Abstract
Neuropathic pain is a debilitating pathological pain condition with a great therapeutic challenge in clinical practice. Currently used analgesics produce deleterious side effects. Therefore, it is necessary to investigate alternative medicines for neuropathic pain. Chinese herbal medicines have been widely used in treating intractable pain. Compelling evidence revealed that the bioactive alkaloids of Chinese herbal medicines stand out in developing novel drugs for neuropathic pain due to multiple targets and satisfactory efficacy. In this review, we summarize the recent progress in the research of analgesic effects of 20 alkaloids components for peripheral neuropathic pain and highlight the potential underlying molecular mechanisms. We also point out the opportunities and challenges of the current studies and shed light on further in-depth pharmacological and toxicological studies of these bioactive alkaloids. In conclusion, the alkaloids hold broad prospects and have the potentials to be novel drugs for treating neuropathic pain. This review provides a theoretical basis for further applying some alkaloids in clinical trials and developing new drugs of neuropathic pain.
Keywords: Chinese herbal medicines, Alkaloids, Peripheral neuropathic pain, Analgesia
Background
Neuropathic pain is a type of chronic pain directly caused by the injuries or dysfunction of the somatosensory nervous system [1], which further triggers anxiety and depression symptoms via worsening sleep, essential daily functioning, and quality of life of the patients. Chronic neuropathic pain has aroused a severe public health concern due to its heavy burden on families and society. The population prevalence of chronic neuropathic pain has been estimated to range from 6.9 to 10% [2–4]. It is noteworthy that the incidence of neuropathic pain is likely to escalate due to the improved survival rate of cancer patients, the aging population, and the aggressively growing incidence of diabetes mellitus.
Generally, neuropathic pain is further subdivided into central and peripheral neuropathic pain. Central neuropathic pain includes central lesions [5] and diseases (e.g., stroke [6], multiple sclerosis [7]), whereas peripheral nerve injuries or pathological changes induce peripheral neuropathic pain [8]. Besides, chemotherapy drugs- and diabetes-induced neuropathy are usually classified as peripheral neuropathic pain [9, 10]. So far, there has not been enough evidence that interventional management is safe and effective for neuropathic pain. Hence, Drug treatment remains a common route for pain relief. The first-line medication recommended by the International Association for the Study of Pain (IASP) includes pregabalin, gabapentin, and tricyclic antidepressants (TCAs), and topical application of lidocaine can relieve pain conditions in 30–50% of the patients [11–13]. However, some clinical overviews reported that review articles and guidelines tend to overstate gabapentin effectiveness [14]. More importantly, those agents generally are accompanied by serious side effects, such as cardiovascular events, sedation, and syncope [15]. A meta-analysis showed that pregabalin significantly increased the risks of adverse events (e.g., somnolence, dizziness, peripheral edema, visual disturbances, ataxia, and euphoria) [16]. Morphine, with the most satisfactory analgesic effect, shall be restricted in the routine clinical management of neuropathic pain due to its abuse risks and the shortcomings of analgesic tolerance [17, 18].
With the difficulties of finding new compounds and the safety-related drug recall, which makes the approval of new analgesic drugs more conservative, the global pharmaceutical industry is currently experiencing a new drug crisis of lacking of promising drug candidates, especially heavyweight drugs. Natural products remain important drug candidates in the development of novel medicines for neuropathic pain [19]. The increasing demand for alternative therapies, such as bioactive components with effective and safe antinociceptive properties in treating neuropathic pain, has been growing throughout the world [20–23]. The alkaloids derived from Chinese herbal medicines, as valuable sources of pharmaceutical products and leading compounds, have been of great significance in the research and development of anti-neuropathic pain drugs. More and more studies have demonstrated that low-dose alkaloids possess potential analgesic effects in various neuropathic pains models [24–30]. The present review focuses on the alkaloids, mainly quinolizidine alkaloids, isoquinoline alkaloids, indole alkaloids, diterpenoid alkaloids, and their analgesic effects on peripheral neuropathic pain.
Alkaloids chemical structure, classification, and sources of Chinese herbal medicines
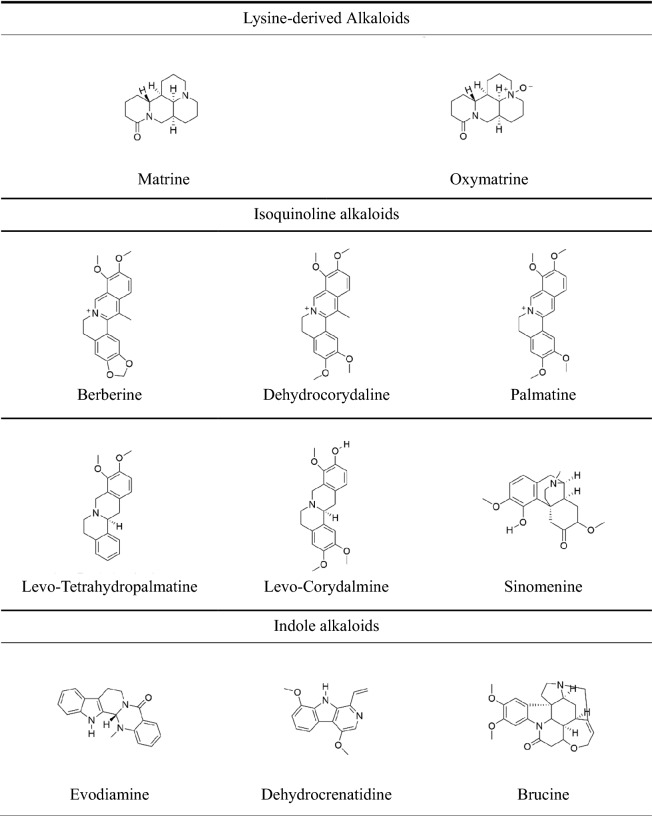
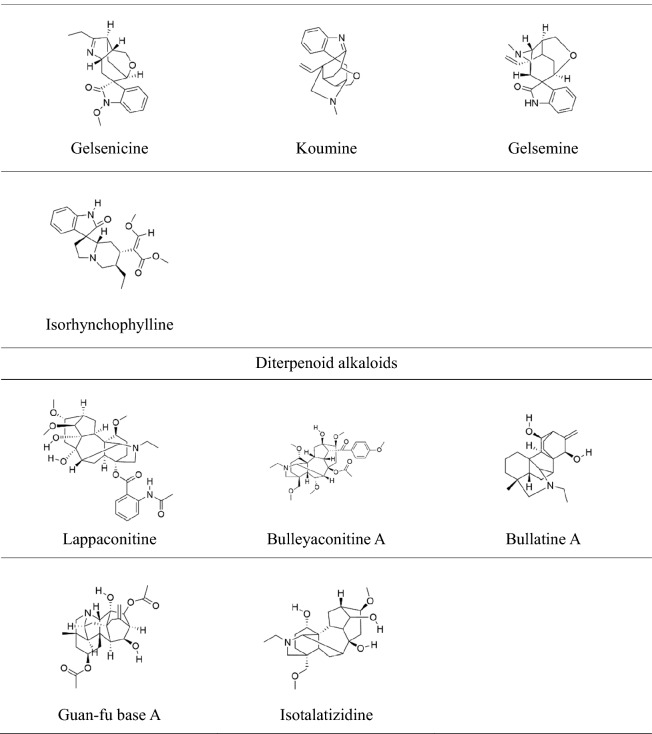
Alkaloids are the largest class of organic compounds containing nitrogen atom with the homophylic properties of alkali [31]. Many classic analgesics, such as morphine, codeine, and aspirin, are isolated from natural products. Therefore, the studies on the analgesic effect and mechanisms of active components and the discoveries of new analgesic drugs from plants provide grounds for innovative researches on analgesic drugs. Upon a literature survey, we identified 20 compounds with significant analgesic activities on peripheral neuropathic pain. The structures of these compounds are shown in Table 1. A compound may exist in various Chinese herbal medicines, and we have summarized all its sources (Table 2).
Table 1.
Chemical structures formula of different sub-groups of alkaloids
Table 2.
Alkaloids with analgesic activity isolated from Chinese herbal medicines
| Alkaloids Sub-groups | Example | CAS | Molecular formula | Sources | Latin name | References |
|---|---|---|---|---|---|---|
| Quinolizidine alkaloids | Matrine | 519-02-8 | C15H24N2O | Baicihua | Sophora davidii | [156] |
| Kudouzi | Sophora alopecuroides | [156–158] | ||||
| Kushen | Sophora flavescens | [156, 159] | ||||
| Shandougen | Sophora subprostrata | [156] | ||||
| Oxymatrine | 16837-52-8 | C15H24N2O2 | Baicihua | Sophora davidii | [156] | |
| Kudouzi | Sophora alopecuroides | [157, 158] | ||||
| Kushen | Sophora flavescens | [159] | ||||
| Shashenhuai | Sophora moorcroftiana | [156] | ||||
| Shandougen | Sophora subprostrata | [156] | ||||
| Isoquinoline alkaloids | Berberine | 2086-83-1 | C20H18NO4+ | Baiqucai | Chelidonium majus | [160] |
| Baiyaozi | Stephania cepharantha | [160] | ||||
| Banruitangsongcao | Thalictrum petaloideum | [160] | ||||
| Huangbai | Phellodendron amurense | [161] | ||||
| Huanglian | Coptis chinensis | [162] | ||||
| Maweilian | Thalictrum foliolosum | [160] | ||||
| Yanguocao | Thalictrum minus | [160] | ||||
| Yanhusuo | Corydalis yanhusuo | [163] | ||||
| Levo-Tetrahydropalmatine | 10097-84-4 | C21H25NO4 | Yanhusuo | Corydalis yanhusuo | [156] | |
| Xiatianwu | Corydalis decumbens | [156] | ||||
| Juhuahuanglian | Corydalis pallida | [156] | ||||
| Chibanyanhusuo | Corydalis remota | [164] | ||||
| Sinomenine | 115-53-7 | C19H23NO4 | Bianfugegen | Menispermum dauricum | [160] | |
| Qingfengteng | Sinomenium acutum | [160, 165] | ||||
| Dehydrocorydaline | 30045-16-0 | C22H24NO4+ | Yanhusuo | Corydalis yanhusuo | [160] | |
| Levo-Corydalmine | 30413-84-4 | C20H23NO4 | Yanhusuo | Corydalis yanhusuo | [160] | |
| Palmatine | 3486–67-7 | C21H22NO4+ | Banruitangsongcao | Thalictrum petaloideum | [164] | |
| Yanguocao | Thalictrum minus | [164] | ||||
| Huangbai | Phellodendron amurense | [164] | ||||
| Huanglian | Coptis chinensis | [164] | ||||
| Haisongzi | Pinus koraiensis | [164] | ||||
| Maweilian | Thalictrum foliolosum | [164] | ||||
| Xiatianwu | Corydalis decumbens | [164] | ||||
| Huanglian | Coptis teetoides | [164] | ||||
| Yanhusuo | Corydalis yanhusuo | [164] | ||||
| Alishanshidagonglao | Mahonia oiwakensis | [166] | ||||
| Qingniudan | Tinospora sagittata | [167] | ||||
| Indole alkaloids | Evodiamine | 518-17-2 | C19H17N3O | Wuzhuyu | Evodia rutaecarpa | [168] |
| Dehydrocrenatidine | 65236-62-6 | C15H14N2O2 | Kumu | Picrasma quassioides | [101] | |
| Brucine | 57-24-9 | C23H26N2O4 | Maqianzi | Strychnos nux-vomica | [169] | |
| Koumine | 1358-76-5 | C20H22N2O | Gouwen | Gelsemium elegans | [170] | |
| Gelsenicine | 82354-38-9 | C19H22N2O3 | Gouwen | Gelsemium elegans | [170] | |
| Gelsemine | 509-15-9 | C20H22N2O2 | Gouwen | Gelsemium elegans | [137, 170] | |
| Isorhynchophylline | 6859-01-4 | C22H28N2O4 | Gouteng | Uncaria rhynchophylla | [171] | |
| Diterpenoid alkaloids | Isotalatizidine | 7633-68-3 | C23H37NO5 | Chuanwu | Aconitum carmichaeli | [172] |
| Bulleyaconitine A | 107668-79-1 | C35H49NO9 | Caowu | Aconitum bulleyanum | [173] | |
| Bullatine A | 1354-84-3 | C21H31NO2 | Xueshangyizhihao | Aconitum brachypodum | [45] | |
| guan-fu base A | 1394-48-5 | C24H31NO6 | Guanbaifu | Aconitum coreanum | [174] | |
| Lappaconitine | 32854-75-4 | C32H44N2O8 | Ganwanwutou | Aconitum finetianum | [156] | |
| Gaowutou | Aconitum sinomontanum | [156] | ||||
| Niubian | Aconitum barbatum | [156] |
Effects of alkaloids on diabetic peripheral neuropathy (DPN)
Diabetic peripheral neuropathy (DPN) is one of the most common and refractory chronic complications of diabetes mellitus [32]. It is accompanied by burning, prickling, numbness, tingling sensation, allodynia, and hyperesthesia [33], which will affect 366 million individuals worldwide by 2030 [34]. Hyperglycemia mediated metabolic disorder is the primary pathogenesis for DPN. Hyperglycemia disturbs several metabolic pathways, such as advanced glycation end products (AGEs) [35, 36], hexosamine [37], polyol [38], protein kinase C (PKC) [39], and poly-ADP ribose polymerase (PARP) pathways [40] in the nervous system.
Koumine dose-dependently attenuated mechanical allodynia. The half-effective dose (ED50) of koumine (i.e., 0.063 mg/kg) is lower than the reported median lethal dose (LD50) of 99 mg/kg [27]. Berberine [41, 42] and palmatine [43] reduced streptozotocin (STZ)-induced mechanical allodynia and thermal hyperalgesia in a dose-dependent manner. Sinomenine [44] and Bullatine A [45] significantly upregulated the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) of STZ mice.
Neuroprotective effects of alkaloids on the sciatic nerve in DPN rodents
A study has reported that STZ could cause significant alterations in C-fibers and Aδ-fibers; alterations in these motor and sensory fibers caused a reduction in nociceptive threshold toward the mechanical and thermal receptors [46]. Also, peripheral nerve axon injuries and myelin degeneration can lead to abnormal sensory nerve conduction velocity (SNCV), which is an early characteristic of neuronal dysfunction in both diabetic neuropathies [47, 48]. SNCV directly reflects the change of axon caliber and myelin integrity induced by hyperglycemia [49], and establishes normal glycemia can be restored [47]. Berberine improved motor nerve conduction velocity (MNCV) and SNCV, which correlated with the upregulated expression of brain-derived neurotrophic factor (BDNF) and insulin-like growth factors I (IGF-I). It is reported that STZ administration caused significant downregulation in both IGF-I messenger ribonucleic acid (mRNA) and protein expression, whereas treatment with insulin significantly upregulated IGF-I mRNA in the sciatic nerve [42]. To sum up, the establishment of normal blood glucose is essential for the recovery of neurological function in diabetic mice, but berberine treatment of NP is not only through establishing normal blood glucose levels, which will be pointed out later. Interestingly, although koumine significantly improved SNCV and decreased myelinated nerve fibers' demyelination in the sciatic nerve, the blood glucose level of STZ-induced rats is not influenced [27]. The number and open characteristics of ion channels are likely to participate in which koumine restored SNCV in DPN mice [50].
Regulation of alkaloids on oxidative stress and neuroinflammation in DPN rodents
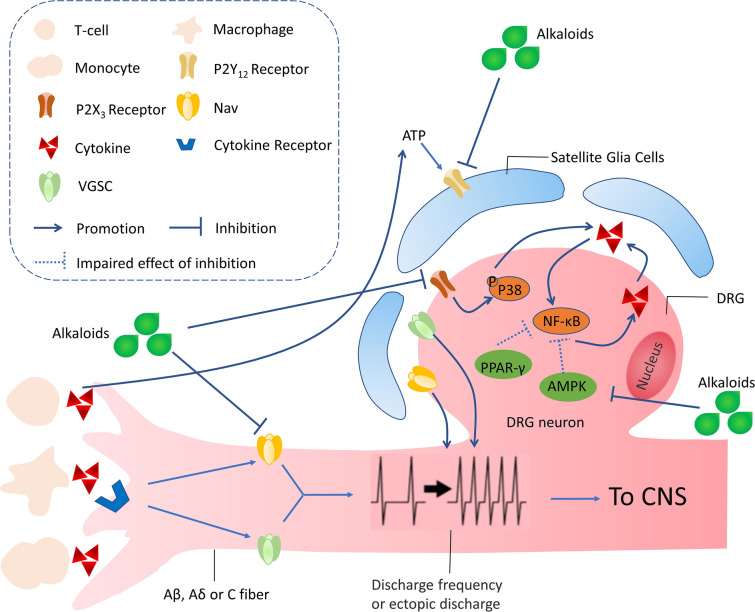
Numerous researches have confirmed that hyperglycemia activates ROS that induces oxidative stress in the nervous system [51, 52]. The mechanism that contributes to increased oxidative stress includes alteration in PKC activity of, decreases Na+K+-ATPase activity, overproduction of prostaglandin, and accumulation of AGEs [53, 54]. The 40 mg/kg dose of berberine treatment significantly reversed STZ-induced oxidative stress and Na+K+-ATPase alteration. Berberine and palmatine are isoquinoline alkaloids stem from Coptis chinensis, which have been widely used to treat intestinal infection [55]. Liu et al. [41] recently revealed that berberine suppressed STZ-induced neuropathic pain via reducing satellite glial cells (SGCs) activation mediated neuroinflammation. The 20 and 40 mg/kg doses of berberine significantly decreased tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) protein levels compared with STZ group rats. It is reported that the activation of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and peroxisome proliferator-activated receptors-γ (PPAR-γ) can reduce the release of inflammatory cytokines [56, 57]. Treatment with berberine remarkably downregulated the protein expression of phosphatase 2Cα (PP2Cα) and upregulated the expression of Thr-172 protein(the site of AMPK phosphorylation) in dorsal root ganglia (DRG) as compared with STZ control rats [42] (Fig. 1). Substantial experimental evidence has supported that the activation of P2X receptors on glial cells are involved in the occurrence and maintenance of chronic pain by stimulating the production and release of TNF-α and IL-1β [58–61]. Besides, P2X7 receptors may be related to the comorbidity of DNP and depression [62]. Palmatine treatment ameliorated the comorbidity of DNP and depression via reducing the expression of TNF-α, IL-1β mRNA, and phosphorylation of extracellular regulates protein kinases 1/2(ERK1/2) protein in the hippocampus of DPN rats [43]. ATP-induced activation of P2X3 receptors might collaborate with the phosphorylation of P38 mitogen-activated protein kinase (P38MAPK) in DRG, causing mechanical and heat hyperalgesia [63]. Sinomenine alleviated high glucose-induced DPN and reduced the mRNA expression of P2X3 and the phosphorylation P38MAPK in DRG (Fig. 1). Furthermore, a molecular docking test revealed that sinomenine had an intense binding with P2X3 receptors [44, 64].
Fig. 1.
The main pharmacological mechanisms of alkaloids on treating DPN. Alkaloids alleviate DPN via inhibiting Nav channels-mediated ectopic discharge in afferent nerve fibers, suppressing purinergic signals in SGC and DRG neurons, and decreasing P38- and NF-κB-mediated peripheral neuroinflammation in DRG
In conclusion, alkaloids attenuated diabetic neuropathy (Table 3) via restoring nerve function, inhibiting oxidative stress, and decreasing neuroinflammatory. In traditional Chinese medicine (TCM), Coptis chinensis have been employed to treat emaciation-thirst disease since ancient China. Berberine and palmatine are the main alkaloids in Coptis chinensis, which can treat many complications of STZ-induced diabetes [65, 66], including diabetic nephropathy [67]. As mentioned above, berberine and the other alkaloids had sound relieving effects on STZ-induced neuropathic pain and had a protective impact on sciatic nerves. Interestingly, berberine promoted axonal and myelin recovery by reversing the high glucose induced-downregulation of BDNF and IGF-I while restoring normal blood glucose levels in DM animals. In contrast, koumine exerted a neuroprotective effect by promoting neurosteroids in the sciatic nerve but did not affect the hyperglycemia level of diabetic animals. Similarly, a previous study that repeated koumine could significantly reduce the elevated total blood cholesterol levels in STZ-induced diabetic rats without affecting blood glucose. Thus, koumine could control cholesterol homeostasis, elevate neurosteroids levels and protect diabetic neuropathy by activating the liver X receptor [68], which may provide a potential novel therapeutic target for diabetic-induced NP. The potencies of koumine on mechanical allodynia, SNCV, and sciatic nerve morphology are different [27], which also suggests that koumine may exert anti-allodynic and neuroprotective effects by different mechanisms. The analgesic effect of berberine is a consequence of etiological treatment; in other words, berberine completely restored the hyperglycemia-induced neuropathic pain by hyperglycemia. Decreasing blood glucose can alleviate the signal activation induced by blood glucose and may be beneficial to the other complications caused by diabetes. Therefore, berberine is an excellent potential drug candidate for DPN. However, berberine should be applied at the appropriate phase of DPN, and the recovery of nerve function is most significant in the initial stage of DPN. In the future, it will be necessary to find out the hypoglycemic mechanism of berberine. Moreover, palmatine and koumine are also advantageous to diabetic-induced peripheral neuropathic pain.
Table 3.
Effects of alkaloids on diabetic painful neuropathy (DPN)
| Alkaloids | Animal/cell | Dose mg/kg (route of administration) | Effects/mechanisms of action | References | |||
|---|---|---|---|---|---|---|---|
| Behavioral evaluation | Histopathological observation | Electrophysiology parameters | Biochemical/Molecular parameters/mRNA | ||||
| Koumine | Male Sprague–Dawley rats |
0.056/0.28/1.4/7 mg/kg s.c 7 days |
→ Blood glucose and body weight ↓Streptozotocin-induced mechanical allodynia |
↑Myelin thickness, axon diameter and number of myelinated nerve fibers | ↑SNCV | – | [27] |
| Berberine | Adult male and female Sprague–Dawley rats |
10/20/40 mg/kg i.g 8 days |
↓Streptozotocin-induced mechanical allodynia and thermal hyperalgesia |
↓Necrosis, edema, inflammatory infiltration, and congestion after intraperitoneal administration of STZ in sciatic nerve ↓Split and disintegrated neurofilaments and swoln axonal mitochondria |
↑MNCV and SNCV |
Reverse Streptozotocin-induced oxido-nitrosative stress (SOD, GSH, MDA, and NO) and Na+K+-ATPase alteration ↑BDNF, IGF-1, PPAR-γ, IL-6, IL-1β and TNF-α |
[42] |
| Berberine | Male C57BL/6 mice |
The dose was not mentioned s.c 14 days |
↓Streptozotocin-induced mechanical allodynia and thermal hyperalgesia | ↓The relative Iba-1-positive area and GFAP-positive area reflected by the Iba-1 and GFAP fluorescence density after the treatment of berberine in spinal cord slices | – | ↓The expression of TNF-α, IL-6, IL-1β, iNOS and COX-2 in spinal cord and DRG | [41] |
| Sinomenine | Male Sprague–Dawley rats HEK293 cells |
40 mg/kg i.p 10 Μm(in vitro) |
↓Streptozotocin -induced thermal hyperalgesia and mechanical hyperalgesia | – | ↓ATP-activated current in HEK293 cells transfected with the pEGFP-hP2X3 plasmid |
↓p-P38MAPK and P2X3 protein in DRG ↓P2X3 mRNA in DRG |
[44, 64] |
| Palmatine | Male Sprague- Dawley rats |
30 mg/kg i.p 14 days |
↓Streptozotocin-induced mechanical hyperalgesia and thermal hyperalgesia ↓Depressive like behavior |
↓Fluorescence intensity of co-localization of P2X7 and GFAP in the hippocampus ↓Positive signal of P2X7 protein in the hippocampus |
– |
↓P2X7, TNF-α and IL-1β mRNA ↓p-ERK1/2 in the hippocampus |
[43] |
| Bullatine A | Male adult Wistar rats |
0.3/1/3/10/30 mg/kg s.c 0.3/1/3/10/30 μg i.t |
↓Mechanical allodynia and thermal hyperalgesia | – | – | – | [45] |
↑: Enhanced/Increased/Upregulate↓: Attenuate/Downregulate/Decrease/Suppress/Inhibit/Prevent
Effects of alkaloids chemotherapy-induced peripheral neuropathy (CIPN)
CIPN is one of the most common complications caused by chemotherapy agents. The typical sensory impairment of CIPN includes numbness, paresthesias, evolving spontaneous hyperalgesia and allodynia to mechanical and thermal stimuli in extremities [69], and motor symptoms such as reduced balance control and distal weakness [70]. Generally, CIPN symptoms arise after repeating chemotherapy for three or four cycles of, mainly depend on the chemotherapeutic agent that is used and the cumulative dose of these drugs; however, the onset of CIPN symptoms has also been observed immediately in some patients after chemotherapy; these symptoms may become permanent and will continue for years [70, 71]. CIPN is suspected to be a complex phenomenon resulting from the interrelation of different mechanisms. It has been observed that anticancer drugs may cause neuronal damage in various ways, such as nuclear and mitochondrial DNA damages, ion channel disturbances (i.e., calcium, sodium, and potassium), impairment of axonal transport, and inflammatory process [72–74]. The effects of alkaloids on CIPN are shown in Table 4.
Table 4.
Effects of alkaloids on chemotherapy-induced peripheral neuropathy (CIPN)
| Alkaloids | Animal/cell | Dose mg/kg (route of administration) | Effects/mechanisms of action | References | |||
|---|---|---|---|---|---|---|---|
| behavioral evaluation | histopathological observation |
Electrophysiology parameters | Biochemical/Molecular parameters/mRNA | ||||
| Berberine | Male Wistar rats |
10/20 mg/kg i.p 8 days |
→ Paclitaxel-induced heat hyperalgesia and cold allodynia |
↑Shrunken and swollen axons with myelin breakdown → Axon number |
– |
↓MDA and GPx levels in the sciatic nerve tissue ↑GSH and SOD levels and Nrf2 mRNA in the sciatic nerve tissue |
[87] |
| Evodiamine | Male Sprague–Dawley rats |
5 mg/kg i.p 3 days |
↓Paclitaxel-induced mechanical and thermal hypersensitivity | ↑IENFs | – |
↓IL-1β, IL-6, TNF-α and MCP-1 ↑PGC-1α, UCP2 and SOD2 mRNA in DRG |
[78] |
| Matrine | Male ICR mice |
15/30/60 mg/kg i.p 11 days |
↓Vincristine-induced mechanical allodynia, cold allodynia, heat hyperalgesia and mechanical hyperalgesia → Motor Coordination → SFI |
↓The loss of myelinated nerve fibers, part of the axons arranged in irregular swelling, rise in the number of vacuoles | ↑Vincristine-induced loss of SNCV and SNAP amplitudes in a dose dependent manner |
↑T-AOC, SOD, GSH-Px and TCA activity ↓MDA and MPO |
[82] |
| Bulleyaconitine A | Male Sprague–Dawley rats |
0.1/0.4/0.8 mg/kg i.g |
↓Mechanical allodynia and thermal hyperalgesia | – |
↓HFS induced LTP ↓The frequency of sEPSCs and mEPSCs in laminar II neurons → The amplitude of sEPSCs and mEPSCs in laminar II neurons |
– | [92] |
| Corydalis saxicola Bunting total alkaloids | Male Sprague–Dawley rats | 30/60/120 mg/kg i.g | ↓Cisplatin-induced mechanical allodynia, thermal hyperalgesia and cold Hyperalgesia | ↓Cisplatin-induced vacuoles, neuron shrinkage, disordered satellite cells, and generally decreased Schwann cells | – |
↓Cisplatin-induced pro-inflammation cytokines release in serum and foot supernatant of rats ↓ Cisplatin-induced p-p38 and TRPV1expression in DRG, TG, Spinal Cord and paw |
[81] |
| Levo-corydalmine |
Male ICR mice Primary astrocytes |
5/10/20 mg/kg i.g 9 days 3/10/30 μM(in vitro) |
Reversed vincristine-induced mechanical allodynia and thermal hyperalgesia | ↓Fluorescence intensity of CXCL1 and p-NF-κB | – | ↓TNF-α, IL-1β, CXCL1 and NF-κB activation in vivo and vitro | [89] |
↑: Enhanced/Increased/Upregulate↓: Attenuate/Downregulate/Decrease/Suppress/Inhibit/Prevent
Neuroprotective effects of alkaloids on intraepidermal nerve fibers and sciatic nerve in CIPN rodents
The loss of intraepidermal nerve fibers (IENFs) is primarily responsible for paclitaxel-induced neuropathic pain in rodent models [75, 76]. Moreover, prevention of the loss of IENFs can effectively ameliorate the sensory disturbance induced by paclitaxel [77]. Evodiamine ameliorated paclitaxel-induced peripheral neuropathy by attenuating IENFs injury [78]. All in all, evodiamine ameliorated paclitaxel-induced peripheral neuropathy by attenuating intraepidermal nerve fiber injuries. The mechanisms might involve the inhibition of peripheral neuroinflammation and activation of mitochondrial antioxidant functions. Corydalis Saxicola Bunting. Total Alkaloids (CSBTA) inhibit cisplatin-induced mechanical hyperalgesia, thermal hyperalgesia, and cold hyperalgesia. The significant loss of small-diameter myelinated or unmyelinated nerve fibers in the epidermis, including Aδ- and C-fibers, plays indispensable roles in cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia in early CIPN [69, 77, 79, 80]. IENFs density is increased at 60 and 120 mg/kg doses of CSBTA, which result has been further confirmed by the histopathological experiments of DRG, revealing that CSBTA ameliorates cisplatin-induced vacuoles, neuron shrinkage, disordered satellite cells, and generally decreased Schwann cells [81].
The peripheral neuropathy model induced by chemotherapy agents is also accompanied by sciatic nerve function changes, such as SNCV and SNAP. However, our previous studies showed that chemotherapy drugs' application could not significantly reduce the sciatic nerve function index (SFI) of mice [82]. Matrine reversed vinorelbine-induced the decrease of SNCV and sensory nerve action potential (SNAP) amplitudes [82], and morphological evidence further support this notion.
Regulation of alkaloids on oxidative stress in CIPN rodents
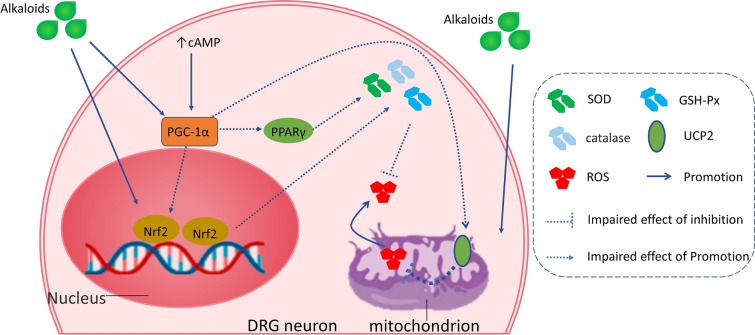
It must be considered that mammalian nerves are especially susceptible to free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), due to their high content in phospholipids and axonal mitochondrion; besides, neuronal antioxidant defenses are weak [83]. Superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) are the major components of the antioxidant system, which maintains stability in DRG by scavenging excessive free radicals [84]. Uncoupling protein 2 (UCP2) on the mitochondrial inner membrane can reduce the electrochemical proton gradient and inhibit ROS generation [85]. Peroxisome proliferator-activated receptor-gamma coactivator 1-α (PGC-1α) can regulate the antioxidant system, and PGC-1 α can increase the level of antioxidant enzymes to protect neurons from the damages of ROS while decreasing glutathione in cells [86]. The treatment of evodiamine remarkably improved paclitaxel-induced mitochondrial dysfunction, evidenced by the restoration of PGC-1α, UCP2, and manganese superoxide dismutase (MnSOD). In vitro, studies found that evodiamine prevented the paclitaxel-induced loss of mitochondrial membrane potential and PGC-1α, UCP2, and MnSOD expression in DRG cells (Fig. 2) [78]. Singh et al. [87] found that berberine was effective in ameliorating the heat hyperalgesia and cold allodynia. Morphometric analysis of sciatic nerves revealed that berberine treatment significantly increased axon diameter and myelin thickness, but the axon number did not be increased compared with the paclitaxel group. Berberine prevented the increase in malondialdehyde level, the decreased GSH, and SOD in the sciatic nerve tissue. In oxidative stress, genes encoding antioxidant defending enzymes are activated, especially the nuclear erythroid 2-related factor 2 (Nrf2) gene, which increases the expression of SOD, glutathione peroxidase (GSH-Px), and catalase genes [88]. Berberine exerted analgesic and neuroprotective effects via upregulating Nrf2 mRNA and further enhancing antioxidant capacity in the sciatic nerve [87].
Fig. 2.
The main pharmacological mechanisms of alkaloids on treating CIPN. Alkaloids alleviate DPN via inhibiting oxidative stress and neuroinflammation in DRG
Regulation of alkaloids on central and peripheral neuroinflammation in CIPN rodents
Administration of levo-corydalmine (L-CDL) combined with vincristine significantly reduced pain hypersensitivity and pro-inflammatory factors, such as TNF-α and IL-1β. In association with these changes, chemokine CXCL1 and its receptor CXCR2 were decreased by L-CDL in spinal astrocytes and neurons. Moreover, nuclear factor kappa-B (NF-κB) was involved in the production of CXCL1 in spinal astrocytes. In cultured astrocytes and primary neurons, CXCL1 was blocked by NF-κB small interfering RNA (siRNA) and was dose-dependently reduced by L-CDL, thus indirectly reducing the increase in CXCR2. (Fig. 2) [89]. Therefore, L-CDL has potential as an analgesic targeting the NF-κB-dependent CXCL1/CXCR2 signaling pathway to relieve vincristine-induced neuropathic pain. p38 is activated in DRG by peripheral inflammation and participates in the maintenance of heat hyperalgesia by regulating levels of the transient receptor potential channel family (TRPV1) [90, 91]. CSBTA also significantly reduced the expression of p38, p-p38, and TRPV1.
Repetitive administration of bulleyaconitine A (BAA) after treatment with paclitaxel produces a long-lasting inhibitory effect on thermal hyperalgesia, but not on mechanical allodynia. Consistently, the spinal synaptic transmission mediated by C-fibers but not by A-fibers is potentiated in paclitaxel-treated rats, and the effect is attenuated by either spinal or intravenous application of BAA. The frequency but not the amplitude of both spontaneous excitatory postsynaptic currents (sEPSCs) and miniature excitatory postsynaptic currents (mEPSCs) recorded in lamina II neurons was enhanced in paclitaxel-treated rats indicates that increase in presynaptic neurotransmitter release may contribute to the effect [92]. Furthermore, BAA could prevent and depress spinal LTP in both naive and paclitaxel-treated rats, but the inhibitory effect was more powerful in paclitaxel-treated animals than that in naïve animals. Treatment of BAA reduced the frequency of sEPSCs and mEPSCs in paclitaxel-treated rats but did not in naïve ones [92]. Taken together, BAA attenuated paclitaxel-induced neuropathic pain and depressed LTP at C-fiber synapses in the spinal dorsal horn, and inhibition of presynaptic transmitter release may be involved in the effect.
Effects of alkaloids on Sciatic nerve chronic constriction injury (CCI) model
CCI-induced neuropathic pain includes several symptoms, such as spontaneous pain (tingling, burning, and electric-shock like), dysesthesia, paresthesias, allodynia, and hyperalgesia [93]. In addition, CCI results in intraneural edema, which produces a more significant reduction of Aβ-fibers, and a vast majority of Aδ-fibers are axotomized while large numbers of C-fibers are intact [94].
Table 5. summarized the effects of alkaloids in alleviating CCI-induced neuropathic pain. Alkaloids matrine [95] reduced among mechanical allodynia, cold allodynia, and thermal hyperalgesia, whereas guanfu base A [96] reduced mechanical and thermal hyperalgesia. Sinomenine [97], levo-tetrahydropalmatine [98], berberine [99], brucine [100], dehydrocrenatidine [101], koumine [102] and isotalatizidine [25] reduced mechanical allodynia. Besides, brucine, koumine, gelsenicine [26], and isorhynchophylline [103] inhibited CCI-induced thermal hyperalgesia. It is reported that matrine [95], levo-tetrahydropalmatine [98], berberine [99], and sinomenine [97] could not affect the ability of spontaneous activity and motor coordination of CCI mice. In other words, these alkaloids did not induce sedation in CCI mice. Sinomenine dose-dependently reversed the increased immobility time in rats receiving CCI but did not affect the duration of immobility in the forced swimming test in healthy animals, suggesting that sinomenine attenuated chronic pain-induced depressive-like behaviors.
Table 5.
Effects of alkaloids on sciatic nerve chronic constriction injury (CCI)
| Alkaloids | Animal/cell | Dose mg/kg (route of administration) |
Effects/mechanisms of action | References | |||
|---|---|---|---|---|---|---|---|
| Behavioral evaluation | Histopathological observation |
Electrophysiology parameters | Biochemical/molecular parameters/mRNA | ||||
| Isotalatizidine |
Female C57BL/6 mice BV2 microglial cells Primary microglial cells |
0.1/0.3/1.0 mg/kg i.t 25 μM(in vitro) |
↓Mechanical allodynia |
↑Fluorescence intensity Dynorphin A in spinal cord | – |
↑p-CREB, p-ERK1/2, dynorphin A in the spinal cord tissue ↑prodynorphin mRNA |
[25] |
| Gelsenicine | Male ICR mice |
0.8/4/20 mg/kg s.c |
↓Thermal hypersensitivity | – | – | – | [26] |
| Dehydrocrenatidine |
Male Sprague–Dawley rats Primary DRG neurons |
50/150/250 µg/kg i.t |
↓Mechanical allodynia | – | ↓TTX-S and TTX-R currents in DRG | – | [101] |
| Matrine | Male ICR mice |
7.5/15/30 mg/kg i.p 8 days |
↓Mechanical allodynia, cold allodynia and thermal hyperalgesia → Motor coordination → Spontaneous locomotor |
– | – | – | [95] |
| Guanfu Base A |
Male Sprague Dawley rats HEK293 cells |
40 mg/kg i.p 14 days |
↓Mechanical and thermal hyperalgesia | ↓Co-expression values of P2Y12 and GFAP | – |
↓The expression of P2Y12 mRNA, P2Y12 protein, TNF-α and p-p38 MAPK in L4 –L6 DRGs ↓ADP-induced cAMP concentration in HEK293 cell |
[96] |
| Sinomenine | Male Sprague–Dawley rats |
10/20/40 mg/kg i.p 2 weeks |
↓Mechanical allodynia ↓Depressive like behavior ↓Locomotor coordination |
– | – | – | [97] |
| Levo-tetrahydropalmatine | Male ICR mice |
20 nmol i.p 2 nmol i.t |
↓Percentage withdrawal response frequency → Motor coordination |
– | – | ↓pNR1 in spinal dorsal horn | [98] |
| Berberine | Male Sprague–Dawley rats |
5/10/20 mg/kg i.p |
↓ Mechanical allodynia and cold allodynia Berberine does not induce sedation |
↓MPO, MDA in Sciatic nerve | [99] | ||
| Brucine | Male C57BL/6 mice | 10/30 mg/kg | ↓Thermal hypersensitivity and mechanical allodynia | – |
↓Action potential (AP) firing activity of DRG neurons ↓TTX-r and TTX-s currents |
– | [100] |
| Isorhynchophylline | Adult maleC57BL/6 J mice |
5/15/45 mg/kg i.g Twice per day for 14 days |
↓Tactile allodynia and thermal → Motor coordination ↓Depression and anxiety like behavior |
– | – |
↑5-HT, NA → 5-HIAA, DA, DOPAC ↓5-HIAA/5-HT ratio, MAO-A |
[103] |
| Oxymatrine | Male ICR mice |
40/80/160 mg/kg i.p |
↓Mechanical allodynia, cold allodynia and thermal hyperalgesia | ↓ NR2B expression in the superficial dorsal horn | – |
↓GAT-1 in spinal cord ↑GABAARα2 |
[109] |
| Oxymatrine | Male ICR mice |
40/80/160 mg/kg i.p |
↓Mechanical allodynia and thermal hyperalgesia |
↑Immunostaining positive neurons of GABAAa2 ↓Immunostaining positive neurons of GAT-1 |
– | ↓NR2B, p-ERK, p-CREB in spinal cord | [115] |
| Koumine |
Male Sprague–Dawley rats Microglial BV2 Microglia |
0.28/1.4/7 mg/kg s.c 7 days 25/50/100/200 μg/ml (in vitro) |
↓Mechanical allodynia | ↓ The fluorescence density of Iba-1, GFAP and TSPO expression in the spinal cord | – |
↓IL-1β, TNF-α and TSPO in the spinal cord ↓ LPS-induced the protein levels and the mRNA expression of M1 markers (CD86, IL-6, IL-1β, and TNF-α) in BV2 cells |
[102] |
| Koumine | Male Sprague–Dawley rats |
0.28/1.4/7 mg/kg s.c twice per day for 7 days |
↓Thermal hyperalgesia and mechanical allodynia | – | – |
↑ Allopregnanolone → pregnenolone |
[130] |
| Koumine | Male adult Sprague–Dawley rats |
0.28/1.4/7 mg/kg s.c twice per day for 7 days |
↓Hyperalgesia and allodynia | ↑Fluorescence density of 3α‑HSOR in the ipsilateral dorsal horn of the lumbar spinal cord (L5–L6) | – | ↑ The level of 3α‑HSOR mRNA and 3α‑HSOR catalytic activity in the spinal cord lumbar region L5–L6 | [131] |
↑: Enhanced/Increased/Upregulate↓: Attenuate/Downregulate/Decrease/Suppress/Inhibit/Prevent
Regulation of alkaloids on excitatory and inhibitory synaptic transmission in CCI rodents
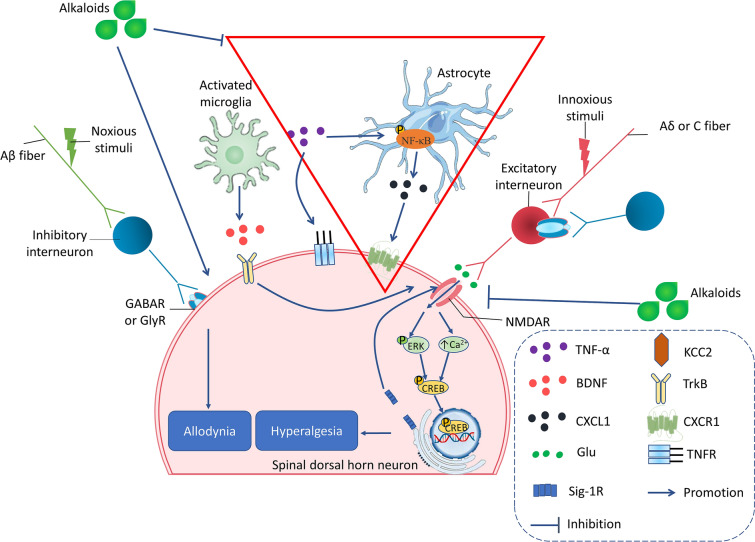
Following a nerve injury, both peripheral and central sensitization act as important pathogenesis of neuropathic pain, including sensitization and hyperexcitability of primary sensory neurons as well as the enhancement of excitatory synaptic transmission or the reduction of inhibitory synaptic transmission in the neurons of the central nervous system [104, 105]. The change of neurotransmitters (e.g., Glutamate and γ-aminobutyric acid (GABA)) within the SDH plays a vital role in the pathogenesis of chronic neuropathic pain [106]. In normal conditions, painful stimuli evoke action potentials in primary afferent neurons (Aδ- or C-fibers) and excite pain transmission in SDH neurons. SDH inhibitory interneurons containing GABA or glycine repress the excitatory interneurons that innervate SDH pain transmission neurons [107]. CCI decreases the inhibitory tone of GABA or glycine interneurons, which results in a relative enhancement of excitatory interneurons activity. The resulting hyperexcitability of pain transmission in neurons contributes to mechanical hypersensitivity. Chemically, although sinomenine is a morphinan analog, its antinociceptive effects are not abolished by an opioid receptors antagonist naloxone [108]. On the contrary, GABAA receptors antagonist bicuculine blocked the antinociceptive effects of sinomenine [97]. Taken together, these shreds of evidence reveal that sinomenine exerts significant antinociceptive effects on CCI-induced neuropathic pain via the GABAA-mediated mechanism. Previous studies in our laboratory found that oxymatrine [109] prevents the development of mechanical allodynia, thermal hyperalgesia, and cold allodynia in CCI mice by reversing CCI-induced the downregulation of GABAARα2 (Fig. 3). The spinal N-methyl-D-aspartate receptors (NMDARs) are known to contribute to the excitatory synaptic transmission within the spinal cord when they are evoked by nociceptive primary afferent stimuli, which plays important roles in the central sensitization of neuropathic pain [110]. Sigma-1 receptor (Sig-1R) is a unique transmembrane receptor that resides in the mitochondria-associated endoplasmic reticulum membrane (MAM) in the nervous system and has been found to play a pronociceptive role in neuropathic pain models [111, 112]. Sig-1R uncouples with immunoglobulin heavy chain binding protein (BiP) and translocates from the MAM to lipid rafts of the cell membrane when the concentration of Ca2+ decreases in MAM, and then modulates NMDAR responses (Fig. 2) [113, 114]. Previous studies have shown that levo-corydalmine alleviated vincristine-induced neuropathic pain. Levo-corydalmine and levo-tetrahydropalmatine are isoquinoline alkaloids isolated from Yanhusuo, levo-tetrahydropalmatine is an analog of levo-corydalmine (L-CDL) in which a methoxy group replaces the phenol hydroxyl group at the C10 position. The pretreatment of levo-tetrahydropalmatine (L-THP) remarkably suppressed mechanical allodynia and upregulated phosphorylation of NR1 in the spinal cord. Then, intrathecal treatment with L-THP combined with BD1047 (Sig-1R antagonist) synergistically reverse CCI-induced mechanical allodynia, indicating that L-THP alleviates CCI-induced neuropathic pain through modulating spinal Sig-1R activation (Fig. 3) [98]. It has been proposed that NMDA activation-induced Ca2+ influx can trigger an early phase of cAMP response element-binding protein (CREB) phosphorylation. However, delayed ERK signal cascade mediates a persistent phase of CREB phosphorylation, which is vital to the development and maintenance of chronic pain. Our study has shown that oxymatrine restores nerve injury-induced neuropathic pain, which could attribute to the inhibition of NR2B and ERK/CREB signaling pathway (Fig. 3) [115].
Fig. 3.
The main pharmacological mechanisms of alkaloids on treating CCI. Alkaloids attenuate mechanical allodynia in the CCI model via upregulating GABA receptor, inhibiting glia-mediated neuroinflammation, and inhibiting the activation of NMDAR
Regulation of alkaloids on peripheral and central neuroinflammation in CCI rodents
In parallel to the changes in the activity of neurons, non-neurons cells, especially glial cells, are increasingly being recognized as essential contributors to the development and maintenance of neuropathic pain [116]. Microglia are activated in response to nerve injuries and then release pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, thus initiating the neuropathic pain process [117]. Microglia are known to promote neuroinflammation not only by interacting with neurons but also by activating adjacent astrocytes [118]. The long-term subcutaneous administration of koumine decreased the fluorescence density of ionized calcium-binding adaptor molecule-1 (IBA-1) (microglia marker) and glial fibrillary acidic protein (astrocyte marker) in the ipsilateral spinal horn of CCI rats. Koumine downregulated the protein expression of IBA-1, Glial fibrillary acidic protein (GFAP), IL-6, IL-1β, and TNF-α by western blot analysis of spinal cord tissues [102]. Schwann cells and SGCs play fundamental and extensive roles in the primary treatment of nerve injuries in the peripheral nervous system. SGCs enwrap primary neurons in the DRG, where the P2Y12 receptor is highly expressed, which is involved in the nociceptive transmission. SGCs activation is characterized by increased expression of GFAP and the increased production of pro-inflammatory substances, such as ATP, ADP cytokines [119, 120], after which P2Y receptors are activated [8]. Guan-fu base A (GFA) decreases the expression of P2Y12 mRNA and protein in the DRG. The decreased co-expression of P2Y12 and GFAP is also observed in the DRG sections, which confirms the inhibitory effect of GFA on P2Y12 [96].
Regulation of alkaloids on other pathological and physiological processes in CCI rodents
DRG modulates noxious stimuli and no-noxious stimuli from the peripheral nervous system through multiple ion channels expressed in DRG. Voltage-gated sodium channels (VGSC) contribute to generating the ectopic action potential in DRG neurons in various peripheral neuropathic pain conditions [121]. It should be noted that CCI-injured DRG neurons display altered expression levels of sodium channel subtypes, such as Nav1.3, Nav1.8, and Nav1.9 [122]. Dehydrocrenatidine (DHCT) is a β-carboline alkaloid from Picrasma quassioides, which suppress both tetrodotoxin-resistant (TTX-R) and sensitive (TTX-S) VGSC currents in DRG neurons with the half-maximal inhibitory concentration (IC50) values of 12.36 µM and 4.87 µM. Furthermore, DHCT prefers to interact with an inactivated state of VGSCs and prolongs the repriming time in both TTX-S and TTX-R VGSCs, transiting the channels into a slow inactivated state from a fast-inactivated state [101]. These data demonstrated that the analgesic effect of DHCT was possibly through inhibition of VGSCs. DHCT suppresses AP generation, suggesting that DHCT could inhibit the neuronal excitability. It shall be noted that various β-carboline alkaloids produce either a sedative, tremorgenic, anxiogenic, or convulsant effect by binding to benzodiazepine receptors acting as full, partial agonists, antagonists, or inverse agonists [123–127]. Whether DHCT interacts with benzodiazepine receptors and contributes to the neuronal excitability is currently unknown. The antinociceptive effect of brucine is related to the inhibition of TTX-R and TTX-S sodium channel directly, but the activation kinetics of Nav channels could not be changed [100].
Growing evidence suggests that endogenous neurosteroids are involved in the modulation of chronic pain. The endogenous biosynthesis of neurosteroids, such as allopregnanolone and pregnenolone, is upregulated in the spinal cord during neuropathic pain [128, 129]. Koumine increased allopregnanolone, but not pregnenolone in the spinal cord of CCI rats. The anti-neuropathic pain activity of koumine is mediated by further upregulation of allopregnanolone to an adequate level against neuropathic pain [130]. The decrease of allopregnanolone attributes to the fact that the key synthetase of allopregnanolone, 3α-hydroxysteroid oxidoreductase (3α-HSOR), is significantly upregulated by koumine [131]. It is worth mentioning that repeated subcutaneous administration of koumine is not associated with adverse effects commonly associated with opioids, such as physical and psychological dependence.
Single or consecutive administration of isorhynchophylline produces an analgesic effect lasting for 1 day or 3 days. Moreover, isorhynchophylline barely induce the change of motor function in the process of pain-related behavior tests. The analgesic effect is relatively rapid while reinitiating 10 days following interruption, indicating that the analgesic effect of isorhynchophylline seems to design neural plasticity changes. The descending monoaminergic projection is a vital pathway of endogenous pain modulation. Isorhynchophylline analgesia attributes to the escalated 5-hydroxytryptamine (5-HT) and the decreased 5-hydroxyindole acetic acid (5-HIAA)/5-HT ratio in the spinal cord. Pharmacological inhibition of spinal 5-HT corroborates that the escalated monoamine tone is responsible for the antinociceptive action of isorhynchophylline. Additionally, isorhynchophylline dose-dependently ameliorates the depressive and anxious conditions in neuropathic mice.
Activated MAPKs induce different intracellular signals and are also involved in maintaining neuropathic pain via regulating downstream cascade responses [132, 133]. Being consistent with this, MAPKs and microglial inhibitors remarkably attenuated neuropathic pain [134]. Shao et al. [25] found that isotalatizidine stimulated p38 and ERK1/2 in a cultured BV-2 cell line or primary microglia, which was completely inhibited by the respective inhibitors. Moreover, isotalatizidine induced phosphorylation of CREB is specifically mediated by the ERK1/2 pathway but not the p38 pathway. Interestingly, isotalatizidine induced the secretion of dynorphin A in the spinal cord tissue in CCI rat and ameliorated neuropathic pain, which can be reversed by the selective ERK1/2 inhibitor or selective CREB inhibitor. Therefore, the antinociception action of isotalatizidine in CCI-induced neuropathic pain was mediated via the activation of the ERK1/2/CREB/dynorphin A axis.
Effects of alkaloids on sciatic nerve ligation (SNL) models
Table 6. summarized the effects of alkaloids in alleviating SNL-induced neuropathic pain. SNL-induced mechanical allodynia and thermal hyperalgesia have been reduced by lappaconitine, bullatine A (BA), bulleyaconitine A (BAA), gelsemine, koumine, and dehydrocorybulbine (DHCB). Lappaconitine antinociceptive activities are similar in mechanical allodynia and thermal hyperalgesia, with cumulative ED50 values of 1.1 mg/kg vs. 1.6 mg/kg and maximum effect (Emax) values of 53 vs. 58% in SNL-induced neuropathic pain, respectively [29]. Moreover, subcutaneous administration of BA refreshed MWT and TWL in the cumulative dose range of 0.3 to 30 mg/kg, and dose–response analysis of BA at one hour after injection showed that Emax is 56.6% and 66.1% on MWT and TWL, respectively, and ED50 is 1.9 mg/kg and 0.7 mg/kg [135]. Both subcutaneous injection and intrathecal injection of BAA could effectively inhibit mechanical pain and thermal radiation pain in rats with Emax values of 60–100% and ED50 values of 42–59 μg/kg (s.c.), 94–126 ng (i.t.) [136]. Gelsemine [137] and koumine [130] can reverse SNL-induced mechanical allodynia and thermal hyperalgesia, and gelsemine at one hour after intrathecal injection exhibited its Emax of 51.2% and ED50 of 0.5 μg. DHCB can effectively reduce SNL-induced neuropathic pain at doses that do not induce sedation [138].
Table 6.
Effects of alkaloids on sciatic nerve ligation (SNL) models
| Alkaloids | Animal/cell | Dose mg/kg (route of administration) |
Effects/mechanisms of action | Biochemical/molecular parameters/mRNA | References | ||
|---|---|---|---|---|---|---|---|
| behavioral evaluation | histopathological observation |
Electrophysiology parameters | |||||
| Lappaconitine |
Male adult Wistar rats Primary neurons, astrocytes, and microglia |
0.3/1/3/10 mg/kg s.c 0.3/1/3/10 μg i.t |
↓Mechanical allodynia and thermal hyperalgesia | – | – |
→The mRNA expression of dynorphin A, prodynorphin in microglia ↓LPS-induced the increase of TNF-α, IL-1β and IL-6 in microglia |
[29] |
| koumine | Male adult Sprague–Dawley rats |
0.28/1.4/7 mg/kg s.c Twice per day for 7 days |
↓Mechanical allodynia and thermal hyperalgesia | – | – | – | [130] |
| Bulleyaconitine A | Male Sprague–Dawley rats |
0.4 mg/kg i.g |
↓Mechanical and thermal sensitivity | – | ↓Morphine induced-the potentiation of spinal LTP at C-fiber synapses | ↓p-PKCγ in SDH | [136] |
| Bullatine A | Male adult Swiss mice |
0.1/0.3/1/3/10/30 mg/kg s.c |
↓Mechanical allodynia and thermal hyperalgesia | – | – | – | [135] |
| Bulleyaconitine A |
Male Sprague–Dawley rats Primary DRG neurons |
– | ↓Mechanical allodynia and thermal hyperalgesia | – | ↓ Na+ channels in the uninjured neurons | – | [151] |
| Bullatine A | Male adult Wistar rats |
0.3/1/3/10/30 mg/kg s.c 0.3/1/3/10/30 μg i.t |
↓Mechanical allodynia and thermal hyperalgesia | ↑Dynorphin A immunofluorescence staining in the ipsilateral SDH | – | → LPS-induced the mRNA expression of TNF-α, IL-1β and IL-6 in microglia | [45] |
| Gelsemine | Male Wistar rats |
0.03/0.1/0.3/1/3/10 μg i.t |
↓Mechanical allodynia | – | – | ↓The level of Gly α3 mRNA and protein | [137] |
| Dehydrocorybulbine | Male 129/sv mice |
10 mg/kg i.p |
↓Mechanical allodynia and thermal hyperalgesia | – | – | – | [138] |
↑: Enhanced/Increased/Upregulate↓: Attenuate/Downregulate/Decrease/Suppress/Inhibit/Prevent
Regulation of alkaloids on endogenous opioid peptides in SNL rodents
The analgesic effects of lappaconitine might attribute to the upregulation of dynorphin A in the central nervous system. The results support that the antinociceptive effects of lappaconitine are entirely blocked by intrathecal injection of the specific dynorphin A antibody and κ-opioid receptor antagonist [29]. Interestingly, it seems that the alkaloids bioactive components from the medicinal plants of Ranunculus can induce the production of dynorphin A in the spinal cord [25, 135, 139]. Huang et al. explored the analgesic mechanism of BA by studying the release of inflammatory factors and microglia dynorphin A. The results showed that BA could not inhibit the expression of inflammatory factors, including TNF-α, IL-1β, and IL-6 induced by SNL. However, it could significantly increase the expression and release of dynorphin A in the spinal cord and primary cultured microglia [45]. BAA's analgesic effects on neuropathic pain rats could be blocked by initial intrathecal injection of microglial activation inhibitor minocycline, dynorphin A antibody, and specific κ-opioid receptors antagonist nor-BNI. At the same time, BAA increased the expression of dynorphin A, which was inhibited by minocycline in primary cultured microglia (not neurons and astrocytes). Therefore, Li et al. believed that BAA's analgesic effect was produced by stimulating microglia in the spinal dorsal horn to release dynorphin A, which then acted on the κ-opioid receptors on the postsynaptic membrane [140].
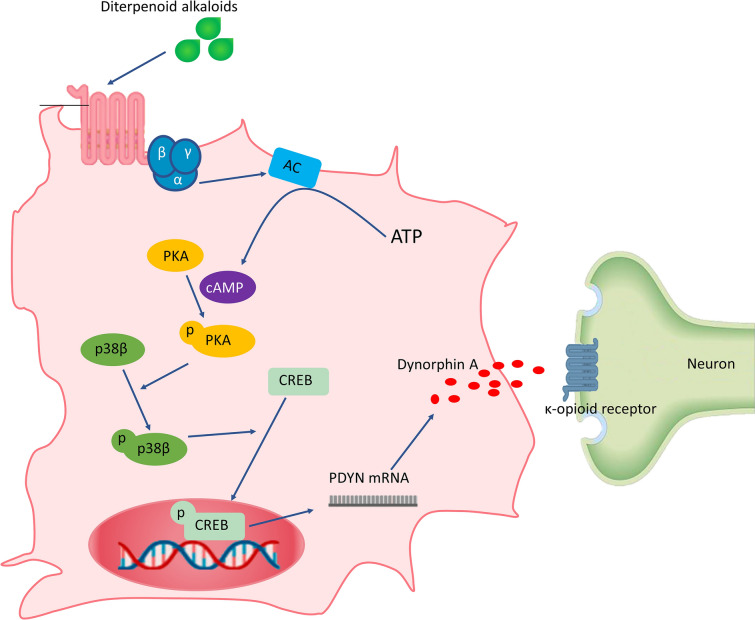
Comparing with the ED50 of these alkaloids (bulleyaconitine A > Lappaconitine = bullatine A) in neuropathic pain rats to EC50 of these diterpenoid alkaloids effects on promoting dynorphin A expression in primary microglia, we found that the analgesic activity is proportional to the effect of dynorphin expression, which further supports that the analgesic activity of these diterpenoid alkaloids is to promote dynorphin A expression. In addition, previous studies have also shown that aconitum extract has analgesic effects by affecting the release of dynorphin A in the spinal cord and acting on κ-opioid receptors [141–143]. Therefore, the molecular basis of diterpenoid alkaloids might stimulate the expression of dynorphin A in spinal cord microglia and further activate κ-opioid receptors on postsynaptic to produce an analgesic effect. The further study of Li et al. found that BAA can promote cAMP production, which could be blocked by G protein-coupled receptors (GPCRs) inhibitors. BAA can activate cAMP-dependent protein kinase A (PKA). PKA specifically upregulated p38 MAPK (not ERK MAPK or JNK MAPK) activity, thus promoting the phosphorylation of CREB and increasing the expression levels of prodynorphin and dynorphin A, further producing an analgesic effect [140]. It is worth noting that p38 activation mediates the release of inflammatory pain induced factors such as TNF-α, IL-1β, and IL-6 by microglia, but BAA also promoted the release of dynorphin A through p38 activation, which seems to be contradictory. In fact, p38 has four subtypes, including α, β, δ, and γ subtypes [144]. Furthermore, p38β siRNA could completely block the release of BAA-induced dynorphin A from microglia, but p38α siRNA did not affect it. These results suggested that p38β activation might be a common mechanism of opioid peptide released from microglia. Based on the above results, it is speculated that the analgesic molecular mechanism of lappaconitine, bullatine A (BA), and even other aconitine analogs are the same as that of BAA, and the expression of dynorphin A is mediated by activating the GPCRs/cAMP/PKA/p38β/CREB signaling pathway of microglia (Fig. 4) [140].
Fig. 4.
Proposed signal transduction pathways for diterpenoid alkaloids-induced analgesia on the SNL model. Following agonism of GPCRs, the cAMP/PKA, p38βMAPK, and CREB signals are successively activated, which mediate dynorphin A expression and subsequent antinociception
Regulation of alkaloids on other pathological and physiological processes in SNL rodents
The specific antinociception of gelsemine in neuropathic pain was blocked by the glycine receptor antagonist strychnine with an apparent half-maximal inhibitory dose (ID50) value of 3.8 μg [170]. In addition, siRNA of glycine receptor α3 subunit was administered intrathecally for seven consecutive days, which completely blocked gelsemine induced-analgesia in neuropathic pain [170]. The results showed that gelsemine produced potent and specific antinociception in chronic neuropathic pain conditions without induction of apparent tolerance by activation of spinal glycine receptor α3 subunit [130]. LTP is abnormal electrical activity in neuropathic pain and morphine tolerance in the spinal cord. L5-SNL and continuous use of morphine can induce LTP lasting for 10 days in the spinal cord [145]. Previous studies have shown that activated protein kinase C-γ (PKCγ) is essential for LTP [146] and morphine tolerance [147] in neuropathic pain. Oral administration of BAA could relieve neuropathic pain and morphine-induced analgesic tolerance. Molecular biology and electrophysiology confirmed that BAA also inhibited LTP and the activation of PKCγ [136]. Interestingly, it has been shown that the spinal PKCγ expressing interneurons is activated only by innocuous inputs conducted by A-fibers [148]. The ectopic discharges mediated by the sodium channels in the primary afferent nerve [149] are significant for developing neuropathic pain [150]. The primary afferent fibers, especially A-fibers, discharge spontaneously following peripheral nerve injuries. BAA inhibited neuropathic pain by blocking the Nav1.7 and Nav1.3 in the peripheral nerve (Fig. 2) [151, 152]. These suggest that the inhibition of sodium channel in A-fibers may be responsible for the BAA-induced analgesia and LTP decrease. It has been found that BA could selectively antagonize P2X7 receptors, inhibit apoptosis of microglia induced by ATP, and inflammatory response mediated by P2X7 receptors [153].
Discussion and conclusion
Most alkaloids are isolated from Chinese herbal medicines. The analgesic activities of various alkaloids are inextricably linked with the traditional application of Chinese herbal medicines. Yanhusuo is a kind of analgesic that is highly praised by Chinese medicine experts. It has the effects of promoting blood circulation, removing blood stasis, regulating qi, and relieving pain. According to the compendium of Materia Medica, Yanhusuo is specially used to treat all kinds of pains in the body. The main active components of Yanhusuo include dehydrocorydaline, levo-Tetrahydropalmatine, and levo-Corydalmine; these alkaloids have shown analgesic effects on neuropathic pain. Besides, Aconitum, such as Aconitum carmichaelii, Aconitum kusnezoffii, and Aconitum sinomontanum, mainly treat rheumatic arthralgia, acute and chronic pain. C18, C19, C20 diterpenoid alkaloids are the main active components of Aconitum and have significant analgesic effects. Previously studies indicated that bulleyaconitine A, lappaconitine, bullatine A, and isotalatizidine have good analgesic effects on neuropathic pain. However, Gouteng had a significant sedative effect in clinical application [154]. Therefore, in the study of alkaloids on neuropathic pain, we should pay more attention to whether alkaloids have sedative effects to avoid the temporary disappearance of pain-related behavior due to central inhibition. Compared to traditional single-target drugs, bioactive natural ingredients derived from herbs may provide additional benefits in preventing chronic neuropathic pain with improved efficacy and lower toxicity, and they represent an important source of drug discovery. In addition, it is a feasible method for researchers to search effective candidate compounds for the treatment of neuropathic pain by starting from the clinical application of traditional Chinese herbal medicines, and it is easier to identify the analgesic effects, toxic effects, and related mechanism of alkaloids.
Although previous studies have reported the pharmacological effects and mechanisms of action of the alkaloids isolated from Chinese herbal medicines, there is no review focusing on bioactive alkaloids in the treatment of peripheral neuropathic pain. Alkaloids inhibited peripheral and central neuroinflammation, reduced oxidative stress damage, and further ameliorated peripheral neuropathic pain. The expression of regulatory factors, including PGC-1α, PPAR-γ, and UCP2, the activation of the Nrf2, and the downstream upregulation of antioxidant enzymes, including SOD, GST, and GPx, have been proposed as common mechanisms underlying the antioxidant effects of these alkaloids. Moreover, most of the studies have suggested that the reversal of oxidative stress plays a critical role in the anti-inflammation effects of these alkaloids. Importantly, alkaloids' mechanism on neuropathic pain is not entirely inconsistent due to the pathological mechanism of different types of neuropathic pain that is different. However, the specific and detailed differences are still unclear. The influence of hyperglycemia and chemotherapy agents on peripheral nerves (e.g., sciatic nerve, epidermal nerve fibers) is serious due to a large number of mitochondria are susceptible to oxygen free radicals in the mammalian nervous system. Therefore, besides removing the etiology, it is necessary to improve the function of mitochondria in the nervous system for the treatment of DPN and CIPN. In addition, alkaloids exert analgesic effects on CCI-induced neuropathic pain via inhibiting the activation of microglia and astrocyte, regulating excitatory and inhibitory synaptic transmission, and regulating endogenous neurosteroids. Moreover, The analgesic mechanism of diterpenoid alkaloids on SNL-induced neuropathic pain is primarily to promote the release of endogenous opioid peptides. Fortunately, bioactive alkaloids have the characteristic of multi-target action on neuropathic pain. Specifically, koumine and berberine exerted analgesic effects on DPN and CCI rodents through different mechanisms. Based on clarifying the pathological mechanism of various neuropathic pain models, it is still necessary to further study the analgesic mechanisms of different alkaloids.
Among the alkaloids mentioned above, diterpenoid alkaloids are the most studied bioactive ingredients but their toxicity is a cause for concern. Researchers generally believe that diterpenoid alkaloids have the most severe toxic effects, such as cardiotoxicity and neurotoxicity, mediated by sodium channels. However, in the previous studies, no similar adverse reactions were found under the treatment dose, and the treatment index of BAA is about seven times that of aconitine. A wider therapeutic index means that the toxic effects and analgesic effects can be separated. Some studies have shown that sodium channel blockers could not reverse the analgesic effects of BAA. These researches further indicated that the analgesic and toxic effects of BAA were separated. In addition, diterpenoid alkaloids can promote the release of dynorphin A from spinal microglia and further exert analgesic effects with synaptic κ-opioid receptors. Direct agonists of κ-opioid receptors, especially synthetic non-peptide agonists, can cause severe irritability and side effects similar to psychosis. Because endogenous dynorphin A produced by diterpenoid alkaloids has a higher potency than exogenous dynorphin A, these alkaloids have no obvious adverse reactions, such as sedation, analgesia tolerance, under the effective treatment doses. In addition, the investigation of the structure–activity relationship is a meaningful way to balance the analgesic effects and toxic effects of alkaloids. At present, the structure–activity relationship of diterpenoid alkaloids has been studied. The following groups for the analgesic effects of C18 and C19 diterpenoid alkaloids are essential: a ring of trivalent N, acetyloxy or ethoxy substitution at the C8 position, aryl substitution at C14 position, and saturation of D ring. In addition, a 3α-hydroxyl group or 5-hydroxy group can enhance the analgesic activity of these compounds [155]. The ester groups at C4, C8, and C14 positions are active groups, as well as toxic groups. However, the introduction of ester groups at the C3 position, can improve the analgesic index of these alkaloids, which may separate the pharmacological activity from the toxicity. Therefore, it is possible to introduce the ester group at the C3 position while hydrolyzing the ester groups at C4, C8, and C14 positions to preserve the effectiveness and reduce toxicity.
In summary, even after taking the above-mentioned obstacles and concerns into account, the alkaloids are valuable drug candidates for treating neuropathic pain. Therefore, there is an urgent need to investigate their pharmacokinetic properties and establish the dose-time-pharmacology-toxicology relationships of alkaloids isolated from Chinese herbal medicines. Further preclinical research and clinical trials are crucial to demonstrate alkaloids' efficacy in alternative analgesia for neuropathic pain. Therefore, future research studies must address the anti-neuropathic pain targets and molecular mechanisms of Chinese herbal medicines and their monomeric compounds, combining laboratory anti-neuropathic pain researches with clinical practices, testing the reliability of Chinese herbal medicines against neuropathic pain, and promoting their application in practical neuropathic pain treatment.
Acknowledgements
Not applicable.
Abbreviations
- TCAs
Tricyclic antidepressants
- DPN
Diabetic peripheral neuropathic pain
- AGEs
Advanced glycation end products
- PKC
Protein kinase C
- PARP
Poly-ADP ribose polymerase
- ED50
Half-effective dose
- LD50
Median lethal dose
- STZ
Streptozotocin
- MWT
Mechanical paw withdrawal threshold
- TWL
Thermal paw withdrawal latency
- SNCV
Sensory nerve conduction velocity
- MNCV
Motor nerve conduction velocity
- BDNF
Brain-derived neurotrophic factor
- IGF-I
Insulin-like growth factors I
- mRNA
Messenger ribonucleic acid
- ROS
Reactive oxygen species
- SGCs
Satellite glial cells
- TNF-α
Tumor necrosis factor-α
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- AMPK
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- PPAR-γ
Peroxisome proliferator-activated receptors-γ
- PP2Cα
Protein phosphatase 2Cα
- DRG
Dorsal root ganglia
- ERK1/2
Regulates protein kinases 1/2
- P38MAPK
P38 mitogen-activated protein kinase
- TCM
Traditional Chinese medicine
- CIPN
Chemotherapy-induced peripheral neuropathy
- IENFs
Intraepidermal nerve fibers
- CSBTA
Corydalis Saxicola Bunting. Total Alkaloids
- SFI
Sciatic nerve function index
- SNAP
Sensory nerve action potential
- RNS
Nitrogen reactive species
- SOD
Superoxide dismutase
- CAT
Catalase
- GSH
Glutathione
- UCP2
Uncoupling protein 2
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator 1-α
- MnSOD
Manganese superoxide dismutase
- Nrf2
Nuclear erythroid 2-related factor 2
- GSH-Px
Glutathione peroxidase
- L-CDL
Levo-corydalmine
- NF-κB
Nuclear factor kappa-B
- siRNA
Small interfering RNA
- TRPV1
Transient receptor potential channel family
- BAA
Bulleyaconitine A
- sEPSCs
Spontaneous excitatory postsynaptic currents
- mEPSCs
Miniature excitatory postsynaptic currents
- LTP
Long-term potentiation
- CCI
Chronic constriction injury
- GABA
γ-Aminobutyric acid
- NMDARs
N-Methyl-D-aspartate receptors
- Sig-1R
Sigma-1 receptor
- MAM
Mitochondria-associated endoplasmic reticulum membrane
- BiP
Immunoglobulin heavy chain binding protein
- L-THP
Levo-tetrahydropalmatine
- CREB
CAMP-response element binding protein
- IBA-1
Ionized calcium-binding adaptor molecule-1
- GFAP
Glial fibrillary acidic protein
- LPS
Lpopolysaccharide
- GFA
Guan-fu base A
- VGSC
Voltage-gated sodium channels
- DHCT
Dehydrocrenatidine
- TTX-R
Tetrodotoxin-resistant
- TTX-S
Tetrodotoxin-sensitive
- IC50
Half maximal inhibitory concentration
- 3α-HSOR
3α-Hydroxysteroid oxidoreductase
- 5-HT
5-Hydroxytryptamine
- 5-HIAA
5-Hydroxyindole acetic acid
- SNL
Spinal nerve ligation
- BA
Bullatine A
- DHCB
Dehydrocorybulbine
- Emax
Maximum effect
- ID50
Half-maximal inhibitory dose
- PKCγ
Protein kinase C-γ
- s.c.
Subcutaneous
- i.g.
Intragastric
- i.p.
Intraperitoneal
- i.t.
Intrathecal
Authors’ contributions
CZ, NL conducted this review and wrote the manuscript. MT, LM, JY, XL retrieved related literature. HM, JN, JY gave some advice for improving the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81960726), the Natural Science Foundation of Ningxia (2019AAC03097), the Key Research and Development Program of Ningxia (2018BFH02001 and 2019BFH02003), and the Major Research and Construction Programs of Ningxia Province (2017BY079).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunhao Zhu and Ning Liu contributed equally to this work
Contributor Information
Hanxiang Ma, Email: mahanxiang@hotmail.com.
Jianguo Niu, Email: nxmcnjg@163.com.
Jianqiang Yu, Email: YujqLab@163.com.
References
- 1.Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Vieira E, Garcia J, Silva AA, Araújo R, Jansen R. Prevalence, characteristics, and factors associated with chronic pain with and without neuropathic characteristics in Sao Luis, Brazil. J Pain Symptom Manage. 2012;44:239–251. doi: 10.1016/j.jpainsymman.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Siddall PJ, Mcclelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 6.Klit H, Finnerup N, Andersen G, Jensen T. Central poststroke pain: a population-based study. Pain. 2011;152:818–824. doi: 10.1016/j.pain.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis—prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21:28. doi: 10.1007/s11916-017-0629-5. [DOI] [PubMed] [Google Scholar]
- 9.Groenen P, van Helmond N, Chapman K. Chemotherapy-induced peripheral neuropathy treated with dorsal root ganglion stimulation. Pain Med. 2018;20:857–859. doi: 10.1093/pm/pny209. [DOI] [PubMed] [Google Scholar]
- 10.Boulton AJM, Gries FA, Jervell J. Guidelines for the diagnosis and outpatient management diabetic peripheral neuropathy. Diabet Med. 1998;15:508–514. doi: 10.1002/(SICI)1096-9136(199806)15:6<508::AID-DIA613>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen T. European federation of neurological societies: EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–1123. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 12.Dosenovic S, Jelicic Kadic A, Miljanović M, Biočić M, Boric K, Cavar M, et al. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg. 2017;125:643–652. doi: 10.1213/ANE.0000000000001998. [DOI] [PubMed] [Google Scholar]
- 13.Wallace MS. Pharmacologic treatment of neuropathic pain. Curr Pain Headache Rep. 2001;5:138–150. doi: 10.1007/s11916-001-0082-2. [DOI] [PubMed] [Google Scholar]
- 14.Goodman C, Brett A. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179:695–701. doi: 10.1001/jamainternmed.2019.0086. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi M, Desaiah D, Ossanna M, Pritchett Y, Brannan S, Detke M. Clinical evidence for serotonin and norepinephrine reuptake inhibition of duloxetine. Int Clin Psychopharmacol. 2008;23:161–169. doi: 10.1097/YIC.0b013e3282f41d7e. [DOI] [PubMed] [Google Scholar]
- 16.Onakpoya I, Thomas E, Lee J, Goldacre B, Heneghan C. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9:e023600. doi: 10.1136/bmjopen-2018-023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell G, Nielsen S, Bruno R, Lintzeris N, Cohen M, Hall W, et al. The Pain and Opioids IN Treatment study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain. 2015;156:231–242. doi: 10.1097/01.j.pain.0000460303.63948.8e. [DOI] [PubMed] [Google Scholar]
- 18.Wilkerson J, Ghosh S, Mustafa M, Abdullah R, Niphakis M, Cabrera R, et al. The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology. 2016;114:156–167. doi: 10.1016/j.neuropharm.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngo LT, Okogun JI, Folk WR. 21st century natural product research and drug development and traditional medicines. Nat Prod Rep. 2013;30:584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg G, Adams JD. Treatment of neuropathic pain with plant medicines. Chin J Integr Med. 2012;18:565–570. doi: 10.1007/s11655-012-1188-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Zhou L, Chen Z, Hu C. Analgesic effect of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on spared nerve injury rat model of neuropathic pain. Pharmacol Biochem Behav. 2012;102:465–470. doi: 10.1016/j.pbb.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Gong Y, Liu S, Liang S. Natural compounds acting at P2 receptors alleviate peripheral neuropathy. Brain Res Bull. 2018;151:125–131. doi: 10.1016/j.brainresbull.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Basu P, Basu A. In vitro and in vivo effects of flavonoids on peripheral neuropathic pain. Molecules. 2020;25:1171. doi: 10.3390/molecules25051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintans J, Antoniolli A, Almeida JR, Santana-Filho V, Quintans-Júnior L. Natural products evaluated in neuropathic pain models—a systematic review. Basic Clin Pharmacol Toxicol. 2013;114:442–450. doi: 10.1111/bcpt.12178. [DOI] [PubMed] [Google Scholar]
- 25.Shao S, Xia H, Hu M, Chen C, Fu J, Shi G, et al. Isotalatizidine, a C(19)-diterpenoid alkaloid, attenuates chronic neuropathic pain through stimulating ERK/CREB signaling pathway-mediated microglial dynorphin A expression. J Neuroinflamm. 2020;17:13. doi: 10.1186/s12974-019-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Shen J, Liu H, Xu Y, Su YP, Yang J, et al. Gelsenicine from Gelsemium elegans attenuates neuropathic and inflammatory pain in mice. Biol Pharma Bull. 2011;34:1877–1880. doi: 10.1248/bpb.34.1877. [DOI] [PubMed] [Google Scholar]
- 27.Ling Q, Liu M, Wu MX, Xu Y, Yang J, Huang HH, et al. Anti-allodynic and neuroprotective effects of koumine, a benth alkaloid, in a rat model of diabetic neuropathy. Biol Pharma Bull. 2014;37:858–864. doi: 10.1248/bpb.b13-00843. [DOI] [PubMed] [Google Scholar]
- 28.Guo Z, Man Y, Wang X, Jin H, Sun X, Su X, et al. Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci Rep. 2014;4:3905. doi: 10.1038/srep03905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun ML, Ao JP, Wang YR, Huang Q, Li TF, Li XY, et al. Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin A expression. Psychopharmacology. 2018;235:2559–2571. doi: 10.1007/s00213-018-4948-y. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, et al. Orally active opioid mu/delta dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J Pharmacol Exp Ther. 2013;348:383–392. doi: 10.1124/jpet.113.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan H, Ain Q, Mubarak M, Pervaiz A. Plant alkaloids as antiplatelet agent: drugs of future in the light of recent development. Front Pharmacol. 2016;7:292. doi: 10.3389/fphar.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tesfaye S, Boulton A, Dickenson A. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown M, Asbury A. Diabetic neuropathy. Ann Neurol. 1984;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- 34.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, et al. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- 37.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97(22):12222. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uehara K, Yamagishi SI, Otsuki S, Chin S, Yagihashi S. Effects of polyol pathway hyperactivity on protein kinase C activity, nociceptive peptide expression, and neuronal structure in dorsal root ganglia in diabetic mice. Diabetes. 2004;53:3239. doi: 10.2337/diabetes.53.12.3239. [DOI] [PubMed] [Google Scholar]
- 39.Cameron NE, Cotter MA. Effects of protein kinase Cβ inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev. 2002;18:315–323. doi: 10.1002/dmrr.307. [DOI] [PubMed] [Google Scholar]
- 40.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Gao L, Zhang N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int J Immunopathol Pharmacol. 2019;33:1–7. doi: 10.1177/2058738419866379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, Yan M, Guo G, Tong N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose Resp. 2019;17:1559325819862449. doi: 10.1177/1559325819862449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Guan S, Ge H, Xiong W, He L, Liu L, et al. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res Bull. 2018;139:56–66. doi: 10.1016/j.brainresbull.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Rao S, Liu S, Zou L, Jia T, Zhao S, Wu B, et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal. 2017;13:227–235. doi: 10.1007/s11302-016-9554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Q, Mao XF, Wu HY, Li TF, Sun ML, Liu H, et al. Bullatine A stimulates spinal microglial dynorphin A expression to produce anti-hypersensitivity in a variety of rat pain models. J Neuroinflamm. 2016;13:214. doi: 10.1186/s12974-016-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauskolb S, Dombert B, Sendtner M. Insulin-like growth factor 1 in diabetic neuropathy and amyotrophic lateral sclerosis. Neurobiol Dis. 2016;97:103–113. doi: 10.1016/j.nbd.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 48.Bordet T, Buisson B, Michaud M, Jean-Louis A, Marchand F, Grist J, et al. Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy. J Pharmacol Exp Ther. 2008;326:623–632. doi: 10.1124/jpet.108.139410. [DOI] [PubMed] [Google Scholar]
- 49.Shi X, Chen Y, Nadeem L, Xu G. Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy. J Neuroinflamm. 2013;10:69. doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizisin A, Bache M, Distefano P, Acheson ANN, Lindsay R, Calcutt N. BDNF attenuates functional and structural disorders in nerves of galactose-fed rats. J Neuropathol Exp Neurol. 1998;56:1290–1301. doi: 10.1097/00005072-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Mizukami H, Yagihashi S. Exploring a new therapy for diabetic polyneuropathy—the application of stem cell transplantation. Front Endocrinol. 2014;5:45. doi: 10.3389/fendo.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Kandhare AD, Mukherjee AA, Bodhankar SL. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018;17:399–419. doi: 10.17179/excli2018-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obrosova I, Ajayi I, Forsell M, Komjati K, Pacher P, Szabo C, et al. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 54.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Yuan X, Zuo H, Li X, Sun Y, Feng A. Berberine induces ZIP14 expression and modulates zinc redistribution to protect intestinal mucosal barrier during polymicrobial sepsis. Life Sci. 2019;233:116697. doi: 10.1016/j.lfs.2019.116697. [DOI] [PubMed] [Google Scholar]
- 56.Freitag C, Miller R. Peroxisome proliferator-activated receptor agonists modulate neuropathic pain: a link to chemokines? Front Cell Neurosci. 2014;8:238. doi: 10.3389/fncel.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novikova DS, Garabadzhiu AV, Melino G, Barlev NA, Tribulovich VG. AMP-activated protein kinase: Structure, function, and role in pathological processes. Biochemistry. 2015;80:127–144. doi: 10.1134/S0006297915020017. [DOI] [PubMed] [Google Scholar]
- 58.Chew BH, Vos R, Mohd Sidik S, Rutten G. Diabetes-related distress, depression and distress-depression among adults with type 2 diabetes mellitus in Malaysia. PLoS ONE. 2016;11:e0152095. doi: 10.1371/journal.pone.0152095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen RR, Nielsen CK, Nasser A, Thomsen SIM, Eghorn LF, Pham Y, et al. P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain. 2011;152:1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett. 2011;504:57–61. doi: 10.1016/j.neulet.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 61.Ursu D, Ebert P, Langron E, Ruble C, Munsie L, Zou W, et al. Gain and loss of function of P2X7 receptors: mechanisms, pharmacology and relevance to diabetic neuropathic pain. Mol Pain. 2014;10:37. doi: 10.1186/1744-8069-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barden N, Harvey M, Gagné B, Shink E, Tremblay M, Raymond C, et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet Part B Neuropsychiatr Genet. 2006;141B:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 63.Krimon S, Araldi D, do Prado FC, Tambeli CH, Oliveira-Fusaro MCG, Parada CA. P2X3 receptors induced inflammatory nociception modulated by TRPA1, 5-HT3 and 5-HT1A receptors. Pharmacol Biochem Behav. 2013;112:49–55. doi: 10.1016/j.pbb.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Yin Q, Xia Y, Wang G. Sinomenine alleviates high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the activation of RhoA/ROCK signaling pathway. Biochem Biophys Res Commun. 2016;477:881–886. doi: 10.1016/j.bbrc.2016.06.152. [DOI] [PubMed] [Google Scholar]
- 65.Ni WJ, Zhou H, Ding HH, Tang LQ. Berberine ameliorates renal impairment and inhibits podocyte dysfunction by targeting the PI3K/Akt pathway in diabetic rat. J Diabetes Invest. 2019;11:2. doi: 10.1111/jdi.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shanshan W, Benhong H, Weijian H, Ninghua W, Liangtao X, Xu W, et al. Berberine alleviates tau hyperphosphorylation and axonopathy-associated with diabetic encephalopathy via restoring PI3K/Akt/GSK3β pathway. J Alzhmer's Disease. 2018;65:1385–1400. doi: 10.3233/JAD-180497. [DOI] [PubMed] [Google Scholar]
- 67.Yu J, Zong GN, Wu H, Zhang KQ. Podoplanin mediates the renoprotective effect of berberine on diabetic kidney disease in mice. Acta Pharmacol Sin. 2019;40:1544–1554. doi: 10.1038/s41401-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cermenati G, Giatti S, Cavaletti G, Bianchi R, Maschi O, Pesaresi M, et al. Activation of the liver x receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J Neurosci. 2010;30:11896–11901. doi: 10.1523/JNEUROSCI.1898-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Taleb O, Bouzobra F, Tekin-Pala H, Meyer L, Mensah-Nyagan AG, Patte-Mensah C. Behavioral and electromyographic assessment of oxaliplatin-induced motor dysfunctions: evidence for a therapeutic effect of allopregnanolone. Behav Brain Res. 2017;320:440–449. doi: 10.1016/j.bbr.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 71.Bakogeorgos M, Georgoulias V. Risk-reduction and treatment of chemotherapy-induced peripheral neuropathy. Expert Rev Anticancer Ther. 2017;17:1045–1060. doi: 10.1080/14737140.2017.1374856. [DOI] [PubMed] [Google Scholar]
- 72.Han F, Smith M. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massicot F, Guillaume H, David L, Chen D, Leuxe C, Garnier-Legrand L, et al. P2X7 cell death receptor activation and mitochondrial impairment in oxaliplatin-induced apoptosis and neuronal injury: cellular mechanisms and in vivo approach. PLoS ONE. 2013;8:e66830. doi: 10.1371/journal.pone.0066830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. doi: 10.3389/fphar.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyette-Davis J, Xin W, Zhang H, Dougherty P. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ko MH, Hu ME, Hsieh YL, Lan CT, Tseng TJ. Peptidergic intraepidermal nerve fibers in the skin contribute to the neuropathic pain in paclitaxel-induced peripheral neuropathy. Neuropeptides. 2014;48:109–117. doi: 10.1016/j.npep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Hu L, Yang Z, Cui WQ, Hu X, Du LX, Mi W, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav Immun. 2017;68:132–145. doi: 10.1016/j.bbi.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Wu P, Chen Y. Evodiamine ameliorates paclitaxel-induced neuropathic pain by inhibiting inflammation and maintaining mitochondrial anti-oxidant functions. Hum Cell. 2019;32:251–259. doi: 10.1007/s13577-019-00238-4. [DOI] [PubMed] [Google Scholar]
- 79.McCarthy B, Hsieh S, Stocks A, Hauer P, Macko C, Cornblath D, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/WNL.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 80.Krukowski K, Ma J, Golonzhka O, Laumet GO, Gutti T, van Duzer JH, et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158:1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuai CP, Ju LJ, Hu PP, Huang F. Corydalis saxicola alkaloids attenuate cisplatin-induced neuropathic pain by reducing loss of IENF and blocking TRPV1 activation. Am J Chin Med. 2020;48:407–428. doi: 10.1142/S0192415X20500214. [DOI] [PubMed] [Google Scholar]
- 82.Gong SS, Li YX, Zhang M-T, Du J, Ma PS, Yao WX, et al. Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem Res. 2016;41:3147–3159. doi: 10.1007/s11064-016-2040-8. [DOI] [PubMed] [Google Scholar]
- 83.Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion—a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox biology. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishii N, Tsubouchi H, Miura A, Yanagi S, Ueno H, Shiomi K, et al. Ghrelin alleviates paclitaxel-induced peripheral neuropathy by reducing oxidative stress and enhancing mitochondrial anti-oxidant functions in mice. Eur J Pharmacol. 2017;819:35–42. doi: 10.1016/j.ejphar.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Chiou CT, Wang KC, Yang YC, Huang CL, Yang SH, Kuo YH, et al. Liu Jun Zi Tang—a potential, multi-herbal complementary therapy for chemotherapy-induced neurotoxicity. Int J Mol Sci. 2018;19:1258. doi: 10.3390/ijms19041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh J, Saha L, Singh N, Kumari P, Bhatia A, Chakrabarti A. Study of nuclear factor-2 erythroid related factor-2 activator, berberine, in paclitaxel induced peripheral neuropathy pain model in rats. J Pharm Pharmacol. 2019;71:797–805. doi: 10.1111/jphp.13047. [DOI] [PubMed] [Google Scholar]
- 88.Cardozo LFMF, Pedruzzi LM, Stenvinkel P, Stockler-Pinto MB, Daleprane JB, Leite M, et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 89.Zhou L, Hu Y, Li C, Yan Y, Ao L, Yu B, et al. Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway. Neuropharmacology. 2018;135:34–47. doi: 10.1016/j.neuropharm.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 91.Kadoi J, Takeda M, Matsumoto S. Prostaglandin E2 potentiates the excitability of small diameter trigeminal root ganglion neurons projecting onto the superficial layer of the cervical dorsal horn in rats. Exp Brain Res. 2007;176:227–236. doi: 10.1007/s00221-006-0608-2. [DOI] [PubMed] [Google Scholar]
- 92.Zhu HQ, Xu J, Shen KF, Pang RP, Wei XH, Liu XG. Bulleyaconitine A depresses neuropathic pain and potentiation at C-fiber synapses in spinal dorsal horn induced by paclitaxel in rats. Exp Neurol. 2015;273:263–272. doi: 10.1016/j.expneurol.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 94.Challa SR. Surgical animal models of neuropathic pain: pros and Cons. Int J Neurosci. 2015;125:170–174. doi: 10.3109/00207454.2014.922559. [DOI] [PubMed] [Google Scholar]
- 95.Haiyan W, Yuxiang L, Linglu D, Tingting X, Yinju H, Hongyan L, et al. Antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury. Pharma Biol. 2013;51:844–850. doi: 10.3109/13880209.2013.767363. [DOI] [PubMed] [Google Scholar]
- 96.Zou L, Yu K, Fan Y, Cao S, Liu S, Shi L, et al. The inhibition by guanfu base a of neuropathic pain mediated by P2Y12 receptor in dorsal root Ganglia. ACS Chem Neurosci. 2019;10:1318–1325. doi: 10.1021/acschemneuro.8b00399. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Q, Zhu J, Fang T, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci Rep. 2014;4:7270. doi: 10.1038/srep07270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang DW, Moon JY, Choi JG, Kang SY, Ryu Y, Park JB, et al. antinociceptive profile of levo-tetrahydropalmatine in acute and chronic pain mice models: role of spinal sigma-1 receptor. Sci Rep. 2016;6:37850. doi: 10.1038/srep37850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HJ. Berberine ameliorates allodynia induced by chronic constriction injury of the sciatic nerve in rats. J Med Food. 2015;18:909–915. doi: 10.1089/jmf.2014.3346. [DOI] [PubMed] [Google Scholar]
- 100.Yu G, Linnan Q, Yu J, Tang M, Wang C, Zhou Y, et al. Brucine alleviates neuropathic pain in mice via reducing the current of the sodium channel. J Ethnopharmacol. 2018;233:56–63. doi: 10.1016/j.jep.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 101.Zhao F, Tang Q, Xu J, Wang S, Li S, Zou X, et al. Dehydrocrenatidine inhibits voltage-gated sodium channels and ameliorates mechanic allodia in a rat model of neuropathic pain. Toxins (Basel) 2019;11:229. doi: 10.3390/toxins11040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin G, He SD, Lin SM, Hong LM, Chen WQ, Xu Y, et al. Koumine attenuates neuroglia activation and inflammatory response to neuropathic pain. Neural Plast. 2018 doi: 10.1155/2018/9347696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao KX, Zhao Q, Wang GR, Yu L, Wu JY, Zhao X. Isorhynchophylline exerts antinociceptive effects on behavioral hyperalgesia and allodynia in a mouse model of neuropathic pain: evidence of a 5-HT(1A) receptor-mediated mechanism. Front Pharmacol. 2020;11:318. doi: 10.3389/fphar.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 106.Craparo E, Bondì M, Pitarresi G, Cavallaro G. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci Ther. 2010;17:670–677. doi: 10.1111/j.1755-5949.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–152. doi: 10.1038/nrn.2018.2. [DOI] [PubMed] [Google Scholar]
- 108.Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ, Xu XJ. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur J Pharmacol. 2013;721:5–11. doi: 10.1016/j.ejphar.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 109.Liu HY, Li YX, Hao YJ, Wang H-Y, Dai XY, Sun T, et al. Effects of oxymatrine on the neuropathic pain induced by chronic constriction injury in mice. CNS Neurosci Ther. 2012;18:1030–1032. doi: 10.1111/cns.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 111.Romero L, Zamanillo D, Nadal X, Sánchez-Arroyos R, Rivera-Arconada I, Dordal A, et al. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol. 2012;166:2289–2306. doi: 10.1111/j.1476-5381.2012.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nieto FR, Cendán CM, Sánchez-Fernández C, Cobos EJ, Entrena JM, Tejada MA, et al. Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. J Pain. 2012;13:1107–1121. doi: 10.1016/j.jpain.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Martina M, Turcotte M-E, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pabba M, Wong A, Ahlskog N, Hristova E, Biscaro D, Nassrallah W, et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci. 2014;34:11325–11338. doi: 10.1523/JNEUROSCI.0458-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H, Li Y, Dun L, Xu Y, Jin S, Du J, et al. Antinociceptive effects of oxymatrine from Sophora flavescens, through regulation of NR2B-containing NMDA receptor-ERK/CREB signaling in a mice model of neuropathic pain. Phytomedicine. 2013;20:1039–1045. doi: 10.1016/j.phymed.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 116.Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron–glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74:3275–3291. doi: 10.1007/s00018-017-2513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain. 2012;8:23. doi: 10.1186/1744-8069-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jasmin L, Vit JP, Bhargava A, Ohara P. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol. 2010;6:63–71. doi: 10.1017/S1740925X10000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Exp Opin Ther Targets. 2017;21:695–703. doi: 10.1080/14728222.2017.1328057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sree Indrani M, Marsat G, Allen K, Williamson D. Differences in sodium channel densities in the apical dendrites of pyramidal cells of the electrosensory lateral line lobe. Front Neural Circuits. 2019;13:41. doi: 10.3389/fncir.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiao WH, Gao H, Li CY, Zhao F, Jiang RW, Wang Y, et al. Quassidines AD, Bis-β-carboline alkaloids from the stems of Picrasma quassioides. J Nat Prod. 2010;73:167–171. doi: 10.1021/np900538r. [DOI] [PubMed] [Google Scholar]
- 124.Haghparast A, Farzin D, Ordikhani-Seyedlar M, Motaman S, Kermani M, Azizi P. Effects of apomorphine and β-carbolines on firing rate of neurons in the ventral pallidum in the rats. Behav Brain Res. 2012;227:109–115. doi: 10.1016/j.bbr.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 125.Hollinshead SP, Trudell ML, Skolnick P, Cook JM. Structural requirements for agonist actions at the benzodiazepine receptor: studies with analogues of 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester. J Med Chem. 1990;33:1062–1069. doi: 10.1021/jm00165a028. [DOI] [PubMed] [Google Scholar]
- 126.Grella B, Teitler M, Smith C, Herrick-Davis K, Glennon RA. Binding of β-Carbolines at 5-HT 2 serotonin receptors. Bioorg Med Chem Lett. 2003;13:4421–4425. doi: 10.1016/j.bmcl.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 127.Glennon RA, Grella B, Tyacke RJ, Lau A, Westaway J, Hudson AL. Binding of beta-carbolines at imidazoline I2 receptors: a structure-affinity investigation. Bioorg Med Chem Lett. 2004;14:999–1002. doi: 10.1016/j.bmcl.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 128.Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: multi-parametric assessment and direct evidence. Pain. 2011;152:170–181. doi: 10.1016/j.pain.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 129.Mensah-Nyagan AG, Kibaly C, Schaeffer V, Venard C, Meyer L, Patte-Mensah C. Endogenous steroid production in the spinal cord and potential involvement in neuropathic pain modulation. J Steroid Biochem Mol Biol. 2008;109:286–293. doi: 10.1016/j.jsbmb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang J, et al. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol Biochem Behav. 2012;101:504–514. doi: 10.1016/j.pbb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 131.Qiu HQ, Xu Y, Jin GL, Yang J, Liu M, Li SP, et al. Koumine enhances spinal cord 3α-hydroxysteroid oxidoreductase expression and activity in a rat model of neuropathic pain. Mol Pain. 2015;11:46. doi: 10.1186/s12990-015-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhuang ZY, Gerner P, Woolf CJ, Ji R-R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 133.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Möller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor: why minocycline is not a specific microglia inhibitor. Glia. 2016;64:1788–1794. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- 135.Huang Q, Sun ML, Chen Y, Li XY, Wang Y. Concurrent bullatine A enhances morphine antinociception and inhibits morphine antinociceptive tolerance by indirect activation of spinal κ-opioid receptors. J Ethnopharmacol. 2016;196:151–159. doi: 10.1016/j.jep.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 136.Mai JZ, Liu C, Huang Z, Mai CL, Zhou X, Zhang J, et al. Oral application of bulleyaconitine A attenuates morphine tolerance in neuropathic rats by inhibiting long-term potentiation at C-fiber synapses and protein kinase C gamma in spinal dorsal horn. Mol Pain. 2020;16:1744806920917242. doi: 10.1177/1744806920917242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang JY, Gong N, Huang JL, Guo LC, Wang YX. Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal α3 glycine receptors. Pain. 2013;154:2452–2462. doi: 10.1016/j.pain.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y, Wang C, Wang L, Parks Gregory S, Zhang X, Guo Z, et al. A novel analgesic isolated from a traditional Chinese medicine. Curr Biol. 2014;24:117–123. doi: 10.1016/j.cub.2013.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang SN, Yang B, Ma L, Huang LT, Ju PJ, Wei J, et al. Bulleyaconitine A exerts antianxiety and antivisceral hypersensitivity effects. Front Pharmacol. 2020;11:328. doi: 10.3389/fphar.2020.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li TF, Fan H, Wang YX. Aconitum-Derived Bulleyaconitine A exhibits antihypersensitivity through direct stimulating dynorphin a expression in spinal microglia. J Pain. 2016;17:530–548. doi: 10.1016/j.jpain.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Antinociceptive effect of Gosha-jinki-gan, a kampo medicine, in streptozotocin-induced diabetic mice. Jpn J Pharmacol. 1999;79:169–175. doi: 10.1254/jjp.79.169. [DOI] [PubMed] [Google Scholar]
- 142.Omiya Y, Goto K, Suzuki Y, Ishige A, Komatsu Y. Analgesia-producing mechanism of processed aconiti tuber: role of dynorphin, an endogenous. KAPPA.Opioid ligand, in the rodent spinal cord. Jpn J Pharmacol. 1999;79:295–301. doi: 10.1254/jjp.79.295. [DOI] [PubMed] [Google Scholar]
- 143.Xu H, Arita H, Hayashida M, Zhang L, Sekiyama H, Hanaoka K. Pain-relieving effects of processed Aconiti tuber in CCI-neuropathic rats. J Ethnopharmacol. 2006;103:392–397. doi: 10.1016/j.jep.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 144.Wu HY, Mao XF, Fan H, Wang YX. p38β mitogen-activated protein kinase signaling mediates exenatide-stimulated microglial β-endorphin expression. Mol Pharmacol. 2017;91:451–463. doi: 10.1124/mol.116.107102. [DOI] [PubMed] [Google Scholar]
- 145.Buesa I, Urrutia A, Bilbao J, Aguilera L, Zimmermann M, Azkue JJ. Non-linear morphine-induced depression of spinal excitation following long-term potentiation of C fibre-evoked spinal field potentials. Eur J Pain. 2008;12:814–817. doi: 10.1016/j.ejpain.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 146.Velázquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol Res. 2007;55:578–589. doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res. 1995;677:257–267. doi: 10.1016/0006-8993(95)00161-I. [DOI] [PubMed] [Google Scholar]
- 148.Rush A, Cummins T, Waxman S. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu X, Eschenfelder S, Blenk KH, Jänig W, Häbler HJ. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84:309–318. doi: 10.1016/S0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 150.Michaelis M, Liu X, Jänig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie MX, Pang RP, Yang J, Shen KF, Xu J, Zhong XX, et al. Bulleyaconitine A preferably reduces tetrodotoxin-sensitive sodium current in uninjured dorsal root ganglion neurons of neuropathic rats probably via inhibition of protein kinase C. Pain. 2017;158:2169–2180. doi: 10.1097/j.pain.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 152.Xie MX, Yang J, Pang R, Zeng WA, Ouyang H, Liu YQ, et al. Bulleyaconitine A attenuates hyperexcitability of dorsal root ganglion neurons induced by spared nerve injury: the role of preferably blocking Nav1.7 and Nav1.3 channels. Mol Pain. 2018;14:174480691877849. doi: 10.1177/1744806918778491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li J, Ren W, Huang XJ, Zou DJ, Hu X. Bullatine A, a diterpenoid alkaloid of the genus Aconitum, could attenuate ATP-induced BV-2 microglia death/apoptosis via P2X receptor pathways. Brain Res Bull. 2013;97:81–85. doi: 10.1016/j.brainresbull.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 154.Chan N, Yu JX, Chen X, Xu RX. Pharmacological actions of Uncaria alkaloids, Rhynchophylline and Isorhynchophylline. Acta Pharmacol Sin. 2003;24:97–101. [PubMed] [Google Scholar]
- 155.Wang JL, Shen XL, Chen QH, Qi G, Wang W, Wang FP. Structure-analgesic activity relationship studies on the C(18)- and C(19)-diterpenoid alkaloids. Chem Pharm Bull. 2009;57:801–807. doi: 10.1248/cpb.57.801. [DOI] [PubMed] [Google Scholar]
- 156.An Editorial Committee of the Administration Bureau of Traditional Chinese Medicine. Chinese Materia Medica. China: Shanghai Science & Technology Press; 2000.
- 157.Zhang YB, Yang L, Luo D, Chen NH, Wu ZN, Ye WC, et al. Sophalines E-I, five quinolizidine-based alkaloids with antiviral activities against the hepatitis B virus from the seeds of Sophora alopecuroides. Org Lett. 2018;20:5942–5946. doi: 10.1021/acs.orglett.8b02637. [DOI] [PubMed] [Google Scholar]
- 158.Wang R, Deng X, Gao Q, Wu X, Han L, Gao X, et al. Sophora alopecuroides L.: an ethnopharmacological, phytochemical, and pharmacological review. J Ethnopharmacol. 2020;248:112172. doi: 10.1016/j.jep.2019.112172. [DOI] [PubMed] [Google Scholar]
- 159.He X, Fang J, Huang L, Wang J, Huang X. Sophora flavescens Ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2015;172:10–29. doi: 10.1016/j.jep.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 160.Sun W. Brief manual for natural active ingredients. China: China Medical Science Press; 1998. [Google Scholar]
- 161.Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of Phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134:234–242. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 162.Yu M, Ren L, Liang F, Zhang Y, Jiang L, Ma W, et al. Effect of epiberberine from Coptis chinensis Franch on inhibition of tumor growth in MKN-45 xenograft mice. Phytomedicine. 2020;76:153216. doi: 10.1016/j.phymed.2020.153216. [DOI] [PubMed] [Google Scholar]
- 163.Chu H, Jin G, Friedman E, Zhen X. Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol. 2008;28:491–499. doi: 10.1007/s10571-007-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen FK, Liu XQ. Determination of active ingredients in traditional Chinese medicine. China: People's Medical Publishing House; 2008. [Google Scholar]
- 165.Zhao Z, Xiao J, Wang J, Dong W, Peng Z, An D. Anti-inflammatory effects of novel sinomenine derivatives. Int Immunopharmacol. 2015;29:354–360. doi: 10.1016/j.intimp.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 166.Chao J, Liao JW, Peng WH, Lee MS, Pao LH, Cheng HY. Antioxidant, analgesic, anti-inflammatory, and hepatoprotective effects of the ethanol extract of Mahonia oiwakensis Stem. Int J Mol Sci. 2013;14:2928–2945. doi: 10.3390/ijms14022928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu X, Hu Z, Shi Q, Zeng H, Shen Y, Jin H, et al. Anti-inflammatory and anti-nociceptive activities of compounds from Tinospora sagittata (Oliv.) Gagnep. Arch Pharmacal Res. 2010;33:981–987. doi: 10.1007/s12272-010-0702-7. [DOI] [PubMed] [Google Scholar]
- 168.Tan Q, Zhang J. Evodiamine and its role in Chronic diseases. Adv Exp Med Biol. 2016;929:315–328. doi: 10.1007/978-3-319-41342-6_14. [DOI] [PubMed] [Google Scholar]
- 169.Lu L, Huang R, Wu Y, Jin JM, Chen HZ, Zhang LJ, et al. Brucine: a review of phytochemistry, pharmacology, and toxicology. Front Pharmacol. 2020;11:377. doi: 10.3389/fphar.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shoaib R, Zhang JY, Mao XF, Wang Y. Gelsemine and koumine, principal active ingredients of Gelsemium, exhibit mechanical antiallodynia via spinal glycine receptor activation-induced allopregnanolone biosynthesis. Biochem Pharmacol. 2019;161:136–148. doi: 10.1016/j.bcp.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 171.Yang W, Ip SP, Liu L, Xian YF, Lin ZX. Uncaria rhynchophylla and its major constituents on central nervous system: a review on their pharmacological actions. Curr Vasc Pharmacol. 2020;18:346–357. doi: 10.2174/1570161117666190704092841. [DOI] [PubMed] [Google Scholar]
- 172.Luo Y, Wang CZ, Sawadogo R, Tan T, Yuan CS. Effects of herbal medicines on pain management. Am J Chin Med. 2020;48:1–16. doi: 10.1142/S0192415X20500019. [DOI] [PubMed] [Google Scholar]
- 173.Xie MX, Zhu HQ, Pang R, Wen BT, Liu X. Mechanisms for therapeutic effect of bulleyaconitine A on chronic pain. Mol Pain. 2018;14:174480691879724. doi: 10.1177/1744806918797243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun J, Peng Y, Wu H, Zhang X, Zhong Y, Xiao Y, et al. Guanfu base A, an antiarrhythmic alkaloid of aconitum coreanum is A CYP2D6 inhibitor of human, monkey, and dog isoforms. Drug Metab Dispos. 2015;43:713–724. doi: 10.1124/dmd.114.060905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.