ABSTRACT
BACKGROUND:
Facial nerve weakness is the most common and most concerning complication after parotidectomy. Risk factors for this complication following surgery for benign diseases remain controversial.
OBJECTIVE:
Review the frequency and prognosis of facial nerve weakness after parotidectomy and analyze potential risk factors.
DESIGN:
Retrospective review of medical records.
SETTINGS:
Two tertiary care centers.
PATIENTS AND METHODS:
We included all parotidectomies performed for benign diseases from January 2006 to December 2018. Details about the development and recovery of postoperative facial weakness were recorded. Patient, disease and surgery-related variables were analyzed using bivariate and multivariate analyses to identify risk factors.
MAIN OUTCOME MEASURES:
Frequency, recovery rates and risk factors for facial nerve weakness
SAMPLE SIZE:
191 parotidectomies, 183 patients, 61 patients with facial weakness.
RESULTS:
The frequency of postoperative facial weakness was 31.9% (61/191 parotidectomies). Among patients with temporary weakness, 90% regained normal facial movement within 6 months. Steroid therapy was not associated with a faster recovery. Postoperative weakness was not associated with age, diabetes, smoking, disease location, use of an intraoperative facial nerve monitor or direction of facial nerve dissection. Risk factors for temporary weakness were total parotidectomy and surgical specimens larger than 60 cubic centimeters. Revision surgery was the only identified risk factor for permanent weakness.
CONCLUSION:
Larger parotid resections increase the risk of temporary facial nerve weakness while permanent weakness is mainly influenced by previous surgeries.
LIMITATIONS:
Retrospective nature, underpowered sample size, selection bias associated with tertiary care cases.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Parotid surgery is indicated for multiple clinical reasons such as benign and malignant tumors, chronic parotitis, and sialolithiasis.1 Most parotid tumors are located in the superficial lobe, with the majority (80%) being benign.2 Complete removal of the disease while maximally preserving facial nerve function remains the main goal of parotid surgery.3 The complications of parotidectomy are well documented, with facial nerve weakness being the most common. Other less common complications include salivary fistulas and sialocele formation and recurrence.4,5 Additionally, parotid surgery can result in long-term sequelae such as Frey's Syndrome (gustatory sweating) and hypoesthesia in the distribution of the greater auricular nerve.4,5 Nevertheless, facial nerve weakness remains the most concerning complication for both patients and surgeons. This devastating complication can affect eye function, appearance, and quality of life.6,7 The reported rate of temporary weakness ranges from 9.3% to 64.6%, while permanent weakness occurs following 0–8% of parotidectomies.4,5,8–12 Malignancy is well established as a risk factor; however, the risk factors for facial nerve weakness following parotidectomy for benign disease remain controversial.11 Old age, diabetes, sialadenitis, large tumors, deep lobe involvement, larger resections, revision surgery, and long operative duration have all been reported as risk factors.1,5,9,10 We conducted this study to review the frequency and prognosis of facial nerve weakness following surgery for benign parotid disease and identify risk factors in our population.
PATIENTS AND METHODS
Following institutional review board approval, we conducted a retrospective review of the medical records of all patients who underwent parotid surgery in two tertiary centers (King Abdulaziz University Hospital and King Fahad Medical City) in Riyadh, Saudi Arabia, from January 2006 to December 2018. We included patients of all ages. Patients who had malignant parotid disease, preoperative facial nerve weakness, or intended facial nerve resection were excluded. The patient-related variables that were recorded included age, gender, body mass index (BMI), diagnosis of diabetes, smoking, and previous parotid surgery. Disease-related variables comprised location and histological diagnosis. Surgery-related variables included side, direction of facial nerve dissection (antegrade vs. retrograde), extent of surgery, volume of surgical specimen, surgery duration, and use of intraoperative facial nerve monitoring (IFNM).
Superficial parotidectomy was defined as the complete dissection of the facial nerve main trunk and branches and removal of the entire superficial lobe. When only a portion of the superficial lobe was removed, it was recorded as a partial superficial parotidectomy. Total parotidectomy was defined as the removal of both superficial and deep parotid lobes. Extracapsular dissection defines the removal of a tumor with a cuff of normal surrounding tissue without prior dissection of the facial nerve. Enucleation was defined as removal of the tumor only without surrounding tissue.13
The development of facial nerve weakness after surgery was recorded as involving the upper division if it was affecting eyebrow elevation or eye closure and the lower division if affecting mouth closure or lip symmetry. The grade of weakness was recorded using the House-Brackmann (H-B) grading scale.14 When weakness resolved completely upon follow up, it was considered temporary, while permanent weakness was defined as the failure to regain normal movement within 12 months. The use of steroid therapy and time-to-resolution (TTR) for temporary weakness were recorded and analyzed. Patients who developed facial nerve weakness but had inadequate follow up were excluded from the subgroup analysis.
The analysis was executed using IBM SPSS for Windows, version 23.0. Descriptive statistical data are presented as the mean and standard deviation (SD), and frequencies and percentages, or the median (interquartile range and minimum and maximum values). Depending on the variable analyzed, the t test, Mann-Whitney U test, or chi-square test were used at the bivariate level of analysis to compare subgroups and identify associations with facial weakness. Significant factors based on the bivariate analysis were selected for multivariate analysis using multiple logistic regression and reported as odds ratios with 95% confidence intervals. P values <.05 were considered statistically significant.
RESULTS
One hundred and ninety-one parotidectomies were performed on 183 patients over the study period. There were 92 females and 91 males with a median follow up (IQR, range) of 10.0 (21, 0–125) months. Pleomorphic adenoma comprised the most common pathological diagnosis (51.8%), followed by Warthin's tumor (23.6%) (Table 1). The most common type of parotidectomy was superficial parotidectomy (73.3%). The remaining were total parotidectomy (13.6%), partial superficial parotidectomy (8.4%), extracapsular dissection (4.2%), and enucleation (0.5%). The majority of the surgeries were primary (94.2%). The surgical duration ranged from 1 to 8.2 hours. IFNM was used in 21.5% of parotidectomies. Most facial nerve dissections were carried out in an antegrade fashion (80.1%). The median specimen volume was 34.6 cubic centimeters (Table 2).
Table 1.
Final pathological diagnosis of surgical specimens (n=191 parotidectomies).
| Chronic sialadenitis | 5 (2.6) |
| Stone (sialolithiasis) | 3 (1.6) |
| Benign cyst | 16 (8.4) |
| Pleomorphic adenoma | 99 (51.8) |
| Warthin's tumor | 45 (23.6) |
| Basal cell adenoma | 4 (2.1) |
| Lipoma | 4 (2.1) |
| Benign lymphadenopathy | 5 (2.6) |
| Other rare benign tumors | 10 (5.2) |
Data are number (%).
Table 2.
Patient- and surgery-related variables.
| Patient-related variable (n=183 patients) | |
| Age (years) | 40.7 (14.3, 2-86) |
| <18 | 10 (5.5) |
| 18 – 60 | 160 (87.4) |
| > 60 | 13 (7.1) |
| Gender | |
| Male | 91 (49.7) |
| Female | 92 (50.3) |
| Body mass index (kg/m2)(median, IQR, range)a | 29 (11-148) |
| ≤ 25 | 55 (30.2) |
| >25 | 127 (69.8) |
| Current smoker | |
| Yes | 59 (32.2) |
| Diabetic | |
| Yes | 28 (15.3) |
| Surgery-related variables (n=191 parotidectomies) | |
| Side of parotid surgery | |
| Right | 100 (52.4) |
| Left | 91 (47.6) |
| Surgery | |
| Primary surgery | 180 (94.2) |
| Revision surgery | 11 (5.8) |
| Use of Intraoperative facial nerve monitor | |
| Yes | 41 (21.5) |
| Deep lobe involvement | |
| Yes | 20 (10.5) |
| Extent of surgery | |
| Enucleation | 1 (0.5) |
| Extracapsular dissection | 8 (4.2) |
| Partial superficial parotidectomy | 16 (8.4) |
| Superficial parotidectomy | 140 (73.3) |
| Total parotidectomy | 26 (13.6) |
| Duration of surgery (hours)b | 3.0 (1.4, 0.125-8.2) |
| Direction of facial nerve dissectionc | |
| Antegrade | 153 (80.1) |
| Retrograde | 33 (17.3) |
| Postoperative facial weakness | |
| Temporary | 46 (24.1) |
| Permanent | 6 (3.1) |
| Undetermined due to short follow-up | 9 (4.7) |
| Duration of follow up (months) median (IQR, range) | 10.0 (21, 0-125) |
| Specimen volume (cc)d(median, range) | 34.6 (41.9, 3-512) |
Data are number (%), mean (standard deviation and range) unless otherwise noted.
Missing in 1 patient;
Missing in 5 cases;
Missing in 5 cases;
Missing in 4 cases.
Postoperative facial weakness
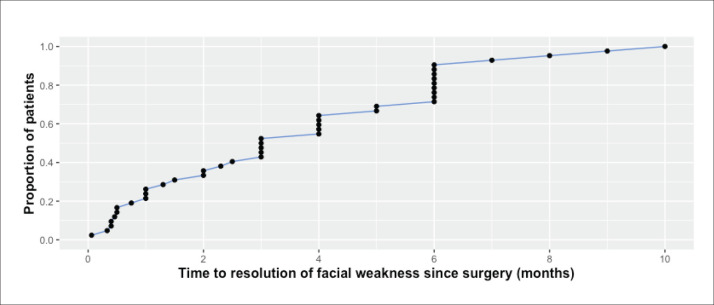
Sixty-one patients developed postoperative facial nerve weakness with an overall frequency of 31.9% (61/191 parotidectomies). None had weakness preoperatively. Forty-six patients had temporary weakness and six patients had permanent weakness, with an overall frequency of 24% and 3.1%, respectively. In the remaining nine patients, the outcome of facial weakness could not be determined due to short follow up (<12 months). Overall, lower division weakness was most common (59%), followed by weakness of both upper and lower divisions (29.5%). Isolated upper division weakness was the least common (11.4%). Most temporary weaknesses (80.4%) were H-B grade III or lower, while most permanent weaknesses (83%) were H-B grades IV–VI (Table 3). Excluding four patients whose exact TTR was not recorded, the median TTR for temporary weakness (n=42) was 3.0 months (interquartile range 4.9, range 0.06–10.0) (Figure 1). Among the remaining 42 patients, 26.2%, 52.4%, 90%, and 100% regained normal facial movement within 1, 3, 6, and 10 months, respectively. Four patients with temporary weakness had no record for TTR and thus were excluded from this specific analysis.
Table 3.
Descriptive analysis of facial weakness subgroups.
| Total (n=61) | Temporary weakness (n=46) | Permanent weakness (n=6) | Undetermined due to short follow up (n=9) | |
|---|---|---|---|---|
| Division | ||||
| Upper division | 7 (11.4) | 4 (8.7) | 0 | 3 (33.3) |
| Lower division | 36 (59.0) | 26 (56.5) | 4 (66.7) | 6 (66.7) |
| Both divisions | 18 (29.5) | 16 (34.8) | 2 (33.3) | 0 |
| Grade of weakness | ||||
| II | 31 (50.8) | 23 (52.3) | 0 | 8 (88.9) |
| III | 15 (24.5) | 14 (31.8) | 1 (16.7) | 0 |
| IV | 8 (13.1) | 6 (13.6) | 1 (16.7) | 1 (11.1) |
| V | 3 (4.9) | 1 (2.3) | 2 (33.3) | 0 |
| VI | 2 (3.2) | 0 | 2 (33.3) | 0 |
| Steroid therapy | ||||
| No | 31 (50.8) | 23 (50.0) | 4 (66.7) | 4 (44.4) |
| Yes | 30 (46.2) | 23 (50.0) | 2 (33.3) | 5 (55.6) |
Data are number (%)
Figure 1.
Time course for resolution of temporary facial weakness.
Specimen volume was highly skewed and not uniformly distributed as assessed by the Shapiro Wilk test (P<.01). The Mann-Whitney U test showed that specimen volume was higher for those who had temporary facial weakness compared those who had no facial weakness (P=.007). There was no significant difference in specimen volume for patients with permanent facial weakness vs those who had no facial weakness (P=.533).
There was no association with other continuous variables such as age, BMI, and duration of surgery. The bivariate analysis of categorical variables (Table 4) revealed that temporary weakness was significantly associated with total parotidectomy (TP) (χ2=9.737, df=1, P=.002) and specimen volume >60 cc (χ2=7.364, df=1, P=.007). Permanent weakness was significantly associated with total parotidectomy (P=.02) and revision surgery (χ2=22.067, df=1, P<.001). There was no difference between those who had superficial parotidectomy and those who had extracapsular dissection or partial superficial parotidectomy (χ2=2.941, df=1, P=.086). Postoperative weakness was not associated with different age groups, gender, diabetes, smoking, deep lobe involvement, use of IFNM, or direction of facial nerve dissection. The multivariate analysis demonstrated that total parotidectomy (OR 3.2, 95% CI 1.2–8.7, P=.02) and specimen volume >60 cc (OR 2.2, 95% CI 1–4.8, P=.04) were risk factors for temporary weakness. For permanent weakness, only revision surgery (OR 17.7, 95% CI 2.4–123.9, P=.01) was identified as a risk factor (Table 5).
Table 4.
Bivariate analysis of factors associated with postoperative facial weakness (includes all patients who developed facial weakness regardless of its final outcome).
| Postoperative facial weakness (n=61) | P valuea | Temporary weakness (n=46) | P valueb | Permanent weakness (n=6) | P valuec | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <18 | 4 (40.0) | .650 | 3 (33.3) | .703 | 1 (14.3) | .329 |
| 18-60 | 53 (32.3) | 40 (26.5) | 5 (4.3) | |||
| >60 | 4 (23.5) | 3 (18.8) | - | |||
| Sex | ||||||
| Male | 28 (28.6) | .306 | 19 (21.3) | .144 | 4 (5.4) | .380 |
| Female | 33 (35.5) | 27 (31.0) | 2 (3.2) | |||
| Body mass index (kg/m2) | ||||||
| ≤25 | 17 (30.4) | .739 | 13 (25.0) | .802 | 3 (7.1) | .307 |
| >25 | 44 (32.8) | 33 (26.8) | 3 (3.2) | |||
| Current smoker | ||||||
| No | 40 (32.3) | .897 | 31 (27.0) | .734 | 4 (4.5) | .918 |
| Yes | 21 (31.3) | 15 (24.6) | 2 (4.2) | |||
| Diabetic | ||||||
| No | 50 (31.2) | .644 | 36 (24.7) | .325 | 5 (4.3) | .932 |
| Yes | 11 (35.5) | 10 (33.3) | 1 (4.8) | |||
| Side of parotid surgery | ||||||
| Right | 30 (30.0) | .641 | 23 (24.7) | .653 | 4 (5.4) | .538 |
| Left | 31 (34.1) | 23 (27.7) | 2 (3.2) | |||
| Surgery | ||||||
| Primary | 55 (30.6) | .098 | 44 (26.0) | .881 | 3 (2.3) | <.001 |
| Revision | 6 (54.5) | 2 (28.6) | 3 (37.5) | |||
| Use of intraoperative facial nerve monitor | ||||||
| No | 49 (32.7) | .679 | 37 (26.8) | .698 | 6 (5.6) | .192 |
| Yes | 12 (29.3) | 9 (23.7) | - | |||
| Extent of surgery | ||||||
| Less than total parotidectomy | 44 (26.7) | <.001 | 35 (22.4) | .002 | 4 (3.2) | .020 |
| Total parotidectomy | 17 (65.4) | 11 (55.0) | 2 (18.2) | |||
| Extent of surgery | ||||||
| Extracapsular dissection + partial superficial parotidectomy | 3 (12.5) | .086 | 2 (8.7) | .084 | 1 (4.5) | .699 |
| Superficial parotidectomy | 41 (29.3) | 33 (25.0) | 3 (2.9) | |||
| Direction of facial nerve dissection | ||||||
| Antegrade | 54 (35.3) | .154 | 40 (28.8) | .313 | 5 (4.8) | .895 |
| Retrograde | 6 (18.2) | 5 (15.6) | 1 (3.6) | |||
| Deep lobe involvement | ||||||
| No | 52 (30.4) | .185 | 40 (25.2) | .366 | 5 (4.0) | .480 |
| Yes | 9 (45.0) | 6 (35.3) | 1 (8.3) | |||
| Concurrent sialadenitis in final pathology | ||||||
| No | 51 (31.3) | .553 | 40 (26.3) | .981 | 4 (3.4) | .165 |
| Yes | 10 (37.0) | 6 (26.1) | 2 (10.5) | |||
| Specimen volume | ||||||
| Less than 60 cc | 36 (26.1) | .001 | 28 (21.5) | .007 | 3 (2.9) | .066 |
| 60 cc and above | 25 (51.0) | 18 (42.9) | 3 (11.1) | |||
Data are number (%). Statistical comparisons:
Development of any postoperative facial weakness vs none;
Development of temporary facial weakness vs none,
Development of permanent facial weakness vs none.
Table 5.
Multivariate analysis of the risk factors for temporary and permanent facial weakness.
| Variable | Temporary facial weakness (n=46)a | Permanent facial weakness (n=6)b | ||||
|---|---|---|---|---|---|---|
| P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | |
| Revision surgery | 0.01 | 17.1 | 2.4-123.9 | |||
| Total parotidectomy | 0.02 | 3.2 | 1.2-8.7 | 0.28 | 3.5 | 0.3-34.9 |
| Specimen Volume (cc) >60 | 0.04 | 2.2 | 1.0-4.8 | 0.57 | 1.8 | 0.2-14.0 |
Dependent variables are temporary weakness vs no weakness (left) and permanent weakness vs no weakness (right).
Omnibus χ2(2)=12.11, P=.002; R2=.068;
Omnibus χ2(3)=11.92, P=.008; R2=.086.
DISCUSSION
In the present study, the rates of temporary and permanent postoperative facial weakness were comparable to those in other studies.11,12 Furthermore, steroid therapy did not hasten recovery of temporary weakness. This finding is supported by a previous randomized trial.15 In addition, Tung et al also found that the use of postoperative steroids did not reduce the rate of permanent weakness.10 Interestingly, in their multivariate analysis, they found that a weakness grade greater than H-B III was a poor prognostic factor for recovery, which is reflected in our finding that most temporary weaknesses (80.4%) were H-B III or lower, while most permanent weaknesses (83%) were greater than H-B III (Table 3).
Our analysis showed that total parotidectomy and larger specimen volumes were risk factors for temporary weakness. This confirms findings from previous studies on the extent of surgery as an important predictor of postoperative facial function.8,12 Over the past decade, multiple publications have suggested that limited parotidectomy techniques, such as extracapsular dissection and partial superficial parotidectomy, do not lead to higher recurrence rates while reducing the postoperative weakness associated with complete superficial parotidectomy.13,16 However, owing to the retrospective nature of and selection bias associated with these publications and the lack of randomized trials, the optimal surgical approach for benign parotid disease remains controversial.16 One potential disadvantage of extracapsular dissection is that it may turn out to be insufficient in cases where the final pathology unexpectedly reveals a malignancy, thereby exposing patients to reoperation or the addition of radiotherapy that could have otherwise been avoided had they had a complete superficial parotidectomy.17 In our study, the frequency of facial weakness was higher in those who had superficial parotidectomy (29.3%) as compared to those who had partial superficial parotidectomy or extracapsular dissection (12.5%). However, this difference did not reach statistical significance (P=.086). This could be due to the percentage of these approaches in our centers being smaller than that in the study by Orabona et al (12.6% vs 76%) which showed a significant difference.16 Furthermore, there was no association between weakness and the direction of facial nerve dissection. Similarly, Stankovic et al found no difference in rates of facial weakness between antegrade and retrograde dissections.18
Among the patient factors, only previous parotid surgery was a significant risk factor as revision surgery significantly increased the risk of permanent weakness (OR=17.7). This finding is consistent with previous studies.3,12 Nevertheless, Guntinas-Lichius et al studied 610 cases and found that age older than 70 years in addition to surgery duration >260 minutes were risk factors for temporary weakness.19 We did not find these factors to be significantly associated in our study. This could be attributed to our smaller sample size (Type II error). In addition, postoperative weakness was not associated with the presence of concurrent sialadenitis in the final pathology. In contrast, sialadenitis significantly increased the risk for postoperative weakness in a study of 162 cases by Nouraei et al.4
The benefit of IFNM is a topic of debate. Two prospective trials have found no benefit in reducing the incidence of postoperative facial weakness.20,21 This is in line with our finding. Nevertheless, in the recent randomized trial carried out by Graciano et al, non-monitored patients had significantly more severe weakness.21 Furthermore, a meta-analysis of 546 patients by Sood et al concluded that IFNM decreases the risk of temporary weakness but does not appear to influence the incidence of permanent facial nerve weakness.22
In revision surgery, where the facial nerve is more difficult to identify and dissect due to scarring and fibrosis, the use of IFNM may be more useful. The rate of immediate weakness in reoperation cases was reported to be statistically lower in the monitored cases.23 However, in a study of revision parotidectomies by Makeieff et al, IFNM did not impact the rate of immediate postoperative facial nerve weakness, but significantly reduced the duration of surgery as well as the degree and the recovery time of weakness.24 Similar results were reported by Liu et al for revision total parotidectomies, but not for revision superficial parotidectomies.25
Generally, parotidectomy for benign diseases have a small impact on general and symptom-specific quality of life (QoL).6 Facial nerve weakness is the most important complication in the early period and affects QoL the most. However, the vast majority recover over time.7 Complications that are less likely to diminish with time are considered more important problems in the long term. Studies on QoL in parotidectomy patients have shown that Frey's Syndrome, hypoesthesia in the distribution of greater auricular nerve, facial contour asymmetry due to loss of parotid tissue and fear from reoperation have more impact on QoL in the long term (>12 months).6,7
Finally, our study is retrospective, and we recognize it may be limited by documentation and prone to subjective bias. Nine patients with facial weakness (14%) were excluded when we analyzed for temporary or permanent weakness due to inadequate follow up, and this could have led to a slight difference in the reported frequency of each subgroup. Furthermore, we used an underpowered sample size that may have introduced a type II statistical error and therefore failed to show significant association with factors identified in larger studies. The availability of only six cases with permanent facial weakness may have limited the validity of the multivariate analysis. In addition, the data were collected from tertiary centers, thus selection bias should be considered.
In conclusion, the overall frequency for facial weakness after surgery for benign parotid diseases was 31.9%. Total parotidectomy and larger resections were risk factors for temporary facial weakness. Only revision parotidectomy significantly increased the risk of permanent facial weakness.
Funding Statement
None.
REFERENCES
- 1.Kim BD, Lim S, Wood J, Samant S, Ver Halen JP, Kim JY.. Predictors of adverse events after parotidectomy: a review of 2919 cases. Ann Otol Rhinol Laryngol. 2015;124(1):35–44. [DOI] [PubMed] [Google Scholar]
- 2.Eneroth CM. Incidence and prognosis of salivary-gland tumours at different sites. A study of parotid, submandibular and palatal tumours in 2632 patients. Acta oto-laryngologica Supplementum. 1969;263:174–8. [DOI] [PubMed] [Google Scholar]
- 3.Mehle ME, Kraus DH, Wood BG, Benninger MS, Eliachar I, Levine HL, et al.. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103(4 Pt 1):386–8. [DOI] [PubMed] [Google Scholar]
- 4.Nouraei SA, Ismail Y, Ferguson MS, McLean NR, Milner RH, Thomson PJ, et al.. Analysis of complications following surgical treatment of benign parotid disease. ANZ J Surg. 2008;78(3):134–8. [DOI] [PubMed] [Google Scholar]
- 5.Ruohoalho J, Makitie AA, Aro K, Atula T, Haapaniemi A, Keski-Santti H, et al.. Complications after surgery for benign parotid gland neoplasms: A prospective cohort study. Head & neck. 2017;39(1):170–6. [DOI] [PubMed] [Google Scholar]
- 6.Ciuman RR, Oels W, Jaussi R, Dost P.. Outcome, general, and symptom-specific quality of life after various types of parotid resection. Laryngoscope. 2012;122(6):1254–1261. [DOI] [PubMed] [Google Scholar]
- 7.Kaya BV, Kılıç C, Özlügedik S, Tuncel Ü, Cömert E.. Long-term effects of parotidectomy. Eur Arch Otorhinolaryngol. 2016;273(12):4579–4583. [DOI] [PubMed] [Google Scholar]
- 8.Marchese-Ragona R, De Filippis C, Marioni G, Staffieri A.. Treatment of complications of parotid gland surgery. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2005;25(3):174–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Henney SE, Brown R, Phillips D.. Parotidectomy: the timing of post-operative complications. Eur Arch Otorhinolaryngol. 2010;267(1):131–5. [DOI] [PubMed] [Google Scholar]
- 10.Tung BK, Chu PY, Tai SK, Wang YF, Tsai TL, Lee TL, et al.. Predictors and timing of recovery in patients with immediate facial nerve dysfunction after parotidectomy. Head & neck. 2014;36(2):247–51. [DOI] [PubMed] [Google Scholar]
- 11.Sethi N, Tay PH, Scally A, Sood S.. Stratifying the risk of facial nerve palsy after benign parotid surgery. J Laryngol Otol. 2014;128(2):159–62. [DOI] [PubMed] [Google Scholar]
- 12.Guntinas-Lichius O, Klussmann JP, Wittekindt C, Stennert E.. Parotidectomy for benign parotid disease at a university teaching hospital: outcome of 963 operations. Laryngoscope. 2006;116(4):534–40. [DOI] [PubMed] [Google Scholar]
- 13.Tweedie DJ, Jacob A.. Surgery of the parotid gland: evolution of techniques, nomenclature and a revised classification system. Clinical otolaryngology : official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34(4):303–8. [DOI] [PubMed] [Google Scholar]
- 14.House JW, Brackmann DE.. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–7. [DOI] [PubMed] [Google Scholar]
- 15.Roh JL, Park CI.. A prospective, randomized trial for use of prednisolone in patients with facial nerve paralysis after parotidectomy. American journal of surgery. 2008;196(5):746–50. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Aversana Orabona G, Bonavolonta P, Iaconetta G, Forte R, Califano L.. Surgical management of benign tumors of the parotid gland: extracapsular dissection versus superficial parotidectomy–our experience in 232 cases. J Oral Maxillofac Surg. 2013;71(2):410–3. [DOI] [PubMed] [Google Scholar]
- 17.Deschler DG. Extracapsular dissection of benign parotid tumors. JAMA Otolaryngol Head Neck Surg. 2014;140(8):770–1. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic P, Wittlinger J, Timmesfeld N, Stephan SH, Georgiew R, Gunzel T, et al.. Anterovs. retrograde nerve dissection in parotidectomy: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(6):1623–30. [DOI] [PubMed] [Google Scholar]
- 19.Guntinas-Lichius O, Gabriel B, Klussmann JP.. Risk of facial palsy and severe Frey's syndrome after conservative parotidectomy for benign disease: analysis of 610 operations. Acta Otolaryngol. 2006;126(10):1104–9. [DOI] [PubMed] [Google Scholar]
- 20.Grosheva M, Klussmann JP, Grimminger C, Wittekindt C, Beutner D, Pantel M, et al.. Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two-center trial. Laryngoscope. 2009;119(12):2299–305. [DOI] [PubMed] [Google Scholar]
- 21.Graciano AJ, Fischer CA, Coelho GV, Steck JH, Paschoal JR, Chone CT.. Facial nerve dysfunction after superficial parotidectomy with or without continuous intraoperative electromyographic neuromonitoring: a prospective randomized pilot study. Eur Arch Otorhinolaryngol. 2018;275(11):2861–8. [DOI] [PubMed] [Google Scholar]
- 22.Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB.. Facial nerve monitoring during parotidectomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;152(4):631–7. [DOI] [PubMed] [Google Scholar]
- 23.Régloix SB, Grinholtz-Haddad J, Maurin O, Genestier L, Lisan Q, Pons Y.. Facial Nerve Monitoring During Parotidectomy:A Two-Center Retrospective Study. Iran J Otorhinolaryngol. 2016;28(87):255–260. [PMC free article] [PubMed] [Google Scholar]
- 24.Makeieff M, Venail F, Cartier C, Garrel R, Crampette L, Guerrier B.. Continuous facial nerve monitoring during pleomorphic adenoma recurrence surgery. Laryngoscope. 2005;115(7):1310–1314. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Wen W, Huang H, Liang Y, Tan X, Liu S, et al.. Recurrent Pleomorphic Adenoma of the Parotid Gland: Intraoperative Facial Nerve Monitoring during Parotidectomy. Otolaryngol Head Neck Surg. 2014;151(1):87–91. [DOI] [PubMed] [Google Scholar]