Abstract
An ultimate goal of neuroscience is to decipher the principles underlying neuronal information processing at the molecular, cellular, circuit, and system levels. The advent of miniature fluorescence microscopy has furthered the quest by visualizing brain activities and structural dynamics in animals engaged in self-determined behaviors. In this brief review, we summarize recent advances in miniature fluorescence microscopy for neuroscience, focusing mostly on two mainstream solutions – miniature single-photon microscopy, and miniature two-photon microscopy. We discuss their technical advantages and limitations as well as unmet challenges for future improvement. Examples of preliminary applications are also presented to reflect on a new trend of brain imaging in experimental paradigms involving body movements, long and complex protocols, and even disease progression and aging.
Keywords: Miniature fluorescence microscopy, Brain imaging, Two-photon microscopy, Neuronal information processing
Introduction
The past few decades have witnessed great expansion and transformation of the neurosciences into the dawning of a new era of brain science. To decipher the principles underlying neuronal information processing at the molecular, cellular, circuit, and system levels, it is imperative to characterize the structural organization and functional dynamics of the brain at different spatial scales. Macroscopic brain imaging modalities include magnetic resonance imaging, positron emission tomography, X-ray computed tomography, and the more recently-invented photoacoustic tomography. For ultrastructural studies, microscopic brain imaging modalities include electron microscopy and different types of super-resolution optical microscopy. However, to bridge animal behaviors with the activity of spines, neurons, and neuronal circuits in specific brain regions, optical imaging technology is the ideal choice because it enables both high spatiotemporal resolution and cell-type specificity in vivo.
However, traditional benchtop optical microscopes are bulky and rigid, and require the animals to be anesthetized or head-fixed while imaging [1–3]. The physical constraints and stresses [4, 5] also limit the duration and complexity of experimental protocols that can be used, precluding real-time imaging paradigms involving body and head movements (e.g., navigation in natural environments, or seizures), social behaviors (e.g., parent-child interactions or fighting), and behaviors of exceeding long duration (e.g., sleep or circadian rhythms). To investigate spatial navigation, although virtual reality technology has been combined with the benchtop two-photon microscope [6, 7], brain activity recorded under this paradigm differs remarkably from that when animals are free to explore real environments [8, 9].
At the turn of this century, Denk and colleagues pioneered a proof-of-principle miniature two-photon microscope with the generic features of a light and small wearable headpiece and a flexible line electrically and optically connecting the headpiece to the rest of the imaging system [10]. Through nearly two decades of innovative efforts made in many laboratories, miniature two-photon microscopy has been improved by reducing the weight and size, improving the spatial and temporal resolution, and extending the penetration depth, field-of-view (FOV), volumetric imaging capacity, and compatibility for imaging GCaMPs [11–15], the most widely-used Ca2+ indicators in the brain science community [16]. Because of these efforts, imaging in freely-behaving animals is considered to be a mature method that can be readily adopted by neuroscientists at large [17].
In this brief review, we summarize recent advances in miniature fluorescence microscopy for neuroscience, focusing mostly on two mainstream solutions – miniature single-photon microscopy and miniature two-photon microscopy. We discuss their technical advantages and limitations as well as unmet challenges for future technical development. Examples of preliminary applications also predict that miniature two-photon microscopy will be the emerging trend in brain science, and will transform the field in the years to come.
Miniature single-photon microscopy
In 2006, Ferezou et al. demonstrated that image guide fiber bundles could be used to image cortical spatiotemporal dynamics with voltage-sensitive dye in freely-moving animals [18]. A customized 300 × 300 fiber bundle of 3 × 3 mm2 directly contacts the cortex and transmits both the emission and excitation light to and from a standard benchtop epifluorescence microscope. In conjunction with a gradient index (GRIN) lens, a fiberscope was also used for deep-tissue imaging with a right-angle micro-prism reflecting a view perpendicular to the apical dendrites [19]. Further, using a compound micro-objective instead of a GRIN lens has been shown to provide better resolution (lateral resolution 2.8 μm–3.9 μm) [20] than the fiber bundle system with no micro-objective. Then, Engelbrecht et al. reported a miniature light-sheet microscope with an axial resolution of 5.1 μm [21]. The light sheet was created with a cylindrical GRIN lens and a right-angle microprism. The fluorescence emission was collected perpendicular to the light sheet through a fiber bundle and detected by a charge-coupled device camera.
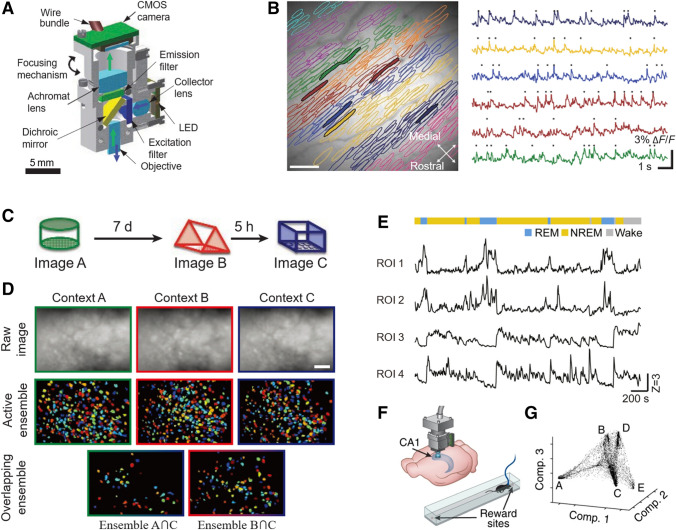
In a landmark work in 2011, Ghosh et al. reported a fully integrated miniature wide-field single-photon fluorescence microscope [22], with a 1.9 g headpiece comprising a micro-optics set, a light-emitting diode (LED) for illumination, and a complementary metal-oxide-semiconductor (CMOS) sensor for image acquisition (Fig. 1A). This system enabled high-speed imaging across ~ 0.5 mm2 at a lateral resolution of 2.5 μm, and was used to simultaneously track Ca2+ spiking in 206 Purkinje neurons across nine cerebellar microzones (Fig. 1B). Recently, some research groups have demonstrated wide-field microscopes with a large FOV of several millimeters. For example, Scott et al. reported a head-mounted wide-field microscope to record large-scale cortical dynamics in freely-moving rats [23]. The system provided a 7.8 × 4 mm2 FOV, and the lateral resolution was 14 μm. To eliminate the influence of hemodynamics on the recorded GCaMP6f signal, 480-nm and 530-nm LED illumination was interleaved and synchronized with the camera for both fluorescence and hemodynamic correction. However, the total weight of the microscope is 33 g, which is too heavy for mice. Senarathna et al. demonstrated a miniature multi-contrast microscope with a fluorescence channel, an intrinsic optical signal channel, and a laser speckle contrast channel [24]. The FOV of the system was 3 × 3 mm2 which permitted, for instance, the interrogation of the entire auditory cortex at a spatial resolution of 5 μm. The complete miniature microscope assembly weighed 9 g and occupied 5 cm3, and a suspension mechanism comprising elastic bands was designed to reduce the effective weight of the microscope assembly to 3 g.
Fig. 1.
Miniature single-photon imaging systems for brain imaging in freely-behaving animals. A Schematic of the integrated headpiece of a wide-field single-photon microscope (LED, light-emitting diode; CMOS, complementary metal-oxide-semiconductor). B Ca2+ spiking dynamics from mouse Purkinje neurons imaged with the system in A (scale bar, 100 μm). Neurons are resolved through computational analysis of their sparse activity amidst a blurred background. Each color denotes one of nine microzones identified. C Experimental design for mice to explore three different contexts (A, B, and C), each for 5 or 10 min separated by either 5 h or 7 days. D CA1 neuronal activity during context exploration according to C. Upper, images of mean fluorescence from each session; middle, ensemble of cells active in each session; lower, cells active in two sessions (scale bar, 100 μm). E Activity of galaninergic neurons in the dorsomedial hypothalamus during sleep. Upper, brain states (color coded); lower, Ca2+ traces (Z scores) from four regions of interest (ROI) in the same field of view. REM, rapid eye movement; NREM, non-REM. F Schematic of the experimental setup. Imaging via a miniature single-photon microscope combined with a GRIN lens. Mice run back and forth on a linear track. G Data of CA1 neuronal activity viewed in the reduced dimensional space of three components, which are the three leading eigenvectors, based on the non-linear dimensionality reduction algorithm Laplacian Eigenmaps. A, B are from [22], C, D from [29], E from [33], F, G from [34].
However, because single-photon imaging lacks optical sectioning capability, its signal-to-background contrast is poor and confounds the optical resolution of thick specimens. Indeed, dendrites and somata can barely be seen only after extensive computational processing of sparse neuronal activation against a hazy background (Fig. 1B). In addition, the penetration depth is typically limited to < 100 μm, restricting its applicability only to superficial layers, or at the tip of a GRIN lens.
It has been shown that light-field microscopy can be adopted to achieve three-dimensional imaging of neuronal activity [25]. By assembling a microlens array in front of the camera to record both the spatial intensity and the direction of light, different imaging depths can be extracted from each frame with a computational de-mixing strategy. In this regard, Skocek et al. demonstrated that a miniature light-field microscope [26] was capable of imaging 810 hippocampal neurons within a volume of 700 × 600 × 360 μm3 at 16 Hz in freely-moving mice. However, because the use of light field compromises the lateral and axial resolution, it can hardly discriminate neurons separated by ~ 15 μm.
These miniature microscopes all used optical fibers and/or electrical wires to transmit signals to and from the headpiece. Recently, Barbera et al. reported a wireless model [27] that contained a miniature microscope body, an image sensor board, a field-programmable gate array, a micro SD card for storage, and a battery backpack, with a total weight of 3.9 g (exclusive of the battery weighted 3.8 g). The wireless design not only resolves the problem of wire entanglement but also removes many other constraints on the design of behavioral paradigms and enables simultaneous recording from multiple freely-moving and socially-interacting mice. Despite these advantages, the battery in its current design can only support 40 min of continuous recording, which prevents its use in long-term imaging paradigms.
Recently, Dussaux et al. reported a miniature differential multipoint-scanning confocal microscope [28] that achieved an axial resolution of 10.5 μm, along with enhanced removal of out-of-focus fluorescence emission. Specifically, the “illumination pinhole matrix” created by intensity modulation using a digital micromirror device was projected onto the entry surface of an image guide, and was scanned rapidly to illuminate the full FOV during one exposure interval of the camera. The fluorescence was collected by a micro-objective and a fiber bundle, of which ~10% was imaged by an sCMOS camera as a wide-field image and the rest was projected onto another part of the digital micromirror device displaying a pattern of the “detection pinhole matrix” to form a confocal image. By simultaneously recording confocal and wide-field images from the same camera, the residual out-of-focus background was subtracted to obtain a background-free confocal image. Using this technology, they demonstrated fast confocal fluorescence imaging (up to 200 Hz) of microvasculature up to 120 μm deep in the mouse cortex.
At present, miniature wide-field single-photon microscopy is technically mature and robust (Table 1). The open-source projects (e.g. UCLA Miniscope [29], Finchscope [30], CHEndoscope [31], and MiniScope [32]) have added a boost to this field, and made the camera-based wide-field single-photon microscope easier to implement and cost-effective. As an emerging trend, it is getting more and more popular among brain science researchers and is being applied to various fields of research, such as memory [29] and sleep [33]. In order to test the memory-allocation hypothesis, Cai et al. recorded memory formation in freely-exploring mice [29]. They used miniature single-photon microscopy to track the Ca2+ dynamics of CA1 neurons, which were selectively activated in several different contexts presented at variable intervals (Fig. 1C). They found that the overlap of active neuronal ensembles in the two different chambers with a shorter interval (5 h) was more than that with a longer interval (7 days) (Fig. 1D). With this and further experiments on fear transfer, extinction, and enhancement, they demonstrated that contextual memories encoded close in time are linked by directing storage into overlapping ensembles. They also found that the processes triggered in the encoding of one memory could modulate the strength of subsequent memories, and they were able to rescue the memory-linking deficit in aged mice by increasing the excitability in a subset of CA1 neurons via designer receptors exclusively activated by designer drugs (DREADD). Chen et al. applied the miniature single-photon microscope to study sleep [33]. They found two subtypes of GABAergic galanin-expressing neurons in the dorsomedial hypothalamusbased on Ca2+ activity: one was selectively activated and the other was suppressed during the rapid eye-movement phase (REM) (Fig. 1E). With virus-assisted circuit tracing and bidirectional optogenetic manipulations, they showed that these two subtypes of neurons projected to the preoptic area and raphe pallidus, respectively, and antagonistically regulated the switch between REM and non-REM sleep.
Table 1.
Summary of features of miniature fluorescence imaging systems
| Systems | Miniature single-photon microscopy | Miniature two-photon microscopy | ||||
|---|---|---|---|---|---|---|
| Integrated miniature headpiece | Fiber-bundle | Miniature confocal system | Fiber-scanning headpiece | MEMS-scanning headpiece | Fiber-bundle | |
| Weight and size |
1.9 g, ~2.4 cm3 [22] 33 g [23] 9 g, 5 cm3 [24] 10 g, 3 cm long [44] |
1.1 g [20] | Not reported [28] |
25 g, 7.5 cm long [10] 5.5 g [11] 0.6 g [12] |
2.9 g, 2.0 × 1.9 × 1.1 cm3 [13] 2.15 g, ~1 cm3 [14] |
2.5 g [15] |
| Signal-to-background contrast | Poor | Good | Excellent | |||
| Resolution of neuronal structures | Dendrites and somas resolved with computational processing of sparse activity against background | Cellular resolution | Cellular resolution |
Cellular resolution [13] Subcellular resolution that resolves spines [14] |
Cellular resolution | |
| Temporal resolution |
36 Hz (640×480 pixels) [22] 30 Hz (758×480 pixels) [23] 15 Hz (640×640 pixels) [24] 900 Hz (32×32 pixels) [44] |
500 Hz [18] 100 Hz [19] 100 Hz [20] |
200 Hz [28] |
2 Hz (128×128 pixels) [10] 11 Hz (64×64 pixels) [11] 25 Hz (128×128 pixels) [12] |
4 Hz (400 × 135 pixels) [13] 40 Hz (256 × 256 pixels) Line scanning: 10,000 Hz Random-access scanning: 128 Hz (4 neurons) [14] |
2.5 Hz [15] |
| kHz-frame-rate imaging | Yes [44] | Yes [18] | Not reported | Not reported | Not reported | Not reported |
| FOV |
0.5 mm2 [22] 7.8 × 4 mm2 [23] 3 × 3 mm2 [24] 4.8 mm2 [44] |
3 × 3 mm2 [18] 685 μm dia. [19] 370 μm dia. [20] |
230 μm dia. [28] |
65 × 65 μm2 [10] ~250 × 250 μm2 * [11] 200 μm dia. [12] |
295 × 100 μm2 [13] 130 × 130 μm2 [14] 420 × 420 μm2 (unpublished) [45] |
240 μm dia. [15] |
| Depth of penetration | 50–100 μm | 120 μm [28] | 250 μm [10] |
~150 μm [14] 400 μm (unpublished) |
180 μm [15] | |
| Volumetric imaging | Yes [26] (miniature light-field microscopy) | Not reported | Not reported | Not reported | Yes [46] | Yes [15] |
| Wireless | Yes [27] | Not feasible | ||||
| Single-neuron precision optogenetics | Not feasible | Feasible | ||||
*Derived from figures in the paper.
Miniature single-photon microscopy has also promoted the development of new research approaches in brain science. For example, Rubin et al. proposed an approach to study the neural mechanisms of behavior without prior assumptions of behavior variables [34]. To verify the rationality of the approach, they made mice run back and forth on a straight track, and used a miniature single-photon microscope to record the neuronal activity in known behavior-related brain regions (Fig. 1F). The unsupervised learning method was applied for dimensionality reduction of the neuronal activity data. Their results revealed a highly-organized internal structure (Fig. 1G), which could be used to reconstruct each neuron’s internal tuning-curve, and revealed the internal representations of neurons, without relying on the behavioral measurements. And they also found that the internal structure of neuronal activity was conserved over time and across mice.
Miniature two-photon microscopy
The two-photon fluorescence microscope invented in 1990 by Denk and Webb [35] has become popular among researchers, particularly neuroscientists, working with tissue (e.g., brain slice) and in vivo imaging. It has two advantages over contemporary single-photon confocal microscopy. First, its penetration depth is about 500–800 μm in the mouse brain [36], and this can be extended to ~ 1.2 mm when using long-wavelength excitation light and with underfilling of the objective [37]. This is because two-photon excitation uses nearly double the wavelength for excitation of a given fluorescent indicator, and tissue scattering, the confounding factor in deep tissue imaging, sharply decreases with wavelength [38]. Second, because two-photon excitation is a quadratic function of light intensity, fluorescence excitation is largely restricted to the focal point and thus two-photon microscopy affords an intrinsic optical sectioning capability. This property is particularly useful when imaging a thick specimen, because it confers high signal-to-background contrast. The out-of-focus photobleaching and photodamage are largely alleviated, which facilitates long-term imaging. Moreover, it is uniquely beneficial when combined with optogenetic manipulation: while single-photon optogenetics indiscriminately activates all neurons along the optical path, two-photon optogenetics can selectively target neurons on the focal plane, allowing for precise manipulation of a subset of neurons in 3D circuits.
Unlike the continuous-wave laser sources used in single-photon imaging, two-photon microscopy needs bulky, expensive, ultrafast (~100 fs) pulsed lasers for much higher peak power by temporally confining the laser pulse. The system also operates in the fast point-scanning mode (microsecond pixel dwell time) to spatially concentrate the light power for efficient nonlinear excitation of the indicator. Thus, the challenges for miniaturizing two-photon microscopy are threefold: how to deliver the high-quality femtosecond laser pulses through a fiber to the live animal, how to devise a light-weight, compact, and fast scanning mechanism to ensure imaging without perturbing animal behavior, and how to collect the fluorescence efficiently to ensure high resolution and contrast imaging. Compared to the single-photon microscope, the two-photon system is more susceptible to motion artifacts owing to its optical sectioning capability and higher spatial resolution, which confers an additional challenge.
The first attempt to miniaturize two-photon microcopy was made in 2001 by Helmchen et al. [10], who reported a system in which the imaging headpiece was 7.5 cm long and weighed 25 g. A single-mode optical fiber was used to transmit near-infrared (820–850 nm) laser pulses. Scanning was achieved by vibrating the fiber tip to image an FOV of 65 × 65 μm2 (128 × 128 pixels) at 2 Hz, and a small photomultiplier tube was incorporated into the headpiece for emission detection. To record the signal from a green Ca2+ indicator (Oregon green BAPTA-1), Sawinski et al. customized a high numerical aperture (NA) mini-objective, and used a single-mode fiber to transmit the 925-nm laser pulse and a large-core, high-NA plastic optical fiber was used to collect the fluorescence [11]. The assembled headpiece, weighing 5.5 g, enabled the recording of Ca2+ transients from the somata of layer II/III neurons in the visual cortex of freely-moving rats with a laser power of 100–150 mW after the objective. Owing to the high dispersion of the conventional silica-based fiber at the two-photon excitation wavelength, the pulse width of the propagating femtosecond laser pulses clearly broadens through several meters of fiber. Normally, a negative pre-chirp is applied to accurately compensate the fiber dispersion. However, this strategy cannot completely solve the problem of pulse distortion caused by an accumulated nonlinear phase shift in the silica fiber core. To overcome this problem, Engelbrecht et al. used a hollow-core photonic crystal fiber (HC-PCF) to deliver 812-nm femtosecond laser pulses [12]. The headpiece was highly compact, weighing only 0.6 g, by using an ultra-thin spiral-pattern fiber scanner. The system achieved micrometer resolution (~ 1 μm laterally and ~ 8 μm axially) at a frame rate of 25 Hz with an FOV of up to 200 μm in diameter. The photonic bandgap created by the periodic air-silica arrays can confine light within a certain wavelength range to the air-core. Thanks to this air-guiding property, intensity-dependent nonlinear effects are cancelled [39].
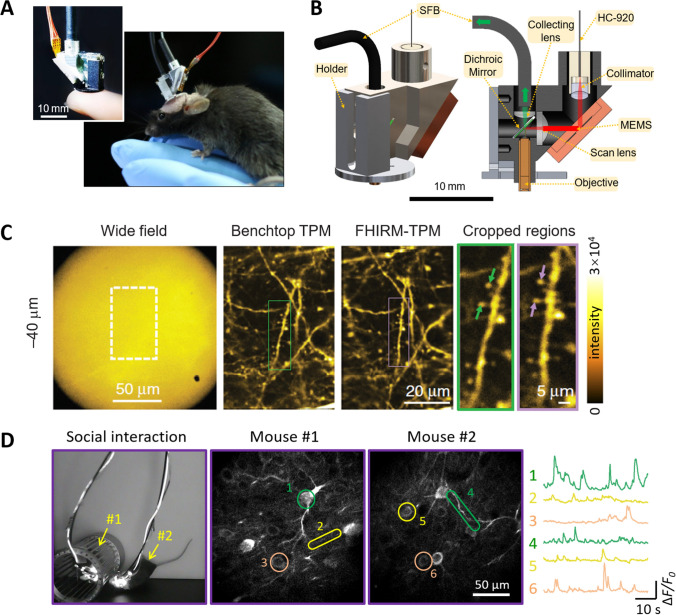
As for the scanning mechanism, Piyawattanametha et al. introduced a microelectromechanical system (MEMS) scanner into miniature two-photon imaging systems [13]. Compared with fiber-tip scanning, the MEMS scanner achieves a much higher response speed because the silicon-based thin-film mirror driven by the MEMS scanner is much smaller than the 10-mm-long fiber driven by a piezoelectric ceramic transducer. However, none of these systems was actually used by any neuroscientists. Aiming at developing a robust miniature two-photon microscope (mTPM) that could be widely accessible to neuroscientists at large, we assembled an interdisciplinary team at Peking University in early 2013, and reported our fast high-resolution, mTPM (FHIRM-TPM) in 2017 [14]. In our system, the headpiece weighs 2.15 g and occupies ~ 1 cm3 (Fig. 2A, B). To make the mTPM efficiently excite GFPs and GCaMPs, the most commonly-used fluorescent markers and Ca2+ indicators, we custom-designed the HC-920, an HC-PCF that delivers 920-nm femtosecond laser pulses with little distortion. The micro-optics design includes a high-NA (0.8) micro-objective lens providing high spatial resolution (0.64 μm laterally and 3.35 μm axially). Its MEMS scanner enables high temporal resolution (40 Hz at 256 × 256 pixels). In its random-access or line-scan imaging modes, it realizes hundred-Hertz frame rates when imaging a few neurons of interest and 10 kHz when line scanning. For emission collection, we abandoned traditional multimode polymer fibers [13] or fusion-type fiber bundles, and designed a new type of supple fiber bundle (SFB) composed of 800 optical fibers fused into a 1.5-mm-diameter cylinder at either end while remaining loose and flexible between the ends. Together with independent rotatable connectors to both the optical and electrical lines, the use of an SFB minimizes the torque and tension on the animal without compromising the photon-collection efficiency. Furthermore, system-level optimization was applied to mitigate motion artifacts. These measures were effective such that the FHIRM-TPM exhibited resolution and contrast comparable to that of a benchtop TPM, and achieved its near-theoretical resolution even in freely-moving animals (Fig. 2C). The robustness and wide applicability of the FHIRM-TPM has been demonstrated by its performance in resolving brain activity at the single synapse and dendrite levels in behavioral paradigms involving tail suspension, stepping down from an elevated platform, and social interaction [14] (Fig. 2D).
Fig. 2.
Miniature two-photon imaging systems for brain imaging in freely-behaving animals. A Photographs of a high-resolution, miniature two-photon microscope (FHIRM-TPM) on a fingertip and mounted on the head of a mouse. B Design of the headpiece FHIRM-TPM headpiece (HC-920, hollow-core photonic crystal fiber for delivering 920-nm femtosecond laser pulses; MEMS, microelectromechanical system; SFB, supple fiber bundle). C The FHIRM-TPM achieves spatial resolution and contrast similar to that of a benchtop TPM. Note the high signal-to-background contrast of two-photon imaging versus single-photon wide-field imaging of the same FOV (leftmost panel). D Simultaneous imaging of prefrontal neuronal activity in a pair of mice during their social interaction. Left, snapshot of two mice interacting while being imaged with a new version of the FHIRM-TPM (NA, 0.7; FOV, 180 × 180 μm; working distance, 390 μm with coverslip correction); middle, GCaMP6s-labeled neuronal somata and dendrites in the prefrontal cortexes of the mice; right, Ca2+ transients from six regions of interest highlighted in the middle panels. A–C are modified from [14].
More recently, Ozbay et al. built a fiber-bundle-based mTPM [15] by a scanning femtosecond pulsed laser onto individual fibers and collecting the fluorescence emission through the same fiber bundle. In order to maintain the ultrashort pulsedurations required for two-photon excitation, the laser pulse was pre-compensated by using a polarization-maintaining single-mode fiber and a grating-pair-based pulse-stretcher to spectrally broaden and apply a negative chirp to the pulse, respectively. An electrowetting tunable lens was integrated into the microscope for axial scanning, enabling 3D imaging with a 180-μm axial focusing range. The main advantage of the fiber-bundle-based system lies in the simplicity of the fiber-optic probe – its light weight and small size. Because of the separation of light scanning and modulatory devices from the fiber-optic probe, it can attain high temporal resolution and flexible illumination light modulation, as well as electromagnetic compatibility (e.g., simultaneous magnetic resonance imaging). However, the limitations of fiber bundles are also evident. Although they can cover a large FOV, their spatial resolution and total pixel number are inherently dictated by the dimensions and total number of fibers used. The large bending radius of stiff image-guide fiber bundles constrains the movement of animals. Besides, the quality of images is further degraded by a honeycomb pixelated pattern due to the packing of the fibers. Two-photon microscopes based on fiber bundles are not only subject to all the above limitations of the bundles and those of single-mode optical fiber excitation such as dispersion and nonlinearity, but also non-uniformity among individual fibers create additional complications.
To fully deliver the promise of mTPM in resolving 3D neuronal circuitry down to the dendrite and spine levels in freely-behaving animals, future efforts should focus on the integration of a number of the most demanding features: (1) redesign of the micro-optics to allow for a long working distance, and a plug-in and plug-out headpiece design compatible with a chronic cranial window; (2) an enlarged FOV and multiple-plane focusing mechanism to image 3D circuits; (3) an ultrafast scanning mechanism to achieve kilohertz frame rates for imaging genetically-encoded voltage sensors [40]; and (4) combination with precision optogenetics. Even deeper penetration (~1.5 mm) could be realized with the miniaturization of three-photon microscopy in the future [41]. We are also expecting the emergence of multi-modality miniature microscopy, e.g., by combining photoacoustic imaging to provide complementary information of regional and even whole-brain activities.
Perspective
Thanks to the development and the convergence of modern micro-optics, MEMS, photonics, and laser physics, we now have several types of miniature imaging systems ready to instigate a new trend of brain science with the feature of imaging neuronal activity in freely-behaving animals. While these different systems co-exist, co-develop and complement each other, we anticipate that miniature two-photon microscopy will have broader applicability, because of its optical sectioning capability, deep penetration, high spatial resolution and contrast, reduced photo-bleaching and photo-toxicity, and the unique ability to combine optogenetic manipulation at single-cell precision (Table 1).
On a related but different front, we are building up the Nanjing Brain Observatory (NBO) which provides the capability of imaging of brain activities at high throughput. With the generous support of Nanjing Jiangbei New Area government, the NBO is now equipped with more than ten setups of mTPMs of different types, alongside ultrasensitive structured illumination microscope [42] and Volumetric Imaging with Synchronized on-the-fly-scan and Readout (VISoR) [43]. A dozen or so early-bird projects are ongoing through collaborations with experts in cortical working memory, sleep, autism, depression, neural pharmacology, and neuronal regeneration. It is our hope that NBO will not only serve the hub to accelerate the idea-to-discovery process for individual scientists, but also establish the core competence for the launch of China and international brain science initiatives, for instance, valuable brain functional atlas for the entire community.
Collectively, these new transformative tools enable neuroscientists to freely explore many novel behavioral paradigms involving body movements (e.g., spatial navigation, social interaction, seizure, fear), long and complex protocols (e.g., circadian rhythms, sleep, neurogenesis and regeneration), and even chronic disease progression (e.g., autism, depression, Alzheimer’s disease) and aging. Along with animal behavioral data, miniature microscopy acquires functional dynamics across scales of synapses, neurons, and neuronal ensembles, with high parallelism (up to thousands of cells) and, in conjunction with genetic markers, cell-type specificity. Parallel developments in imaging processing methods, computational neuroscience, and network theory are also necessary to generate activity-based mapping of neuronal types, to identify engram of learning and memory at the synapse and population levels, and to investigate their long-term stability and plasticity at supra-synapse level, all critical to deciphering the working principles of the brain.
Acknowledgments
We thank Dr. Zhe Zhao and Dr. Haitao Wu for helping with the experiments for Fig. 2D, and Dr. Weijian Zong for discussion. This work was supported by grants from the National Natural Science Foundation of China (31327901, 31570839, 61975002, 31830036, 31821091, and 8182780030), the Major State Basic Research Program of China (2016YFA0500400 and 2016YFA0500403), and the National Postdoctoral Program for Innovative Talents of China (BX20190011).
References
- 1.Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 2.Weisenburger S, Tejera F, Demas J, Chen B, Manley J, Sparks FT, et al. Volumetric Ca2+ imaging in the mouse brain using hybrid multiplexed sculpted light microscopy. Cell. 2019;177:1050–1066. doi: 10.1016/j.cell.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Y, Li L, Sun L, Yu J, Hu Z, Lian K, et al. In vivo two-photon calcium imaging in dendrites of rabies virus-labeled v1 corticothalamic neurons. Neurosci Bull 2019: 1–9. [DOI] [PMC free article] [PubMed]
- 4.Li R, Wang M, Yao J, Liang S, Liao X, Yang M, et al. Two-photon functional imaging of the auditory cortex in behaving mice: from neural networks to single spines. Front Neural Circuit. 2018;12:33. doi: 10.3389/fncir.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aharoni DB, Hoogland T. Circuit investigations with open-source miniaturized microscopes: past, present and future. Front Cell Neurosci. 2019;13:141. doi: 10.3389/fncel.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghajan ZM, Acharya L, Moore JJ, Cushman JD, Vuong C, Mehta MR. Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci. 2015;18:121–128. doi: 10.1038/nn.3884. [DOI] [PubMed] [Google Scholar]
- 9.Acharya L, Aghajan ZM, Vuong C, Moore JJ, Mehta MR. Causal influence of visual cues on hippocampal directional selectivity. Cell. 2016;164:197–207. doi: 10.1016/j.cell.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope: high-resolution brain imaging in freely moving animals. Neuron. 2001;31:903–912. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 11.Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JND. Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci U S A. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelbrecht CJ, Johnston RS, Seibel EJ, Helmchen F. Ultra-compact fiber-optic two-photon microscope for functional fluorescence imaging in vivo. Opt Express. 2008;16:5556–5564. doi: 10.1364/oe.16.005556. [DOI] [PubMed] [Google Scholar]
- 13.Piyawattanametha W, Cocker ED, Burns LD, Barretto RPJ, Jung JC, Ra H, et al. In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror. Opt Lett. 2009;34:2309–2311. doi: 10.1364/ol.34.002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong W, Wu R, Li M, Hu Y, Li Y, Li J, et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods. 2017;14:713–719. doi: 10.1038/nmeth.4305. [DOI] [PubMed] [Google Scholar]
- 15.Ozbay BN, Futia GL, Ming M, Bright VM, Gopinath JT, Hughes EG, et al. Three dimensional two-photon brain imaging in freely moving mice using a miniature fiber coupled microscope with active axial-scanning. Sci Rep. 2018;8:8108. doi: 10.1038/s41598-018-26326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 17.Method of the Year 2018: Imaging in freely behaving animals. Nat Methods 16, 1 (2019). 10.1038/s41592-018-0292-8. [DOI] [PubMed]
- 18.Ferezou I, Bolea S, Petersen CCH. Visualizing the Cortical Representation of Whisker Touch: Voltage-Sensitive Dye Imaging in Freely Moving Mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Murayama M, Pérez-Garci E, Lüscher HR, Larkum ME. Fiberoptic System for Recording Dendritic Calcium Signals in Layer 5 Neocortical Pyramidal Cells in Freely Moving Rats. J Neurophysiol. 2007;98:1791–1805. doi: 10.1152/jn.00082.2007. [DOI] [PubMed] [Google Scholar]
- 20.Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RPJ, Ko TH, et al. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelbrecht CJ, Voigt F, Helmchen F. Miniaturized selective plane illumination microscopy for high-contrast in vivo fluorescence imaging. Opt Lett. 2010;35:1413–1415. doi: 10.1364/OL.35.001413. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott BB, Thiberge SY, Guo C, Tervo DGR, Brody CD, Karpova AY, et al. Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron. 2018;100:1045–1058. doi: 10.1016/j.neuron.2018.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senarathna J, Yu H, Deng C, Zou AL, Issa JB, Hadjiabadi DH, et al. A miniature multi-contrast microscope for functional imaging in freely behaving animals. Nat Commun. 2019;10:1–13. doi: 10.1038/s41467-018-07926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevedel R, Yoon YG, Hoffmann M, Pak N, Wetzstein G, Kato S, et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat Methods. 2014;11:727–730. doi: 10.1038/nmeth.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skocek O, Nöbauer T, Weilguny L, Traub FM, Xia CN, Molodtsov MI, et al. High-speed volumetric imaging of neuronal activity in freely moving rodents. Nat Methods. 2018;15:429–432. doi: 10.1038/s41592-018-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbera G, Liang B, Zhang L, Li Y, Lin DT. A wireless miniScope for deep brain imaging in freely moving mice. J Neurosci Methods. 2019;323:56–60. doi: 10.1016/j.jneumeth.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dussaux C, Szabo V, Chastagnier Y, Fodor J, Léger JF, Bourdieu L, et al. Fast confocal fluorescence imaging in freely behaving mice. Sci Rep. 2018;8:16262. doi: 10.1038/s41598-018-34472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberti WA, III, Perkins LN, Leman DP, Gardner TJ. An open source, wireless capable miniature microscope system. J Neural Eng. 2017;14:045001. doi: 10.1088/1741-2552/aa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob AD, Ramsaran AI, Mocle AJ, Tran LM, Yan C, Frankland PW, et al. A compact head-mounted endoscope for in vivo calcium imaging in freely behaving mice. Curr Protoc Neurosci. 2018;84:e51. doi: 10.1002/cpns.51. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Liang B, Barbera G, Hawes S, Zhang Y, Stump K, et al. Miniscope GRIN lens system for calcium imaging of neuronal activity from deep brain structures in behaving animals. Curr Protoc Neurosci. 2019;86:e56. doi: 10.1002/cpns.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen KS, Xu M, Zhang Z, Chang WC, Gaj T, Schaffer DV, et al. A hypothalamic switch for REM and non-REM sleep. Neuron. 2018;97:1168–1176. doi: 10.1016/j.neuron.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Rubin A, Sheintuch L, Brande-Eilat N, Pinchasof O, Rechavi Y, Geva N, et al. Revealing neural correlates of behavior without behavioral measurements. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-019-12724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 36.Mittmann W, Wallace DJ, Czubayko U, Herb JT, Schaefer AT, Looger LL, et al. Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nat Neurosci. 2011;14:1089–1093. doi: 10.1038/nn.2879. [DOI] [PubMed] [Google Scholar]
- 37.Kondo M, Kobayashi K, Ohkura M, Nakai J, Matsuzaki M. Two-photon calcium imaging of the medial prefrontal cortex and hippocampus without cortical invasion. Elife. 2017;6:e26839. doi: 10.7554/eLife.26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Wu C, Sinefeld D, Li B, Xia F, Xu C. Comparing the effective attenuation lengths for long wavelength in vivo imaging of the mouse brain. Biomed Opt Express. 2018;9:3534–3543. doi: 10.1364/BOE.9.003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung ELM, Schnitzer MJ. Fiber-optic fluorescence imaging. Nat Methods. 2005;2:941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villette V, Chavarha M, Dimov IK, Bradley J, Pradhan L, Mathieu B, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell. 2019;179:1590–1608. doi: 10.1016/j.cell.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takasaki K, Abbasi-Asl R, Waters J. Superficial bound of the depth limit of Two-Photon imaging in mouse brain. eNEURO, 2020, 7(1). 10.1523/ENEURO.0255-19.2019 [DOI] [PMC free article] [PubMed]
- 42.Huang X, Fan J, Li L, Liu H, Wu R, Wu Y, et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat Biotechnol. 2018;36:451–459. doi: 10.1038/nbt.4115. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Zhu Q, Ding L, Shen Y, Yang CY, Xu F, et al. Scalable volumetric imaging for ultrahigh-speed brain mapping at synaptic resolution. Natl Sci Rev. 2019;6:982–992. doi: 10.1093/nsr/nwz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osman A, Park JH, Dickensheets D, Platisa J, Culurciello E, Pieribone VA. Design constraints for mobile, high-speed fluorescence brain imaging in awake animals. IEEE T BIOMED CIRC S. 2012;6:446–453. doi: 10.1109/TBCAS.2012.2226174. [DOI] [PubMed] [Google Scholar]
- 45.Obenhaus HA, Zong W, Rose T, Donato F, Jacobsen RI, Chen L, et al. Functional network topography of the medial entorhinal cortex revealed by miniaturized large-field-of-view two-photon microscopy in freely moving mice. Program No. 604.09. 2019 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience 2019. Online.
- 46.Zong W, Chen L. Advanced Miniature Microscopy for Brain Imaging. In: Kao FJ, Keiser G, Gogoi A. (eds) Advanced Optical Methods for Brain Imaging. Progress in Optical Science and Photonics, 2019, Vol 5. Springer, Singapore. 10.1007/978-981-10-9020-2_9.