Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of ventilator-associated pneumonia (VAP). Patients with VAP have poorly functioning neutrophils, related to increased levels of the complement fragment C5a. The antibiotic linezolid has been useful in controlling MRSA-related VAP infections; however clinical benefit does not always correlate with antimicrobial effect, suggesting the possibility of immunomodulatory properties. Here the effects of linezolid on healthy and dysfunctional neutrophils (modelled by C5a-induced injury) was investigated. Functional assays (killing, phagocytosis, transmigration, and respiratory burst) were used to assess the effects of pre-, co- and post-incubating linezolid (0.4–40 mg/L) with healthy neutrophils relative to those with C5a-induced injury. C5a decreased neutrophil killing, and phagocytosis of MRSA. Furthermore, C5a significantly decreased neutrophil transmigration to IL-8, but did not affect respiratory burst. Co-incubation of linezolid significantly improved killing of MRSA by dysfunctional neutrophils, which was supported by concomitant increases in phagocytosis. Conversely linezolid impaired killing responses in healthy neutrophils. Pre- or post-incubation of linezolid prior or following C5a induced injury had no effect on neutrophil function. This study suggests that linezolid has immunomodulatory properties that protect human neutrophils from injury and provides insight into its mode of action beyond a basic antibiotic.
Subject terms: Infectious diseases, Respiratory tract diseases, Cell biology, Microbiology, Infectious diseases, Innate immune cells, Inflammation, Acute inflammation
Introduction
Ventilator-associated pneumonia (VAP) is an important infection acquired in the intensive care unit (ICU) and can occur in up to 20% of patients mechanically ventilated for periods greater than 48 h1. The 2016 annual European report on healthcare-associated infections showed that of 12,735 patients staying more than 2 days in ICU, 6% developed pneumonia (where 97% of these were intubated), with Staphylococcus aureus the causative organism in 17.8% of cases (30% being methicillin-resistant S. aureus (MRSA))2. Treatments for MRSA pneumonia have relied on vancomycin and teicoplanin until the introduction of linezolid in 2000, which has been particularly successful at treating MRSA pneumonia3–5. While it is clear that linezolid has a clinical3–5 and economic benefit6,7 in treating MRSA pneumonia, the apparent treatment effect is not due to its antimicrobial activity alone3,8, suggesting potential host-specific effects, such as immunomodulation.
The underlying illness in critically ill patients, including those with VAP, is often associated with major deficiencies in the innate immune system9 specifically, neutrophil dysfunction, an inability to phagocytose or produce a respiratory burst10,11. Our previous studies found that VAP patients’ neutrophils had 36% lower phagocytic capacity than healthy volunteers, and was significantly and negatively correlated with serum C3a des-Arg (C3a breakdown product) and positively correlated with neutrophil cell surface expression of the C5a receptor (CD88). In vitro modelling in healthy volunteer neutrophils found that C5a treatment could mimic impaired phagocytosis and down-regulate CD8812–14.
The effect of linezolid on dysfunctional human neutrophils has not been studied to date. However, numerous studies have addressed the effects of linezolid on neutrophils from healthy volunteers. For instance, linezolid at concentrations of 10–160 mg/L, had no negative effects on chemotaxis or respiratory burst in either a pure drug or intravenous injection formulation15. Similarly, pre-incubation of healthy neutrophils with linezolid (2–20 mg/L) had no effect on phagocytosis of methicillin-resistant or -susceptible S. aureus, or Enterococcus faecalis (vancomycin-resistant or -susceptible)16. Other studies have shown small decreases in phagocytosis in response to certain strains of Escherichia coli17. Interestingly, Pascual and co-workers have shown that linezolid penetrates the neutrophil rapidly as intracellular concentrations greater than those of the external environment are reached within 20 min18. This ability to cross biological membranes is reflected in its high concentrations in lung epithelial lining fluid (ELF). These studies suggest that linezolid in concentrations far in excess of its MIC (4 mg/L)19 are not cytotoxic to healthy neutrophils using a variety of functional assays.
Using our established model of C5a-induced neutrophil dysfunction12,13, we investigated the effect of linezolid on both healthy and clinically relevant C5a-impaired neutrophils in a variety of relevant functional assays.
Results
Killing response of neutrophils to VAP39 resulted in ~ 50% decrease in colony counts over the first 2 h of infection (Fig. 1A). MRSA incubated without neutrophils doubled in number over the same period showing strain viability in our assay media. Incubation of neutrophils with increasing doses of C5a (1–100 ng/ml) resulted in a significant increase in the survival of MRSA at the highest dose of C5a (100 ng/ml) demonstrating dysfunctional killing responses. Consistent with this result a significant decrease (p < 0.05) was detected in neutrophil phagocytosis of VAP39 (Fig. 1B and Supplementary Image 2) over 2 h. Thus C5a (100 ng/ml) decreased neutrophil killing which is associated with attenuated phagocytosis (Fig. 1A,B).
Figure 1.
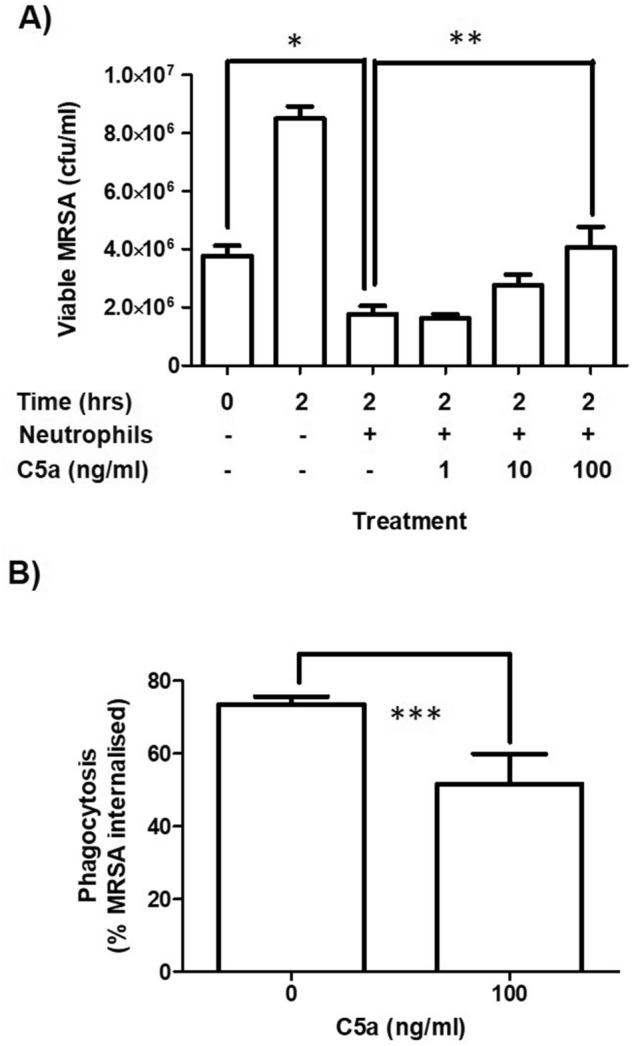
Effects of C5a on neutrophil function: killing and phagocytosis. Purified neutrophils were treated with or without C5a (1–100 ng/ml) for 16 h prior to functional assay. (A) Killing expressed as viable counts of MRSA (cfu/ml). (B) Phagocytosis expressed as the percentage of neutrophils containing MRSA. Data are expressed as the mean ± SEM of 4 separate donors. *Represents a significant difference between viable counts of MRSA at t = 0 versus t = 2 with neutrophils present. **Represents a significant difference between viable counts of MRSA at t = 2 with neutrophils present versus t = 2 with neutrophils present treated with C5a at 100 ng/ml. ***Represents a significant difference in phagocytosis between neutrophils treated and not treated with C5a.
The effect of C5a on the ability of neutrophils to transmigrate across membrane filters in response to IL-8 was investigated (Fig. 2A). IL-8 induced a significant increase (p < 0.05) in neutrophil transmigration (Fig. 2 and Supplementary image 3). Addition of C5a caused a dose-dependent decrease in IL-8-induced transmigration which reached significance (P < 0.05) at the highest dose of C5a (100 ng/ml). In contrast, while neutrophil respiratory burst (Fig. 2B) was significantly induced (p < 0.05) using PMA it was independent of C5a over the dose range studied. Collectively these results (Figs. 1 and 2) demonstrate that functional responses (without C5a) and dysfunctional responses (with C5a at 100 ng/ml) can be produced in killing, phagocytosis and transmigration. Neutrophils are viable as confirmed by full respiratory burst in healthy and dysfunctional neutrophils (Fig. 2B). Neutrophil metabolic activity measurements using alamar blue confirmed that cellular reducing power decreased up to 10% and 45% in functional and dysfunctional neutrophils respectively (data not shown) and is consistent with a recent study confirming the effects of C5a on cellular respiration20. Using these two conditions (with and without C5a at 100 ng/ml) the effect of linezolid on functional and dysfunctional neutrophils was investigated.
Figure 2.
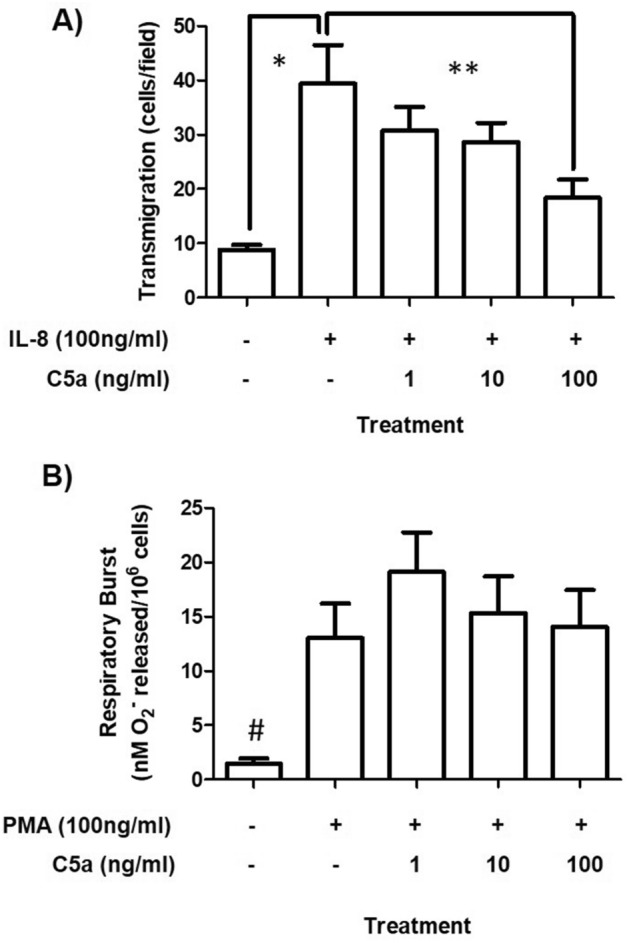
Effects of C5a on neutrophil function: transmigration and respiratory burst. (A) Transmigration is expressed as the number of cells counted per high power field. (B) Respiratory burst is expressed as the amount of superoxide release per million cells. Data are expressed as the mean ± SEM of 4 separate donors. Differences between groups were calculated using ANOVA with a Tukey’s post-hoc test where p < 0.05 considered significant. *Represents a significant difference between neutrophil transmigration with and without IL-8. **Represents a significant difference between IL-8 stimulated neutrophils treated with and without C5a. #Represents a significant difference in respiratory burst between neutrophils stimulated with PMA and those without PMA.
Functional (grey bars) and dysfunctional neutrophils (black bars) were generated and co-incubated with linezolid during the C5a incubation period (Fig. 3). Functional neutrophils killed MRSA over 2 h (white bar vs. first grey bar in Fig. 3A), whereas linezolid had no effect on killing by functional neutrophils (Fig. 3A, grey bars). This result also confirms there was no anti-bacterial effect from linezolid carried over inside neutrophils. In contrast, linezolid significantly improved (at 40 mg/L) killing of VAP39 in dysfunctional neutrophils (Fig. 3A, black bars). Indeed, the killing response was no different to that produced initially in functional neutrophils (first grey bar vs. final black bar) showing that linezolid could prevent C5a-induced injury in neutrophils when co-incubated with C5a. Further investigation confirmed that linezolid, co-incubated with C5a could also attenuate the defect induced in phagocytosis of MRSA (Fig. 3B). Thus, linezolid appears to improve killing by dysfunctional neutrophils by improving phagocytosis. This mechanism was only observed during co-incubation and not when linezolid was incubated prior to or after the C5a-injury period (Supplementary Figs. 2 and 3). Further assessment of neutrophil metabolic activity by alamar blue confirmed that linezolid decreased (10–20%) the reducing power of neutrophils after 1 h (pre and post-incubation protocols) and by 10–30% over 16 h (co-incubation) (Supplementary Fig. 4) when given alone. In the absence and presence of C5a, the reducing power of the cell decreased 10 and 50% respectively and is consistent with a recent study confirming the effects of C5a on cellular respiration20.
Figure 3.
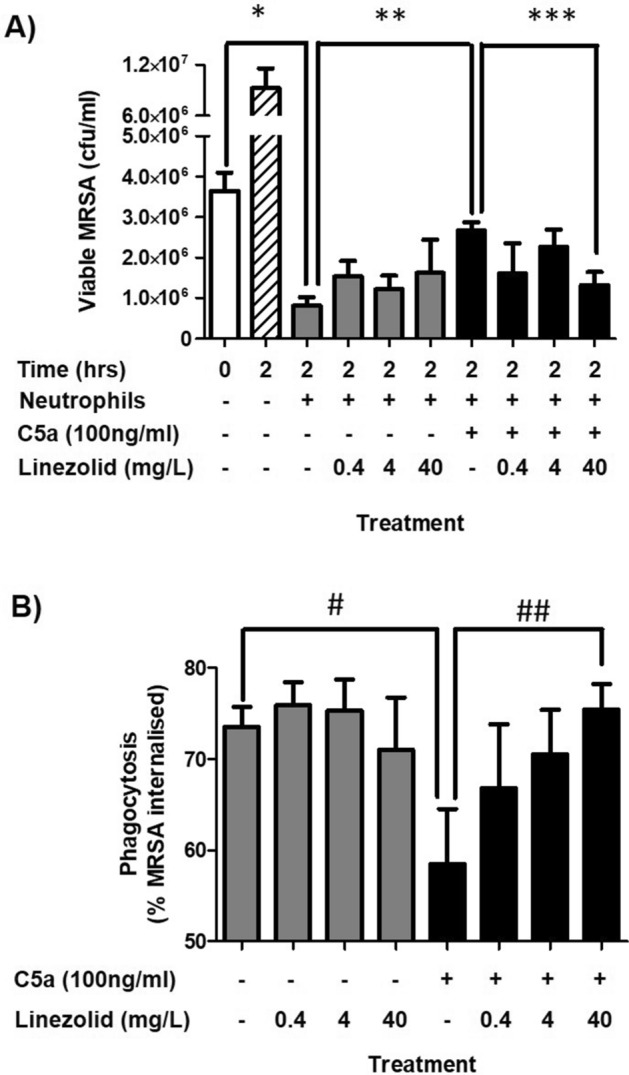
Effects of linezolid on functional and dysfunctional neutrophil killing and phagocytosis. (A) Killing expressed as viable counts of MRSA (cfu/ml). (B) Phagocytosis expressed as the percentage of neutrophils containing MRSA. Data are expressed as the mean ± SEM of 4 separate experiments. White bar represents viable MRSA at t = 0. Hatched bar represents viable MRSA at t = 2 without neutrophils. Grey and black bars represent neutrophils at t = 2 without and with C5a respectively. *Represents a significant difference between viable counts of MRSA at t = 0 versus t = 2 with neutrophils present. **Represents a significant difference between viable counts of MRSA treated with functional and dysfunctional neutrophils at t = 2. ***Represents a significant difference between viable counts of MRSA exposed to dysfunctional neutrophils treated with or without linezolid. # Represents a significant difference in phagocytosis of MRSA by functional and dysfunctional neutrophils. ## Represents a significant difference in the phagocytosis of MRSA by dysfunctional neutrophils with or without linezolid.
The effect of linezolid on IL-8-induced transmigration was then assessed by co-incubating functional and dysfunctional neutrophils with linezolid during C5a incubation period (Fig. 4). In functional neutrophils, linezolid caused a significant dose dependent decrease in transmigration (Fig. 4, grey bars), while having no effect on the transmigration of dysfunctional neutrophils (Fig. 4, black bars). Furthermore, pre-incubation or post-incubation with linezolid prior or after the C5a injury period had no significant effects on transmigration, (Supplementary Fig. 5A and B).
Figure 4.
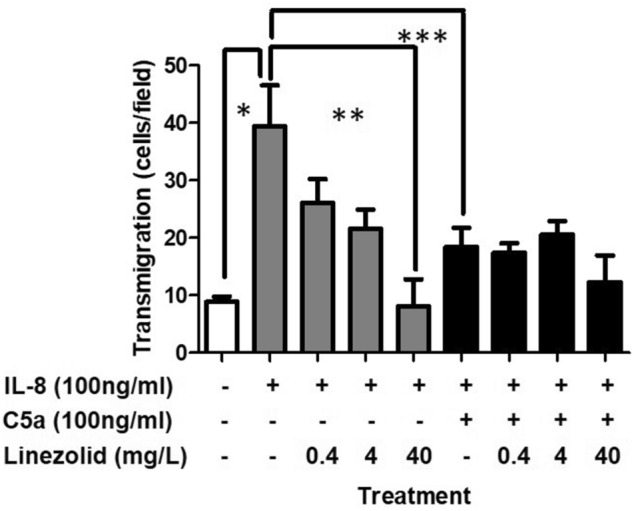
Effects of linezolid on functional and dysfunctional neutrophil IL-8 induced transmigration. Grey and black bars represent functional and dysfunctional neutrophils, respectively. Data expressed as the mean ± SEM of 4 separate experiments. *Represents a significant difference between the transmigration of functional and dysfunctional neutrophils. **Represents a significant difference between the transmigration of neutrophils treated with linezolid (40 mg/L) and those without.
Finally, the effects of linezolid on respiratory burst (Fig. 5) were assessed by adding PMA to functional and dysfunctional neutrophils respectively. A significant respiratory burst response to PMA could be generated in functional (Fig. 5, grey bars) and in dysfunctional neutrophils (Fig. 5, black bars) but linezolid had no effect on this PMA induced response (Fig. 5). Samples that were not stimulated by PMA but included combinations with and without C5a or linezolid were not significantly increased compared to negative control in Fig. 5. The fact that a full respiratory burst could be induced in dysfunctional neutrophils, albeit independent of linezolid, encouraged us to investigate whether pre-incubation and post-incubation of linezolid prior to and after C5a induced injury may be affected (Supplementary Fig. 6). The results showed increased trends in respiratory burst at lower doses of linezolid (0.4 and 4 μg/ml) but not at the highest dose (40 mg/L) for both functional and dysfunctional neutrophils.
Figure 5.
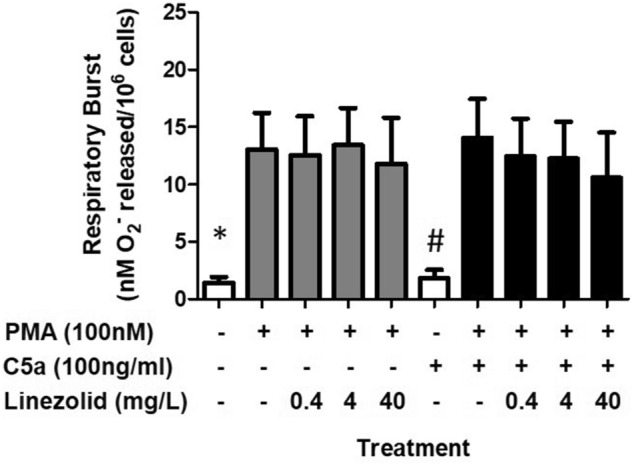
Effects of linezolid on functional and dysfunctional neutrophil respiratory burst. Grey and black bars represent functional and dysfunctional neutrophils, respectively following treatment with PMA and linezolid. Data expressed as the mean ± SEM of 4 separate experiments. *Represents a significant difference between PMA treated and untreated functional neutrophils (grey bars). # Represents a significant difference between PMA treated and untreated dysfunctional neutrophils (black bars).
Discussion
The current study extends our previous model of C5a-induced dysfunction in response to P. aeruginosa12,13 and S. epidermidis biofilm accumulation in whole blood21 to investigate the potential of the oxazolidinone, linezolid, to modulate important innate immune responses in healthy and dysfunctional neutrophils. Concentrations of C5a measured in BAL during lung infection range from 20 to 180 ng/ml22,23. The current study used 1–100 ng/ml C5a to induce dysfunction in MRSA killing, phagocytosis and transmigration, but retain a functional respiratory burst. This is in keeping with our previous work on S. epidermidis biofilm where C5a concentrations of up to 80 ng/ml were generated ex vivo21. Taken together our current and previous neutrophil killing and phagocytosis results using P. aeruginosa, S. aureus and S. epidermidis suggest that neutrophil sensitivities to clinically relevant C5a concentrations may well be dependent on bacterial species when investigating killing and phagocytosis responses.
There is now very clear scientific evidence, defining the metabolic and signalling pathways underlying functional neutrophils24 and those subjected to C5a-induced dysfunction12,13,20,25. Thus, ligation of C5aR1 (CD88) by C5a, activates its inherent G-protein activity resulting in phosphoinositide 3-kinase delta (PI3Kδ) activation inhibiting activation of the small GTPase RhoA, actin polymerisation and phagocytosis13. At the time of writing Wood and co-workers confirmed that C5a induced decreases in the phagocytosis of S. aureus are associated with impaired phagosomal maturation by phosphoproteomic remodelling through selective impairment of phagosomal protein phosphorylation, including endosomal marker ZFYVE16 and V-ATPase protein channel component ATPV1G125. In addition, Denk and co-workers proposed that C5aR1 ligation also leads to selective activation of the Na+/H+ exchanger causing increased intracellular alkalinisation (pHi), increased glycolytic flux, glucose uptake and lactate and proton excretion20. In the current study, alamar blue assessment of the reducing power of the cells also confirmed 50% decreased in response to C5a. This is consistent with broad metabolic defects observed in immune cells (immunoparalysis) and characterised by a switch from oxidative phosphorylation to aerobic glycolysis26. Indeed, a deficit in energy status (nutrition delivered—energy expenditure) has been reported as a risk factor for the development of S. aureus VAP27. These effects also appear reversible, as GM-CSF can reverse the effects of C5a-induced injury by reactivating RhoA at the cellular level13 and can improve neutrophil phagocytosis in critically ill patients28. Furthermore, others have shown that IFN-γ can partially restore function in immunoparalysed leukocytes26.
The present study showed that C5a has little effect on oxygen radical killing mechanisms, unlike a study by Huber–Lang which demonstrated C5a-induced impairment of ROS production and failure of NADPH oxidase assembly29. Indeed our previous work could not demonstrate C5a impairment of ROS either, but did find that it could be negatively correlated with C3a des-Arg (C5a degradation product) levels in the serum of critically ill patients12,13. This may be attributed to functional differences in rat and human neutrophils. Clearly, the C5a effect we observe is independent on the method of superoxide radical production. In our previous study, neutrophils were primed with 100 nM platelet-activating factor (PAF) and stimulated with 100 nM formyl methionyl leucyl phenylalanine (fMLP), whilst in the current study a single PMA induction was used. Alternatively, killing mechanisms in the current study may be protease-mediated, as we have shown previously for neutrophil dysfunction in the lung30.
Choosing appropriate doses of linezolid was important during experimental design of this study. There were three major considerations behind our rationale; firstly, to approximate cellular exposure such as in the epithelial lining fluid (ELF), intracellularly and serum concentrations in humans; secondly, to take into account antibiotic concentrations in health and disease; and thirdly to consider defined MIC breakpoints for antibiotic and pathogen. Numerous pharmacokinetic studies and reviews have addressed these issues31–34. For example pharmacokinetic studies in humans confirmed that 600 mg of oral linezolid resulted in mean concentrations of 7.6, 24.3 and 1.4 mg/L 12 h later in plasma, ELF and alveolar cells respectively31. The EUCAST breakpoint for linezolid on MRSA is 4 mg/L19. Thus, our decision to use linezolid at 0.4, 4 and 40 mg/L was well justified as these concentrations are very likely to contact neutrophils in both injured and uninjured lung.
Three linezolid incubation conditions were used to investigate effects on functional and dysfunctional neutrophils and included, pre-, co-, and post C5a incubation period strategies. The rationale being that protection, inhibition and restoration of neutrophil function could be measured respectively. The current study showed very clearly that linezolid’s effects were produced solely using the co-infection strategy. Thus, speculation on linezolid’s targets must draw evidence from C5a signalling processes and emphasise the importance of the C5aR1 (CD88) cell surface receptor, the PI3Kδ-RhoA pathway, phagosome maturation and modulators of glycolytic flux such as glucose transporters and pHi12,13,20,25. Critically, Wood et al. alluded to the timing of exposure to C5a as important in downstream effects25. They find that C5a only impairs phagocytosis if the cells are exposed to it before they encounter S. aureus—not at the same time or after initial exposure. This effect is independent of CD88. In the current study it seems reasonable to assume that co-infection with linezolid must influence this critical C5a signalling period and its effect is independent of cell surface CD88, but dependent on effects produced intracellularly on pHi or maturation of the phagosome20,25.
Further insight into the cellular effects of linezolid may be gained when viewed anatomically. There is good evidence for linezolid penetration into the intracellular compartment of cells33, thus binding and biological activity at the level of organelles is likely. Interestingly, sufficient patient data now exist on the inhibitory effect of linezolid on protein synthesis in the mitochondria leading to anaerobic glycolysis and cellular acidosis35–38. Specifically with respect to the neutrophil, the mitochondrion has evolved a specific function in apoptosis (not ATP production) and contains numerous apoptotic proteins39. There is an intriguing possibility for linezolid to modify neutrophil function in a state-dependent (function/dysfunction) manner as shown in this work. However a recent study by Akinnusi and co-workers could not show any effect of linezolid on neutrophil apoptosis in a MRSA pneumonia mouse model40.
In healthy neutrophils, linezolid produced a significant dose-dependent decrease in transmigration. This is partly supported by other studies which showed either no effect on neutrophil functions15,16 or mild toxic responses in phagocytosis17 over similar dose ranges (0–160 mg/L) to our study (0.4–40 mg/L). The trends towards enhanced respiratory burst observed in this study may be due to the much shorter incubation times (1 h) which may generate a priming effect as with a PAF/fMLP combination shown previously12. One strength of the current study is extension of this work to dysfunctional cells which are more likely to be present in the critically ill patient who receives linezolid. Two major findings are particularly pertinent; (1) linezolid inhibited C5a-induced dysfunction in neutrophil killing; (2) linezolid inhibited C5a induced dysfunction in neutrophil phagocytosis. Firstly, these results suggest that the effects of C5a are reversible. Secondly, they support previous observations in clinical studies, where clinical benefit could not be explained by antimicrobial activity alone3,8. We speculate that linezolid’s inhibition of C5a-induced dysfunction (under co-incubation conditions only) could be responsible for a large proportion of the underlying mechanism on clinical benefit. Thus, less neutrophils would become dysfunctional in the presence of C5a. Indeed, if this were the case the inhibitory effect that linezolid has on functional neutrophil transmigration may now come into play and also reduce the excessive inflammation seen in the lung during pneumonia. This is compelling as there are already numerous reports that suggest linezolid modulates cytokine cascades41–46. Furthermore, such effects on healthy and dysfunctional neutrophils raise the interesting novel possibility that linezolid’s action could be dependent on the state of the cell. This is analogous to antibiotics having activity on dividing cells but not on dormant bacteria47.
There is a growing literature that suggests that linezolid has an advantage over glycopeptides such as vancomycin and teicoplanin in the treatment of proven MRSA pneumonia3–5,48,49. At least four potential reasons underlie this effect. Firstly, concentrations in ELF can consistently exceed the MIC breakpoint (4 mg/L) needed for adequate antimicrobial activity31,34,48,50 with concentrations reported to stay above MIC levels 100% of the time31. Secondly, vancomycin is associated with renal toxicity and neutropenia51. Thirdly, protein synthesis inhibition by linezolid produces a ‘non-bacteriolytic’ action and reduces virulence factor expression52–54. Finally, linezolid may affect host cell functions and cytokine networks41–46. Indeed, a recent experimental infection model of MRSA pneumonia and clinical studies in community-acquired MRSA pneumonia suggest protective immunomodulatory effects of linezolid55,56. These studies lend support to the hypothesis that linezolid has ‘additional’ antimicrobial efficacy through mechanisms involving immunomodulation.
We accept that this study is not without limitations. Firstly, we did not investigate the effects of glycopeptides (such as vancomycin) on neutrophils. We did investigate vancomycin as a potential control early in this study but found that dosing was inconsistent especially when combined with C5a in the dysfunctional model and thus did not pursue this further. However, the effects of vancomycin on neutrophils have been well studied57–60, and it is interesting to note that dosing remains a difficulty following 60 years of use61. Secondly, this study used one strain of MRSA (VAP 39) from our previous studies12, but selected appropriately with justification from a choice of 6 strains (Table 1 and supplementary Fig. 1). This allowed the current study to focus on intricate cell biology. Future work will investigate these effects across the wider S. aureus species. Thirdly, we also undertook preliminary experiments with cellular inhibitors (e.g. cytochalasin D and MAP kinase inhibitors) as we have done previously12,13,21,62, however the use of three reagents (C5a, linezolid and inhibitor) resulted in overwhelming cell toxicity (95–100% cell death) and were discontinued. Fourth, studies using C5a to investigate its effects in assays of neutrophil function/dysfunction should take care in design as chemotactic agents can have contrasting effects and lead to neutrophil priming/de-priming responses63–65. Finally, studies such as this could be applied to the next generation of novel oxazolidinone and anti-staphylococcal agents, such as tedizolid, which has potential for treating MRSA infections66.
Table 1.
VAP and non VAP MRSA strains.
| Organism(s) | Strain | Islolated from | CFU/ml | VAP (+ / −) | Antibiotic MIC (μg/ml) | |
|---|---|---|---|---|---|---|
| Linezolid | Vancomycin | |||||
|
H. influenzae S. aureus (MRSA) |
VAP 025 | BAL |
104 103 |
+ | 4 (S) | 1 (S) |
| S. aureus (MRSA) | VAP 026 | BAL | 102 | − | 4 (S) | 0.5 (S) |
| S. aureus (MRSA) | VAP 032 | ETA | 106 | − | 4 (S) | 1 (S) |
|
H. influenzae S. aureus (MRSA) |
VAP 034 | BAL + ETA |
104 104 |
+ | 4 (S) | 1 (S) |
| S. aureus (MRSA) | VAP 039 | BAL | 104 | + | 4 (S) | 1 (S) |
|
Aspergillus spp. S.aureus (MRSA) |
VAP 040 | BAL |
102 103 |
− | 4 (S) | 1 (S) |
Six MRSA strains were used and clinically defined as VAP or non VAP from a previous study12. Column 1 shows isolates detected in the original study with MRSA isolated and sub-cultured to produce the work in this manuscript. The strain used throughout this study, VAP39, is highlighted in bold.
To conclude, this study confirms that linezolid has immunomodulatory properties that protect human neutrophils from injury and provides insight into its mode of action beyond a basic antibiotic. These results will go some way in explaining why the therapeutic/treatment effect of linezolid is greater than its antimicrobial activity.
Materials and methods
All methods were carried out in accordance with relevant guidelines and regulations.
Bacterial strains and determination of MIC
Six clinical MRSA isolates obtained from bronchoalveolar alveolar fluid (BAL) in our previous studies were used (Table 1)12,13, and methicillin-sensitive S. aureus Cowan 1 was used as a control strain. Linezolid and vancomycin MICs were determined by using the limiting dilution method. Briefly, MRSA isolates were grown in tryptic soy broth ((TSB); Becton Dickinson, Cockeysville, USA)) overnight at 37 °C, then washed and resuspended to an OD600 = 0.1 (~ 1 × 107 cfu/ml). MRSA isolates (5 × 105 cfu) were then added to a 96-well plate containing a dilution series of linezolid (0.25–256 mg/L final) in TSB and incubated overnight at 37 °C. Plates were examined the following day and the lowest concentration of linezolid inhibiting visible growth determined. Antimicrobial sensitivity was compared to current EUCAST guidelines19.
Selection of MRSA strain VAP 39
To select an appropriate MRSA strain for these studies all six MRSA isolates from our previous studies were tested for their sensitivity to linezolid and vancomycin (Table 1). Strains were isolated from the bronchoalveolar alveolar fluid (BAL) of patients, with 3 coming from patients with bacterial growth > 104 colony-forming units/ml (CFU/ml) of lavage fluid, and 3 with growth below this conventional cut-off for the diagnosis of VAP. All strains had consistent MIC values for linezolid (4 μg/ml) and for vancomycin (1 μg/ml) except VAP26 (0.5 μg/ml). Thus, all MRSA strains were sensitive to linezolid and vancomycin by EUCAST guidelines19. Further consideration (Table 1) of the specificity of the source (BAL only) confirmed VAP39 as the test isolate for further studies. In addition, VAP39 was appropriate for downstream leukocyte assays, as comparisons between VAP39, VAP26 and laboratory reference, methicillin-sensitive S. aureus Cowan 1 strain showed no differences in killing and phagocytosis assays (Supplementary Fig. 1A–D). Therefore, VAP39 was selected for use in the remaining experiments.
Bacterial culture for functional assays
One colony of MRSA VAP39 was inoculated into TSB and incubated overnight at 37 °C. One millilitre of overnight culture was centrifuged at 9677 g and the supernatant removed. Pellets were resuspended in 1 ml of Iscove’s Modified Dulbecco’s Medium (IMDM, Thermofisher) and washed once before measuring the OD600 and adjusted to OD600 = 0.1 (~ 1 × 107 cfu/ml).
Isolation of human neutrophils
Whole blood from healthy volunteers was isolated using the vacuette blood collection system (5–9 ml) on the day of the experiment. Volunteers gave written informed consent. The project (Reference 13/WA/0190) was reviewed and the procedures and protocols approved by the local research ethics committee, Wales REC 6 (E-mail: Wales.REC6@nhs.uk). Healthy volunteer neutrophils were isolated and purified as previously described67. Freshly drawn blood was collected into citrated (light blue tops) tubes and mixed by gentle inversion prior to centrifugation at 350 g for 20 min. The platelet rich plasma was aspirated (for serum generation) and the leukocytes remaining in the cellular layer separated from red blood cells through dextran (1.25% final concentration) sedimentation. Leukocytes were removed with a Pasteur pipette and washed with warm saline, before centrifugation at 350 g for 6 min. The leukocyte cell pellet was resuspended in 3 ml of 55% isotonic percoll (GE Healthcare). Then, a tube containing overlaid solutions of percoll was prepared comprising 3 ml each of 81%, 70% and the leukocyte suspension in 55% percoll. Then, percoll gradients were centrifuged at 720 g for 20 min. Granulocytes were harvested at the 70/81% interface using a Pasteur pipette, washed in PBS without calcium and magnesium prior to centrifugation at 230 g for 6 min. Granulocytes were resuspended in buffer appropriate for functional assay. Neutrophil were only used at > 95% purity. Neutrophils counted by trypan blue staining after treatment with C5a/linezolid alone or in combination had viabilities ranging from 90 to 98%.
Generation of neutrophil dysfunction
Purified neutrophils were resuspended to 1 × 107 viable cells/ml in IMDM, then diluted to 1 × 106/ml in IMDM containing 3% autologous serum and recombinant human C5a (R and D Systems, Abingdon) at 1–100 ng/ml or untreated control12. Neutrophils were incubated by rotation (10 rpm) at 37 °C for 16 h prior to functional assays. Neutrophil viable counts were re-assessed by trypan blue exclusion and adjusted once again to 1 × 106 viable cells/ml.
Linezolid incubation conditions
Linezolid was used at concentrations (0.4–40 mg/ml) consistent with tissue and cellular levels of the drug in humans31–34. Three incubation strategies were used to study the effect of linezolid on neutrophils.
To study protection against injury: Linezolid (0.4–40 mg/L) was added 1 h prior to C5a injury and washed out before addition of C5a;
To study inhibition of injury: Linezolid (0.4–40 mg/L) was added in combination with C5a for 16 h;
To study restoration of function: Linezolid (0.4–40 mg/L) was added for 1 h following C5a incubation.
After each treatment, neutrophils were centrifuged at 300 g for 5 min, the supernatant removed and cells gently re-suspended in appropriate buffer for functional assays. Linezolid was removed prior to all assays involving MRSA so that it would have no direct antimicrobial effect on the bacteria.
Neutrophil functional assays
Phagocytosis and killing assays
MRSA (OD600 = 1) was pre-opsonised with 100% autologous serum for 30 min at 37 °C and then corrected to an OD600 = 0.1 in 3% autologous serum/IMDM. Treated (C5a and linezolid as above) and untreated neutrophils at twice normal concentration were exposed for 2 h to pre-opsonised MRSA (multiplicity of infection = 1). Incubation was carried out at constant rotation (10 rpm) at 37 °C. Then 80 μl of MRSA-infected neutrophils was used for cytospin preparations (300 rpm for 3 min), air dried and stained with Hemacolor according to the manufacturer’s instructions (Millipore Ltd, Watford). For a quantitative measure of phagocytosis, light microscopy was used to determine the number of neutrophils containing bacteria within phagosomes with the results expressed as a percentage (Supplementary Image 1 and 2). The remaining infected neutrophils were used to assess viable counts by gentle lysis in 0.1% Triton X100 for 1 min to release intracellular bacteria. Lysates were diluted, plated on TSB agar and incubated overnight at 37 °C prior to determination of viable counts.
-
2.
Superoxide assay
Neutrophil superoxide was assayed by cytochrome c reduction assay as described previously68. Briefly, treated (C5a and linezolid) and untreated neutrophils were re-suspended in 1 ml Hank’s Balanced Salt Solution (HBSS; Thermofisher) with calcium at 1 × 107/ml. Then in separate tubes 50 μl of neutrophils (i.e. 500,000 cells), 800 μl of cytochrome C (1.25 mg/ml, Sigma Aldrich) and 100 µl of phorbol myristol acetate (PMA, final concentration 100 nM; Sigma Aldrich) were combined and incubated at 37 °C for 15 min. The reaction was terminated by centrifuging at 300×g for 5 min at 4 °C, and the OD550 of the supernatant determined. The ‘nM of O2− released per million cells’ was calculated from:
-
3.
Transmigration assay
The ability of neutrophils to migrate was assessed by using a Transwell transmigration assay system (pore size 3 μm supplied by VWR, Lutterworth, UK), where IL-8 (1–100 ng/ml) was placed in the lower chamber and 1 × 105 treated (C5a and linezolid as above) or untreated neutrophils added to the top chamber. Following 90 min incubation at 37 °C the membrane of the top chamber was wiped very gently with a cotton bud to remove cells that had not migrated and fixed for 10 min in 100% methanol. The Transwell filter was then stained with Hemacolor, cut from the Transwell casing and mounted on a microscope slide with a drop of DPX (Sigma Aldrich, Gillingham). When dry, transmigration was quantified by counting the number of neutrophils (Supplementary Image 3) using a light microscope (100 × oil immersion).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) as assessed in Excel. Plots were generated using GraphPad Prism software (V5.00 for Windows, GraphPad Software, San Diego California USA). Data were subjected to a Shapiro–Wilk normality test and then a one way-analysis of variance (ANOVA) with Tukey’s post-hoc test. In Supplementary Fig. 1 a two-way ANOVA and Bonferonni post-hoc test was used. Differences between treatment groups were considered statistically significant if p < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr Jan Chesham, (Medical Affairs Advisor, Pfizer Essential Health) for advice and support during the project.
Author contributions
T.S.W., D.M. and L.G.H. conceived the idea and secured funding for the work. T.S.W., R.E.J. and S.J. wrote the manuscript. S.J.E., L.G.H. and A.R. carried out the practical work. A.C.M. and J.S. provided expert opinion in clinical V.A.P. All authors reviewed the manuscript. Pfizer have approved the manuscript but did not contribute to analysis of the results.
Funding
This work was funded by a competitive European Aspire Award given to TSW and DM by Pfizer (Pfizer Tracking Number; WS1810005).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72454-0.
References
- 1.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.ECDC. Surveillance Report: Annual epidemiological report for 2016. Healthcare-associated infections in intensive care units. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-HAI_0.pdf (2016).
- 3.Wunderink RG, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin. Infect. Dis. 2012;54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 4.Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin. Ther. 2003;25:980–992. doi: 10.1016/S0149-2918(03)80118-2. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study Clin. Infect. Dis. 2001;32(402):412. doi: 10.1016/S0149-2918(03)80118-2. [DOI] [PubMed] [Google Scholar]
- 6.Niederman MS, et al. Health economic evaluation of patients treated for nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: secondary analysis of a multicenter randomized clinical trial of vancomycin and linezolid. Clin. Ther. 2014;36:1233–1243. doi: 10.1016/j.clinthera.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Patel DA, et al. Modeling the economic impact of linezolid versus vancomycin in confirmed nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Crit. Care. 2014;18:R157. doi: 10.1186/cc13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wunderink RG, et al. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest. 2008;134:1200–1207. doi: 10.1378/chest.08-0011. [DOI] [PubMed] [Google Scholar]
- 9.Wunderink RG. Nosocomial pneumonia, including ventilator-associated pneumonia. Proc. Am. Thorac. Soc. 2005;2:440–444. doi: 10.1513/pats.2005080-83JS. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann I, et al. Polymorphonuclear leukocyte dysfunction syndrome in patients with increasing sepsis severity. Shock. 2006;26:254–261. doi: 10.1097/01.shk.0000223131.64512.7a. [DOI] [PubMed] [Google Scholar]
- 11.Simms HH, D'Amico R. Polymorphonuclear leukocyte dysregulation during the systemic inflammatory response syndrome. Blood. 1994;83:1398–1407. doi: 10.1182/blood.V83.5.1398.1398. [DOI] [PubMed] [Google Scholar]
- 12.ConwayMorris A, et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am. J. Respir. Crit. Care Med. 2009;180:19–28. doi: 10.1164/rccm.200812-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway Morris A, et al. C5a-mediated neutrophil phagocytic dysfunction is RhoA-dependent and predicts nosocomial infection in critically ill patients. Blood. 2011 doi: 10.1182/blood-2010-08-304667. [DOI] [PubMed] [Google Scholar]
- 14.Conway Morris A, et al. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018;44:627–635. doi: 10.1007/s00134-018-5247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naess A, Stenhaug Kilhus K, Nystad TW, Sornes S. Linezolid and human polymorphonuclear leukocyte function. Chemotherapy. 2006;52:122–124. doi: 10.1159/000092539. [DOI] [PubMed] [Google Scholar]
- 16.Ballesta S, Pascual A, Garcia I, Perea EJ. Effect of linezolid on the phagocytic functions of human polymorphonuclear leukocytes. Chemotherapy. 2003;49:163–166. doi: 10.1159/000071139CHE2003049004163. [DOI] [PubMed] [Google Scholar]
- 17.Gruger T, et al. Negative impact of linezolid on human neutrophil functions in vitro. Chemotherapy. 2012;58:206–211. doi: 10.1159/000338390. [DOI] [PubMed] [Google Scholar]
- 18.Pascual A, Ballesta S, Garcia I, Perea EJ. Uptake and intracellular activity of linezolid in human phagocytes and nonphagocytic cells. Antimicrob. Agents Chemother. 2002;46:4013–4015. doi: 10.1128/AAC.46.12.4013-4015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. https://www.eucast.org (2019).
- 20.Denk S, et al. Complement C5a functions as a master switch for the pH balance in neutrophils exerting fundamental immunometabolic effects. J. Immunol. 2017;198:4846–4854. doi: 10.4049/jimmunol.1700393. [DOI] [PubMed] [Google Scholar]
- 21.Al-Ishaq R, et al. Effects of polysaccharide intercellular adhesin (PIA) in an ex vivo model of whole blood killing and in prosthetic joint infection (PJI): a role for C5a. Int. J. Med. Microbiol. 2015;305:948–956. doi: 10.1016/j.ijmm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins H, Stull T, Von Essen SG, Robbins RA, Rennard SI. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989;95:1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- 23.Hair PS, et al. Complement effectors, C5a and C3a, in cystic fibrosis lung fluid correlate with disease severity. PLoS ONE. 2017;12:e0173257. doi: 10.1371/journal.pone.0173257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curi R, et al. The critical role of cell metabolism for essential neutrophil functions. Cell Physiol. Biochem. 2020;54:629–647. doi: 10.33594/000000245. [DOI] [PubMed] [Google Scholar]
- 25.Wood AJT, et al. C5a impairs phagosomal maturation in the neutrophil through phosphoproteomic remodelling. JCI Insight. 2020 doi: 10.1172/jci.insight.137029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SC, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 2016;17:406–413. doi: 10.1038/ni.3398. [DOI] [PubMed] [Google Scholar]
- 27.Faisy C, Candela Llerena M, Savalle M, Mainardi JL, Fagon JY. Early ICU energy deficit is a risk factor for Staphylococcus aureus ventilator-associated pneumonia. Chest. 2011;140:1254–1260. doi: 10.1378/chest.11-1499. [DOI] [PubMed] [Google Scholar]
- 28.Pinder EM, et al. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax. 2018;73:918–925. doi: 10.1136/thoraxjnl-2017-211323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber-Lang MS, et al. Complement-induced impairment of innate immunity during sepsis. J. Immunol. 2002;169:3223–3231. doi: 10.4049/jimmunol.169.6.3223. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson TS, et al. Ventilator-associated pneumonia is characterized by excessive release of neutrophil proteases in the lung. Chest. 2012;142:1425–1432. doi: 10.1378/chest.11-3273. [DOI] [PubMed] [Google Scholar]
- 31.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 2002;46:1475–1480. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boselli E, et al. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit. Care Med. 2005;33:1529–1533. doi: 10.1097/01.CCM.0000168206.59873.80. [DOI] [PubMed] [Google Scholar]
- 33.Honeybourne D, Tobin C, Jevons G, Andrews J, Wise R. Intrapulmonary penetration of linezolid. J. Antimicrob. Chemother. 2003;51:1431–1434. doi: 10.1093/jac/dkg262dkg262. [DOI] [PubMed] [Google Scholar]
- 34.Kiem S, Schentag JJ. Interpretation of epithelial lining fluid concentrations of antibiotics against methicillin resistant Staphylococcus aureus. Infect. Chemother. 2014;46:219–225. doi: 10.3947/ic.2014.46.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrabou G, et al. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob. Agents Chemother. 2007;51:962–967. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vriese AS, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006;42:1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 37.Soriano A, Miro O, Mensa J. Mitochondrial toxicity associated with linezolid. N. Engl. J. Med. 2005;353:2305–2306. doi: 10.1056/NEJM200511243532123. [DOI] [PubMed] [Google Scholar]
- 38.Palenzuela L, et al. Does linezolid cause lactic acidosis by inhibiting mitochondrial protein synthesis? Clin. Infect Dis. 2005;40:e113–116. doi: 10.1086/430441. [DOI] [PubMed] [Google Scholar]
- 39.Maianski NA, et al. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. 2004;11:143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 40.Akinnusi ME, Hattemer A, Gao W, El-Solh AA. Does linezolid modulate lung innate immunity in a murine model of methicillin-resistant Staphylococcus aureus pneumonia? Crit. Care Med. 2011;39:1944–1952. doi: 10.1097/CCM.0b013e31821bd79e. [DOI] [PubMed] [Google Scholar]
- 41.Yoshizawa S, Tateda K, Saga T, Ishii Y, Yamaguchi K. Virulence-suppressing effects of linezolid on methicillin-resistant Staphylococcus aureus: possible contribution to early defervescence. Antimicrob. Agents Chemother. 2012;56:1744–1748. doi: 10.1128/AAC.05430-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pichereau S, et al. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 2012;67:123–129. doi: 10.1093/jac/dkr417. [DOI] [PubMed] [Google Scholar]
- 43.Lambers C, et al. Early immunomodulatory effects of linezolid in a human whole blood endotoxin model. Int. J. Clin. Pharmacol. Ther. 2010;48:419–424. doi: 10.5414/CPP48419. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi G, et al. Effect of linezolid on cytokine production capacity and plasma endotoxin levels in response to lipopolysaccharide stimulation of whole blood. J. Infect. Chemother. 2010;1.6:94–99. doi: 10.1007/s10156-009-0012-5. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Roca P, Mancilla-Ramirez J, Santos-Segura A, Fernandez-Aviles M, Calderon-Jaimes E. Linezolid diminishes inflammatory cytokine production from human peripheral blood mononuclear cells. Arch. Med. Res. 2006;37:31–35. doi: 10.1016/j.arcmed.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Danin J, Linder L, Lundqvist G, Wretlind B. Cytokines in periradicular lesions: the effect of linezolid treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;96:492–498. doi: 10.1016/S1079210403000593. [DOI] [PubMed] [Google Scholar]
- 47.Mascio CT, Alder JD, Silverman JA. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 2007;51:4255–4260. doi: 10.1128/AAC.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. doi: 10.1016/S0012-3692(15)33412-7. [DOI] [PubMed] [Google Scholar]
- 49.Kollef MH, Rello J, Cammarata SK, Croos-Dabrera RV, Wunderink RG. Clinical cure and survival in gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30:388–394. doi: 10.1007/s00134-003-2088-1. [DOI] [PubMed] [Google Scholar]
- 50.Cruciani M, et al. Penetration of vancomycin into human lung tissue. J. Antimicrob. Chemother. 1996;38:865–869. doi: 10.1093/jac/38.5.865. [DOI] [PubMed] [Google Scholar]
- 51.Bruniera FR, et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur. Rev. Med. Pharmacol. Sci. 2015;19:694–700. [PubMed] [Google Scholar]
- 52.Coyle EA, Cha R, Rybak MJ. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob. Agents Chemother. 2003;47:1752–1755. doi: 10.1128/AAC.47.5.1752-1755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernardo K, et al. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob. Agents Chemother. 2004;48:546–555. doi: 10.1128/aac.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gemmell CG, Ford CW. Virulence factor expression by gram-positive cocci exposed to subinhibitory concentrations of linezolid. J. Antimicrob. Chemother. 2002;50:665–672. doi: 10.1093/jac/dkf192. [DOI] [PubMed] [Google Scholar]
- 55.Jacqueline C, et al. Linezolid dampens neutrophil-mediated inflammation in methicillin-resistant Staphylococcus aureus-induced pneumonia and protects the lung of associated damages. J. Infect. Dis. 2014;210:814–823. doi: 10.1093/infdis/jiu145. [DOI] [PubMed] [Google Scholar]
- 56.Bhan U, et al. Linezolid has unique immunomodulatory effects in post-influenza community acquired MRSA pneumonia. PLoS ONE. 2015;10:e0114574. doi: 10.1371/journal.pone.0114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capodicasa E, et al. In-vitro effects of teicoplanin, teicoplanin derivative MDL 62211 and vancomycin on human polymorphonuclear cell function. J. Antimicrob. Chemother. 1991;27:619–626. doi: 10.1093/jac/27.5.619. [DOI] [PubMed] [Google Scholar]
- 58.Moran FJ, et al. Activity of vancomycin and teicoplanin against human polymorphonuclear leucocytes: a comparative study. J. Antimicrob. Chemother. 1991;28:415–418. doi: 10.1093/jac/28.3.415. [DOI] [PubMed] [Google Scholar]
- 59.Pedrera MI, Barriga C, Rodriguez AB. Intracellular activity of both teicoplanin and vancomycin against Staphylococcus aureus in human neutrophils. Comp. Immunol. Microbiol. Infect. Dis. 1995;18:123–128. doi: 10.1016/0147-9571(95)98853-A. [DOI] [PubMed] [Google Scholar]
- 60.Van der Auwera P, Bonnet M, Husson M. Influence of teicoplanin and vancomycin on degranulation by polymorphonuclear leucocytes stimulated by various agonists: an in-vitro study. J. Antimicrob. Chemother. 1990;26:683–688. doi: 10.1093/jac/26.5.683. [DOI] [PubMed] [Google Scholar]
- 61.Rodvold KA. 60 plus years later and we are still trying to learn how to dose vancomycin. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciz467. [DOI] [PubMed] [Google Scholar]
- 62.John DA, Williams LK, Kanamarlapudi V, Humphrey TJ, Wilkinson TS. The bacterial species campylobacter jejuni induce diverse innate immune responses in human and avian intestinal epithelial cells. Front. Microbiol. 2017;8:1840. doi: 10.3389/fmicb.2017.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitayama J, Carr MW, Roth SJ, Buccola J, Springer TA. Contrasting responses to multiple chemotactic stimuli in transendothelial migration: heterologous desensitization in neutrophils and augmentation of migration in eosinophils. J. Immunol. 1997;158:2340–2349. [PubMed] [Google Scholar]
- 64.Luu NT, Rainger GE, Nash GB. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J. Immunol. 2000;164:5961–5969. doi: 10.4049/jimmunol.164.11.5961. [DOI] [PubMed] [Google Scholar]
- 65.Vogt KL, Summers C, Chilvers ER, Condliffe AM. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Investig. 2018;48(Suppl 2):e12967. doi: 10.1111/eci.12967. [DOI] [PubMed] [Google Scholar]
- 66.Hall RG, 2nd, Smith WJ, Putnam WC, Pass SE. An evaluation of tedizolid for the treatment of MRSA infections. Expert Opin. Pharmacother. 2018;19:1489–1494. doi: 10.1080/14656566.2018.1519021. [DOI] [PubMed] [Google Scholar]
- 67.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 68.Condliffe AM, Chilvers ER, Haslett C, Dransfield I. Priming differentially regulates neutrophil adhesion molecule expression/function. Immunology. 1996;89:105–111. doi: 10.1046/j.1365-2567.1996.d01-711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


