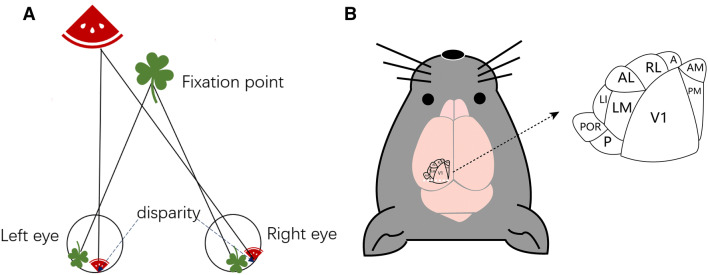
Binocular disparity, resulting from the differences between the retinal images of the two eyes, plays a fundamental role in the formation of stereoscopic vision and depth perception [1]. Generally, a greater difference (disparity) between the two images indicates that the objects are closer to each other (Fig. 1A). The brain uses binocular disparity to extract depth information from the two-dimensional retinal images, and the subtle differences between the images received by each eye allow us to perceive stereoscopic depth, which is important for the visual perception of the three-dimensional space [2].
Fig. 1.
The paradigm of binocular disparity and extrastriate visual cortical areas surrounding V1. A Schematic of binocular disparities. An object away from a fixation point has different retinal images between the two eyes and result in disparity. Generally, the closer the object, the larger the disparity. B Schematic of the nine higher visual areas around V1. A, anterior; AL, anterolateral; AM, anteromedial; LI, laterointermediate; LM, lateromedial; P, posterior; PM, posteromedial; POR, postrhinal; RL, rostrolateral.
It has been reported that almost all vision-related brain regions, such as V1, V2, V3, V3A, ventral posterior, middle temporal (V5), medial superior temporal, and superior colliculus, contain neurons that respond to binocular disparity [3]. Cortical area V1, which is at a preliminary processing stage for the analysis of stereoscopic depth, was shown to encode binocular disparity long ago. Poggio and Fischer found that 84% of the cells recorded from areas V1 and V2 in awake monkeys responded differentially to stimuli presented at a variety of depths [4], indicating that V1 and V2 are enriched in disparity-selective neurons. Furthermore, some of the extrastriate areas may also be responsible for the generation of neuronal representations that underlie the perception of binocular depth. Wang and Burkhalter demonstrated the existence of at least nine extrastriate visual cortical areas in mice, including the lateromedial (LM), anterolateral, rostrolateral (RL), anteromedial, posteromedial, laterointermediate, posterior, postrhinal, and anterior areas [5], based on the topographic projections from V1 (Fig. 1B). Neurons in each of these areas are selective for specific features of visual stimuli within their receptive fields (RFs) [6], and the extrastriate visual areas have consistent biases in visual field coverage, indicating that these are potential areas for investigating basic features such as disparity sensitivity.
Recently, a report by Chioma and colleagues published in Current Biology revealed an area-specific mapping of binocular disparity in the higher visual areas LM and RL [7]. In this study, using in vivo two-photon imaging, dichoptic gating stimulation, and random dot stereograms, which induce inter-ocular disparity, demonstrated that, simultaneously across areas V1, LM, and RL, neurons have similar disparities and tuning properties. Then, by calculating the disparity selectivity index of each cell in these areas, and comparing the degree and distribution of disparity selectivity, they found a similarity among V1, LM, and RL. Next, they compared the preferred disparity of the disparity-sensitive neurons in these areas, and found that area RL contained a significantly higher fraction of neurons tuned to negative (near) disparity than areas V1 and LM, indicating that area RL may specifically encode near disparity. Considering V1, RL, and LM cover partially different regions of the visual fields in elevation (Fig. 2), the authors further explored the relationship between preferred disparity and RF elevation, and concluded that the preferred disparities of disparity-tuning neurons in V1 and RL have no direct correlation with RF position in elevation, but cells with RFs in the lower visual field are on average tuned to more negative disparities than cells with RFs in the upper visual field, suggesting that the binocular disparity encoded in the RL area is not simply related to its retinotopic representation of the lower visual field, but is likely mediated by area-specific disparity processing.
Fig. 2.
Regulation of binocular disparity in V1, LM, and RL. The V1, LM, and RL areas receive inputs from both eyes and represent different RF elevations as described on the left. The RL area, where the RF elevation is lower than in V1, covers the lower visual field and encodes negative disparities (curve closer to the left). In contrast, the V1 and LM areas have higher RF elevations and more positive disparities (curve closer to the right). Abbreviations as in Fig. 1.
This report by Chioma et al. is the first step in the exploration of binocular disparity in higher visual areas, supporting the idea that the higher visual area RL indeed plays a specific role in encoding binocular disparity. It remains unclear, however, whether the disparities in RL have an encoding pattern distinct from other visual areas, and the cross-correlation between RL and V1 is unknown. It is also unclear whether the neurons that encode orientation selectivity overlap with disparity-tuning neurons in RL, V1, and other areas. The orientation tuning of the binocular neurons in the visual cortex shows differences between the two eyes. It is worth noting that the differences in orientation sensitivity between the RFs of the two eyes may be regarded as a neural mechanism for depth perception. Although some evidence shows that the modulation of disparity tuning is independent of the preferred orientation in some disparity-tuning neurons, the higher visual areas, which contain a distinct visuotopic representation and encode a unique combination of spatiotemporal features, may be different. For instance, neurons in almost all areas, in addition to RL, have stronger orientation selectivity than V1. In this case, these specific features may endow RL or other areas the ability to modulate disparity tuning.
Binocular disparity is regarded as a beneficial outcome of binocular integration, which contributes to the formation of binocular vision and stereopsis [8]. The mechanisms of binocular disparity, either in V1 or in other higher visual areas, have not been completely elucidated. It has been reported that the shift of binocularity (after monocular deprivation) decreases disparity sensitivity and disrupts binocular disparity in mouse V1 binocular neurons [9], similar to the effects of monocular deprivation on ocular dominance and binocular matching. Furthermore, it is reported that in the brain regions downstream of V1, such as RL and anteromedial, neurons also have binocular visual fields as in V1, suggesting that disparity tuning may also rely on the changes of binocularity.
In addition, exploration of binocular disparity has gradually focused on the location, structure, and properties of RFs, including response amplitude, RF width, and optimal spatial frequency. It has been found that binocular disparity may be caused by the differences between the two monocular RF positions of cortical binocular simple cells, or by differences in RF organization [10]. In addition, it has also been reported that the differences in RF structure between the two eyes are the critical determinant of a cell’s relative depth selectivity. However, the structure of RFs in extrastriate areas such as RL, which has retinotopic map locations and RF properties different from V1, may encode disparities in a specific way. Chioma et al. showed that RL encodes near disparity partially due to its RF elevation, underlying the potential roles of higher area RF properties for processing disparity tuning.
In conclusion, binocular disparity provides a clue for the depth perception of objects, in which the encoding process may extend across all the visual cortices, but is not fully understood. Recently, RL and other higher visual areas, which contain specific orientation selectivity and higher temporal frequency properties than V1, have become candidates for exploring the downstream processing of binocular disparity. Chioma et al. present clear differences in the preferred disparities across visual areas, suggesting that the higher visual area RL is specialized for encoding near disparity, which may shed light on the roles that higher visual areas play in encoding specific binocular disparity processing.
Acknowledgements
This highlight article was supported by the National Natural Science Foundation of China (31872764 and 81800862), a Shanghai Municipal Science and Technology Major Project (2018SHZDZX01) and ZJLab, and the Shanghai Science and Technology Committee Rising-Star Program (19QA1401600).
References
- 1.Gonzalez F, Perez R. Neural mechanisms underlying stereoscopic vision. Prog Neurobiol. 1998;55:191–224. doi: 10.1016/S0301-0082(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 2.Parker AJ. Binocular depth perception and the cerebral cortex. Nat Rev Neurosci. 2007;8:379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- 3.Hubel DH, Wiesel TN, Yeagle EM, Lafer-Sousa R, Conway BR. Binocular stereoscopy in visual areas V-2, V-3, and V-3A of the macaque monkey. Cereb Cortex. 2015;25:959–971. doi: 10.1093/cercor/bht288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poggio GF, Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol. 1977;40:1392–1405. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- 6.Garrett ME, Nauhaus I, Marshel JH, Callaway EM. Topography and areal organization of mouse visual cortex. J Neurosci. 2014;34:12587–12600. doi: 10.1523/JNEUROSCI.1124-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Chioma A, Bonhoeffer T, Hubener M. Area-specific mapping of binocular disparity across mouse visual cortex. Curr Biol. 2019;29(2954–2960):e2955. doi: 10.1016/j.cub.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen S, Tanabe S, Cumming B. Disparity processing in primary visual cortex. Philos Trans R Soc Lond B Biol Sci 2016, 371. [DOI] [PMC free article] [PubMed]
- 9.Scholl B, Pattadkal JJ, Priebe NJ. Binocular disparity selectivity weakened after monocular deprivation in mouse V1. J Neurosci. 2017;37:6517–6526. doi: 10.1523/JNEUROSCI.1193-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao DY, Conway BR, Livingstone MS. Receptive fields of disparity-tuned simple cells in macaque V1. Neuron. 2003;38:103–114. doi: 10.1016/S0896-6273(03)00150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]