENSIONAHA.108.123455. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson J.C., Hingorani A.D., Ashby M.J. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet. 1999;353:2008–2013. doi: 10.1016/s0140-6736(98)07614-4. [DOI] [PubMed] [Google Scholar]
- 40.Keenan K., Hayen A., Neal B.C. Long term monitoring in patients receiving treatment to lower blood pressure: analysis of data from placebo controlled randomised controlled trial. BMJ. 2009;338:b1492. doi: 10.1136/bmj.b1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta A.K., Arshad S., Poulter N.R. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 42.Kizilirmak P., Uresin Y., Yildiz O.B. The efficacy and safety of triple vs dual combination of angiotensin II receptor blocker and calcium channel blocker and diuretic: a systematic review and meta-analysis. J Clin Hypertens. 2013;15:193–200. doi: 10.1111/jch.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de la Sierra A., Barrios V. Blood pressure control with angiotensin receptor blocker-based three-drug combinations: key trials. Adv Ther. 2012;29:401–415. doi: 10.1007/s12325-012-0019-7. [DOI] [PubMed] [Google Scholar]
- 44.Webster R., Salam A., de Silva H. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018;320:566–579. doi: 10.1001/jama.2018.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lung T., Jan S., de Silva H.A. Fixed-combination, low-dose, triple-pill antihypertensive medication versus usual care in patients with mild-to-moderate hypertension in Sri Lanka: a within-trial and modelled economic evaluation of the triumph trial. Lancet Glob Health. 2019;7:e1359–e1366. doi: 10.1016/S2214-109X(19)30343-2. [DOI] [PubMed] [Google Scholar]