Abstract
Neuronal apoptosis is one of the essential mechanisms of early brain injury after subarachnoid hemorrhage (SAH). Recently, HLY78 has been shown to inhibit apoptosis in tumor cells and embryonic cells caused by carbon ion radiation through activation of the Wnt/β-catenin pathway. This study was designed to explore the anti-apoptotic role of HLY78 in experimental SAH. The results demonstrated that HLY78 attenuated neuronal apoptosis and the neurological deficits after SAH through the activation of low-density lipoprotein receptor-related protein 6 (LRP6), which subsequently increased the level of phosphorylated glycogen synthesis kinase 3 beta (p-GSK3β) (Ser9), β-catenin, and Bcl-2, accompanied by a decrease of p-β-catenin, Bax, and cleaved caspase 3. An LRP6 small-interfering ribonucleic acid reversed the effects of HLY78. In conclusion, HLY78 attenuates neuronal apoptosis and improves neurological deficits through the LRP6/GSK3β/β-catenin signaling pathway after SAH in rats. HLY78 is a promising therapeutic agent to attenuate early brain injury after SAH.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00532-4) contains supplementary material, which is available to authorized users.
Keywords: Subarachnoid hemorrhage, HLY78, LRP6, Neuronal apoptosis
Introduction
SAH accounts for 5% of strokes with a mortality of ~30%, which imposes a heavy burden on society [1–3]. Neuronal apoptosis plays a crucial role in early brain injury following SAH [4, 5]. Thus, inhibition of neuronal apoptosis is a potential therapeutic strategy to attenuate brain injury following SAH.
LRP6, a co-receptor for the Wnt family, is involved in the regulation of Wnt/β-catenin signal transduction [6, 7]. In the canonical Wnt/β-catenin pathway, the Wnt ligand binds to its receptor complex composed of Frizzled and LRP5/6, then recruits the intracellular Axin-GSK3 complex to the receptors, which subsequently promotes the phosphorylation of LRP5/6 and inhibits the phosphorylation of β-catenin, thereby stabilizing β-catenin [8, 9]. The canonical Wnt/β-catenin pathway has been reported to modulate the cell apoptosis in multiple diseases. Previous studies of tumors have reported that inhibition of the Wnt/β-catenin pathway via knockdown of LRP6 leads to the apoptosis of tumor cells [10–12]. It has also been validated that the initiation of the Wnt/β-catenin pathway by recombinant human Wnt1 (rhWnt1) reduces the cortical cell apoptosis after SAH [13]. Furthermore, activation of the Wnt/β-catenin signaling pathway by simvastatin reduces neuronal apoptosis and promotes functional and pathological recovery after spinal cord injury. Thus, apoptosis following SAH may be inhibited by activation of the LRP6-dependent Wnt/β-catenin signaling pathway.
HLY78 is a small molecular lycorine derivative, which targets the DIX domain of Axin and promotes the Axin–LRP6 association, thus promoting LRP6 phosphorylation and Wnt signal transduction [9]. Previous studies have shown that HLY78 inhibits the carbon ion radiation-induced apoptosis of zebrafish embryos [14]. Moreover, the anti-apoptotic effect of HLY78 has also been demonstrated in tumors [15]. However, the role of HLY78 in SAH has not yet been explored.
In this study, we hypothesized that the novel agent HLY78 could inhibit neuronal apoptosis and early brain injury after SAH, and that the underlying mechanism is mediated through the LRP6/GSK3β/β-catenin signaling pathway.
Materials and Methods
Animals
Adult male Sprague-Dawley rats weighing 280–310 g were used for SAH induction. All the procedures were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University and were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The animals were kept in a humidity- and temperature-controlled room with a 12-h light/dark cycle and free access to food and water.
Experimental Design
Four separate experiments were performed in a rat model of SAH (Fig. S1).
Experiment 1
The time-course of endogenous p-LRP6 expression in the ipsilateral hemisphere at 3, 6, 12, 24, and 72 h after SAH was analyzed by Western blot. Furthermore, the cellular localization of p-LRP6 at 24 h after SAH was assessed via double immunofluorescence staining. Forty-two rats were divided into 6 groups: sham (n = 9), SAH-3 h (n = 6), SAH-6 h (n = 6), SAH-12 h (n = 6), SAH-24 h (n = 9) and SAH-72 h (n = 6).
Experiment 2
The effects of HLY78 on short-term neurobehavioral function and neuronal apoptosis were assessed at 24 h. The modified Garcia scale and beam-balance test were used to assess the short-term behavior (Experiment 2.1). Thirty rats were randomly divided into five groups (n = 6/group): sham, SAH + Vehicle, SAH + HLY78 (0.2 mg/kg), SAH + HLY78 (0.6 mg/kg), and SAH + HLY78 (1.8 mg/kg). Six rats were used to analyze the efficiency of intranasal administration of HLY78 (0.6 mg/kg) by Western blot (n = 3/group): Naïve + Vehicle and Naïve + HLY78 (0.6 mg/kg). Then, based on the results of the modified Garcia scale and beam-balance test, an additional 12 rats were divided into the following groups for TUNEL staining (Experiment 2.2) (n = 4/group): sham, SAH + Vehicle, and SAH + HLY78 (0.6 mg/kg). The rats used for Western blot analysis of apoptosis were shared with the groups for the short-term behavioral test (Experiment 2.1): sham, SAH + Vehicle, and SAH + HLY78 (0.6 mg/kg).
Experiment 3
The effects of HLY78 on long-term neurobehavioral function were assessed using the rotarod test and Morris water maze. Twenty-seven rats were randomly divided into three groups (n = 9/group): sham, SAH + Vehicle, and SAH + HLY78 (0.6 mg/kg).
Experiment 4
The mechanism underlying the anti-apoptotic effect of HLY78 was analyzed by Western blot. Twenty-four rats were divided into the following 6 groups: naïve + Scrambled siRNA (n = 3), naïve + LRP6 siRNA (n = 3), SAH + Scrambled siRNA (n = 3), SAH + LRP6 siRNA (n = 3), SAH + HLY78 + Scrambled siRNA (n = 6), and SAH + HLY78 + LRP6 siRNA (n = 6). In addition, samples of the following three groups were shared with Experiment 2.1: sham (n = 6), SAH + Vehicle (n = 6), and SAH + HLY78 (0.6 mg/kg) (n = 6).
SAH Model
As reported previously, the SAH model was induced by endovascular perforation [16, 17]. Briefly, each rat was intubated and maintained with 3% isoflurane mixed with oxygen in a ratio of 7 to 3 by a rodent ventilator (Harvard Apparatus, Holliston, MA, USA). The rats were placed in the supine position and the skin and subcutaneous tissues of the neck were opened along the midline. After the appropriate vessels were separated, a sharpened 4-0 nylon suture was inserted into the left internal carotid artery from the external carotid artery stump until resistance was felt. Then the suture was further inserted to perforate the bifurcation of the left anterior and middle cerebral arteries followed by immediate withdrawal. Finally, the incision was cleaned and sutured. In the sham group, the same procedures were performed except for the perforation with suture. Physiological parameters were continuously monitored from 10 min before SAH to 60 min after its induction and again at 24 h after SAH or before euthanasia.
Drug Administration
The HLY78 (MedChemExpress, Monmouth Junction, NJ, USA) was dissolved in dimethyl sulfoxide and diluted with normal saline. To explore a more accurate and optimal dose of HLY78, different doses (0.2, 0.6, and 1.8 mg/kg) were administered via intranasal injection at 1 h post-SAH based on the results of preliminary experiments (Fig. S2). A total volume of 30 μL was administered alternately into the left and right nares, 5 μL per naris every 5 min for 30 min. Rat-derived LRP6 siRNA (500 pmol/5 μL; OriGene Technologies, Rockville, MD, USA) or scrambled siRNA (500 pmol/5 μL; OriGene Technologies) was injected via intracerebroventricular injection (i.c.v.) at 48 h prior to surgery (1.0 mm anterior, 1.0 mm lateral to bregma, and 1.7 mm deep in the left hemisphere).
SAH Grading
The SAH grading score was blindly evaluated after euthanasia at 24 h after SAH, as previously described [18]. Briefly, the basal cistern was divided into six segments, and according to the amount of subarachnoid blood clot in the section, each section was allotted a score from 0 to 3. Rats with a score < 8 were excluded from this study.
Assessment of Short-term Neurological Function
The modified Garcia scale and beam-balance test were used to evaluate short-term neurological function [19]. The maximum value of the modified Garcia scale is 18, composed of the following six parts: spontaneous activity, spontaneous movement of forelimbs, forelimb outstretch, vibrissa touch, body proprioception, and climbing capacity. According to the performance of animals walking on a narrow wooden beam in 1 min, the score of the beam-balance test was graded from 0 to 4. The average of two consecutive trials was defined as the final score.
Assessment of Long-term Neurological Function
The long-term effects of HLY78 on neurological function were evaluated by the rotarod test and Morris water maze. The rotarod test was used to assess the motor-sensory deficits as previously described [20]. Briefly, rats were placed on a rotating horizontal cylinder (7 cm diameter) and led to walk forward. The cylinder started at a speed of 5 revolutions per minute (rpm) and 10 rpm with an acceleration of 2 rpm every 5 s. The time rats stayed on the rotating wheel was recorded and averaged from 2 trials.
The Morris water maze was blindly performed at 21–26 days after SAH to assess spatial learning and memory. A circular pool (diameter, 110 cm) was filled with water at 24 ± 1 °C, while a platform (diameter, 10 cm) was placed at a fixed position at the center of one of the quadrants. On the first day, rats were allowed to find the platform or guided to it if they did not find it within 60 s. In the next five days, each rat performed four trials every day with a 10 min inter-trial interval. On the sixth day, the platform was removed, and rats were allowed to swim freely for 60 s. The escape latency, swim distance, swim path, and probe quadrant duration were recorded by a computerized tracking system (Noldus Ethovision, Tacoma, WA, USA).
Immunofluorescence
Immunofluorescence staining was performed as previously described [21]. After deep anesthesia, rats were perfused with phosphate buffer solution, followed by perfusion with 10% formalin. Then the brain was removed intact and fixed in 10% formalin for 24 h, followed by 30% sucrose for 72 h at 4 °C. Frozen brains were cut into 10-μm coronal sections on a cryostat (CM3050S, Leica Microsystems, Bannockburn, IL, USA) and stored at –80 °C. After re-warming to room temperature, sections were blocked with 5% donkey serum for 1 h and incubated with the following primary antibodies at 4 °C overnight: p-LRP6 (1:50, MBS462070, MyBioSource Inc., San Diego, CA, USA), NeuN (1:200, ab104224, Abcam, Cambridge, MA, USA), GFAP (1:200, ab53554, Abcam), and Iba1 (1:200, ab5076, Abcam). Then the sections were incubated with appropriate fluorescence-conjugated secondary antibodies at room temperature for 2 h before DAPI staining (Vector Laboratories, Inc., Burlingame, CA, USA). Fluorescence microscopy and LASX software were used to observe and capture images (Leica DMi8, Leica Microsystems, Wetzlar, Germany).
TUNEL Staining
Neuronal apoptosis at 24 h after SAH was assessed by TUNEL staining with an Apoptosis Detection Kit (C1090, Beyotime, China) according to the manufacturer’s instructions. The number of TUNEL-positive neurons in the ipsilateral cortex was counted, and the final data were expressed as the ratio of TUNEL-positive neurons (%).
Western Blot
Western blotting was performed as previously described [22]. Samples were collected from the ipsilateral hemisphere at 24 h after SAH. Equal amounts of protein samples were loaded into each lane of the SDS-PAGE gel, followed by electrophoresis and transfer onto a nitrocellulose membrane. The membranes were blocked with non-fat milk for 2 h and incubated with the primary antibody at 4 °C overnight. The following primary antibodies were used: p-LRP6 (1:500, MBS462070, MyBioSource), LRP6 (1:1000, PA5-89161, ThermoFisher Scientific, Waltham, MA, USA), p-GSK-3β (Ser9) (1:1000, #9323, Cell Signaling Technology, Danvers, MA, USA), GSK-3β (1:1000, 22104-1-AP, Proteintech, Wuhan, China), LRP5 (1:200, ab36121, Abcam, Cambridge, MA, USA), p-LRP5 (1:200, ab203306, Abcam), Frizzled 4 (1:1000, ab77724, Abcam), p-β-Catenin (1:200, sc-57535, Santa Cruz Biotechnology, Dallas, TX, USA), β-Catenin (1:1000, 51067-2-AP, Proteintech), Bcl-2 (1:1000, ab59348, Abcam), Bax (1:1000, ab59348, Abcam), cleaved caspase-3 (1:500, ab49822, Abcam), β-actin (1:3000, sc-517582, Santa Cruz Biotechnology). After incubation with appropriate secondary antibodies (1:5000, Santa Cruz Biotechnology) for 2 h at room temperature, the membrane was developed with an ECL reagent (Amersham Biosciences UK Ltd., Chalfont, UK). Detected blot bands were quantified using ImageJ software (National Institutes of Health, MD, USA).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism (GraphPad Software, San Diego, CA, USA). Data that satisfied normality and homogeneity of variance were expressed as the mean ± standard deviation (SD) and analyzed with one-way analysis of variance (ANOVA) followed by multiple comparisons using Tukey’s post hoc analysis. When the data did not conform to the normality distribution, the Kruskal-Wallis test followed by the Dunn post hoc test was applied, and the median (interquartile range) was used for expression. Two-way ANOVA was applied to analyze the data on long-term neurological functions. Statistical difference was defined as P <0.05.
Results
Mortality and SAH Grade
In total, 173 rats were used. Based on the SAH grading score, 11 were excluded (Fig. 1A). The mortality rate of rats subjected to SAH was 15.79 % (21/133), and no rats in the sham and naïve groups died (Fig. 1A). The rats subjected to SAH showed blood clots around the basal cistern (Fig. 1B) and significantly higher grading scores than the sham group (P <0.05, Fig. 1C). No significant difference was found in SAH grading scores among the SAH-induced groups (P >0.05, Fig. 1C).
Fig. 1.
Mortality and SAH grade. A Animal usage and mortality in groups. B Representative images showing subarachnoid blood clots in the circle of Willis at 24 h after SAH. C SAH grade scores expressed as the median with interquartile range using the Kruskal-Wallis test followed by the Dunn post hoc test (*P <0.05 vs sham group; n = 6 per group; SAH, subarachnoid hemorrhage; HLY78, 4-ethyl-5-methyl-5, 6-dihydro-[1, 3]-dioxolo-[4, 5-j] phenanthridine; LRP6, low-density lipoprotein receptor-related protein 6; siRNA, small-interfering ribonucleic acid; Scr siRNA, scrambled siRNA).
Endogenous Expression and Cellular Location of p-LRP6 After SAH
Western blot results showed that the endogenous expression of p-LRP6 was significantly increased after SAH and reached a peak at 24 h after SAH (P <0.05, Fig. 2A, B). The immunofluorescence staining showed that the p-LRP6 receptor was abundantly expressed in neurons (NeuN), while little was expressed in astrocytes (GFAP) (Fig. 2C).
Fig. 2.
Temporal expression and cellular localization of p-LRP6 in the ipsilateral hemisphere after SAH. A, B Representative Western blot bands and quantitative analysis of the p-LRP6 expression level in the ipsilateral hemisphere within 72 h after SAH. Data are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test (*P <0.05 vs sham group; n = 6 per group). C Representative images of co-immunofluorescence staining of p-LRP6 (green) with neurons (NeuN, red), astrocytes (GFAP, red), and microglia (Iba-1, red) in the ipsilateral cortex at 24 h after SAH. Nuclei are stained with DAPI (blue) (scale bars, 50 µm; n = 3 per group).
Short-term Effects of HLY78 on Neurobehavioral Function and Neuronal Apoptosis After SAH
Compared to the sham group, rats given vehicle showed significant neurobehavioral deficiency (Fig. 3A, B). After treatment with HLY78 at 0.6 and 1.8 mg/kg, the modified Garcia and beam balance scores were significantly improved compared with the SAH + Vehicle group (Fig. 3A, B). The dose of 0.6 mg/kg was used for the subsequent experiments. And the intranasal delivery efficiency of HLY78 (0.6 mg/kg) was validated by Western blot analysis. After administration of HLY78, the expression of p-LRP6 in the ipsilateral hemisphere was significantly higher than in the Naïve + vehicle or SAH + vehicle group (Fig. 3C, D), which indicated that HLY78 was successfully delivered into the brain via intranasal administration at 0.6 mg/kg and was sufficient to significantly increase the phosphorylation of LRP6. Monitoring the physiological parameters showed no significant differences among groups at each time point (Table S1).
Fig. 3.
Effects of HLY78 on short-term neurological functions at 24 h after SAH. Treatment with HLY78 at 0.6 or 1.8 mg/kg significantly improved the modified Garcia score (A) and beam-balance score (B) at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; n = 6 per group). C, D Representative Western blot bands (C) and quantitative analysis (D) of p-LRP6 expression in the ipsilateral hemisphere after administration of HLY78 (0.6 mg/kg) in Naïve and SAH groups (@P <0.05 vs Naïve + Vehicle group; $P <0.05 vs SAH + Vehicle group; n = 3 per group). Data are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test.
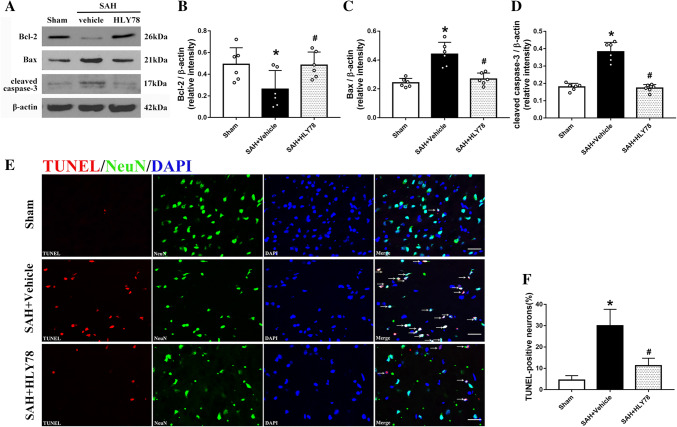
Western blot analysis of apoptosis showed that the level of Bcl-2 in the ipsilateral hemisphere was significantly decreased at 24 h after SAH compared with the sham group (Fig. 4A, B), while the level of Bax and cleaved caspase 3 was markedly increased (Fig. 4A, C, D). HLY78 treatment reversed the changes of the Bcl-2, Bax, and cleaved caspase 3 levels (Fig. 4A–D). The TUNEL staining results showed few TUNEL-positive cells in the sham group, while they were significantly more numerous in the SAH + Vehicle group at 24 h after SAH (Fig. 4E, F). And HLY78 (0.6 mg/kg) attenuated the number of TUNEL-positive cells compared with the SAH + Vehicle group (Fig. 4E, F).
Fig. 4.
Effects of HLY78 on neuronal apoptosis at 24 h after SAH. A Representative Western blot bands of Bcl-2, Bax, and cleaved caspase 3. B–D Quantitative analysis of the levels of Bcl-2, Bax, and cleaved caspase 3 in the ipsilateral hemisphere at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; n = 6 per group). E, F Representative micrographs and quantitative analysis of TUNEL-positive neurons in the ipsilateral cortex at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; n = 4 per group; scale bars, 50 µm). Data are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test.
Long-term Effects of HLY78 on Neurobehavioral Function After SAH
Results of the rotarod test showed that the falling latency in both the 5 and 10 rpm trials were significantly decreased compared with the sham group at 7, 14, and 21 days after SAH (Fig. 5A, B). This was significantly extended by treatment with HLY78 compared with the SAH + Vehicle group (Fig. 5A, B). In the water maze test, the latency and swimming distance before rats found the platform were significantly higher in blocks 2–5 in the SAH + Vehicle group than in the sham group (Fig. 5C, D). However, a significantly shorter latency in blocks 3–4 and a reduced swimming distance in blocks 2–4 were found in the SAH + HLY78 group compared with the SAH + Vehicle group (Fig. 5C, D). After removing the platform, the rats in the SAH + Vehicle group spent less time in the target quadrant than the sham group, and this was improved by HLY78 treatment (Fig. 5E, G). The swimming speed did not differ among the groups (Fig 5F).
Fig. 5.
Effects of HLY78 on long-term neurological functions after SAH. A, B Results of the rotarod test at 7, 14, and 21 days after SAH. C, D Escape latency and swimming distance before rats found the platform in the Morris water maze test at 21 to 25 days after SAH. E Quantification of probe quadrant duration in the probe trial. F Swimming speed in the probe trial. G Representative heatmaps of the probe trial (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; n = 9 per group). Probe quadrant duration and swimming speed are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test; other data are expressed as two-way ANOVA followed by Tukey’s post hoc test.
Effects of HLY78 on Frizzled 4 and LRP5/6 Expression After SAH
The endogenous protein levels of the receptors of the Wnt ligand in the ipsilateral hemisphere were detected by Western blot. Following SAH, the expression of p-LRP5 and p-LRP6 were significantly increased, while Frizzled 4 decreased (Fig. 6A–D). HLY78 administration further augmented the expression of p-LRP5 and p-LRP6 at 24 h after SAH (Fig. 6A–C). However, it did not affect the level of Frizzled 4 (Fig. 6D).
Fig. 6.
Effects of HLY78 on LRP5/6 and Frizzled 4 expression, the depletion efficiency of LRP6 siRNA, and its effects on short-term neurobehavioral functions after SAH. A–D Representative Western blot bands and quantitative analysis of the expression of p-LRP5/6, LRP5/6, and Frizzled 4 in the ipsilateral hemisphere at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; n = 6 per group). E, F Representative Western blot bands and quantitative analysis of the level of LRP6 after administration of LRP6 siRNA in the ipsilateral hemisphere of the naïve and SAH groups (@P <0.05 vs Naïve + Scr siRNA group; $P <0.05 vs SAH + Scr siRNA group; n = 3 per group). G, H LRP6 siRNA significantly inhibits the beneficial effects of HLY78 on the modified Garcia score (G) and beam-balance score (H) at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; &P <0.05 vs SAH + HLY78 + Scr siRNA group; n = 6 per group). Data are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test.
The Depletion Efficiency of LRP6 siRNA and Its Effects on Short-term Neurobehavioral Function After SAH
Based on the above findings, we speculated that HLY78 acts mainly on the LRP5/6 receptor, and applied LRP6 siRNA in the following experiments. The depletion efficiency of LRP6 siRNA was validated by Western blot. Administration of LRP6 siRNA significantly inhibited the expression of LRP6 in the ipsilateral hemisphere in both the naïve and SAH groups relative to the scrambled siRNA group (Fig. 6E, F). The neurobehavioral functions of rats were assessed again after the administration of LRP6 siRNA, and the results showed the beneficial effects of HLY78 were markedly reversed (Fig. 6G, H).
LRP6 siRNA Reverses the Effects of HLY78 on Neuronal Apoptosis After SAH
Western blot data from the mechanistic study showed that the endogenous expression levels of p-LRP6, p-GSK-3β, p-β-catenin, Bax, and cleaved caspase 3 were significantly higher at 24 h after SAH than in the sham group (Fig. 7A, B, D, E, H, I), while the expression of β-catenin and Bcl-2 was markedly lower (Fig. 7A, F, G). Treatment with HLY78 further increased the expression of p-LRP6 and p-GSK-3β relative to the SAH + Vehicle group, which subsequently increased the levels of β-catenin and Bcl-2 accompanied by decreased p-β-catenin, Bax, and cleaved caspase 3 expression (Fig. 7A–I). When compared to the SAH + HLY78 + Scrambled siRNA group, administration of LRP6 siRNA significantly inhibited the expression of LRP6 at 24 h after SAH, which resulted in significant reduction of p-LRP6, p-GSK-3β, β-catenin, and Bcl-2 expression, accompanied by increased p-β-catenin, Bax, and cleaved caspase 3 (Fig. 7A–I).
Fig. 7.
Inhibition of LRP6 abolishes the beneficial effects of HLY78 on apoptosis at 24 h after SAH. A–I Representative Western blot bands and quantitative analysis of p-LRP6, LRP6, p-GSK3β, GSK3β, p-β-catenin, β-catenin, Bcl-2, Bax, and cleaved caspase 3 in the ipsilateral hemisphere at 24 h after SAH (*P <0.05 vs sham group; #P <0.05 vs SAH + Vehicle group; &P <0.05 vs SAH + HLY78 + Scr siRNA group; n = 6 per group). Data are expressed as the mean ± SD using one-way ANOVA followed by Tukey’s post hoc test.
Discussion
In the present study, our data demonstrated that the level of endogenous p-LRP6 was significantly increased in the ipsilateral hemisphere after SAH and reached a peak at 24 h. The p-LRP6 receptor was primarily expressed in neurons, while a little was expressed in astrocytes. Treatment with HLY78 at 0.6 mg/kg significantly attenuated the short-term and long-term neurobehavioral deficits, as well as the neuronal apoptosis after SAH. Mechanistic study showed that HLY78 treatment significantly increased the expression of p-LRP6, p-GSK3β (Ser9), β-catenin, and Bcl-2 accompanied by decreased p-β-catenin, Bax, and cleaved caspase 3 in the ipsilateral hemisphere at 24 h after SAH. Knockdown of LRP6 with siRNA abolished the beneficial effects of HLY78 on neurobehavioral functions and neuronal apoptosis. Taken together, HLY78 improved the neurobehavioral deficits after SAH by attenuating the neuronal apoptosis, which may be mediated by the LRP6/GSK3β/β-catenin signaling pathway (Fig. 8).
Fig. 8.

Schematic showing that HLY78 inhibits neuronal apoptosis through the LRP6/GSK3β/β-catenin signaling pathway after SAH.
Early brain injury is the primary cause of morbidity and mortality within 72 h after SAH, and neuronal apoptosis plays a vital role in the early brain injury [4, 23, 24]. Neuronal apoptosis after SAH has been mainly reported in the cerebral cortex and hippocampus [25]. Emerging studies have shown that Wnt/β-catenin signaling is involved in cell survival [13, 26]. Wnt/β-catenin signaling activated by Simvastatin has been shown to inhibit the neuronal apoptosis following spinal cord injury [27]. In experimental SAH, the Wnt/β-catenin signaling pathway activated by rhWnt1 also promotes the survival of damaged neurons [13]. Therefore, activating the Wnt/β-catenin signaling pathway is a promising therapeutic strategy to alleviate the early brain injury after SAH. The Wnt family proteins function through several signaling pathways, including the canonical Wnt/β-catenin pathway, the non-canonical Wnt/β-catenin pathway, and the Wnt-Ca2+ pathway [28]. The canonical Wnt/β-catenin pathway is activated by Wnt ligands binding to the Frizzled receptor and LRP5/6 co-receptors, which subsequently promotes the phosphorylation of LRP6 and inhibits the activity of GSK3β, thus inhibiting the degradation of β-catenin [6].
HLY78 is a synthetic lysine derivative with a molecular weight of 267.32 [9]. Previous studies have shown that it acts synergistically with the Wnt ligands to activate the Wnt/β-catenin signal pathway [9, 29]. In gastric cancer cells, HLY78 inhibits mitochondrial apoptotic events and the apoptotic cell death induced by oxysterol-binding protein-related protein 8 [15]. However, Yakisich et al. reported that HLY78 can also potentiate the Nigericin-induced inhibition of the viability of cancer cells, which may be attributed to the promotion of β-catenin translocation into the nucleus by HLY78 [30].
Since the effects of HLY78 on other organs are unknown, to avoid its systemic effects and explore an optimal dose, HLY78 was delivered intranasally in three doses. The results showed that the rats treated with HLY78 at both 0.6 and 1.8 mg/kg exhibited a significant increase in the modified Garcia score and beam-balance score. Therefore, we used 0.6 mg/kg in the subsequent experiments. The quantitative analysis of TUNEL-positive neurons and the Western blot data for Bcl-2, Bax, and cleaved caspase 3 demonstrated that neuronal apoptosis in the ipsilateral cortex of the SAH+HLY78 group was significantly alleviated compared with the vehicle-treated group. In Wnt signing, Axin interacts with LRP6 and is required to phosphorylate it [31]. HLY78 promotes the association of Axin with LRP5/6 [9]. Consistent with previous studies, our data showed that HLY78 significantly increased the phosphorylation of LRP5 and LRP6, but had no effect on Frizzled 4.
In keeping with our results, Abe et al. demonstrated that LRP6 acts as an endogenous neuroprotective receptor in ischemic stroke, alleviating the post-ischemic brain damage through suppressing GSK3β activity [32]. However, it is worth noting that our results exhibited some differences. First, our results from immunofluorescence staining showed that a few p-LRP6 receptors were expressed in astrocytes as well, while Abe et al. reported that no LRP6 is expressed in astrocytes after ischemic stroke. Since p-LRP6 appeared to be expressed on the processes of astrocytes, there was a high probability of false-negative results. Second, our results showed that HLY78 treatment significantly increased the level of p-GSK-3β (Ser 9) in the ipsilateral hemisphere at 24 h after SAH and was accompanied by an increase of β-catenin. However, in ischemic stroke, inhibition of GSK3β does not affect the β-catenin level. This may be due to differences in the downstream transduction signals that dominate the two models.
Moreover, our results suggested that LRP6, the receptor of Wnt family proteins, plays an essential role in Wnt signaling and is involved in apoptosis mediation. It is well known that GSK-3β has two common phosphorylation sites: the inhibitory site Ser 9 and the activating site Y206. Endogenous p-GSK-3β (Ser 9) has been reported to increase at 1, 6, and 24 h in the cerebral cortex after experimental SAH, and it is beneficial by reducing the early brain injury caused by SAH [33]. However, significant apoptotic cell death can still be detected at 24 h after SAH [34–35]. Thus, the level of endogenous p-GSK-3β (Ser 9) might be not sufficient to prevent neuronal apoptosis after SAH. The results of the mechanistic study showed that the endogenous p-LRP6 level was significantly increased after SAH and further augmented after the administration of HLY78, which resulted in an increase of p-GSK-3β (Ser 9), β-catenin, and Bcl-2 accompanied by inhibition of p-β-catenin, Bax, and cleaved caspase 3. And LRP6 siRNA abolished this effect. Therefore, the anti-apoptotic effect of HLY78 acts at least partly through the LRP6/GSK-3β/β-catenin pathway, resulting in the inhibition of caspase-dependent apoptosis.
There are some limitations to our study. Previous studies have reported that LRP6 is involved in the promotion of angiogenesis and neurite growth [36, 37]. In this study, we only investigated the anti-apoptotic effect of LRP6 after SAH. Further studies are needed to elucidate the other effects of LRP6 after SAH and the underlying signaling mechanisms. In addition, there is no reference for the dose of HLY78 in vivo, and research on the pharmacokinetics of HLY78 has not yet been completed. Thus, the metabolism and safety of HLY78 in other organs need to be investigated.
In conclusion, HLY78 attenuates neuronal apoptosis and improves the neurological deficits after SAH through the LRP6/GSK3β/β-catenin signaling pathway. Therefore, HLY78 may be a promising therapeutic agent to attenuate the early brain injury after SAH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81771961 and 81401505) and the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (201959).
Conflict of interest
The authors declare no potential conflict of interest.
Contributor Information
Yuan Cheng, Email: chengyuan023@aliyun.com.
Zongyi Xie, Email: zyxieneuro2013@yahoo.com.
References
- 1.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377:257–266. doi: 10.1056/NEJMcp1605827. [DOI] [PubMed] [Google Scholar]
- 2.Nath S, Koziarz A, Badhiwala JH, Almenawer SA. Predicting outcomes in aneurysmal subarachnoid haemorrhage. BMJ. 2018;360:k102. doi: 10.1136/bmj.k102. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 4.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu HM, Li B, Wang XD, Guo YS, Hui H, Zhang HP, et al. Fluoxetine is neuroprotective in early brain injury via its anti-inflammatory and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Neurosci Bull. 2018;34:951–962. doi: 10.1007/s12264-018-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab. 2013;24:31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Yin J, Chen D, Nie F, Song X, Fei C, et al. Small-molecule modulation of Wnt signaling via modulating the Axin-LRP5 interaction. Nat Chem Biol. 2013;9:579–585. doi: 10.1038/nchembio.1309. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Yue L, Xu H, Li N, Li J, Zhang Z, et al. Curcumin suppresses osteogenesis by inducing miR-126a-3p and subsequently suppressing the WNT/LRP6 pathway. Aging (Albany NY) 2019;11:6983–6998. doi: 10.18632/aging.102232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D, et al. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumor metastasis. Nat Commun. 2015;6:6906. doi: 10.1038/ncomms7906. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Li B, Zhou L, Yu S, Su Z, Song J, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci U S A. 2016;113:13150–13155. doi: 10.1073/pnas.1616336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Bao DJ, Xu B, Cheng CD, Dong YF, Wei XP, et al. Neuroprotection mediated by the Wnt/Frizzled signaling pathway in early brain injury induced by subarachnoid hemorrhage. Neural Regen Res. 2019;14:1013–1024. doi: 10.4103/1673-5374.250620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Si J, Zhou R, Zhao B, Xie Y, Gan L, Zhang J, et al. Effects of ionizing radiation and HLY78 on the zebrafish embryonic developmental toxicity. Toxicology. 2019;411:143–153. doi: 10.1016/j.tox.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Zhang L, Fan Y, Zhang D, Qin L, Dong S, et al. Oxysterol-binding protein-related protein 8 inhibits gastric cancer growth through induction of ER stress, inhibition of Wnt signaling, and activation of apoptosis. Oncol Res. 2017;25:799–808. doi: 10.3727/096504016X14783691306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, et al. Recombinant netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARγ/NFκB signaling pathway after subarachnoid hemorrhage in rats. Brain Behav Immun. 2018;69:190–202. doi: 10.1016/j.bbi.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Li S, Ma J, Wang C, Chen A, Xin Z, et al. Effect of gastrodin on early brain injury and neurological outcome after subarachnoid hemorrhage in rats. Neurosci Bull. 2019;35:461–470. doi: 10.1007/s12264-018-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Wu P, Budbazar E, Zhu Q, Sun C, Mo J, et al. Mitophagy reduces oxidative stress via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke. 2019;50:978–988. doi: 10.1161/STROKEAHA.118.021590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Zhao G, Ding Y, Wang T, Flores J, Ocak U, et al. Rh-IFN-α attenuates neuroinflammation and improves neurological function by inhibiting NF-κB through JAK1-STAT1/TRAF3 pathway in an experimental GMH rat model. Brain Behav Immun. 2019;79:174–185. doi: 10.1016/j.bbi.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, et al. Intranasal administration of recombinant Netrin-1 attenuates neuronal apoptosis by activating DCC/APPL-1/AKT signaling pathway after subarachnoid hemorrhage in rats. Neuropharmacology. 2017;119:123–133. doi: 10.1016/j.neuropharm.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Li X, Matei N, McBride D, Tang J, Yan M, et al. Ezetimibe, an NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp Neurol. 2018;307:12–23. doi: 10.1016/j.expneurol.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 24.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 25.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, et al. TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J Neurosci. 2017;37:1772–1784. doi: 10.1523/JNEUROSCI.2459-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao K, Shen Z, Yuan Y, Han D, Song C, Guo Y, et al. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/beta-catenin signaling pathway after spinal cord injury. J Neurochem. 2016;138:139–149. doi: 10.1111/jnc.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, et al. MiR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287:42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yakisich JS, Azad N, Kaushik V, O’Doherty GA, Iyer AK. Nigericin decreases the viability of multidrug-resistant cancer cells and lung tumorspheres and potentiates the effects of cardiac glycosides. Tumour Biol. 2017;39:1010428317694310. doi: 10.1177/1010428317694310. [DOI] [PubMed] [Google Scholar]
- 31.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Abe T, Zhou P, Jackman K, Capone C, Casolla B, Hochrainer K, et al. Lipoprotein receptor-related protein-6 protects the brain from ischemic injury. Stroke. 2013;44:2284–2291. doi: 10.1161/STROKEAHA.113.001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–2146. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Wang Z, Liu C, Shen H, Chen Z, Yin J, et al. Pramipexole-induced hypothermia reduces early brain injury via PI3K/AKT/GSK3β pathway in subarachnoid hemorrhage rats. Sci Rep. 2016;6:23817. doi: 10.1038/srep23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Peng J, Pang J, Wu Y, Huang X, Li Y, et al. Apolipoprotein E-mimetic peptide COG1410 promotes autophagy by phosphorylating GSK-3β in early brain injury following experimental subarachnoid hemorrhage. Front Neurosci. 2018;12:127. doi: 10.3389/fnins.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, et al. The Wnt antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, Ko HM, Kwon KJ, Lee J, Han SH, Han DW, et al. tPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014;49:199–215. doi: 10.1007/s12035-013-8511-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.