Abstract
Importance
An observant Chinese doctor Li Wenliang became the first physician to alert the world about COVID-19. Being an ophthalmologist himself, he has put the additional onus on us. The fact that the ocular manifestation could be the first presenting feature of novel coronavirus pneumonia should not be ignored and the possibility of spread of SARS-CoV-2 through the ocular secretions cannot be ruled out. However, with breakthroughs still evolving about this disease, the calls are now louder for closer examination on the pathogenesis of conjunctivitis associated with it. Hence, we conducted a scoping review of all available literature till date to fill in the “potential” gaps in currently available knowledge on ocular manifestations of SARS-CoV-2 infection in an attempt to establish continuity in the “chain of information” from December 2019 till April 2020. We also summarize a possible hypothesis on much less understood and highly debated topics on regard to the etiopathogenesis of ocular involvement in SARS-CoV-2 based on either presence or absence of ACE2 receptor in the ocular surface.
Methods
We conducted a scoping review search of published and unpublished SARS-CoV-2-related English language articles from December 2019 till mid of April 2020 from the online databases. The findings were summarized using text, tables, diagrams, and flowcharts.
Results
The commonest ocular manifestation in SARS-CoV-2 infection is follicular conjunctivitis and has been the first manifestation of SARS-CoV-2 infection in 3 reported cases till date. The ocular surface inoculated with the SARS-CoV-2 leads to the facilitation of the virus to the respiratory system via the lacrimal passage. RT-PCR analysis of the ocular secretions has shown the presence of the SARS-CoV-2 nucleotides indicating the possibility of infection of ocular secretions. ACE2 receptors and its expression on the ocular mucosal surface are linked behind the etiopathogenesis of conjunctivitis.
Conclusion
Conjunctivitis can be the presenting manifestation but may go unnoticed due to its mild nature. The ocular surface could serve as the entry gateway for the virus and ocular secretions could play a role in virus shed. The eye care personnel, as well as the general people, need to be more vigilant and adopt protective eye measures.
Keywords: ACE2 receptor, conjunctiva, coronavirus, COVID-19 infection, ocular, ophthalmic, 2019-nCOV, SARS-CoV-2
Introduction
The World Health Organization (WHO) initially used the term 2019 novel coronavirus to refer to the coronavirus disease that affects the lower respiratory tract of patients causing severe pneumonia in Wuhan, China in December 2019 and revised it officially as COVID-19.1–3 The virus has received the final reference name and is now also commonly known as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is due to its similarity with severe acute respiratory syndrome coronavirus (SARS-CoV).4–6
COVID-19 outbreak turned to be pandemic hitting the 210 countries and its territories around the world and 2 international conveyances over the past 14 weeks till 17th April 2020.7 SARS-CoV-2 is capable of active replication in the upper respiratory tissues, with continuous pharyngeal shedding of the virus. Though the respiratory system is the primary site of insult, various reports have suggested that the eyes can be affected in the early or late stage of the disease process.8–10
Currently, our understanding of the possible ocular involvement in SARS-CoV-2 infection is very limited and gradually expanding. Nevertheless, the fact that the ocular manifestation could be the first presenting feature of the novel coronavirus pneumonia should not be neglected by the general population, health professionals as well as policymakers and teams.8,9,11,12 There are recent reports even on the nosocomial SARS-CoV-2 infection with conjunctivitis in a nurse, suggesting eye as the potential route of nosocomial transmission of the SARS-CoV-2.10
Multiple respiratory droplets may be released when an infected person coughs, sneezes or speaks. These respiratory droplets contact host ocular mucus membranes. Upon contact with an epithelial cell, the virus particle gains entry inside using the SARS-CoV-2 spike (S) glycoprotein which binds to the cell membrane protein angiotensin-converting enzyme 2 (ACE2). The S glycoprotein is composed of 2 subunits S1 and S2. The S2 subunit is shown to be highly conserved with a fusion peptide, a transmembrane domain and a cytoplasmic domain.13
Whether the eyes are one of the preliminary sites of virus transmission from the environment to the lungs via the lacrimal passage or the virus reaches the eyes in retrograde fashion through the airways passage via the lacrimal system to eyes is still an issue to look upon. The presence or absence of Angiotensin-Converting Enzyme (ACE) 2 receptors on the ocular surface is still a topic of debate. The conjunctiva is directly exposed to the extraocular pathogens, and the mucosa of the ocular surface and upper respiratory tract is connected by nasolacrimal duct and may share some common entry receptors for these various respiratory viruses.14
Given the rapid worldwide spread of the novel coronavirus and its high impacts on morbidity and mortality on the human health, the ophthalmic professionals have to focus upon the research to understand the pathogenesis of conjunctivitis in COVID-19 infection and to identify its impact on the eyes and shedding of the virus via ocular secretions. The knowledge on the sensitivity and specificity of RT-PCR in ocular secretion, the prevalence of SARS-CoV-2 nucleotides in the eye, and the viral load still needs full exploration.
To fulfill this gap of knowledge, we aimed to conduct a scoping review to summarize and critically analyze all the published scientific articles regarding the SARS- CoV-2 virus and its infection in the eye till the mid of April 2020. This review aims to provide the possible hypothesis on the etiopathogenesis of the ocular involvement, routes of entry of the virus in the eye, presence/absence of ACE2 receptors in ocular surface, RT-PCR analysis in ocular secretions, review of various articles related with SARS-CoV-2 infections and brief preventive and protective measures of SARS-CoV-2 infection in ophthalmic practices to avoid the occupational hazard.
Methods
We planned a scoping review following the methodological framework suggested by Arksey and O’Malley.15 The following five stages were followed to conduct this scoping review: i) identifying clear research objectives and search strategies, ii) identifying relevant research articles, iii) study selection of research articles, iv) charting and extraction of data, and v) discussing, collating, summarizing and reporting the results.
Literature Search Strategies
To achieve a comprehensive literature search (published and unpublished articles) and reviews, we adopted a strategy that involved searching the following online databases: PubMed, Google scholar medRxiv, bioRxiv, Medline, CNKI and WanFang Data (the two primary databases for biomedical research in mainland China). Also, some white papers published online by the National Health Commission of the People’s Republic of China, Centre for Disease Control (CDC) and the World Health Organization were included in the analysis. We searched scientific publications from 1st January till the mid of April 2020. The search terms were ‘SARS-CoV’, ‘SARS-CoV-2nCoV’, ‘2019 novel coronavirus’, “2019-nCoV”, ‘novel coronavirus’, “Pneumonia”, ‘COVID-19ʹ, “Ocular surface involvement”, “Conjunctivitis”, “RT-PCR” and “ACE2 Receptor”. We included all the relevant scientific publications written in English in the review (Prospective/retrospective studies, Case-series, Case report, Letter to the editor). Around 1/3rd of these papers were at the press (preprint version) and rest were published in high impact peer-reviewed journals, including The New England Journal of Medicine, JAMA, and the Lancet. We checked the bibliographies of these studies found through the database searches to ensure they can be included in the scoping exercise.
Data Extraction from the Included Studies
The articles were scrutinized and selected by the common consent of the authors after the evaluation of its information regarding ocular involvement in SAR-CoV-2 infection. The data were summarized using text, tables, diagrams, and flow charts.
This scoping review was approved by the local ethical committee of Birat Eye Hospital and adhere to the Declaration of Helsinki.
Results and Discussion
The ocular manifestations reported till now during SARS-CoV-2 infection are conjunctivitis, including conjunctival hyperemia, chemosis, foreign body sensation, increased secretions, and epiphora.8 Normal visual acuity, intact corneal epithelium, quiescent anterior chamber, and no tenderness or enlargement of the preauricular lymph node has been mentioned along with the conjunctival findings.10
Epiphora and Conjunctival redness had been the first manifestation of SARS-CoV-2 infection in 3 reported cases till date which includes a member of National expert on pneumonia during his visit to endemic areas of Wuhan and an anesthesiologist contracting the virus from a known patient of novel coronavirus pneumonia during intubation in Italy; similarly, it was reported in a nurse working in the emergency department of ophthalmology who presented with viral conjunctivitis and watering as a first sign.12,16 All reported subjects were fully gowned with personal protective equipment but with no or dislocated protective eyewear. These incidences of anesthesiologist and nurse are only some of the proof of the nosocomial spread of SARS-CoV-2 in the eyes.3,8,9,12,14,16 Dr. Li Wenliang, the Chinese ophthalmologist and the first whistleblower about this disease himself is assumed to get infected by SARS-CoV-2 from an asymptomatic glaucoma patient and died on February 7, 2020.17 Similarly, Dr. Jay M Galst, a renowned ophthalmologist lost his life on 12th April due to SARS-CoV-2 infection. Both have put the additional onus on us ophthalmologists. The clinical presentation of conjunctivitis is usually characterized by signs and symptoms of viral conjunctivitis. It was recently reported that in a hospitalized patient of COVID-19, who complained of ocular redness and watering post 13 days of illness showed bilateral moderate conjunctival injection along with watery discharge and inferior palpebral conjunctival follicles which resembles signs of acute viral conjunctivitis. His conjunctival swabs were also tested positive for SARS-CoV-2 RNA. He was treated with ribavirin eyedrops four times a day and showed symptomatic and clinical resolution of conjunctivitis within 5 days.18 Present understanding shows that SARS-CoV-2 conjunctivitis has no specific manifestation, can be follicular, and can present in one eye or both. In the early stage, it might appear as common conjunctival hyperemia with fewer watery secretions and akin to thin mucus. Occasionally conjunctival hemorrhage in few is also mentioned.9 The ocular symptoms of the patients can be mild and self-healing such that many COVID-19 patients might not bother to report it. So the symptoms may be noted in the early or late phase of the disease. However, correlations between conjunctivitis and COVID-19 could also be confounded by co-correlation with unobserved factors.9 The presence of binding receptors in the ocular surface, the binding affinity between the binding receptors and SARS-CoV-2 virus, the viral tropism and viral load all are responsible for the manifestations in the form of conjunctivitis. It has also been proven that rhesus monkeys can be infected with SARS-CoV-2 via the conjunctival route viral load was comparatively high in the nasolacrimal system and lesions in the lung were relatively mild and local when the SARS-Co-2 entered via the conjunctival route.19
However, at present, there has been no proven literature to support that the ocular surface abnormalities in COVID-19 patients are related to primary eye infection or secondary eye infection.
Hence, our knowledge and understanding about the SARS-CoV-2 virus, modes of entry to the eye, hypothesis on the interaction with the Renin-Angiotensin System (RAS) system and ACE2 receptor and ocular pathogenesis and RT PCR analysis from the ocular secretions have been summarized below using text, tables, diagrams, and flowcharts. We also tabulated 12 articles’ papers related to the ocular manifestation of SARS-CoV-2 infection and compared the ocular and laboratory findings with the past SARS outbreak.
SARS-CoV-2 Virus Genomics
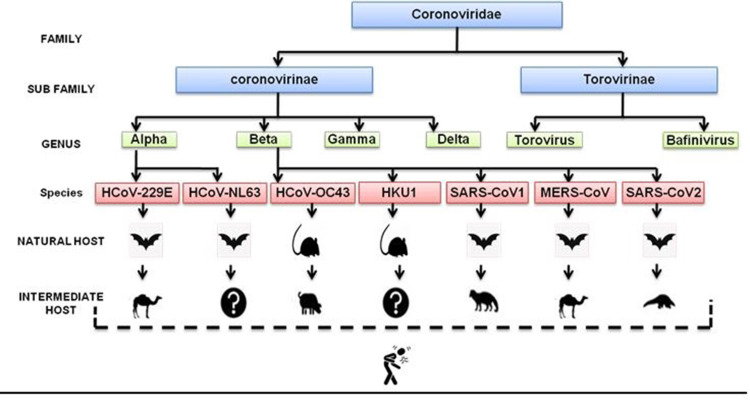
Coronaviruses (CoV) belong to the subfamily Coronavirinae, in the family Coronaviridae of the order Nidovirales. There are four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. As far now only seven viruses of this family including SARS-CoV-2 were reported to cause human infection21 (Figure 1).
Figure 1.
Flow chart classification and origin of coronaviruses.
Coronaviruses are enveloped single-stranded positive-sense RNA possessing the largest genome of RNA viruses (26–32 KB bp in size) with varying G + C contents (32% to 43%).19 The genomes are polyadenylated at the 3ʹ end and those are the largest genome among RNA virus. Through genomic study, it is established that SARS-CoV-2 and SARS-CoV share the same cell receptor in the human while MERS-CoV uses dipeptidyl peptidase 4 (DPP4) as a gateway to enter into human cells.22 Over two decades, the virus form Beta Coronovirinae has been causing many epidemics and pandemic outbreak. These viruses are believed to be zoonotically transmitted and cause secondary transmission from human-to-human. Previously known coronaviruses like Human Coronavirus (HCoV) −229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1 were known to cause mild self-limiting symptoms until the identification of three more opportunistic strands that can cause severe pneumonia named acute respiratory syndrome (SARS)-CoV-1 in 2002, Middle Eastern respiratory syndrome (MERS)-CoV in 2012 and more recently SARS-CoV-2 in December 2019 as shown in Table 1.
Table 1.
A Comparative Chart of SARS, MERS and SARS-CoV-2 Outbreak
| Virus | Year | Incubation Time (Days) | Community Attack Rate (%) | Hospitalization Rate | Globally Infected Rate |
|---|---|---|---|---|---|
| SARS-CoV-1 | 2002–2004 | 2–7 | 30–40 | Most cases | 8098 |
| MERS | 2012 | 6 | 4–13 | Most cases | 320 |
| SARS-CoV-2 | 2019–Ongoing | 4–14 | 30–40 | Unknown | Ongoing |
Abbreviation: SARS, severe acute respiratory syndrome; MERS, Middle East respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The morphology and the properties of the viral protein of SARS-CoV-2 virus and its replication after binding with the target ACE2 receptor of the target cell are depicted in Figures 2 and 3.
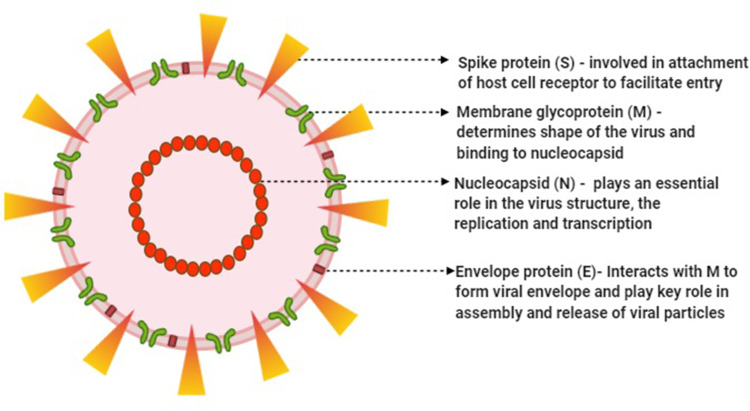
Figure 2.
Morphological structure of SARS-CoV-2 and steps of replication in the target human cell.
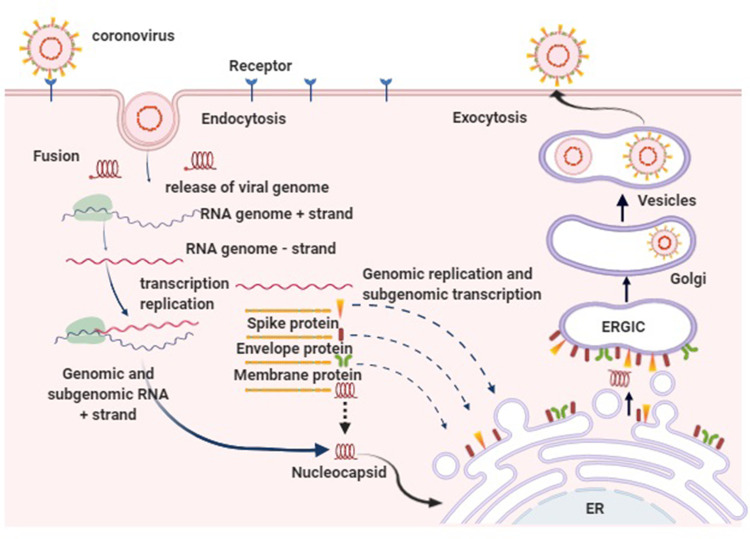
Figure 3.
Figure illustrating the virus binding to the target receptor (ACE-2) on the surface of the target cell with its spike proteins. The viral particle is then internalized by endocytosis by the fusion of S protein of the viral envelope with cell membrane followed by the translation of the viral genomic RNA to produce a virus-specific RNA-dependent RNA polymerase which transcribes a full-length complementary minus-strand RNA which then interacts within the cytoplasm of the cell with the viral nucleocapsid protein to form helical nucleocapsids. These nucleocapsids bud in the compartment through the membranes of the rough endoplasmic reticulum (ER) and the Golgi apparatus in areas that contain the viral glycoprotein (ERGIC). Mature virion synthesized are then transported in the vesicles to the cell periphery for the release upon the cell lysis.
Modes of Transmission of SARS-CoV-2 Through the Ocular Route
The exposed ocular surface can serve as a gateway to acquiring respiratory viruses. After a detailed review of the various manuscripts, we hypothesize the following probable entry routes of the virus to the eye which is shown in Table 2.
Table 2.
Possible Routes for SARS-CoV-2 Virus in the Eye
| Serial Number |
Routes of Virus Entry Into the Eye | Mechanism | Remarks |
|---|---|---|---|
| 1 | Droplets transmission | Respiratory droplets from infected person coughs/sneezes) →virus inoculate into the exposed ocular mucosal surface | → Causes the local infection or directly enters the lacrimal passage → reach upper & lower respiratory system |
| 2 | Aerosol transmission | High dose of contaminated aerosols → virus into enter the exposed ocular mucosal surface | |
| 3 | Hand to eye contact transmission | Contamination of hand after touching virus contaminated surface/object → direct touch the eyes → virus inoculated in the exposed ocular mucosal surface | |
| 4 | Nosocomial transmission | Use of virus contaminated ophthalmic instruments during gonioscopy, tonometry, ocular imaging etc → virus inoculated in the exposed ocular mucosal surface | |
| 5 | Retrograde/Reflux transmission | Nasopharynx, the primary site of viraemia → reflux of nasopharyngeal secretion → virus enter to the lacrimal passage → enter ocular mucosal surface | Reverse entry from the upper respiratory tract to the eye |
| 6 | Blood borne | Virus and the released cytokines can → enter the blood circulation → reach the ocular surface via ocular blood supply | Viraemia |
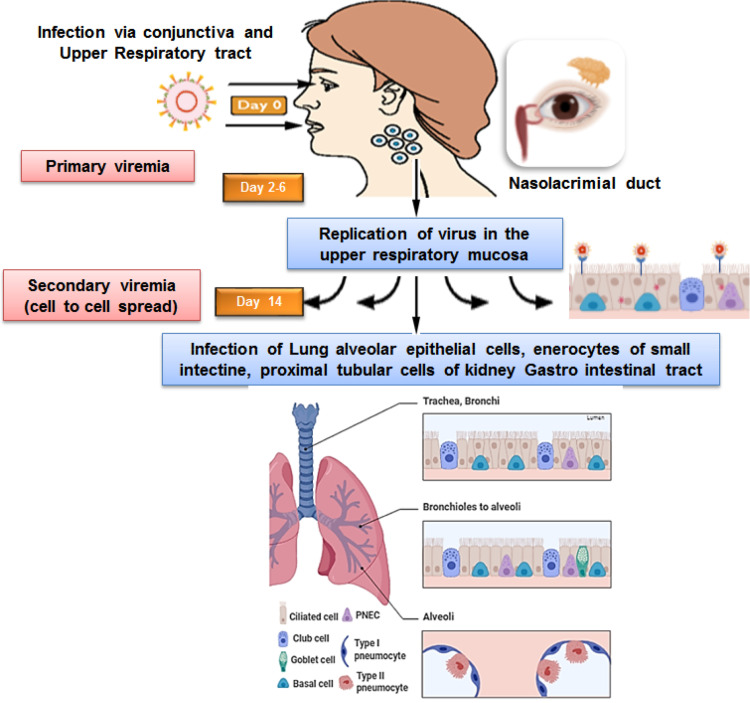
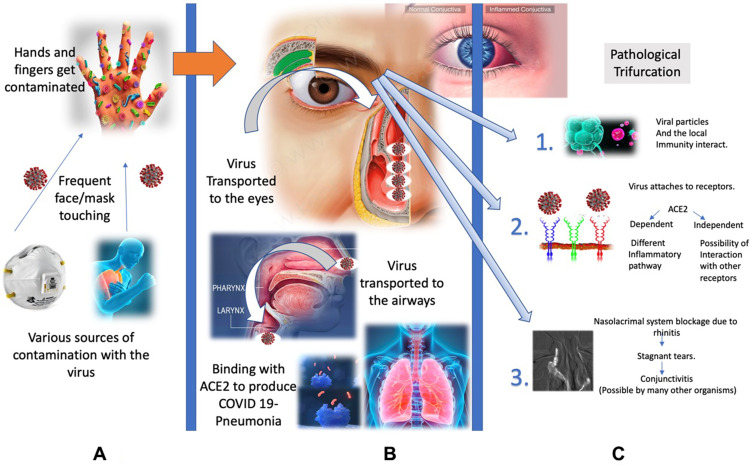
Besides the nasal mucosa, the SARS-CoV-2 virus may have alternate assess to the lungs through the eyes where they can manifest the ocular disease or further travel down to the lungs over a week as shown in Figure 4.
Figure 4.
Modes of entry and pathogenesis of the SAR-CoV-2 infection.
In the primary stage of the infection, the virus coming into the contact with mucosal region of the eye could be absorbed by the attachment of viral spike protein to the host cell receptor present in the contact region forming antigen receptor complex. This complex is proteolytically processed by type 2 transmembrane protease (TMPRSS2) leading to subsequent cleavage of ACE-2 receptor and activation of viral spike protein thus facilitating the viral entry.22–24 The virus may be partly absorbed through conjunctiva or cornea via the nasolacrimal duct or directly enter into the host cells of the upper respiratory tract. This causes primary viremia. The virus replicated in the upper respiratory system subsequently infects the adjacent cells causing secondary viremia by similar mode of binding of virus spike protein to ACE2 receptor in type 2 pneumocytes in the lungs triggering a cascade of inflammation in the lower respiratory systems consisting of nasopharynx and trachea or reach gastro intestinal tract.24
Renin-Angiotensin System (RAS) and Angiotensin-Converting Enzyme 2 Receptor – Do They Have a Role in the Ocular Manifestation of SARS-CoV-2 Infection?
The efficacy of virus entry into host cells depends on three points: the invasiveness of the virus, viral receptors on the host cell membrane, and the immune status of the host. The virus invades the host cell by binding to the Angiotensin-Converting Enzyme 2 (ACE 2) receptor via viral spike proteins. The type II transmembrane serine protease (TMPRSS2) binds and cleaves the ACE-2 receptor.22
The renin-angiotensin system (RAS) plays an important role in the regulation of blood pressure mainly via fluid and electrolyte homeostasis.25
Besides this, RAS is also responsible for the tissue-specific regulatory system and is accountable for long term “adaptive” regional changes. These are also found in various glands of the body including intricate regulation of homeostasis in the special sensory -the eye.26
Although RAS is associated with various enzymes and peptides, Angiotensin-converting enzyme 2 has gained special interest recently. This has been studied well and was found to be the “portal” of entry for the SARS-Co-V virus during the outbreak of 2003–04.27
ACE 2 is mainly found in the smooth muscles of the upper and lower respiratory tracts including the alveolar wall. While many of the previous literature agree on the presence and its distribution in certain tissues of the eye, the growing debate of its presence in the conjunctiva has become controversial.9 And with this, the pathogenesis of conjunctivitis in SARS-CoV-2 infection seems to be much less understood.25
If the virus were to inflict the cascade of inflammatory reaction on the conjunctiva as per our understanding of its pathogenesis in the respiratory system, one would, without doubt, expect the presence of ACE2 receptors on the conjunctiva. Some reports also agree to the fact that ACE2 receptors are present in the conjunctiva and hence make the above theory more convincing.9,25 But after the reports from the Centre for evidence-based medicine (CEBM) suggested that conjunctiva is free of the ACE2 membrane protein, the theory seems to dismantles itself.28 With reports now suggesting that the virus merely get shed from tears, the enigma only gets more difficult.11,29-31
The Hypothesis and Requirement of Further Research to Understand the Pathology
Based on the knowledge from various published articles, we propose few hypotheses which can be validated in the future which could justify many of the questions that currently remain unanswered.11,25,31,32
If ACE2 Receptors are Present in the Conjunctiva
Hypothesis 1: Past studies during 2004 during the SARS outbreak have found that the tears of SARS patients tested positive for viral nucleic acid.33 So far, we know that the chromosome of SARS‐CoV‐2 is 82% similar to that of SARSCoV.34 If the ACE2 receptor exists in conjunctival mucosa, the interaction between the SARS-CoV-2 virus and the conjunctiva can produce conjunctivitis with features of hyperemia, chemosis, and watery secretion. But the nature of inflammation of the conjunctiva could be milder than the respiratory tract. Some reports have predicted the presence of the SARS-CoV-2 virus in conjunctival samples as early as 2–3 days of systemic disease onset.13,31 Though present, the positive expression of ACE2 in the human ocular surface might be much less than in human lung and kidney tissues.13
Thus, in short, if the ACE2 receptor exists in conjunctival mucosa, the interaction between the SARS-CoV-2 virus and the conjunctiva can produce conjunctivitis with features of hyperemia, chemosis, and watery secretion.
Hypothesis 2: It is known that even the swab from the oropharynx, despite having ACE2 receptors is not a very good site for collecting specimens for detecting the virus - rather it’s the nasopharynx.9,35 Contemplating, same could hold true and the conjunctiva may not be a proper specimen to analyze for detection of the virus. The transmigration of the virus could make the virus an “intrinsic” pathogen and hence may not be present on the conjunctival surface or even in the tears. The squamous cells which are shed from the conjunctiva or a conjunctival tissue may harbor the virus and could be studied in much detail.25,32
If ACE2 Receptors are Absent in the Conjunctiva
Hypothesis 3: Even if ACE2 receptor may be absent in the conjunctiva, the ocular surface may harbor the SARS-CoV-2 virus due to transmission of the virus to the eye through the contaminated hand, finger or object from where the tears can further aid in the transport of the virus from the nasolacrimal duct into the airway passage, where ACE2 receptors are present and produce respiratory symptoms.
Thus in short, if we assume that conjunctival mucosa lacks ACE2 receptors, these ACE2 receptors are also found on the various cell lineages of the macrophages and other immune cells including the eye. If such cells are activated, the degranulation process which follows with the release of inflammatory markers could produce conjunctivitis-like symptoms which share the common pathogenesis with allergic rhinitis and conjunctivitis. Based upon the literature published till mid of April 2020, the hypotheses of entry of the virus into the eyes of its interaction with the target tissue are summarized in Figure 4.
Hypothesis 4: Even if we assume that conjunctival mucosa lack ACE2 receptors, these ACE2 receptors are found on the various cell lineages of the macrophages and other immune cells- including the eye.36 If such cells are activated, the degranulation process which follows with the release of inflammatory markers could produce conjunctivitis like symptoms which share the common pathogenesis with allergic rhinitis and conjuncitivitis.
These hypotheses of entry of the virus into the eyes, its interaction with the target tissue are summarized in Figure 5.
Figure 5.
The figure illustrates the various modes of SARS-CoV-2 entry to the human eye (A), passage to through the nose, mouth, and eyes to the respiratory system (B) and replication with the target receptor of the target cell surface leading to inflammation in the infected organ or cells in the lungs and eyes (C).
SAR-CoV-2 and Eye
We have analyzed and tabulated the 12 articles published about the ocular involvement and outcome of ocular fluid sampling in SARS- CoV-2 Outbreak from Dec 2019 till mid of April 2020. We have compared their ocular and laboratory findings with a SARS article published in 2003 (Table 3).
Table 3.
Review of Published Articles on Ocular Prospective of SARS-CoV 2 Infection
| S No. | Article Title and Authors |
Published Journal | Article Type/Design | Study Period | Place of Study | Mean Age/Range Years | Novel Corona Pneumonia cases | Presence of Ocular Features | Ocular Sample RT PCR + | Ocular Viral load |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Clinical Characteristics of Coronavirus Disease 2019 in China (Guan et al)40 |
The New England Journal of Medicine | Original article/Cohort Study | 11th Dec 2019–29th Jan 2020 | 47 (Range, 35–58) | 1099 (926 non severe and 173 severe patients) | 9/1099 (0.8%) 5(0.5% in Non severe) and 4(2.3% in Severe) cases |
Not Mentioned | Low | |
| 2 |
There may be virus in conjunctival secretion of patients with COVID-19 (Liang L et al)41 |
Acta Ophthalmologica 2020 (Perspective In Ophthalmology) |
Cohort Study | Not Mentioned | Yichang Central People’s Hospital, China | Not Mentioned | 37 (12 severe, 15 mild cases) | 3 | 1 conjunctival sac secretion nucleic acid test +ve | Low |
| 3 |
Comparisons of nucleic acid conversion time of SARS-CoV-2 of different samples in ICU and non-ICU patient (Fang et al)42 |
Journal of Infection | Letter to the Editor | Jan–Feb 2020 | Central Hospital of Xiangtan |
34–54 years | 32 (8 ICU & 24r non-ICU) | Not Mentioned | The positive rate of tears (15.6%, 5/32) | Not Mentioned |
| 4 |
Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China (Wu P et al)8 |
JAMA Ophthalmology | Brief Report/Prospective | 9–15th Feb 2020 | Yichang Central People’s Hospital, China | 65.8± 16.6 | 38 | 12 (31.6%) | 2 patients (5.2%;95% CI, 0.617.8) in conjunctival specimen | Not Mentioned |
| 5 |
Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection (Xia J et al)31 |
Journal of Medical Virology | Prospective, Case-series | 26th Jan–9th Feb | Hospital of Zhejiang University, China | 54.50±14.17 | 30 | 1(3.3%) | 1 (3.3%) in conjunctival swab | Not Mentioned |
| 6 |
Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva (Zhou Y et al)9 |
MedRxiv | Retrospective cohort study | 17−28th Jan 2020 | Renmin Hospital of Wuhan University, China | 35.7± 10.6 | 67 | 1(1.5%) | 3 (4.4%) 1 Positive 2 Probable Positive |
Not mentioned |
| 7 |
Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals (Seah I et al)30 |
Ocular Immunology And Inflammation | Invited Review | Not Mentioned | Singapore | Feline& Murine eyes | Live Corona virus isolated in cats and mice eyes | – | – | – |
| 8 |
Ocular manifestation, eye protection, and COVID-19 (Mungmungpuntipantip R et al)43 |
Graefe’s Archive for Clinical and Experimental Ophthalmology | Letter to Editor | March | 26 Medical Center, Bangkok, Thailand | Not mentioned | 48 | No Ocular findings | 0% | 0 |
| 9 |
Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear (Li JO et al)44 |
British Journal of Ophthalmology | Editorial | Not Mentioned | Not Mentioned | Not Mentioned | Not Mentioned | Not Mentioned | Not Mentioned | Not Mentioned |
| 10 |
The evidence of SARS-CoV-2 infection on ocular surface (Zhang X et al)10 |
Ocular Surface | Cohort study | Not Mentioned | Tongji Hospital, Wuhan, China | Not Mentioned | 72 | 2 (2.78%) | 1 | Not mentioned |
| 11 |
Screening for novel coronavirus related conjunctivitis among the patients with corona virusdisease-19 (Lan QQ et al)45 |
Chinese Medical Association Publishing House Ltd. | Prospective, Case-series | Not Mentioned | Guangxi Zhuang Autonomous Region, Nanning | Not Mentioned | 81 | 3 | 0 | Not mentioned |
| 12 |
Assessing Viral Shedding and Infectivity of Tears in Coronavirus Disease 2019 (COVID-19) Patients (Seah IYJ et al)37 |
Ophthalmology | Prospective, Case-series | Not Mentioned | National University Hospital, Singapore, Republic of Singapore | Not Mentioned | 17 | 1 | 0 | Not Mentioned |
Univariate analysis by few authors has shown that the patients with ocular symptoms were more likely to have higher white blood cell, neutrophil counts, procalcitonin, C-reactive protein, and lactate dehydrogenase than the patients without ocular symptoms.8 They also suggested that ocular manifestations are more common amongst COVID-19 patients with more severe pnuemonia.8
Besides conjunctivitis, other ocular surface abnormalities like dry eyes, pterygium, corneal abrasions, and ulcerations due to SARS-CoV-2 tropism are still an area to explore in the future. The evaluation of retinal involvement along with the impact of retinal and choroidal circulation in COVID-19 cases with ocular involvement is an arena to work upon.
The Outcome of PCR Assay in Ocular Specimen
The conventional RT-PCR techniques using primer probes targeting the envelope (E) gene for screening and amplification targeting the RNA-dependent RNA polymerase (RdRp) genes for the confirmation are considered as the gold standard method for diagnosis of SARS-CoV-2 in respiratory secretions and this may hold true for the ocular samples from conjunctival secretion and tears.2,36
There are reports mentioning the concentration of SARS-CoV-2 nucleotide in the nasopharynx and throat to be of 104–1010 RNAs/Swab but the literature to document the exact concentrations in the ocular secretions is limited.35 Hence, the knowledge on the sensitivity and specificity of RT-PCR in ocular secretion, the prevalence of SARS-CoV-2 nucleotides in the eye and the viral load still needs to be explored in the coming days.
The present understanding of the outcome of the RT-PCR analysis in the ocular samples is mainly based on the following-
Conjunctival specimen PCR analysis – One report has mentioned low prevalence (5.2%; 95% CI, 0.617.8) of SARS-CoV-2 nucleotides in conjunctival specimens of patients with COVID-19 but many have reported negative presence.8,28-30
Tear film PCR analysis – few reports have documented the presence of SARS-CoV-2 in tear fluid while others have not detected SARS-CoV-2 nucleotides in the tear specimen.28-31,37
The reports of nil prevalence to an extremely low positive rate of SARS-CoV-2 nucleotides in the tears and conjunctival secretions from the COVID-19 patients need to be analyzed cautiously. The inappropriate timing of ocular sample collection (too early or too late of systemic disease), poor specimen site, faulty technique, unknown sensitivity of current RT-PCR technique in ocular fluid, local ocular immune system activation with a significant increase in lactoferrin and secretory IgA levels in tears could be some of the factors contributing to the low positive rate of RT-PCR analysis of the ocular samples.
Since reports have suggested that conjunctivitis in SARS-CoV-2 infection cases could be both early or late presentation, we suggest that it would be better to perform the RT-PCR analysis of the ocular secretions in both early and late stage of infection to minimize the chance of missing the false negative report of RT-PCR results in tears and conjunctival secretions. The lower of the viral concentration and diverse genome fraction in the eye compared to the tracheal aspirates could be one of the contributing factor.10
However, the study conducted among the rhesus monkey eyes showed that after direct inoculation of the SARS-CoV-2 via ocular conjunctival inoculation; the viral load was detectable in several nasolacrimal system associated-tissues, especially in the conjunctiva, lacrimal gland, nasal cavity and throat, which drew the outline of the anatomical bridge between ocular and respiratory tissues.19 Thus, lacrimal duct serving as a conduit to collect and drain the SAR-CoV-2 from ocular to respiratory tract tissues via inferior meatus.19
Until proven via large-scale analysis, it is always better to urge on the worst side and consider the ocular secretions as an infectious and potential source for the spread of contamination. The infectivity of the tears and conjunctival secretion from affected patients may have impacts not only in the daily ophthalmic practice but also on the universal infection control measures adopted by the general public and health-care professionals as it may have the potential to catalyze the spread.39 Hence, a safe ophthalmic practice is needed for the ophthalmologists and their patients to protect themselves.15
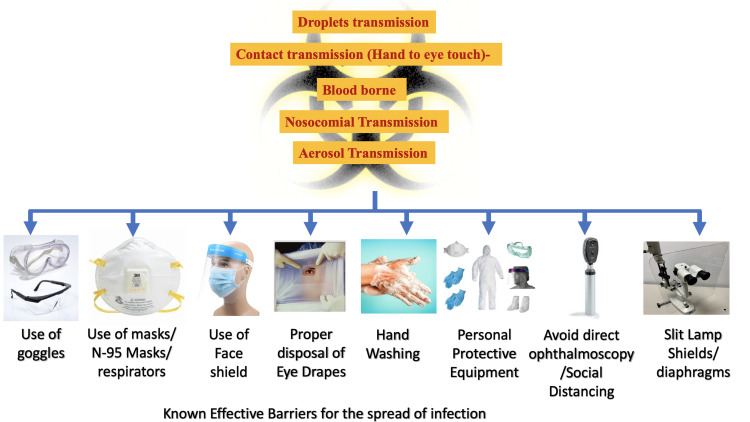
Thus, the best option, for now, is the adoption and improvisation of the precaution measures to break the chain of the spread of theSARS-CoV-2 virus and infection in the community and ophthalmic practices.46 Some of the effective measures are shown in Figure 6.
Figure 6.
Preventive and protective measures to avoid the entry of contaminated droplets and aerosols in the eyes.
Conclusion
The ocular surface mucosa could represent a target organ and a “portal” of entry or transporter of SARS-CoV-2 to infect the respiratory tract. The presence or absence of the ACE2 receptors in the conjunctival tissue and nasolacrimal passage is still debatable but may not even be required for producing ocular manifestations. The possibility of lesser affinity to the receptors to the eye compared to other organs such as nose and airway passage could also mean an “unpreferred” gateway for SARS-CoV-2. The ocular involvement either as the first manifestation or late feature during SARS-CoV-2 infection should also not be under looked. Therefore, it may be advisable to perform RT–PCR analysis of ocular secretion in both the early and late phases.
At the same time, the infectivity of the ocular secretions of the COVID-19 infection should not be ignored. Due to close doctor-patient distance in ophthalmic practice, social distancing is virtually impossible with greater apt for transmitting SARS-CoV-2 virus by droplets, aerosols, ocular secretions, and contaminated ocular instruments. Thus, hand hygiene and personal protection are recommended for health care workers to avoid hospital-related viral transmission during ophthalmic practice.
Since there are newer reports of SARS-CoV-2infections coming with recurrence, it would be better to instruct the patients are instructed to report early if they have any ocular problem, however trivial for being a harbinger of more severe disease.
Acknowledgment
We would like to thank Dr. Gunjan Prasai for proofreading and the linguistic support.
Funding Statement
There is no funding to report.
Abbreviations
ACE, Angiotensin-Converting Enzyme; 2019-nCoV, 2019 novel coronavirus; MERS, Middle East Respiratory Syndrome; NCP, Novel coronavirus pneumonia; RT-PCR, Reverse Transcriptase Polymerase Chain Reaction; SARS, Severe Acute Respiratory Syndrome; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; WHO, World Health Organization.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization World Health. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. WHO; 2020. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical -guidance/laboratory-guidance. Accessed February9, 2020. [Google Scholar]
- 3.CDC. 2019 Novel coronavirus. Wuhan, China; 2020. Available from: https://wwwcdcgov/coronavirus/2019-nCoV/summaryhtml. February. [Google Scholar]
- 4.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. Novel Coronavirus (2019-Ncov) Situation Report-5. 25January 2020 Geneva, Switzerland: 2020. [Google Scholar]
- 6.Enserink M. Update: ‘A bit chaotic.’ Christening of new coronavirus and its disease name create confusion. Science. 2020. doi: 10.1126/science.abb2806 [DOI] [Google Scholar]
- 7.Worldometer website. Updated April15 Available from: https://www.ajronline.org/doi/full/10.2214/AJR.20.22969 RM. COVID-19 coronavirus outbreak. wwwworldometersinfo/coronavirus/. Accessed April15, 2020.
- 8.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575. doi: 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Zeng Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv. 2020;2002(2011):20021956. [Google Scholar]
- 10.Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Elsevier. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C-W, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jan DXXN. Peking University Hospital Wang Guangfa disclosed treatment status on Weibo and suspected infection without wearing goggles. Cited January24 Available from: http://wwwbjnews comcn/news/2020/01/23/678189html.
- 13.Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun CB, Wang YY, Liu GH, Liu Z. Role of the Eye in Transmitting Human Coronavirus: What We Know and What We Do Not Know. Front Public Health. 2020;8:155. doi: 10.3389/fpubh.2020.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 16.Bacherini D, Biagini I, Lenzetti C, Virgili G, Rizzo S, Giansanti F. The COVID-19 pandemic from an ophthalmologist’s perspective. Trends Mol Med. 2020;26:529–531. doi: 10.1016/j.molmed.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2020 AAoOAF. Coronavirus kills Chinese whistleblower ophthalmologist; 2020. Available from: https://wwwaaoorg/headline/coronavirus-kills-chinese-whistleblower-ophthalmol.
- 18.Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. British J Ophthalmol. 2020;104:748–751. doi: 10.1136/bjophthalmol-2020-316304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W, Bao L, Gao H, et al. Rhesus macaques can be effectively infected with SARS-CoV-2 via ocular conjunctival route. bioRxiv. 2020. [Google Scholar]
- 20.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan Y, Graham R, Baric R, Li F. An analysis based on decade-long structural studies of SARS 3, JVI Accepted Manuscript Posted Online 29 January 2020. J Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T. Jahn O and Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holappa M, Vapaatalo H, Vaajanen A. Many faces of renin-angiotensin system-focus on eye. Open Ophthalmol J. 2017;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giese MJ, Speth RC. The ocular renin–angiotensin system: a therapeutic target for the treatment of ocular disease. Pharmacol Ther. 2014;142:11–32. doi: 10.1016/j.pharmthera.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 27.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.medicine (CEBM) Cfe-b. Spreading SARS-CoV-2 through ocular fluids; March 2020 Available from: https://wwwcebmnet/covid-19/spreading-sars-cov-2-through-ocular-fluids/.
- 29.Jun ISY, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). StatPearls. 2020. [PubMed] [Google Scholar]
- 33.Peiris JS, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. New England J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498 [DOI] [PubMed] [Google Scholar]
- 34.Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes Infections. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver C, Jones N. Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID-19. Oxford COVID-19 evidence service team centre for evidence based medicine. 2020. [Google Scholar]
- 36.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–979. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loon S, Teoh S, Oon L, et al. The severe acute respiratory syndrome coronavirus in tears. British J Ophthalmol. 2004;88:861–863. doi: 10.1136/bjo.2003.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadhu S, Agrawal R, Pyare R, et al. (2020) COVID-19: limiting the risks for eye care professionals. Ocul Immunol Inflamm. doi: 10.1080/09273948.2020.1755442 [DOI] [PubMed] [Google Scholar]
- 40.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L Iang L, Wu P. There may be virus in conjunctival secretion of patients with COVID-19. Acta Ophthalmol. 2020;98(3):223. doi: 10.1111/aos.14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mungmungpuntipantip R, Wiwanitkit V. Ocular manifestation, eye protection, and COVID-19. Graefe’s Archive Clin Exp Ophthalmol. 2020;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JO, Lam DSC, Chen Y, Ting DSW. Novel Coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol. 2020;104(3):297–298. doi: 10.1136/bjophthalmol-2020-315994 [DOI] [PubMed] [Google Scholar]
- 45.Lan QQ, Zeng SM, Liao X, Xu F, Qi H, Li M. Screening for novel coronavirus related conjunctivitis among the patients with corona virus disease-19. Zhonghuayanke Za Zhi. doi: 10.3760/cma.j.cn112142-20200322-00213 56:E009 [DOI] [PubMed] [Google Scholar]
- 46.Khatri A, Kharel M, Chaurasiya B, Ashma KC, Khatri BK. COVID-19 and ophthalmology: An underappreciated occupational hazard. InfectControl Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.344 [DOI] [PMC free article] [PubMed] [Google Scholar]