Abstract
Transcranial direct current stimulation (tDCS) is a promising method for altering cortical excitability with clinical implications. It has been increasingly used in neurodevelopmental disorders, especially attention-deficit hyperactivity disorder (ADHD), but its efficacy (based on effect size calculations), safety, and stimulation parameters have not been systematically examined. In this systematic review, we aimed to (1) explore the effectiveness of tDCS on the clinical symptoms and neuropsychological deficits of ADHD patients, (2) evaluate the safety of tDCS application, especially in children with ADHD, (3) model the electrical field intensity in the target regions based on the commonly-applied and effective versus less-effective protocols, and (4) discuss and propose advanced tDCS parameters. Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses approach, a literature search identified 14 empirical experiments investigating tDCS effects in ADHD. Partial improving effects of tDCS on cognitive deficits (response inhibition, working memory, attention, and cognitive flexibility) or clinical symptoms (e.g., impulsivity and inattention) are reported in 10 studies. No serious adverse effects are reported in 747 sessions of tDCS. The left and right dorsolateral prefrontal cortex are the regions most often targeted, and anodal tDCS the protocol most often applied. An intensity of 2 mA induced stronger electrical fields than 1 mA in adults with ADHD and was associated with significant behavioral changes. In ADHD children, however, the electrical field induced by 1 mA, which is likely larger than the electrical field induced by 1 mA in adults due to the smaller head size of children, was sufficient to result in significant behavioral change. Overall, tDCS seems to be a promising method for improving ADHD deficits. However, the clinical utility of tDCS in ADHD cannot yet be concluded and requires further systematic investigation in larger sample sizes. Cortical regions involved in ADHD pathophysiology, stimulation parameters (e.g. intensity, duration, polarity, and electrode size), and types of symptom/deficit are potential determinants of tDCS efficacy in ADHD. Developmental aspects of tDCS in childhood ADHD should be considered as well.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00501-x) contains supplementary material, which is available to authorized users.
Keywords: Transcranial direct current stimulation, Attention-deficit hyperactivity disorder, Dorsolateral prefrontal cortex, Executive function, Systematic review, Brain modeling, Non-invasive brain stimulation, Pediatric
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly-diagnosed neurodevelopmental disorders. It is characterized by the symptoms of inattention, hyperactivity, impulsivity [1], and various cognitive dysfunctions [2, 3]. A precise description of the neuropathology underlying ADHD is difficult due to its neuropsychological heterogeneity [4] and the substantial overlap between children with ADHD and typically developing children [5]. However, based on neuroimaging and neuropsychological findings, distinct brain regions and networks have been identified to account for the hallmark symptoms [6, 7] and subtypes [8] of ADHD.
Poor inhibitory control resulting from deficient executive resources (i.e., the inhibition-based model) and impulse control deficits that lead to hyperactivity (i.e., the motivational dysfunction model) are influential theories of ADHD pathophysiology. According to the first model, the decisive factor in ADHD pathophysiology is impaired executive functions [9, 10], which are associated with hypoactivation of the dorsolateral prefrontal cortex (dlPFC) [11, 12] and hyperactivation of some subcortical regions [13]. Some executive functions play more critical roles in the ADHD symptoms and deficits, including cognitive flexibility, inhibitory control, and working memory [14, 15]. In the “motivational dysfunction theory” [16, 17], hyperarousal to environmental stimuli leads to an inability of the executive functioning system to control the respective stimuli [18, 19]. This theory attributes these symptoms to the medial frontal cortex, orbital and ventromedial prefrontal areas [7], and subcortical regions such as the caudate nucleus, amygdala, nucleus accumbens, and thalamus [20].
Such large-scale brain abnormalities in ADHD pathophysiology have encouraged researchers to look for novel treatment options that target the symptoms by modulating, altering, and remediating deficient brain functions. Recent studies highlight the relevance of non-invasive brain stimulation for modulating cortical excitability [21]. Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that uses a weak electrical current and can modulate cortical excitability [22]. Anodal stimulation increases cortical excitability, while cathodal stimulation usually decreases it [23] although an excitatory-enhancing effect on motor cortical excitability [24] and on non-motor areas [25] has recently been reported. TDCS has been shown to improve impaired components of executive functions not only in ADHD [7, 26] but also in other disorders accompanied by impaired executive functions such as depression [27], obsessive-compulsive disorder [28], anxiety disorders [29], and drug addiction [30], as well as in healthy populations [31–34], depending on the stimulation parameters. The safety of tDCS in adults [35] and children has been documented in previous studies [36] and confirmed by recent large dataset [37] although more evidence is needed for its safe application in pediatric populations.
Application of tDCS in pediatrics, especially children with neurodevelopmental deficits, has gained attention in recent years and has been suggested to be a promising tool for their rehabilitation and/or treatment [38, 39]. In ADHD with a typical onset of symptoms in childhood, early interventions that may modify the atypical neural circuits and networks involved in its pathophysiology might be promising. Several studies have shown the efficacy of tDCS for improving cognitive and behavioral functioning in both children and adults with ADHD, such as inhibitory control [40–43], visual attention [44], declarative and working memory [40, 44–46], and clinical symptoms [47, 48].
Most of the studies so far have investigated the effects of tDCS on neuropsychological symptoms (i.e., response inhibition and working memory) [26] and others were limited to behavioral and clinical symptoms [48, 49]. Furthermore, knowledge of the tDCS efficacy and safety in the clinical pediatric population, including ADHD, is still relatively limited and warrants further investigation [39, 50, 51]. Recently, several reviews have discussed non-invasive brain stimulation in neurodevelopmental disorders including ADHD [52–54]. These reviews however are not specifically dedicated to tDCS or ADHD. Furthermore, they do not provide an objective and comprehensive picture of tDCS efficacy in improving both the clinical symptoms and cognitive deficits with respect to effect size. Moreover, systematic investigation of tDCS safety, computational modeling of the electrical current flow in the head that is associated with neurophysiological modulation [55], and a detailed discussion of potential stimulation parameters are still missing.
Here, we used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) method to systematically review the studies that investigated the effects of tDCS on the clinical symptoms and cognitive-behavioral impairments in both childhood and adult ADHD in order to (1) evaluate the efficacy of this technique in improving the clinical symptoms and neuropsychological deficits, (2) calculate the effect sizes and the magnitude of changes in outcome variables, (3) investigate the safety aspects of tDCS in clinical pediatric populations, (4) model the electrical fields induced by the commonly-used tDCS protocols in ADHD, and (5) discuss and propose advanced tDCS parameters and montages for ADHD treatment.
Methods
Data for this systematic review were collected in accordance with the PRISMA checklist [56]. Our checklist can be found in the supplementary information.
Eligibility Criteria
Only peer-reviewed published studies were included in our analysis. The inclusion criteria were (1) randomized placebo (sham)-controlled or baseline-controlled design and open-label studies with sham or baseline control, (2) published in English, (3) targeting the clinical symptoms and/or cognitive deficits of ADHD, (4) no comorbidity with other developmental disorders/disabilities (e.g. autism, learning disabilities, conduct disorder, or oppositional defiant disorder) in childhood ADHD and psychiatric disorders in adult ADHD, and (5) published as empirical and not review or methods articles. Having a history of or under stimulant medication was allowed as long as the regimen was stable. In Munz et al. [41] (n = 14) and Breitling et al. [42] (n = 21), 3 patients had comorbid conduct disorders. No studies were excluded based on the participants’ age, and both adult and childhood ADHD were included. In addition, a clinical diagnosis of ADHD based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV/V) confirmed by a psychiatrist, and/or meeting accepted cut-off values on validated ADHD symptom rating scales were required (see supplementary information for details of rating scales).
Information Sources
The primary sources of information for identifying studies were the PubMed and Scopus databases. We also used other widely-used search engines, such as Google Scholar, and reference lists from the identified articles.
Search Strategy and Study Selection
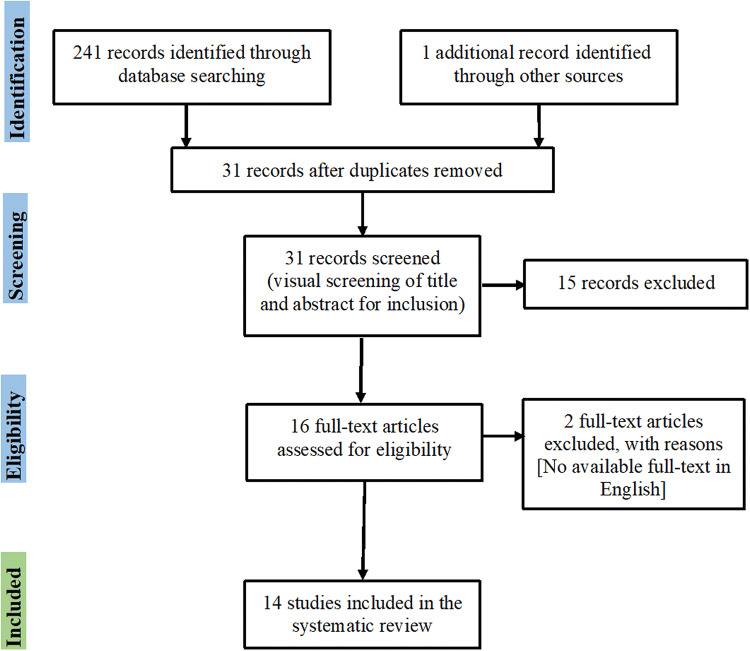
A comprehensive literature search was conducted by two independent data extractors [the first (MAS) and fourth (AM) authors] with the final search updated on January 30, 2020. The search terms were: attention-deficit hyperactivity disorder, ADHD, attention disorders, transcranial direct current stimulation, tDCS, transcranial electrical stimulation, and tES. Furthermore, a manual search of the reference sections of the retrieved studies and review articles was carried out. The final search identified a total of 241 articles, which were reduced to 31 after removing duplicates. The titles and abstracts of the remaining records were screened for eligibility, which led to the exclusion of 15 for either being animal model studies or with an inappropriate scope. Afterwards, the full text of each publication was assessed and eligible studies according to the inclusion criteria were selected. In this step, 2 more articles were excluded since the main text was not written in English. In sum, 17 (15 + 2) articles were excluded for not meeting the inclusion criteria after the abstract and full-text screening. Thus 14 articles remained for full-text assessment and data extraction (Fig. 1).
Fig. 1.
PRISMA flow diagram of included studies investigating the effects of transcranial direct current stimulation in ADHD.
Outcome Variables
Major clinical symptoms (inattention, hyperactivity, and impulsivity) and executive dysfunctions were the main outcome measures. Clinical symptoms were measured by DSM-IV/V diagnostics and/or the scores on ADHD self-reports and other-report scales [i.e., Adult ADHD Self-Report Scale (ASRS), Conner’s Adult ADHD Rating Scale–Self Report, Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Rating Scale, Swanson, Nolan and Pelham Rating Scale–IV (SNAP-IV), German Adaptive Diagnostic Checklist for ADHD (FBB-ADHD), and Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime (K-SADS-PL)], or performance in behavioral tests like the QbTest [57], or Conners Continous Performance Test [58]. Neuropsychological and cognitive deficits included response inhibition, working memory, interference control, attention, and cognitive flexibility. Response inhibition and interference control were measured with Go/No-Go, Stroop, Flanker, and Neuropsychological Development Assessment (NEPSY II). For working memory, the following tasks were included: (1) N-back, (2) Digit Span, and (3) Corsi block-tapping test. [59]. Cognitive flexibility was measured by the Wisconsin Card Sorting Test [60]. Details of the outcome measures examined in each task are presented in the supplementary materials.
Risk of Bias
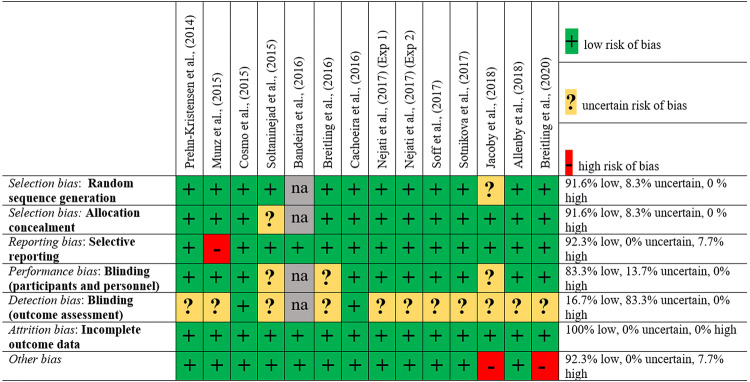
Risk of bias assessment was performed using the Cochrane Collaboration’s tool [61]. For each study, the authors judged the risk of selection, performance, detection, attrition, reporting, and other biases. Risk of bias was categorized as low, high, or uncertain. The evaluation of each study is displayed in Fig. 2.
Fig. 2.
Bias assessment of included studies using the Cochrane risk of bias tool (na, not applicable).
Results
Inter-rater reliability for the inclusion and exclusion of studies was high (Cohen’s k = 0.84). In total, 14 separate studies published between 2014 and the end of January 2020 were included [40–49, 62–64]. Details of the studies are summarized in Table 1 and Fig. 3.
Table 1.
Characteristics of included studies investigating the effects of tDCS in ADHD.
| Reference | Study type (Blinding) | n (mean age ± SD); gender (m/f) | ADHD type (inattentive/hyperactive/combined) | Medication intake (n) | Task (measure) | Expected outcome | Current intensity; duration | Repetition rate | Polarity/electrode size | Control | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prehn-Kristensen et al. [45] | RCT (double blind) | ADHD children n = 12 (12 ± 1.4, 10–14 years)/Healthy control n = 12; 24/0 | 4/0/8 | Off stimulants 48 h (n = 12) | Declarative memory | Change in score in the retrieval phase | So-tDCS (0–250 µA, 0.75 Hz); 5 × 5 min | Single session | Anodal F3- cathodal M1 & anodal F4 - cathodal M2/0.503 cm2 (Ag/AgCl sintered skin electrodes) | Sham | Enhanced memory consolidation and retrieval following active tDCS vs sham tDCS |
| Munz et al. [41] | RCT (double blind) successful blinding | n = 14 (12.3 ± 1.39, 10–14 years); 14/0 | 6/0/8 | Off stimulants 48 h (n = 10) | Go/No-Go (response inhibition), motor memory task | Change in the RT and accuracy of tasks | So-tDCS (0–250 µA, 0.75 Hz); 5 × 5 min | Single session | Anodal F3- cathodal M1 & anodal F4 – cathodal M2/0.503 cm2 (Ag/AgCl sintered skin electrodes) | Sham | Significantly improved RT after active stimulation vs sham in Go/No-Go and motor memory tasks but no significant improvement in the correct responses. |
| Cosmo et al. [43] | RCT (double blind) successful blinding | n = 60 (32.25 ± 10.96,18–65 years); 35/25 | 13/3/44 | On stimulants (n = 11) | Go/No-Go (response inhibition) | Change in the Go/No-Go task performance | 1 mA; 20 min | Single session | Anodal F3-cathodal F4/5 × 7 cm | Sham | No significant differences in inhibitory control between active tDCS vs sham tDCS |
| Soltaninejad et al. [62] | RCT (single-blind) | n = 20 (16.40 ± 1.09, 15–17 years); 20/0 | NR | Off medication | Go/No-Go and Stroop (response inhibition, attention, interference inhibition) | Change in Go/No-Go & Stroop tasks performance | 1.5 mA; 15 min | Single session |
(1) anodal F3 - cathodal Fp2 (2) cathodal F3, anodal Fp2 /5 × 7 cm |
Sham | Anodal left dlPFC tDCS vs sham, did not improve response inhibition in the Go-No/Go task. Cathodal left dlPFC Tdcs vs sham, improved inhibition accuracy in the Go-No/Go task. Noprotocols improved selective attention |
| Bandeira et al. [44] | Open-label study | n = 9 (11.11 ± 2.08, 6–16 years); 8/1 | NR | Off medication 1 week before | TAVIS 3, Corsi Cube test, NEPSY II & Digit Span (selective attention, WM, response inhibition) ADHD symptoms via SNAP-IV | Changes in selective attention, WM, IC tasks, and SNAP-IV | 2 mA; 30 min | Five sessions | Anodal F3 cathodal Fp2/5 × 7 cm | Baseline Control | Significantly reduced omission errors in selective attention task, shorter RT and reduced errors in the switching but not inhibition phase. Clinical symptoms slightly improved according to the parent’s response in the SNAP-IV |
| Breitling et al. [42] | RCT (single blind) unsuccessful blinding | ADHD children n = 21 (14.33)/Healthy control n = 21 (14.24); 21/0 | 5/0/6 | Off stimulants 24 h (n = 11) | Flanker task (interference control, response inhibition) (online) | Changes in the Flanker task | 1 mA; 20 min | Three sessions | Anodal-cathodal-shamF8, reference electrode over left mastoid/5 × 7 cm | Sham | No significantly improved interference control in ADHD patients. Only anodal tDCS, if applied in the first session, significantly diminished commission errors in ADHD group vs healthy controls (learning effect). |
| Cachoeira et al. [48] | RCT (double blind) successful blinding | n = 17 (32.37 ± 4.91, 18–45 years); 8/9 | 7/0/10 | Off stimulants | ASRS (ADHD symptoms) & SDS | Change in the ASRS and SDS scores | 2 mA; 20 min | Five sessions | Anodal F4- cathodal F3/5 × 7 cm | Sham | Bilateral dlPFC tDCS (anodal right) improved symptoms in adult ADHD. This improvement persisted after the end of the stimulation |
| Nejati et al. [46] (Exp 1) | RCT (double-blind) | n = 15 (10 ± 2.3, 8–15 years); 15/0 | 6/0/9 | Off stimulants | Go/No-Go (response inhibition)/1-back (WM)/Stroop (interference control)/WCST (cognitive flexibility) | Change in the Go/No-Go, 1-back, Stroop & WSCT tasks performance | 1 mA; 15 min | Two sessions | Anodal F3- cathodal F4/5 × 5 cm | Sham | Bilateral dlPFC tDCS (anodal left) improved executive control function (WM, interference control) but not prepotent response inhibition and cognitive flexibility |
| Nejati et al. [46] (Exp 2) | RCT (double-blind) | n = 10 (9 ± 1.8, 7–12 years); 5/5 | 5/5 | Off stimulants | Go/No-Go (response inhibition)/1-back (WM)/WCST (cognitive flexibility) | Change in the Go/No-Go, 1-back, & WSCT tasks performance | 1 mA; 15 min | Three sessions | (1) Anodal F3-cathodal Fp2 (2) cathodal F3-anodal Fp2/ 5 × 5 cm | Sham | Anodal left dlPFC tDCS improved WM accuracy and RT. Cathodal left dlPFC tDCS improved response inhibition. Both anodal and cathodal left dlPFC tDCS improved cognitive flexibility. |
| Soff et al. [47] | RCT (double-blind) successful blinding |
n = 15 (14.20 ± 1.2, 12–16 years); 12/3 |
patients were combined or hyperactive type | Off stimulants 96 h (n = 5) | FBB-ADHD &QbTest | Change in the inattention, hyperactive and impulsivity scales | 1 mA; 20 min | Five sessions | Anodal F3, cathodal Cz/5 × 7 cm | Sham | Anodal tDCS significantly reduced symptoms of inattention (measured by FBB-ADHD), and hyperactivity/inattention(measured by the Qbtest) in adolescents with ADHD compared to sham stimulation |
| Sotnikova et al. [40] | RCT (double-blind) successful blinding |
n = 13 (14.33 ± 1.32, 12–16 years); 11/2 |
patients were combined or hyperactive type | Off stimulants 96 h (n = 5) | Q-b Test (WM) | Changes in the Qb test scales | 1 mA; 20 min | Two sessions | Anodal F3, cathodal Cz/5 × 7 cm | Sham | Moderate effect of tDCS. Anodal tDCS led to significantly less increased RT and RT variability but also more errors. Increased neuronal activation and connectivity in the left dlPFC and other remote brain regions |
| Jacoby et al. [63] | RCT (single-blind) | ADHD group n = 21/Healthy control n = 16 (23.03 ± 2.54, 19–29 years); 23/12 | NR | On stimulants | MOXO-CPT (Attention, hyperactivity) | Changes in the CPT performance | 1.8 mA; 20 min | Two sessions | Double anodal bilateral tDCS (anodal 1 F3, anodal 2 F4, cathodal cerebellar cortex) | Sham | tDCS did not improve attention, timing, and impulsivity. The only measure which was improved by tDCS was hyperactivity |
| Allenby et al. [49] | RCT (double-blind) unsuccessful blinding | n = 37 (18–65); 26/11 | 21/0/16 | On stimulants (n = 17) | CPT, SST | Change in the CPT false error and SST accuracy and RT | 2 mA; 20 min | Three sessions | Anodal F3-cathodal Fp2/5 × 5 | Sham | Anodal left dlPFC tDCS improved impulsivity symptoms in ADHD compared to sham tDCS |
| Breitling et al. [64] | RCT (double-blind) | ADHD children n = 14 (13.3 ± 1.9)/Healthy control n = 15 (13.3 ± 1.8); 25/4 | 4/0/10 | Off stimulants 24 h (n = 5) | 2-back (WM) (online) and right after tDCS during EEG/K-SADS-PL (symptoms) | Change in 2-back task performance |
1 mA (conventional) 0.5 mA (4 × 1 montage); 20 min |
Three sessions | (1) Anodal F8, cathodal Fp1/5 × 7 cm; (2) 1 cm diameter electrodes in 4 × 1 montage (HD-tDCS) | Sham | No effect of conventional or HD-tDCS on WM performance. Numerically higher rate of responders for 4 × 1 (50%) than conventional (35%) tDCS. Higher N200 and P300 amplitudes after both protocols |
atDCS, transcranial direct current stimulation; So-tDCS, Slow-oscillating tDCS; ADHD, attention-deficit hyperactivity disorder; RCT, randomized control study; SD, standard deviation; RT, response time; m, male; f, female; F3, left dorsolateral prefrontal cortex (dlPFC); F4, right dlPFC; F8, right inferior frontal gyrus (rIFG); M1, A1 in 10-20- EEG system (left mastoid); M2, A2 in 10-20 EEG system (right mastoid); IFG, inferior frontal gyrus; Fp1, left supraorbital area; Fp2, right supraorbital area; NR, not reported; TAVIS 3, computerized test of visual attention; WM, working memory; ASRS, Adult ADHD Self-Report Scale Symptom Checklist 3; SDS, Sheehan Disability Scale; CPT, Conners Continuous Performance Task; SST, Stop Signal Task; FBB-ADHD, German adaptive ADHD Diagnostic Checklist; QbTest, Quantified Behavior Test; SNAP-IV, Swanson, Nolan, and Pelham–IV; K-SADS, Schedule for affective disorders and schizophrenia for school-age children; WSCT, Wisconsin Card Sorting Test.
Fig. 3.
Stimulation parameters of included studies investigating the effects of tDCS in ADHD (tDCS, transcranial direct current stimulation; toDCS, transcranial slow-oscillating direct current stimulation; Fp1, left supraorbital area; Fp2, right supraorbital area; F3, left dorsolateral prefrontal cortex; F4, right dorsolateral prefrontal cortex; F8, right inferior frontal gyrus; A1, left mastoid; A2, right mastoid; Cz, vertex; Pz, cerebellar cortex).
Risk of Bias
Risk of bias as judged by the authors is represented in Fig. 2. Generally, the risk of bias was very low with no indication of selection, performance, or attrition bias. In Jacoby et al. [63] the sources of other biases include no formal diagnosis for 3 participants in the ADHD group, recruitment of 3 participants from the student community, and no randomization/counterbalancing of the task in each session. In Breitling et al. [64], the sources of other biases are different experimental procedures in the control and ADHD groups, and reduction of stimulation intensity to 50% in 3 out of 14 participants due to low tolerability of the standard current intensity. Three studies used a single-blind design [42, 62, 63] yielding a potential detection bias as the experimenter was not blind to the tDCS condition. A blinding efficacy check was not explicitly reported in 6 studies [44–46, 62–64], hence these were categorized as uncertain for selection bias. No other biases were found.
Clinical Efficacy
Three of the included studies specifically investigated the effects of tDCS on the clinical symptoms of ADHD [47–49]. The results of these studies show that anodal tDCS can improve impulsivity and inattention and suggest that tDCS over the dlPFC might be suitable as a potential therapeutic approach in ADHD. While anodal left dlPFC tDCS was applied in two studies [47, 49], anodal right dlPFC tDCS was used in the Cachoeira et al. study. In the Allenby et al. study, the stimulation protocol was selected based on cognitive control and attention networks, which are more closely related to left dlPFC activation [65, 66] and task-based functional patterns in ADHD [7]. Similarly, in the Soff et al. study, the montage was selected based on the targeted cognitive deficit, which was working memory that strongly depends on left dlPFC activation. In the Cachoeira et al. study, no specific cognitive deficit was targeted. Based on the right hemisphere dysfunction in the frontal network, including the dlPFC, in ADHD [67], they applied anodal right dlPFC tDCS with the reference electrode over the left dlPFC and symptoms were monitored using self-report scales rather than behavioral tasks. Details of these studies are displayed in Table 1 and Fig. 3.
Neuropsychological and Cognitive Effects
The remaining studies [40–46, 62–64] mainly investigated the effects of tDCS on specific neuropsychological and cognitive deficits in ADHD (e.g., working memory, inhibitory control, attention, executive functions) (study details are summarized in Table 1). Briefly, the results show that dlPFC tDCS – specifically over the left dlPFC – improved the response inhibition, attention, working memory, and cognitive flexibility in ADHD patients. To be more specific, anodal left dlPFC tDCS, but not cathodal stimulation, improved working memory in ADHD [46]. Anodal right IFG (r-IFG) stimulation did not improve working memory [64]. With regard to inhibitory control, anodal left or right dlPFC tDCS [41, 45, 46], compared to bilateral dlPFC tDCS[43, 46] and anodal r-IFG tDCS [42], had a larger impact on response inhibition in ADHD. For those executive function domains that involve motivational and emotional processing [e.g. the Wisconsin Card Sorting Task (WCST)], tDCS over both prefrontal and frontopolar areas was more effective than dlPFC-only tDCS [46]. In other words, only when both the dlPFC and orbitofrontal cortex (OFC) were stimulated, did performance in the WCST improve [46].
Size of Effects
We calculated the effect sizes (Cohen’s d) of the outcome measures reported in the included studies (Table 2). Due to the heterogeneity of outcome measures, calculation of cumulative overall effect size (meta-analysis) is not accurate and informative. Therefore, we separately analyzed the effects of tDCS for each clinical symptom and neuropsychological deficit. A recently published meta-analysis on the effects of tDCS on response inhibition and working memory in ADHD showed a significant tDCS effect (especially anodal dlPFC tDCS) on response inhibition with a small effect size, but no significant effect on working memory, except for performance speed, with a medium effect size [26]. In the present study, we calculated Cohen’s d not only for clinical symptoms, but also for other cognitive/neuropsychological measures reported in the tDCS studies, including selective attention and cognitive control (Table 2). For cognitive control, the results show that the acquired effect sizes strongly depend on the stimulation protocol. With regard to tDCS effects on ADHD clinical symptoms, a large effect size for the inattention subscale was revealed [47, 48] and a medium effect size for hyperactivity/impulsivity [47, 49]. However, a definite conclusion with respect to the clinical efficacy of tDCS based on the results of these three studies is not possible.
Table 2.
Effect size of the outcome measures (both significant and non-significant) of included studies.
| Study | Outcome variable | P | Cohen’s d | Size | |
|---|---|---|---|---|---|
| Prehn-Kristensen et al. [45] | Working memory | Digit span* | 0.004 | −0.575 | Medium |
| Munz et al. [41] | Response inhibition | RT* | 0.03 | 0.91 | Large |
| RT variability* | 0.04 | 0.855 | Large | ||
| Cosmo et al. [43] | Response inhibition (letters) | Correct responses | 0.69 | 0.073 | Very small |
| Omission error | 0.89 | 0.272 | Small | ||
| Commission error | 0.98 | −0.069 | Very small | ||
| Response inhibition (fruits) | Correct responses | 0.78 | −0.104 | Small | |
| Omission error | 0.79 | −0.133 | Small | ||
| Commission error | 0.68 | −0.035 | Very small | ||
| Soltaninejad et al. [62] | Response inhibition (Go/NoGo) | Go accuracy | 0.07 | −0.028 | Very small |
| No-go accuracy* | 0.03 | 0.054 | Very small | ||
| RT | 0.09 | 0.249 | Small | ||
| Response inhibition (Stroop) | accuracy | 0.10 | 0.594 | Medium | |
| RT | 0.31 | 0.249 | Small | ||
| Bandeira et al. [44] 1 | Selective attention | Omission errors* | 0.03 | 0.376 | Small-to-medium |
| Response inhibition (NEPSY-II) | Total error* | 0.012 | 0.165 | Small | |
| Completion time* | 0.016 | 0.588 | Medium | ||
| Digit span forward | Accuracy | 0.125 | −0.82 | Very small | |
| Digit span backward | Accuracy | 0.531 | −0.356 | Medium | |
| Corsi cube forward | Accuracy | 0.281 | −0.401 | Medium | |
| Corsi cube backward | Accuracy | 0.813 | 0.125 | Very small | |
| Breitling et al. [42] | Response inhibition (Flanker task) – anodal stimulation | Omission error | n/r | −0.088 | Very small |
| Commission error* | 0.02 | 0.476 | Medium | ||
| RT | n/r | −0.122 | Small | ||
| RT variability* | 0.05 | 0.153 | Small | ||
| Response inhibition (Flanker task) – cathodal stimulation | Omission error | n/r | −0.58 | Very small | |
| Commission error | n/r | 0.187 | Small | ||
| RT | n/r | 0.152 | Small | ||
| RT variability | n/r | 0 | – | ||
| Cachoeira et al. [48] | ADHD symptoms (ASRS) | Inattention* | 0.001 | 0.85 | Large |
| Hyperactivity/impulsivity | 0.01 | 0.60 | Medium | ||
| total | 0.003 | 0.81 | Large | ||
|
Nejati et al. [46] (Exp 1) Anodal left DLPFC – cathodal right DLPFC |
Response inhibition (Go/NoGo) | Go accuracy | 0.66 | 0.157 | Small |
| No-go accuracy | 0.32 | 0.112 | Small | ||
| RT | 0.14 | 0.263 | Small | ||
| Response inhibition (Stroop) | Accuracy* | 0.01 | 0.726 | Large | |
| RT* | 0.02 | 1.119 | Large | ||
| Working memory | Accuracy | 0.65 | 0.109 | Small | |
| RT* | 0.01 | 1.42 | Large | ||
| Cognitive control / flexibility (WCST) | Completion time* | 0.01 | 0.577 | Medium | |
| Perseverative errors | 0.86 | 0.063 | Very small | ||
| Categories completed | 0.81 | 0.063 | Very small | ||
| Total errors | 0.69 | 0.115 | Small | ||
|
Nejati et al. [46] (Exp 2) – Anodal left DLPFC tDCS |
Response inhibition (Go/NoGo) | Go accuracy | 0.16 | 0.451 | Medium |
| No-go accuracy | 0.50 | 0.699 | Medium | ||
| RT | 0.15 | −0.196 | Small | ||
| Working memory - anodal stimulation | Accuracy* | 0.01 | 1.197 | Large | |
| RT* | 0.04 | 1.003 | Large | ||
| Cognitive control / flexibility (WCST) - anodal stimulation | Completion time | 0.07 | 0.915 | Large | |
| Perseverative errors* | 0.01 | −2.545 | Large | ||
| Categories completed* | 0.01 | 1.666 | Large | ||
| Total errors* | 0.01 | −2.579 | Large | ||
| Cathodal left DLPFC tDCS | Response inhibition (Go/NoGo) - cathodal stimulation | Go accuracy | n/r | 0.451 | Medium |
| No-go accuracy* | 0.01 | 1.248 | Large | ||
| RT | n/r | −0.64 | Medium | ||
| Working memory - cathodal stimulation | accuracy | 0.345 | 0.578 | Medium | |
| RT | 0.108 | 0.587 | Medium | ||
| Cognitive control / flexibility (WCST) - cathodal stimulation | Completion time | n/r | −0.465 | Medium | |
| Perseverative errors* | 0.04 | −0.943 | Large | ||
| Categories completed* | 0.01 | 0.960 | Large | ||
| Total errors* | 0.01 | −1.157 | Large | ||
| Soff et al. [47] 1,2 | ADHD symptoms (FBB-ADHD) | Inattention* | 0.03 | 1.241 | Large |
| Hyperactivity | n/r | 0.694 | Medium | ||
| Impulsivity | n/r | 1.177 | Large | ||
| ADHD symptoms (QB-test) | Inattention* | 0.05 | 0.221 | Small | |
| Hyperactivity* | 0.03 | 0.336 | Small-to-medium | ||
| Impulsivity | n/r | 0.125 | Small | ||
| Sotnikova et al. [40] | Working memory – 1-back | Accuracy | n/r | −0.96 | Large |
| RT | n/r | −0.022 | Very small | ||
| RT variability | n/r | 0.214 | Small | ||
| Working memory – 2-back | Accuracy* | 0.001 | −1.11 | Large | |
| RT* | 0.021 | 0.682 | Medium | ||
| RT variability* | 0.026 | 1.094 | Large | ||
| Response inhibition (Go/NoGo) | Accuracy* | 0.013 | −0.652 | Medium | |
| RT | n/r | 0.271 | Small | ||
| RT variability | n/r | −0.017 | Very small | ||
| Jacoby et al. [63] | ADHD symptoms (MOXO CPT) | Attention RT | 0.86 | 0.011 | Very small |
| Hyperactivity* | 0.013 | 0.483 | Medium | ||
| Timing score | n/r | 0.104 | Small | ||
| Allenby et al. [49] | Response inhibition (CPT) | False positive error* | 0.01 | 0.425 | Medium |
| True positive error | 0.05 | -0.051 | Very small | ||
| Response inhibition (SST) | Response time | > 0.05 | −0.101 | Small | |
| RT | > 0.05 | −0.177 | Small | ||
|
Breitling et al. [64] Conventional montage |
Working memory (2-back) | Accuracy | n/r3 | −0.240 | Small |
| RT | n/r3 | 0.009 | Very small | ||
| Accuracy | n/r3 | −0.088 | Very small | ||
| 4 × 1 electrode-montage | RT | n/r3 | 0.172 | Small | |
Cohen’s d was calculated (using mean and average SDs) between active vs sham tDCS for each outcome measure; *significant measures reported in the studies are bold. n/r, no report of P value; ASRS, Adult ADHD Self-Report Scale Symptom Checklist; WCST, Wisconsin Card Sorting Test; QbTest, Quantified Behavior Test; FBB-ADHD, German adaptive ADHD Diagnostic Checklist; CPT, Conners Continuous Performance Task; SST, Stop Signal Task. 1, Cohen’s d in these studies are calculated based on the pre-post measurement difference according to the reported data analysis; 2, in the Soff et al. study [47], the difference is calculated based on baseline vs 7-day post-measurement; 3, in Breitling et al. [64], P value is reported for the ANOVA results (0.570 for WM accuracy and 0.100 for RT) and no P-value for t-tests is reported.
Adverse Effects
Of 14 studies included in the present review, 10 were conducted in patients with childhood ADHD. Of 747 sessions of tDCS applied in 278 ADHD patients, 449 sessions of tDCS were conducted in 143 ADHD children and none of them reported serious adverse effects during or after tDCS. No adverse effects were reported by the participants during or after tDCS in the Prehn-Kristensen et al. [45] and Munz et al. [41] studies. Soltaninejad et al. [62] used a side-effect survey after tDCS to assess potential side-effects and reported there were none. In the Bandeira et al. study [44], the authors reported that adverse effects were mostly mild; however, they reported a feeling of ‘‘shock’’ during tDCS in some patients. Breitling et al. [42] reported no adverse effect beyond skin sensations (tingling and itching) during active and sham stimulation, with trend-wise stronger sensations during cathodal stimulation. In their recent report [64], medium- intensity tDCS-related sensations were reported for itching, pain, fatigue, low headache, and phosphenes. In both experiments of Nejati et al. [46] no severe adverse effects were reported, but mild tingling and itching under the surface of the electrodes were mentioned. Similarly, adolescent participants in the Soff et al. study [47] tolerated the stimulation without any problem, except for one case of headache after anodal tDCS, and mild tingling and itching under the electrodes. Last, in the Sotnikova et al. study [40], no signs of fatigue, burning, pain, or other uncomfortable sensations during stimulation were reported. Only one participant felt nervous or overexcited during stimulation and another reported headache.
In four studies conducted in adult patients with ADHD, no major adverse effects of tDCS were reported. Cosmo et al. [43] assessed the safety and potential side-effects via open questions based on the tDCS adverse events questionnaire [68] and reported none. In the Cachoeira et al. study [48] only one participant experienced an acute mood change, sadness, hypobulia, and tension 5 h after stimulation. No more side-effects were reported. All participants in the Jacoby et al. study [63] tolerated tDCS well and no adverse effects were reported. Finally, Allenby et al. [49] reported that the average side-effects experienced during tDCS were generally mild, rated below 3 out of 10. Tingling (Mean = 1.9), burning sensation (Mean = 2.8), and difficulty concentrating (Mean = 2) were the most prominent experiences.
Electrical Current Flow in the Head
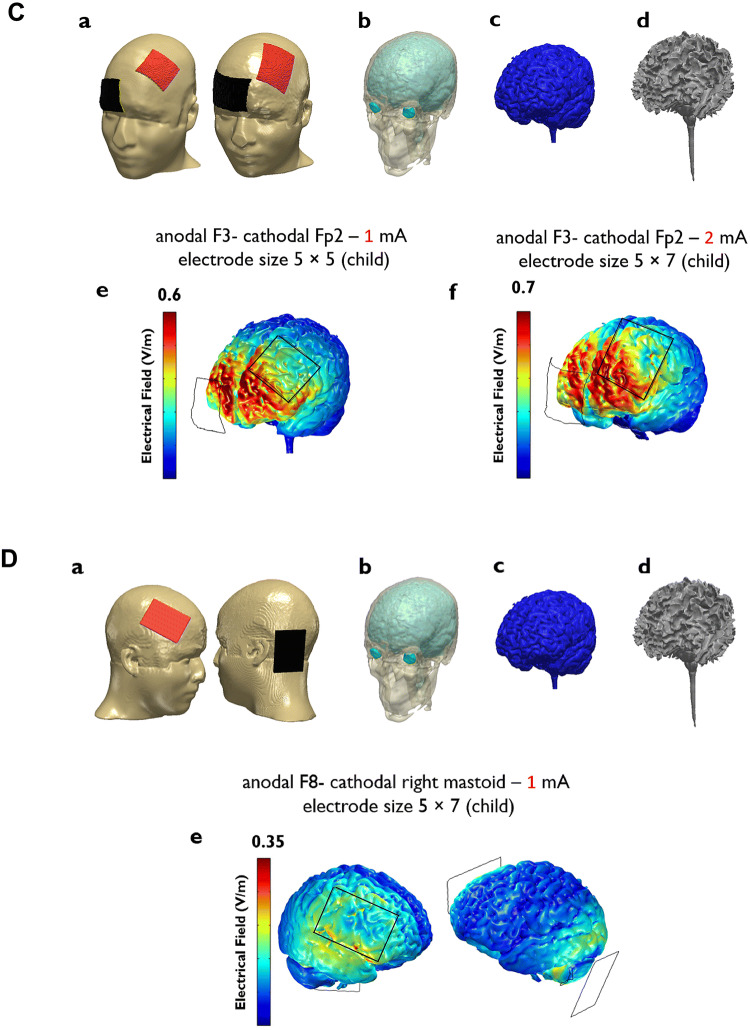
Neurophysiological modulations induced by tDCS are associated with electrical field strength [55]. The flow of electrical current in the head depends on stimulation intensity, electrode size, angle, and the developmental stage of the participant (child or adult). Modeling current flow in the head can mathematically indicate how much cortical/subcortical regions are affected by the current and allow evaluation of the protocol-induced modulation of activity in targeted regions. In 12 studies, the anodal electrode was placed over the left dlPFC, and in these studies, bilateral dlPFC tDCS and anodal left dlPFC – cathodal right supraorbital tDCS were the most used protocols (Table 1) in both adults and children. We modeled the current flow induced by these two commonly-used protocols at current intensities of 1 mA and 2 mA for both adults and children with ADHD (Fig. 4). We also modeled the r-IFG protocol [42], which found null effect of r-IFG stimulation on executive control in ADHD (Fig. 4D). Cosmo et al. [43] and Cachoeira et al. [48] used bilateral dlPFC tDCS with 35 cm2 electrodes and 1 mA and 2 mA, respectively, in adult ADHD patients (Fig. 4A). While Cosmo et al. found no significantly superior effect of active tDCS compared to sham tDCS, Cachoeira et al. reported improved ADHD symptoms. This difference could be due to the repetition rate of stimulation in the latter study; however, modeling of the current flow induced by these two protocols shows that the current flow induced in cortical regions by 1 mA is approximately half (0.4 V/m) of that by 2 mA (0.8 V/m) in adults (Fig. 4). We also modeled bilateral dlPFC tDCS with 25 cm2 electrodes and 2 mA to compare the current flow induced by smaller electrode size (Fig. 4Ag).
Fig. 4.
Three-dimensional models of electrical current flow in the head induced by common montages applied to ADHD patients with the head sizes of an adult (1 and 2; the New York (ICBM-NY) head [69]) and a 9 year-old child (3 and 4; open-source ABIDEII-OHSU child MR datasets). MR images were first segmented into 6 tissue types: gray matter (GM), white matter (WM), CSF, skull, scalp, and air cavities using the SPM8 software package (Welcome Trust Center for Neuroimaging, London, UK) with an improved tissue probability map. A custom MatLab script was then used to correct for segmentation errors made by SPM [70]. Then, a 3D model of the segmented images, with addition of the electrodes and saline-soaked sponges, was designed using the Simpleware software package version 5 (Synopsys, Mountain View, CA). Finally, the current flow distribution inside the head was calculated based on the finite element method using COMSOL Multiphysics software package version 5.2 (COMSOL Inc., Burlington, MA). The conductivity values used for each tissue type were as follows (in S/m): GM: 0.276; WM: 0.126; CSF: 1.65; skull: 0.015; scalp: 0.465; air: 2.5 × 10−14; saline-soaked sponge: 1.5; electrode rubber: 29 [71, 72]. Electrical fields throughout the gray matter are shown for stimulation intensities of 2.0 mA (Ae) and 1.0 mA (Af) with an F3 anodal–F4 cathodal montage and electrode sizes of 35 cm2 and 25 cm2 (Ag), 2.0 mA with an F3 anodal–Fp2 cathodal montage (Be), 1.0 mA (Ce) and 2 mA (Cf) with an F3 anodal–Fp2 cathodal montage in children and electrode sizes of 25 cm2 and 35 cm2, respectively, and 1 mA with an F8 anodal–A1 cathodal montage (De). F3, left dorsolateral prefrontal cortex; F4, right-left dorsolateral prefrontal cortex; Fp2, right supraorbital area; F8, right inferior frontal gyrus; A1, left mastoid. The figures also depict electrical fields induced in adjacent non-target areas, which should not mislead the interpretation of the electrical field under the target electrodes. The current flow under the electrode area is of interest.
The current flow induced by 2 mA anodal left dlPFC – cathodal right supraorbital tDCS through 25 cm2 electrodes in the adult population reported in the Allenby et al. study [49] found a significant reduction of impulsivity scores induced by tDCS (Fig. 4B). The same protocol (anodal F3–cathodal Fp2, 25 cm2 electrodes) with 1 mA and 2 mA was used for ADHD children in studies by Nejati et al. [46] (2nd experiment) and Bandiera et al. [44], respectively (Fig. 4C). The first important point to note here is the resulting electrical field in children compared to adults. 1 mA (anodal F3–cathodal Fp2) induces a field strength of 0.6 V/m in the brain of children (Fig. 4Ce), which has been shown to be sufficient to modulate target regions, while the same intensity (anodal F3–cathodal F4) in adults induces a field strength of 0.4 V/m (Fig. 4Af) which was insufficient to alter performance in the studies. Second, it is important to take into account that the resultant electrical field is determined not only by current strength, but by the combination of electrode size, stimulation intensity, and distance between electrodes. In the Bandiera et al. study, the current intensity was 2 mA, which results in an induced electrical field of ~ 0.7 V/m, while in the study of Nejati et al., 1 mA induces an electrical field of 0.6 V/m in the target region. A major reason for this lower than expected difference between resultant electrical fields is that in the Bandiera et al. study, the distance between electrode edges was smaller than that in the study by Nejati et al., due to the larger electrode size used in the former study, and the relatively close positions of the targeted areas in the heads of children, which increases the shunting of current between the electrodes through the skin. This shows the importance of electrode size and the distance between electrodes for the effects of tDCS; this is especially relevant in children, where the distance to target regions is smaller than in adults due to the smaller head size. The electrical field induced by the anodal r-IFG tDCS protocol at 1 mA is shown in Fig. 4D (last row) [42]. No significant tDCS-specific effect on ADHD symptoms was reported. As our modeling suggests, the electrical field induced by this electrode arrangement does not properly target the r-IFG (the area under the electrode), which could be one reason for the reported null effects. We discuss the impact of stimulation parameters on tDCS efficacy in more detail in the next section.
Discussion
The results of this systematic review show that tDCS at least partially improves the symptoms of ADHD, especially the cognitive deficits. All of the included studies, except for three experiments, reported significant improving effects of tDCS on some of the target variables (Table 1). However, tDCS efficacy and importantly its clinical utility in ADHD cannot be concluded yet and warrant further investigation with optimized designs. One source of variability in the findings was the heterogeneous stimulation parameters that may not be well-suited for all ADHD subtypes. In what follows we discuss how these parameters could yield larger effects, and the clinical and methodological implications for future studies. Given that the majority of studies were conducted on childhood ADHD, we discuss the developmental aspects of tDCS separately.
Stimulation Parameters
Stimulation Site
Three cortical regions were targeted by tDCS in the studies included in this review: the dlPFC [40, 41, 43–49, 62, 63], the OFC (supraorbital cortex) [44, 46, 49, 62], and the r-IFG[42, 64]. In 13 experiments, the dlPFC was the primary target of stimulation, while the r-IFG was stimulated in only two experiments. For stimulation over the dlPFC, both left and right dlPFCs were targeted in different studies, but left dlPFC tDCS was applied more frequently (Table 1).
The rationale for targeting the dlPFC, r-IFG, or OFC was based on the involvement of these regions in ADHD symptoms and pathophysiology. Recent meta-analyses of neuroimaging studies in ADHD showed bilateral hypoactivity of the dlPFC [73], reduced activity of the right inferior and dorsolateral PFC [67], and reduced left and medial frontal cortex activation [74] during the response inhibition, working memory, and attention tasks. For some dlPFC-related deficits, such as in working memory, an association between the stimulation site (i.e., left dlPFC) and deficit-specificity was established. In other deficits such as response inhibition, both the left and right dlPFC are involved and tDCS effects differ depending on the type of task applied and the outcome measure (i.e., commission versus omission error, reaction time). Nevertheless, the right dlPFC, which seems to be more relevant to ADHD with impaired inhibitory control, has not yet been studied enough. A recent repetitive transcranial magnetic stimulation study showed involvement of the right prefrontal cortex in alleviating symptoms of adult ADHD [75]. The importance of right dlPFC is partly due to its connections with the r-IFG.
The r-IFG has reduced activity in individuals with poor inhibitory control [76, 77] and is therefore regarded as a potential region of interest in ADHD pathophysiology [78, 79]. Only two studies in the database targeted the r-IFG with anodal tDCS and reported no significant improving effect on interference control [42] and working memory [64]. As our modeling results suggest (Fig. 4D), the electrode arrangement in the first Breitling et al. study [42] was likely suboptimal, and did not target the r-IFG sufficiently. According to the modeling results, placing the reference electrode on a more superior region rather than the mastoid could increase the electrical field in the r-IFG. Moreover, since this region is anatomically deeper than the dlPFC, it might be necessary to increase the stimulation intensity to achieve relevant results in that region. In their recent study, Breitling et al. [64] compared the 4 × 1 electrode montage (0.5 mA) with the conventional montage (1 mA) over the r-IFG in ADHD patients (Table 1). Although they reported more responders to the focal stimulation montage than the conventional montage, the protocols had no significant effect on behavioral performance. The use of large electrodes (5 × 7 cm2) in the conventional montage, relatively low stimulation intensity in the 4 × 1 electrode montage, small sample size, and use of a working memory task that may be more closely related to dlPFC than r-IFG activation could be reasons for their null effects. The null effect of r-IFG tDCS could also be due to contributions of different prefrontal regions to different aspects of response inhibition (i.e., cognitive vs motor aspects) shown in previous studies [78–80]. Accordingly, it is reasonable to speculate that ADHD subtypes, which differ regarding cognitive and motor-related symptoms (i.e., inattentive vs hyperactive types), and specific components of response inhibition (i.e., motor vs cognitive response inhibition), also affect the efficacy of tDCS over the respective target areas. This hypothesis was partially supported by the Breitling et al. study [64] where it was shown that in patients with fewer hyperactive symptoms the focal stimulation over the r-IFG had larger positive effects. Nevertheless, the involvement of the r-IFG in ADHD should be further explored in future tDCS studies.
Other potentially involved but less-explored sites are the ventromedial prefrontal cortex and OFC that are involved in motivational/emotional processes and social cognition [25, 33, 81], which are impaired in ADHD. These areas are parts of the reward network and show reduced activation during tasks involving “hot” executive functions (involving emotional/motivational components) in ADHD [7, 82] and their activity differs in ADHD depending on the type of deficit, task, and ADHD subtype [83, 84].
Stimulation Polarity
The pathophysiology of ADHD includes under-activation of the lateral, orbital, and inferior prefrontal cortex. Along this line, anodal tDCS-generated excitability enhancement was used in most of the tDCS studies of ADHD (Table 1). Although cognition-enhancing and symptom-alleviating effects of anodal tDCS in ADHD are partially supported in this review, it might be that cathodal tDCS over pathologically hyperactive regions, especially areas involved in emotion processing or in hyperactive ADHD subtypes, is also beneficial. However, this has not been systematically explored so far. In this context, a recent neuroimaging study showed that the connectivity of ADHD-related brain networks differs between subtypes, with more hyperconnectivity in the default mode network in the combined, but not the inattentive subtype [8].
Another relevant point is the laterality of the stimulation protocols. As a caveat, most of the tDCS studies in ADHD so far applied bipolar stimulation over areas relevant to ADHD. Conceptually, unilateral protocols that merely target a region of interest without positioning the return electrode over a potentially similarly involved region might show better efficacy, especially if the region over which the cathodal return electrode is placed shows pathological hypoactivity. In this case, the effects of the cathodal return electrode might compromise performance, and thus partially antagonize anodal tDCS effects over the target region. One last point about cathodal tDCS is that changing conventional stimulation parametrs can add complexity or even reverse to the expected tDCS aftereffects which should be considered (for a review on this see [85]).
Stimulation Intensity, Duration, and Repetition Rate
The stimulation intensities applied in the included studies ranged from 1 mA (seven experiments) to 2 mA (three experiments) and two experiments used 1.5 mA and 1.8 mA. As our 3D modeling of the electrical fields showed (Fig. 3), the field density induced by 1 mA is approximately half of that induced by 2 mA tDCS, and might have been too low to induce relevant effects in the hypo-active targeted region in the adult ADHD population. This is in line with previous findings from other clinical populations, for example in tinnitus [86], cognitive impairment in Parkinson’s disease [87], and cognitive performance and auditory hallucinations in schizophrenia [88, 89]. In children, however, increasing the stimulation intensity should be considered cautiously as shown in our computational modeling of current flow in the brain. Furthermore, results of titrating anodal [90] and cathodal [24] stimulation intensity show that increasing intensity does not always enhance neuroplastic aftereffect, especially in cathodal tDCS and should be considered with respect of duration and repetition rate [85].
The effects of different stimulation durations have not been addressed systematically in ADHD studies so far. Prolonging stimulation to increase the efficacy of the intervention is similar to enhancing the intensity and depends on the targeted cortical area [91, 92]. One advantage of increasing the duration rather than the intensity is that it does not increase the probability of side-effects like itching and tingling [93, 94], which might be specifically relevant for studies in children. However, similar to the intensity [95], the non-linear relation of duration and the effects on cortical excitability should be taken into consideration [85, 92, 96]. An overview of the stimulation polarity, intensity, and duration used in the studies included in the present review is presented in Fig. 3. Repetition rate is another parameter relevant to tDCS efficacy which was explored in only 3 tDCS studies [44, 47, 48], all of which reported significant reduction in ADHD symptoms or improvement of cognitive deficits. This is in line with previous studies that showed that tDCS efficacy is boosted by repeated tDCS sessions over motor and prefrontal regions [97, 98].
Electrode Size
Electrode size is another parameter that has an impact on the effects of tDCS. Motor cortex tDCS studies have shown that larger electrodes (e.g. 35 cm2) result in greater changes in motor cortical excitability than smaller electrodes (e.g. 16 cm2) [98], but such an electrode-size effect has not been found in other studies [99, 100]. Computational modeling of electrical current has shown that electrode size and inter-electrode distance affect the current density at the level of the brain, focality, and current shunting through the scalp [98, 101]. These parameters can explain the modulatory effects of tDCS and have been found to be associated with the electrical field strength [55]. Larger electrodes generate less focal electrical fields at the brain level. In the included studies, 35 cm2 and 25 cm2 were most commonly used in both adults and children. These electrode sizes do not significantly differ with respect to the current density generated over the motor area in the adult brain [98]. However, the target areas in ADHD are prefrontal regions, and slight differences in electrode size might be important over this functionally more heterogeneous region. According to our modeling results, the electrical field induced by bilateral dlPFC protocol with 35 cm2 electrodes was slightly larger (0.50) than with 25 cm2. The use of smaller electrodes increases the focality of stimulation effects [99, 101] but whether enhanced current focality is indeed helpful for increasing clinical efficacy is still an unanswered question and depends on the pathophysiological networks involved in ADHD. A recent study [64] that compared 4 × 1 (1 cm diameter) and conventional stimulation montages found no significant differences between these protocols on working memory, which speaks against a different effect and should be taken with caution, because in that study neither protocol significantly altered performance. Systematic investigation of different electrode sizes in ADHD symptoms and cognitive deficits is needed. Finally, the distance between electrodes, which is also determined by electrode size, might be another important parameter. As a rule of thumb, there should be at least 6 cm between electrode edges to prevent excessive current shunting via the skin [102]. This parameter might be more critical in childhood ADHD because of the smaller head size of children. As shown in our modeling results (Fig. 4Ce,f), the larger electrode size, and decreased distance between the electrodes in the Bandeira et al. study (2 mA) caused an electrical field of 0.7 V/m, which is almost identical to that induced by 1 mA (0.6 V/m) in the Nejati et al. study with smaller electrodes.
Sham Methodology
Establishing an effective sham condition is challenging in brain stimulation studies. All studies included in this review, except for two, used sham-controlled designs for the control condition. In two studies, including one with 2 mA intensity [49], participants could discern sham from active stimulation. A potential solution for ensuring proper sham control, especially for intensities > 1 mA, is the application of anesthetic cream under the stimulation area, as suggested in previous studies [96, 103].
Childhood ADHD and Developmental Aspects of tDCS
Most of the included studies were conducted on childhood ADHD. There are unique practical considerations about the application of tDCS in the developing brain. It is crucial to consider the developmental aspects of tDCS in childhood ADHD, which might also have implications for other neurodevelopmental disorders and pediatric populations. Here, we discuss the stimulation parameters outlined above in childhood ADHD.
Previous studies have suggested that stimulation parameters should be adjusted in children and adolescents [104]. Beyond the targeted cortical regions (stimulation sites) discussed above, it is of foremost importance to consider that the developing brain in childhood ADHD is believed to be more plastic than the adult ADHD brain [51]. This might make the effects of plasticity-inducing interventions stronger, especially during sensitive developmental periods [105]. Moreover, the smaller head size of children and adolescents likely results in a stronger electrical field than in adults. Since non-linear effects of tDCS have been found dependent on stimulation intensity and duration [24, 92, 96], adapting stimulation parameters is therefore critical.
Along this line, stimulation intensity, duration, and repetition rate should be carefully considered. As our modeling results show, the stimulation intensity required to generate an electrical field comparable to that achieved in adult ADHD (2 mA, 0.8 V/m) is almost half in children (1 mA, 0.6 V/m), under otherwise identical conditions. Therefore, stimulation intensity might have to be adjusted in children to achieve effects similar to those in adults, also in light of the consideration that higher stimulation intensities might modulate areas beyond the target electrode, with possibly unintended effects on the clinical symptoms being tackled. However, a reduction of intensity might not be warranted for all targets. The intensity that modulates the dlPFC, which is relatively near the surface, might not be sufficient to reach deeper regions, such as the r-IFG. This might be one reason why the studies that targeted r-IFG found null effect in childhood ADHD [42, 64].
Regarding polarity-specific effects, cathodal tDCS at 1 mA, which has excitability-diminishing effects in adults, has excitatory effects in children and adolescents, with otherwise identical stimulation parameters [106]. Such a conversion of the directionality of tDCS after-effects has been described in adults when the intensity is enhanced to 2 mA [96]. This conversion of after-effects might be beneficial in ADHD when the return electrode is positioned over prefrontal regions, which are hypo-active in this disease. If an excitability-diminishing effect of the relevant electrode cannot be ruled out, extracephalic positioning might be advantageous.
Electrode size is also an important aspect in this respect. A smaller electrode has the principal advantage of generating the current density aimed for at the brain level with a lower current intensity [101], and with higher focality, which is relevant for tDCS application in pediatrics because of the smaller head size compared to adults. The distance between electrodes should be sufficient to minimize current shunting through the skin. Using 35 cm2 electrodes in children can reduce the current intensity reaching the brain if target regions are close. As our modeling shows, the use of 35 cm2 electrodes over the left dlPFC and right Fpz or bilateral dlPFC does not guarantee the minimum required distance between electrodes, resulting in current shunting (Fig. 4D, Cf), and therefore 25 cm2 electrodes are preferable. Finally, there are important ethical and practical challenges regarding the application of tDCS in childhood ADHD due to the relatively small number of available studies, which warrants further systematic investigation. For a relevant and detailed discussion of ethics in childhood ADHD tDCS, see Sierawska et al. [107].
Clinical and Methodological Implications
Optimized and Personalized Stimulation Protocols in ADHD
The results of the included studies show that the effects of tDCS on ADHD symptoms and deficits are strongly dependent on not only the nature of the symptoms/deficits but also the stimulation parameters (i.e., site, polarity, intensity, duration, and repetition rate). This is in line with the therapeutic guidelines for tDCS in other neuropsychiatric disorders [94], which suggest the adaptation of stimulation parameters to symptoms and deficits, as well as individually, if applicable. Considering the heterogeneous pathophysiology, symptoms, deficits, and treatment response of ADHD, it might be beneficial to adopt this approach in future tDCS studies in ADHD and shape the stimulation protocols to the relevant factors. ADHD subtype, for example, is an important but under-studied inter-individual factor which is identified by specific deficit patterns and their pathophysiological foundations [74]. In addition, symptoms and especially cognitive impairments are heterogeneous in ADHD [4, 7], which suggests adapting the stimulation protocols accordingly.
Combined Interventions
Recent studies have shown that combining tDCS with other interventions such as cognitive training [108, 109], pharmacological treatment [110, 111], and psychological interventions [112, 113] can boost and prolong its clinical efficacy. These findings could have implications for ADHD treatment. For example, tDCS can be combined with pharmacological interventions, which sometimes only have short-lasting effects and suffer from partial or no response in some patients[114], or cognitive training and neurofeedback, major non-pharmacological interventions in ADHD that have yielded inconsistent effects when applied alone [115]. Physical exercise, especially in a cognitively-demanding context has stronger effects on children’s executive functions [116] and has recently been proposed as a novel cognitive enhancement paradigm in ADHD [117]; its combination with tDCS could be promising.
Limitations and Future Directions
The following limitations should be taken into account. First, from the currently available studies, it is difficult to rate the clinical utility of tDCS in ADHD due to a lack of systematic titration of stimulation parameters. Most of the studies reported so far are under-dosed regarding these parameters. Second, individualization and adaptation of stimulation protocols based on symptom subtypes, ADHD subtypes, specific cognitive deficits, and neuroanatomical differences are lacking in currently available studies. Future studies should: (1) systematically investigate stimulation parameters at the group level, (2) explore the effects of tDCS in different neurodevelopmental periods to determine the optimal developmental window in which to initiate interventions, especially in childhood ADHD, and (3) develop personalized stimulation approaches.
Conclusions
The findings of this systematic review suggest at least a partial improvement of symptoms and cognitive deficits in ADHD by tDCS. They further suggest that stimulation parameters such as polarity and site are relevant to the efficacy of tDCS in ADHD. Anodal tDCS over the dlPFC, compared to cathodal stimulation, seems to have a superior effect on both the clinical symptoms and cognitive deficits. However, the routine clinical application of this method as an efficient therapeutic intervention cannot yet be recommended based on these studies, but requires optimizing the stimulation parameters to improve clinical efficacy, and the exploration of clinical symptoms in addition to surrogate parameters.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This review was supported by the Department of Psychology and Neurosciences, Leibniz-Institut für Arbeitsforschung, Ministry of Science, Research and Technology, Deputy of Scholarship and Students Affairs, Iran (95000171) and the German Ministry of Research and Education (German Center for Brain Stimulation grant number 01EE1403C).
Conflict of interest
Michael A. Nitsche is a member of the Scientific Advisory Boards of Neuroelectrics and NeuroDevice. The other authors declare no competing interests.
Contributor Information
Mohammad Ali Salehinejad, Email: salehinejad@ifado.de.
Vahid Nejati, Email: nejati@sbu.ac.ir.
Michael A. Nitsche, Email: nitsche@ifado.de
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub, 2013.
- 2.Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Roy A, Oldehinkel AJ, Hartman CA. Cognitive functioning in adolescents with self-reported ADHD and depression: Results from a population-based study. J Abnorm Child Psychol. 2017;45:69–81. doi: 10.1007/s10802-016-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci 2018, 12. [DOI] [PMC free article] [PubMed]
- 8.Qian X, Castellanos FX, Uddin LQ, Loo BRY, Liu S, Koh HL, et al. Large-scale brain functional network topology disruptions underlie symptom heterogeneity in children with attention-deficit/hyperactivity disorder. NeuroImage. 2019;21:101600. doi: 10.1016/j.nicl.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dongen EV, von Rhein D, O’Dwyer L, Franke B, Hartman CA, Heslenfeld DJ, et al. Distinct effects of ASD and ADHD symptoms on reward anticipation in participants with ADHD, their unaffected siblings and healthy controls: a cross-sectional study. Mol Autism. 2015;6:48. doi: 10.1186/s13229-015-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samea F, Soluki S, Nejati V, Zarei M, Cortese S, Eickhoff SB, et al. Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neurosci Biobehav Rev. 2019;100:1–8. doi: 10.1016/j.neubiorev.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapport MD, Orban SA, Kofler MJ, Friedman LM. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. 2013;33:1237–1252. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin Psychol Rev. 2012;32:605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda NJ, Cepeda ML, Kramer AF. Task switching and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2000;28:213–226. doi: 10.1023/a:1005143419092. [DOI] [PubMed] [Google Scholar]
- 17.Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Keune PM, Wiedemann E, Schneidt A, Schönenberg M. Frontal brain asymmetry in adult attention-deficit/hyperactivity disorder (ADHD): Extending the motivational dysfunction hypothesis. Clin Neurophysiol. 2015;126:711–720. doi: 10.1016/j.clinph.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2010;16:1147. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Polanía R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174–187. doi: 10.1038/s41593-017-0054-4. [DOI] [PubMed] [Google Scholar]
- 22.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 24.Mosayebi Samani M, Agboada D, Jamil A, Kuo MF, Nitsche MA. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex. 2019;119:350–361. doi: 10.1016/j.cortex.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Salehinejad MA, Nejati V, Nitsche MA. Neurocognitive correlates of self-esteem: from self-related attentional bias to involvement of the ventromedial prefrontal cortex. Neurosci Res. 2019 doi: 10.1016/j.neures.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Salehinejad MA, Wischnewski M, Nejati V, Vicario CM, Nitsche MA. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits. PLoS One. 2019;14:e0215095. doi: 10.1371/journal.pone.0215095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salehinejad MA, Ghanavai E, Rostami R, Nejati V. Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): Evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC) J Affect Disord. 2017;210:241–248. doi: 10.1016/j.jad.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Brunelin J, Mondino M, Bation R, Palm U, Saoud M, Poulet E. Transcranial direct current stimulation for obsessive-compulsive disorder: a systematic review. Brain Sci. 2018;8:37. doi: 10.3390/brainsci8020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vicario CM, Salehinejad MA, Felmingham K, Martino G, Nitsche MA. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2019;96:219–231. doi: 10.1016/j.neubiorev.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Alizadehgoradel J, Nejati V, Movahed FS, Imani S, Taherifard M, Mosayebi-Samani M, et al. Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: A randomized, double-blind, parallel-group study. Brain Stimul. 2020;13:582–593. doi: 10.1016/j.brs.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Imburgio MJ, Orr JM. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia. 2018;117:156–166. doi: 10.1016/j.neuropsychologia.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Ghanavati E, Salehinejad MA, Nejati V, Nitsche MA. Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Sci Rep. 2019;9:3700. doi: 10.1038/s41598-019-40273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nejati V, Salehinejad MA, Nitsche MA. Interaction of the left dorsolateral prefrontal cortex (l-DLPFC) and right orbitofrontal cortex (OFC) in hot and cold executive functions: Evidence from transcranial direct current stimulation (tDCS) Neuroscience. 2018;369:109–123. doi: 10.1016/j.neuroscience.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 34.Ghanavati E, Nejati V, Salehinejad MA. Transcranial direct current stimulation over the posterior parietal cortex (PPC) enhances figural fluency: Implications for creative cognition. J Cogn Enhanc. 2018;2:88–96. [Google Scholar]
- 35.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8:76–87. doi: 10.1016/j.brs.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zewdie E, Ciechanski P, Kuo HC, Giuffre A, Kahl C, King R, et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: Prospective single center evidence from 3.5 million stimulations. Brain Stimul 2020, 13: 565–575. [DOI] [PubMed]
- 38.Vicario C, Nitsche M. Non–invasive brain stimulation for the treatment of brain diseases in childhood and adolescence: state of the art, current limits and future challenges. Front Syst Neurosci 2013, 7. [DOI] [PMC free article] [PubMed]
- 39.Ciechanski P, Kirton A. Chapter 5 - Transcranial Direct-Current Stimulation (tDCS): Principles and Emerging Applications in Children. Pediatric Brain Stimul 2016: 85–115.
- 40.Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial direct current stimulation modulates neuronal networks in attention deficit hyperactivity disorder. Brain Topogr. 2017;30:656–672. doi: 10.1007/s10548-017-0552-4. [DOI] [PubMed] [Google Scholar]
- 41.Munz MT, Prehn-Kristensen A, Thielking F, Mölle M, Göder R, Baving L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci. 2015;9:307. doi: 10.3389/fncel.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breitling C, Zaehle T, Dannhauer M, Bonath B, Tegelbeckers J, Flechtner HH, et al. Improving interference control in ADHD patients with transcranial direct current stimulation (tDCS) Front Cell Neurosci. 2016;10:72. doi: 10.3389/fncel.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosmo C, Baptista AF, de Araújo AN, do Rosário RS, Miranda JGV, Montoya P, et al. A randomized, double-blind, sham-controlled trial of transcranial direct current stimulation in attention-deficit/hyperactivity disorder. PLoS One 2015, 10: e0135371. [DOI] [PMC free article] [PubMed]
- 44.Bandeira ID, Guimarães RSQ, Jagersbacher JG, Barretto TL, Jesus-Silva JRd, Santos SN, et al. Transcranial direct current stimulation in children and adolescents with attention-deficit/hyperactivity disorder (ADHD):A pilot study. J Child Neurol 2016, 31: 918–924. [DOI] [PubMed]
- 45.Prehn-Kristensen A, Munz M, Göder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy Controls. Brain Stimul. 2014;7:793–799. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 46.Nejati V, Salehinejad MA, Nitsche MA, Najian A, Javadi AH. Transcranial direct current stimulation improves executive dysfunctions in ADHD: Implications for inhibitory control, interference control, working memory, and cognitive flexibility. J Atten Disord. 2017 doi: 10.1177/1087054717730611. [DOI] [PubMed] [Google Scholar]
- 47.Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm. 2017;124:133–144. doi: 10.1007/s00702-016-1646-y. [DOI] [PubMed] [Google Scholar]
- 48.Cachoeira CT, Leffa DT, Mittelstadt SD, Mendes LST, Brunoni AR, Pinto JV, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder A pilot randomized controlled study. Psychiatry Res. 2017;247:28–32. doi: 10.1016/j.psychres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Allenby C, Falcone M, Bernardo L, Wileyto P, Rostain A, Ramsay JR, et al. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018;11:974–981. doi: 10.1016/j.brs.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Hagrassy MM, Jones F, Rosa G, Fregni F. CNS non-invasive brain stimulation. Adult Pediatr Neuromodulation 2018: 151–184.
- 51.Rubio B, Boes AD, Laganiere S, Rotenberg A, Jeurissen D, Pascual-Leone A. Noninvasive brain stimulation in pediatric attention–deficit hyperactivity disorder (ADHD): A review. J Child Neurol. 2016;31:784–796. doi: 10.1177/0883073815615672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finisguerra A, Borgatti R, Urgesi C. Non-invasive brain stimulation for the rehabilitation of children and adolescents with neurodevelopmental disorders: A systematic review. Front Psychol. 2019;10:135. doi: 10.3389/fpsyg.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda F, Nakajima S, Miyazaki T, Tarumi R, Ogyu K, Wada M, et al. Clinical effectiveness of repetitive transcranial magnetic stimulation treatment in children and adolescents with neurodevelopmental disorders: A systematic review. Autism. 2019;23:1614–1629. doi: 10.1177/1362361318822502. [DOI] [PubMed] [Google Scholar]
- 54.Rivera-Urbina GN, Nitsche MA, Vicario CM, Molero-Chamizo A. Applications of transcranial direct current stimulation in children and pediatrics. Rev Neurosci. 2017;28:173. doi: 10.1515/revneuro-2016-0045. [DOI] [PubMed] [Google Scholar]
- 55.Antonenko D, Thielscher A, Saturnino GB, Aydin S, Ittermann B, Grittner U, et al. Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stimul. 2019;12:1159–1168. doi: 10.1016/j.brs.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 56.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Reh V, Schmidt M, Lam L, Schimmelmann BG, Hebebrand J, Rief W, et al. Behavioral assessment of core ADHD symptoms using the QbTest. J Atten Disord. 2015;19:1034–1045. doi: 10.1177/1087054712472981. [DOI] [PubMed] [Google Scholar]
- 58.Conners C. Conners Continuous Performance Test 3rd edition™(Conners CPT 3™). 2014.
- 59.Kessels RPC, van Zandvoort MJE, Postma A, Kappelle LJ, de Haan EHF. The Corsi block-tapping task: Standardization and normative data. Appl Neuropsychol. 2000;7:252–258. doi: 10.1207/S15324826AN0704_8. [DOI] [PubMed] [Google Scholar]
- 60.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test (WCST): Manual: Revised and Expanded. Psychological Assessment Resources (PAR), 1993.
- 61.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord. 2019;23:325–332. doi: 10.1177/1087054715618792. [DOI] [PubMed] [Google Scholar]
- 63.Jacoby N, Lavidor M. Null tDCS effects in a sustained attention task: The modulating role of learning. Front Psychol 2018, 9. [DOI] [PMC free article] [PubMed]
- 64.Breitling C, Zaehle T, Dannhauer M, Tegelbeckers J, Flechtner HH, Krauel K. Comparison between conventional and HD-tDCS of the right inferior frontal gyrus in children and adolescents with ADHD. Clin Neurophysiol. 2020 doi: 10.1016/j.clinph.2019.12.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, et al. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex. 2015;73:62–72. doi: 10.1016/j.cortex.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 68.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Intl J Neuropsychopharmacol. 2011;14:1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y, Parra LC, Haufe S. The New York Head-A precise standardized volume conductor model for EEG source localization and tES targeting. Neuroimage. 2016;140:150–162. doi: 10.1016/j.neuroimage.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y, Dmochowski JP, Su Y, Datta A, Rorden C, Parra LC. Automated MRI segmentation for individualized modeling of current flow in the human head. J Neural Eng. 2013;10:066004. doi: 10.1088/1741-2560/10/6/066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(201–207):e201. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: A computer-based human model study. NeuroImage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 73.Lei D, Du M, Wu M, Chen T, Huang X, Du X, et al. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology. 2015;29:874–881. doi: 10.1037/neu0000200. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol Med. 2014;44:869–880. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- 75.Alyagon U, Shahar H, Hadar A, Barnea-Ygael N, Lazarovits A, Shalev H, et al. Alleviation of ADHD symptoms by non-invasive right prefrontal stimulation is correlated with EEG activity. NeuroImage 2020: 102206. [DOI] [PMC free article] [PubMed]
- 76.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004, 8: 170–177. [DOI] [PubMed]
- 77.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Depue BE, Burgess GC, Willcutt EG, Ruzic L, Banich MT. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: Evidence from deficits in individuals with ADHD. Neuropsychologia. 2010;48:3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morein-Zamir S, Dodds C, Hartevelt TJ, Schwarzkopf W, Sahakian B, Müller U, et al. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Human Brain Mapping. 2014;35:5141–5152. doi: 10.1002/hbm.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006;26:2424. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vicario CM, Nitsche MA, Hoysted I, Yavari F, Avenanti A, Salehinejad MA, et al. Anodal transcranial direct current stimulation over the ventromedial prefrontal cortex enhances fear extinction in healthy humans: A single blind sham-controlled study. Brain Stimul 2019. [DOI] [PubMed]
- 82.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “Hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 83.Ma I, van Holstein M, Mies GW, Mennes M, Buitelaar J, Cools R, et al. Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex. 2016;82:225–236. doi: 10.1016/j.cortex.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Plichta MM, Vasic N, Wolf RC, Lesch K-P, Brummer D, Jacob C, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Salehinejad MA, Ghanavati E. Complexity of cathodal tDCS: Relevance of stimulation repetition, interval, and intensity. J Physiol. 2020;598:1127–1129. doi: 10.1113/JP279409. [DOI] [PubMed] [Google Scholar]
- 86.Shekhawat GS, Sundram F, Bikson M, Truong D, De Ridder D, Stinear CM, et al. Intensity, duration, and location of high-definition transcranial direct current stimulation for tinnitus relief. Neurorehabil Neural Repair. 2016;30:349–359. doi: 10.1177/1545968315595286. [DOI] [PubMed] [Google Scholar]
- 87.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 88.Hoy KE, Arnold SL, Emonson MRL, Daskalakis ZJ, Fitzgerald PB. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr Res. 2014;155:96–100. doi: 10.1016/j.schres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Andrade C. Transcranial direct current stimulation for refractory auditory hallucinations in schizophrenia. J Clin Psychiatry. 2013;74:e1054–1058. doi: 10.4088/JCP.13f08826. [DOI] [PubMed] [Google Scholar]
- 90.Agboada D, Mosayebi Samani M, Jamil A, Kuo MF, Nitsche MA. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci Rep. 2019;9:18185. doi: 10.1038/s41598-019-54621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) J Neurophysiol. 2010;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- 92.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Brunoni A, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 95.Samani MM, Agboada D, Jamil A, Kuo MF, Nitsche MA. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex. 2019;119:350–361. doi: 10.1016/j.cortex.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 96.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety. 2006;23:482–484. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- 98.Ho K-A, Taylor JL, Chew T, Gálvez V, Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: Evidence from single and repeated sessions. Brain Stimul. 2016;9:1–7. doi: 10.1016/j.brs.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 100.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimul. 2013;6:644–648. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 101.Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8:066017. doi: 10.1088/1741-2560/8/6/066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guleyupoglu B, Febles N, Minhas P, Hahn C, Bikson M. Reduced discomfort during high-definition transcutaneous stimulation using 6% benzocaine. Front Neuroeng 2014, 7. [DOI] [PMC free article] [PubMed]
- 104.Moliadze V, Schmanke T, Andreas S, Lyzhko E, Freitag CM, Siniatchkin M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin Neurophysiol. 2015;126:1392–1399. doi: 10.1016/j.clinph.2014.10.142. [DOI] [PubMed] [Google Scholar]
- 105.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 106.Moliadze V, Lyzhko E, Schmanke T, Andreas S, Freitag CM, Siniatchkin M. 1 mA cathodal tDCS shows excitatory effects in children and adolescents: Insights from TMS evoked N100 potential. Brain Res Bull. 2018;140:43–51. doi: 10.1016/j.brainresbull.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 107.Sierawska A, Prehn-Kristensen A, Moliadze V, Krauel K, Nowak R, Freitag CM, et al. Unmet needs in children with attention deficit hyperactivity disorder—can transcranial direct current stimulation fill the gap? Promises and ethical challenges. Front Psychiatry 2019, 10. [DOI] [PMC free article] [PubMed]
- 108.Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25:122–126. doi: 10.1097/WNR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 109.Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res. 2012;219:363–368. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- 110.Valiengo L, Benseñor IM, Goulart AC, Oliveira JF, Zanao TA, Boggio PS, et al. The sertraline versus electrical current therapy for treating depression clinical study (select-TDCS): results of the crossover and follow-up phases. Depress Anxiety. 2013;30:646–653. doi: 10.1002/da.22079. [DOI] [PubMed] [Google Scholar]
- 111.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trialsertraline vs electrical current therapy. JAMA Psychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 112.Bajbouj M, Padberg F. A perfect match: noninvasive brain stimulation and psychotherapy. Eur Arch Psychiatry Clin Neurosci. 2014;264:27–33. doi: 10.1007/s00406-014-0540-6. [DOI] [PubMed] [Google Scholar]
- 113.Nejati V, Salehinejad MA, Shahidi N, Abedin A. Psychological intervention combined with direct electrical brain stimulation (PIN-CODES) for treating major depression: A pre–test, post–test, follow–up pilot study. Neurol Psychiatry Brain Res. 2017;25:15–23. [Google Scholar]
- 114.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8(4):4. [PMC free article] [PubMed] [Google Scholar]
- 115.Catalá-López F, Hutton B, Núñez-Beltrán A, Mayhew AD, Page MJ, Ridao M, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: protocol for a systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2015;4:19. doi: 10.1186/s13643-015-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Best JR. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Dev Rev. 2010;30:331–351. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salehinejad M, Nejati V. Cognition-engaging physical exercise for improving cognitive impairments in attention-deficit hyperactivity disorder: A behavioral medicine approach. Heart Mind. 2019;3:73–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.