Abstract
Current scenario depicts that world has been clenched by COVID-19 pandemic. Inevitably, public health and safety measures could be undertaken in order to dwindle the infection threat and mortality. Moreover, to overcome the global menace and drawing out world from moribund stage, there is an exigency for social distancing and quarantines. Since December, 2019, coronavirus, SARS-CoV-2 (COVID-19) have came into existence and up till now world is still in the state of shock.At this point of time, COVID-19 has entered perilous phase, creating havoc among individuals, and this has been directly implied due to enhanced globalisation and ability of the virus to acclimatize at all conditions. The unabated transmission is due to lack of drugs, vaccines and therapeutics against this viral outbreak. But research is still underway to formulate the vaccines or drugs by this means, as scientific communities are continuously working to unravel the pharmacologically active compounds that might offer a new insight for curbing infections and pandemics. Therefore, the topical COVID-19 situation highlights an immediate need for effective therapeutics against SARS-CoV-2. Towards this effort, the present review discusses the vital concepts related to COVID-19, in terms of its origin, transmission, clinical aspects and diagnosis. However, here, we have formulated the novel concept hitherto, ancient means of traditional medicines or herbal plants to beat this pandemic.
Keywords: SARS-CoV-2, Herbal plants, Natural therapeutics, Traditional medicine, Immune-System Rebooting, PAK-1 Blockers
Abbreviations: ACE2, angiotensin-converting enzyme; ACTH, adrenocorticotrophic hormones; APC, antigen presenting cell; ARC, artepillin C; ARDS, acute respiratory distress syndrome; ASCs, antibody-secreting cells; AYUSH, ayurvedic, yoga and naturopathy, unani, siddha and homeopathy; C-1, Caspase-1; CAPE, caffeic acid phenyl ester; CHM, Chinese herbal medicine; GCSF, granulocyte-colony stimulating factor; ICTV, international committee on taxonomy of viruses; IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; MAPK, mitogen activated protein kinases; MCP1, monocytechemoattractant protein 1; MERS-CoV, middle east respiratory syndrome; MIP1, macrophage inflammatory protein 1; MT, metalloprotease; MVD, molegro virtual docker; NF, neuro fibromatosis; NK, natural killer; NLR, neutrophil-to-lymphocyte ratio; NLRP3, NOD-like receptor proteins; PDB, protein data bank; R0, basic reproduction number; RBD, receptor-binding domain; RNA, ribonucleic acid; SARS-CoV, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TCM, traditional Chinese medicines; Tfh, follicular helper T-cells; TNF, tumor necrosis factor; WHO, World Health Organisation
Graphical abstract
1. Introduction
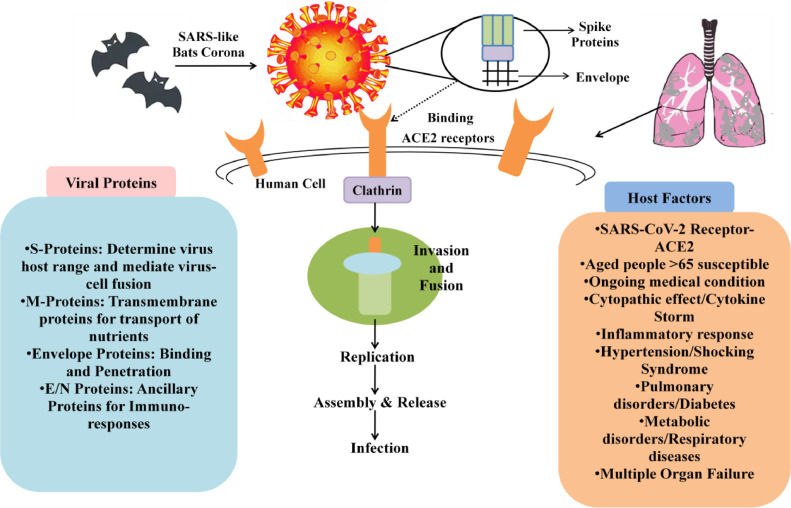
Pandemic diseases are of global concern in the present era, to cause gigantic morbidity and transience, regardless of, extensive medical facilities. Particularly, anti-viral therapies have been fraught because of surfacing of mutants competent enough to subdue the drugs targeting viral elements (Ahmad et al., 2020a). Most importantly, enhanced universal travel and swift urbanization has led to contagious outbreak by rising or re-emergence of viruses, posing a serious menace towards communal health and safety, specifically in such unprecedented times where there are no preventive vaccines available. With the advent of twenty first century, our planet has observed the incidence of catastrophic viral outbreaks namely, SARS-CoV (Severe Acute Respiratory Syndrome) and MERS-CoV (Middle East Respiratory Syndrome) within human populace (Markotić and Kuzman, 2020). At present, COVID-19 (Coronavirus) is the third most important disease of animal origin, which is prevailing in almost four corners of the world by getting initiated from a single place. Nearly, 213 countries of all continents have been affected in less than three months by this pernicious virus (Acharya, 2020). After studying its clinical characteristics, experts affirmed that it is quite similar to pneumonia and therefore, named as Novel Coronavirus. However, in the second week of March, 2020, COVID-19 was stated as pandemic by World Health Organisation (WHO) (Gautret et al., 2020). As of August, 24th, 2020, there have been reported 23,584,259 COVID-19 cases with 812,517 deaths globally as depicted by WHO (WHO, 2020). The causal agent of COVID-19 is SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) as per officially named by ICTV (International Committee on Taxonomy of Viruses) (Gautret et al., 2020). After incessant investigations, it has been acquired that SARS-CoV resembles sequence homology with bat coronavirus. Though, its spike glycoproteins have highest affinity for human angiotensin-converting enzyme 2 (ACE2) receptors, that enable them to invade and transmit through human to human (Guo et al., 2020). Even though it shares resemblance with SARS-CoV, yet its spreading ability and diagnosis is relatively different. The distinctive feature is most likely the nucleotide pattern of spike proteins along with its receptor binding domains (Wan et al., 2020). Moreover, belonging to the category of β-coronaviruses, a highly prevalent virus family in nature, it has several hosts, natural, intermediate as well as final. Henceforth, it is a colossal issue to prevent and cure the outbreak of this viral infection owing to its greater transmitting and infecting power.
Looking into the present scenario, WHO worked really very hard on a plan to battle against this monstrous virus. For instance, they have strategized to minimize physical contact among each other, isolate and screen the infected people during initial stages and recognise and dwindle the spread from the animal sources. Additionally, focus have been made to address the critical mysteries related to virus in order to conduct awareness among citizens (WHO, 2020). In essence, it has been known to transmit through droplets such assaliva or nose or even through air-borne transmission. This has vexed the scientists all around the globe to find out best prevention against this pandemic until the suitable vaccine is discovered. However, manufacturers are progressively working on manufacturing masks and sanitizers to prevent disease incidence, lucrative for both health professionals as well as common people (Balachandar et al., 2020). At this point of time, it is immensely imperative to unravel viral pathogenisis to design the drugs or vaccines. It is a gloomy instance that, there is a lack of a verified treatment, however, many drugs are in a pipeline of clinical trials, yet no medically significant results have come out. Till that time, existing antiviral drugs like Lopinavir, Chloroquine, Nitazoxanide, Ritonavir, Hydroxuchloroquine, Tocilizumab and Azithromycin have been practiced that tend to reduce the replication and viral load (Gautret et al., 2020). As a research standpoint, scientists are in a quick pace to achieve their targets in order to safeguard the public. Moreover, envisaging steroids, monoclonal antibodies, peptides, interferons, oligonucleotides, enzyme inhibitors are suggested in curbing the disease (Mehta et al., 2020; Li and Clercq, 2020). Hitherto, there are still unproven antiviral drugs, vaccines and other alternatives available that are being tried to target SARS-CoV-2, consequently, accentuation is being imposed on precautionary measures and symptomatic cure (Jean et al., 2020). Yet, new discoveries require several trials for which few months to years can pass for the development. Having said that, there is an urgency to beat the COVID-19 outbreak, for which natural products, herbal plants and their formulations should be sent to this battlefield. Because of their feasibility, cost-effectiveness, eco-friendliness, efficacious nature and zilch side-effects, they need to be promoted for this warfare.
In this milieu, medicinal herbs are 'Gifted Gods' for healing, supporting and rehabilitating patients. Even though, no substantiation is present, but different studies on herbal plants are being conducted that have the ability to strengthen immune system and cope up with this virus. To expedite, certain phyto-compounds are being recognised to characterise the herbs in mitigating the incidence of infection. Ayurveda, Unani, Siddhi, Homeopathy, Romanian, Persian, Chinese traditional medicinal plants for example, are being currently exploited to check the effectivenesson this virus (Nikhat and Fazil, 2020; Yang et al., 2020). Since decades, herbal plants have been utilised in aboriginal health services as well as conventional medicines to combat diseases. The natural products provide an ancillary steer to unlock different mysteries behind the sickness. The exploitation of antiviral mechanisms of these natural compounds could shed light on their modes of action towards viral life-cycle, invasion, penetration, replication, assemblage and release. Moreover, customary acquaintance about plant sources and their usage is chiefly indispensable to employ it appropriately under right conditions. There have been more than 25,000 herbal formulations used in folk remedies in Ayurveda alone (Pundarikakshudu and Kanaki, 2019). Generally, these medications are disregarded and underrated in research and development due to contemporary medicines. Perhaps, they are ambiguous, but have broader demand nowadays in Western technology (Yuan et al., 2016). One single herbal species comprises plentiful phyto-constituents that single-handedly or collectively generate a pharmacological effect (Parasurman et al., 2014). Subsequently, these natural constituents are isolated and modulated as drug formulations against different diseases. Medicinal herbs are by far, are the life-saving drugs these days and research is being conducted on them to promote their usage in treatment of COVID-19 patients due to their potential of possessing anti-inflammatory, antioxidant and antiviral properties. Although, clinical trials are conducted to repurpose their value, for innovative treatment to defeat its transmission. During this period of global fretfulness, it is pertinent to locate long-lasting measures to avert the spread of this pandemic. Hence, its need of the hour to collaborate and counteract against COVID-19 by exercising social distancing along with maintaining hygienic surroundings (Balachander et al., 2020). The present review discusses the general overview, transmission, clinical approaches and immune-responses of coronavirus along with herbal immune-boosters to combat this infectious and pandemic disease that has created panic all over. By this review, we suggest that herbal or medicinal plant formulations could be essential alternative strategy, a step ahead to battle these awful viruses.
2. A general overview on coronavirus
SARS-CoV-2, provisionally known as 2019-novel coronavirus, is an enveloped positive-sense single-stranded RNA virus that belongs to the subfamily Orthocoronavirinae, and the family Coronaviridae (Huang et al., 2020; Gorbalenya et al., 2020). Subfamily, Orthocoronavirinae, includes four genera, namely alpha-, beta-, delta- and gammacoronavirus. Predominantly, alpha-and beta- CoVs infect mammals, whereas the main targets of delta- and gamma-CoVs are avian species (Fan et al., 2019). With the outbreak of recent SARS-CoV-2 total of seven human-susceptible CoVs strains have been currently identified that can infect human population. Most of these viruses tend to cause mild infections; however, SARS-CoV identified in 2002, MERS-CoV identified in 2012 and ongoing pandemic caused by SARS-CoV-2 have emerged as fatal CoVs capable of causing severe respiratory tract infections (Guo et al., 2020). Genomic sequencing analyses have revealed the close evolutionary relationship of SARS-CoV-2 with other beta-CoVs. It resembles more with Sarbecovirus subgenus that comprises of SARS-CoV than that of MERS-CoVs of Merbecovirus subgenus origin. At the nucleotide level, SARS-CoV-2 shares 79% homology with SARS-CoV, whereas only 50% with MERS-CoVs (Peng et al., 2020; Zhang and Holmes, 2020). Moreover, SARS-CoV-2 just like SARS-CoV utilizes the same ACE2 receptors to infect its hosts (Guo et al., 2020). Thus, the sites where ACE2 protein is mainly expressed are the potential target sites for SARS-CoV-2 respectively. These regions are belong to type II alveolar cells of the lungs and enterocytes of the small intestine (Hamming et al., 2004; Zheng, 2020). Nevertheless, there are some remarkable biological differences between the SARS-CoV-2 and the other beta-CoVs, which probably make it more infectious. Consequently, the epidemiological dynamics of SARS-CoV-2 is different from previous human-CoV outbreaks having striking local and global spread (Zhang and Holmes, 2020; Zheng, 2020).
Although, SARS-CoV-2 shows greater human-to-human transmission efficiency, its crude fatality rate (0.25% to 5%) is comparatively far less than that of SARS-CoV which is approx. 10%. Furthermore, SARS-CoV-2 has R0 (basic reproduction number) of 4.7 to 6.6. This highly contagious nature of SARS-CoV-2 is supported by the fact that its spike (S) protein possess 10 to 20 time's greater affinity for ACE2 receptors than SARS-CoV (Zheng, 2020). S-protein is the surface glycoprotein that assists the virus in the attachment to the host cells through its receptor-binding domain (RBD). S-protein has several domains, one of the sections termed as ectodomain has two subunits, S1 and S2, which form a crown-like structure around the virus (Vellingiri et al., 2020). Besides, S-protein of SARS-CoV-2 contains a furin-like cleavage site at the S1–S2 junction, missing in other members of its sister clade. This additional cleavage site might also be responsible for greater pathogenicity of SARS-CoV-2 as it also occurs in highly infectious form of influenza virus but lacking in less pathogenic ones (Coutard et al., 2020; Zhang and Holmes, 2020).
3. Origin and transmission of coronavirus
The natural reservoir for both the SARS-CoV and MERS-CoV were bats; however, these viruses infect humans through an intermediate host. Palm civets are supposed to be the intermediate host of SARS-CoV while dromedary camels are of MERS-CoV (Yin and Wunderink, 2018).Unfortunately, the exact zoonotic origin of SARS-CoV-2 is still elusive. Since SARS-CoV-2 shares 88% nucleotide homology with two SARS-like CoV found in bats (bat-SL-CoVZC45 and bat-SL-CoVZXC21) and 96% with RaTG13 virus found in horseshoe bat (Rhinolophus affinis), bats are considered to be its natural host (Lu et al., 2020b; Zhou et al., 2020b). Despite the 96% nucleotide similarity, the RBD of both the viruses varies significantly (Mackenzie and Smith, 2020). However, due to the ecological separation of the bats from the humans and the requirement of some necessary mutations in the virus genome to cross the species barrier, SARS-CoV-2 likely has one or more mammalian intermediate hosts for efficient animal-to-human transmission (Zhang and Holmes, 2020). Analyses of interactions between RBD in SARS-CoV-2 and ACE2 receptors in different hosts indicate that pangolins, snakes, and turtles may be the immediate host of SARS-CoV-2 (Liu et al., 2020a) (Fig. 1 ). Recent phylogenetic studies on the genome of Pangolin-CoV found from dead Malayan pangolins, which are illegally imported in China revealed that its genome is about 91% identical to SARS-CoV-2 and around 90% to RaTG13 (Bat-CoV). Besides, five of the six amino acids (contact residues) on RBD, vital for binding to ACE2 receptors on hosts are consistent between SARS-CoV-2 and Pangolin-CoV, but only four between Pangolin-CoV and RaTG13 and only one out of six between SARS-CoV and SARS- CoV-2 (Zhang et al., 2020a; Andersen et al., 2020). These findings suggest the probability of pangolins as natural reservoirs of SARS-CoV-2 (Zhang et al., 2020a).More and more studies with a wider sampling of mammals from China's wildlife markets or that are in close contact with humans like these are needed to resolve intermediate hosts of SARS- CoV-2 (Zhang and Holmes, 2020).
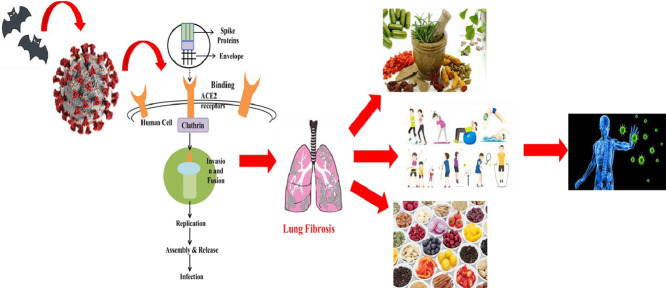
Fig. 1.
Schematic Presentation of COVID-19 Binding to Host Cells, Invasion, Replication, Assemblage, Release and Infection Along with the Associated Host and Viral Factors. Fig. illustrates the origin of coronavirus from bats; the viral structure comprises of spike proteins that enable attachment and virus is enveloped in nature for protection as well as facilitation of viral entry; the viral particles bind onto ACE2 receptors of host cells; clathrin is associated with coating membranes for endocytosis from the plasma membrane; viral particles are invaded and fused followed by replication; assembly and release of viral particles occurs to initiate the infection process.
3.1. Human-to-human transmission routes
Person-to-person transmission is primarily reported in families, communities, and hospitals (Guo et al., 2020). Droplet transmission is considered as the main route of person-to-person transmission (Han et al., 2020). The infection also spreads through direct contact and via fomite exposure i.e., direct contact to eyes, nose, and mouth after touching surfaces and objects contaminated by an infected individual (Peng et al., 2020; Morawskaa and Cao, 2020). In general, human-CoVs can remain infectious on some material surfaces for even 9 days (Kampf et al., 2020). Besides, SARS-CoV-2 also spreads through contact with asymptomatic carriers (Peng et al., 2020). Since SARS-CoV-2 infection is spreading at an unprecedented pace, airborne transmission too warrants meticulous determination (Morawskaa and Cao, 2020). In addition, SARS-CoV-2 was found in a stool sample, gastrointestinal tissue even urine and saliva of an infected person, suggests the possibility of intestinal and fecal-oral transmission (Xiao et al., 2020; Guan et al., 2020). Although no case of mother to child transmission has been reported yet, infection in two newborns of Wuhan, China raises the question for vertical transmission (Han et al., 2020). Apart from this, there are recent studies that reveal that there is a possibility of sexual transmission of COVID-19 infection. There are an array of evidences for this transmission such as through faecal-oral route via gastrointestinal infection (Peng et al., 2020). This is most probably due to ACE2 that enable virus entry and ACE2 mRNA is overexpressed in gastro-intestinal system as revealed through immunofluorescent analysis of rectal epithelial cells (Hindson, 2020). Moreover, RNA analysis of SARS-CoV-2 also revealed that the viral particles can infect these cells therefore, it is quite predictable that sexual intercourse could be the possible way of contagion. There have been cases when person shows negative results on nasopharyngeal swabs, while, positive on rectal swabs, depicting that sexual transmission might be the possibility (Xu et al., 2020). Henceforth, the physicians as well as doctors in particular recommend a strong message to discourage sexual practices if in any case of COVID-19 infection. Hence, comprehensive possible means of SARS-CoV-2 transmission warrants further study.
4. Clinical attributes of COVID-19
A study conducted on 1099 patients (926 non-severe and 173 severe) from 30 provinces of China from December 11, 2019, to January 29, 2020, revealed that dominant clinical manifestations of SARS-CoV-2 infection include fever, cough, fatigue and sputum production. While, symptoms such as shortness of breath, myalgia, sore throat, headache, chills, are common, whereas the gastrointestinal symptoms such as vomiting and diarrhea along with anosmia and hypogeusia are rare in pateints (Guan et al., 2020). Later, meta-analysis of total 43 studies from the period of January 24, 2020, to February 28, 2020, including 3600 patients conducted by Fu and others (2020), ascertains that fever, cough, and fatigue are most frequent, while congestion, sore throat, rhinorrhea, and diarrhea are uncommon clinical manifestations of SARS-CoV-2. Moreover, lymphocytopenia, and increased lactate dehydrogenase, C-reactive protein levels and ground-glass opacities are other common abnormalities among patients. Furthermore, the comprehensive study also suggests this novel SARS-CoV-2 affects elderly people with coexisting other medical complications more seriously. These results are consistent with previous studies conducted on severely ill patients which also advocates high mortality rate in elderly people with acute respiratory distress syndrome (ARDS) and comorbidities such as hypertension, diabetes, chronic obstructive lung disease and coronary heart disease (Yang et al., 2020; Zhou et al., 2020a). The most common complications associated with SARS-CoV-2 infection are sepsis, respiratory and heart failure, ARDS, and septic shock (Zhou et al., 2020a). Even though children of all ages face risk of SARS-CoV infection, the serious progression of infection and morbidity is rare in children and adolescents relative to the adults (Lu et al., 2020a).
5. Perspectives on immune responses and immunopathology
SARS-CoV-2 infection is often categorized into three stages: first, asymptomatic phase; second, non-severe symptomatic phase; and third, severe respiratory symptomatic phase (Shi, 2020). Usually, a small number of patient's progress to the severe stage and develop ARDS and/or multiorgan failure (Cao et al., 2020).
Host's immune responses initiate as soon as SARS-CoV-2 binds to ACE2 receptors and releases viral RNA for replication. Both the innate and adaptive immune response could be triggered in response to the SARS-CoV-2 infection (Cao et al., 2020). However, immune responses are different between severely and moderately infected persons. In a blood sample of symptomatic hospitalised patients with mild to moderate SARS-CoV-2 infection before resolution of symptoms, immunological changes such as increase in the number of activated CD4+ helper T cells and CD8+ killer T cells, follicular helper T (Tfh) cells, antibody-secreting cells (ASCs) and antibodies particularly IgG (Immunoglobulin G)and IgM (Immunoglobulin M) were detected (Thevarajan et al., 2020). On the other hand, in severely infected patients, lymphocytopenia is a common denominator with substantial fall in numbers of natural killer cells, B cells, CD3+ T cells, CD4+ helper T cells, CD8+ killer T cells along with the increase in neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein levels. Additionally, in comparison to the non-severe patients, pro-inflammatory cytokines and chemokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-2, IL-6, IL-7, IL-8, IL-10, Granulocyte-colony stimulating factor (GCSF), monocytechemoattractant protein 1 (MCP1) and macrophage inflammatory protein 1-alpha (MIP1-alpha) are often reported to be elevated in serum levels of critically ill patients (Huang et al., 2020; Qin et al., 2020; Wang et al., 2020a). The elevated ratio of NLR, which is a biomarker of systemic inflammatory response syndrome, points to the devastated inflammatory state of ICU patients (Salciccioli et al., 2015). Moreover, uncontrolled levels of cytokines and chemokines cause over-active inflammatory responses or cytokine storm. This hyperactive immune response along with impaired adaptive immune response may trigger pulmonary injury, ARDS, viral sepsis and organ failure like complications, and eventually death in some cases (Prompetchara et al., 2020).
6. Traditional cure for COVID-19: a possible room for ayurveda
The traditional Chinese medicines and Ayurveda since the Vedic period (1500-500 BCE) provides globally with potential remedies to lessen the severity of the illnesses caused by microorganisms (Chattopadhyay et al., 2015; Jhadav et al., 2012). Ayurveda is considered as the world's oldest medical network, which is believed to manage wide array of infections without causing any side effects. It is well equipped with diverse treatment modules for multifaceted noxious diseases (Goothy et al., 2020). The ayurvedic specialists and health care professionals have been aware of subsistence of wide range of microorganisms as well as infections caused by them (Panda, 2005). The Ayurveda and the Siddha practices originally arose in India and still extensively practiced for curing plethora of infections (Vellingiri et al., 2020). By the identification and isolation of bioactive phytochemicals and compounds, their effectual characterization in the medicinal plants might help in combating such deadly infections. The repurposing the ancient medicinal plants, provides a new approach for defeating the viral infections and their transmission. At present time of global trepidation, it is vital to stumble on solutions for the long sprint and to prevent further transmission of such a pandemic (Balachandar et al., 2020). Currently, there are no vaccinations or any kind antiviral treatment modules developed for the treatment of COVID-19, hence the employment of traditional medicines, which were used in previous epidemic out-break are taken into consideration (Luo et al., 2020). Also the Chinese herbal medicine (CHM) is one of the greatest herbal medicine modules and it is an imperative component of traditional Chinese medicines (TCM). They have been suggested to alleviate contagions in the form of warm water extracts for almost 2000 years from about 10,000 herbal medicines since the ancient times (Lin et al., 2016).
Romanian flora similarly, is anticipated to have about 3500 to 4500 cormophyte (the plants which can be differentiated into root, shoots and leaves) species of which 283 have been considered to be medicinally significant and smaller amount from them have been investigated for their potential to counteract certain kinds of infections (Murariu et al., 2002; Chiru et al., 2020). In the past three decades the use of these traditional medicinal plants in Romania has been scarce (Pieroni et al., 2015; Papp et al., 2011, 2014; Gilca et al., 2018). They are typically registered from small traditional areas, which have preserved discrete features in spite of their common history and living space (Pieroni et al., 2015). The ayurvedic and traditional healing is becoming a substitute to usual conventional medicines due to the fact of easily availability, no side effects and not as much cost. Recent reports on COVID-19 research suggest drug repurposing and employment of TCM as the treatment alternative for COVID-19 out-break, used in the patient of varied countries (Lem et al., 2020). However, there is a lack of direct evidences of role of ayurvedic medicines in curing COVID-19 infections, some of the traditional and herbal medications have proven immunomodulatory potential and can be employed as a preventive medicine to counter the symptoms of COVID-19 (Mamidi and Gupta, 2017; Panda et al., 2020).
6.1. Plant based-therapeutic approaches against COVID-19
The traditional medicines have been in general disregarded in the novel research and expansion of contemporary drugs due to the fact that their translational ability is commonly underrated. These medicines are considered vague in the context of their usage in the non-western medical technologies (Yuan et al., 2016). Wide array of phytochemical components is extracted from a single herb that may function unaided or in amalgamation with other components to yield preferred pharmacological effects (Parasuraman et al., 2014). It has been frequently indicated that 70-80% of the people belonging to the developing countries are directly dependent on the herbal drug for their primary healthcare in comparison to the modern synthetic drugs (Hamilton, 2004). Beneficial impact of the medicinal plants lies in their bioactive constituent's specifically secondary metabolites viz. steroids, alkaloids, diterpenes, triterpenes, aliphatics and glycosides etc. (Chikezie et al., 2015). The exploration for innovative phytochemical with antiviral bioactivity has frequently been substandard and inefficient due to adaptive viral resistance accompanied by viral latency and persistent infections in patients with compromised immunity (Sumithira et al., 2012). Most of the antiviral therapeutics modules are non-specific in their action towards viruses (Jiang et al. 2015). The progression in development of novel antiviral mediators is the foremost concern of the medical research at present. The antiviral bioactivities of plethora of medicinal plants plays a remarkable role in diverse stages of virus growth (Akram et al., 2018).
The traditional Indian medicines network is one of the oldest health modules since the human existence and plays a crucial role in combating and fulfilling the needs of the global healthcare system (Ravishankar and Shukla, 2007). These traditional practices include ayurveda, siddha, yoga and unani, homeopathy and naturopathy and they are lucratively practiced for healing varied infectious disorders (Gomathi et al., 2020). These modules employ plants, animal products and minerals for treatment of wide range of diseases (Tabuti et al., 2003). Approximately, twenty-five thousand plant based formulations and extracts have been used in folk medication in the south Asian subcontinents (Pundarikakshudu and Kanaki, 2019). Moreover, recently total medicinal plants in India were estimated to be 3000, nevertheless traditional ayurveda practioners use around 8000 varied species of plant for the treatments (Pundarikakshudu and Kanaki, 2019).
Recently, in India, it was suggested by the Ministry of AYUSH (Ayurvedic, Yoga and Naturopathy, Unani, Siddha and Homeopathy), to drink Kadha as a booster of immunity and lowering the tenderness caused during COVID-19 catastrophe (AYUSH Advisory, 2020). A Kadha is an extract prepared from less juicy or dry ingredients like spices and herbs. The Ministry of AYUSH with its conventional acquaintance has an extensive custom of maintenance of nation's health and its participation has augmented manifolds in this COVID-19 pandemic crisis (AYUSH Advisory, 2020). All ayurvedic healthcare professional generally recommend classical ayurveda medicine, however AYUSH-64 a novel formulation prepared by CCRAS provides resistance against malaria and other fevers. The decoction of sunthi (Zingiber officinaleRoscoe.), lavanga (Syzygium aromaticum) and maricha (Piper nigrum) have been recommended to the healthy as well as COVID-19 infected person, as it provides support in the humoral and cell mediated responses and also lowers the air way hyper responsiveness and nasal congestions (Carrasco et al., 2009; Kim and Lee, 2009; Bui et al., 2019). Various ayurvedic products and fatty acids in the form of ghee are implicated in the up-regulation of resistance. The resistance is enhanced in a pleiotropic manner and the bioactive compounds participate in various procedures of adaptive as well as innate immune responses (Shukla et al., 2014). Similarly, the bioactive constituent in Curcuma longa Linn. i.e. curumin, is identified to block cytokine release, specifically interleukin-1, interleukin-6, pro-inflammatory cytokines and tumor necrosis factor-α and is directed to be consumed with milk (Omara et al., 2010). Inhibition of the cytokine discharge is one of the prime clinical development associated with experimental modules of flu and other infectious diseases and have also been compared to COVID-19 where similar cytokine storm play an imperative role in transience (Sordillo and Helson, 2015). Moreover, AYUSH has recommended certain preventive and medicinal plants for prevention and prophylactic of COVID-19 including warm extracts of Tinospora cordifolia (advised for chronic fever), Andrograhis paniculata (advised for fever and cold), Cydonia oblonga, Zizyphus jujube and Cordia myxa (enhancing antioxidant, immune-modulatory, anti-allergic, smooth muscle relaxant, anti-influenza activity) and Arsenicum album 30 (found effective against SARS-CoV-2, immune-modulator). The symptomatic management of COVID-19 was suggested to be acquired from Agastya Haritaki (prevention of upper respiratory infections and Anuthaila (sesame oil drops) recommended to prevent respiratory infections (Vellingiri et al., 2020).
TCM clinical trials and studies indicate the amelioration of symptoms of mild to moderate COVID-19 infections. The seriously infected patients tormented from hypoxia, were competent to ease the symptoms including high fever, breathing problems and coughing (Toots et al., 2019). Furthermore, a report by Song et al. (2019) revealed the bioactive potential of extracts Sanctellaria baicalensis containing baicalin which is considered as one of the prime TCM herbal constituent as well as hesperetin a bioactive constituent present in tangerine peel, have been employed in curing the symptoms associated with COVID-19 (Panda et al., 2020). Another TCM medicine viz. Xuebijing Injections has been widely affirmed to lower the hazards of community-transmission of pneumonia as well as it reduces the time required for ventilating a severe patient (Song et al., 2019).
One of the commonly accepted traditional Chinese medicines is Kombucha, which is a concoction prepared from the culture of Acetobacter and yeast. This concoction is prepared in black tea and Chinese herbal extract comprised of liquorice, green tea, chrysanthemum and Grosvenor momordia, which acts as an effective probiotic (Greenwatt et al., 2000). Similar extract has been employed in treatment of foot and mouth disease virus. Moreover, their exist a wide array of reports suggesting utilization of herbal extracts in TCM such as extracts prepared from Azadirachta indica (Kumar and Navaratnam, 2013; Gupta et al., 2017), Camellia sinensis (Song et al., 2005; Kuzuhara et al., 2009; Li et al., 2015), Ocimum sanctum and Agremone mexicana (Varshney et al., 2013), Zingiber officinale (Shah and Krishnamurthy, 2013), Tinospora cordifolia (Shah and Krishnamurthy, 2013), Alium sativum (Kim et al., 2005; Wang et al., 2006) and Ocimum basilicum (Kubiça et al., 2014) etc. for their antimicrobial potential. A corroborative suggestion was made by Su et al. (2020), that traditional Chinese herb i.e. Exocarpium Citri grandis was found to be effective in preventing and treating COVID-19 pandemic. A novel approach to TCM is combination therapy in which the old traditional practices are mixed together to develop an effective formulation for treating various diseases. Combination treatment has been employed in suppression of viral hepatisis. Also liquid fermented broth of Ganoderma lucidum in combination with extract of Radix Sophorae flavescentis, was affirmed to be effective against hepatitis B virus. Another vital example is glycyrrhizin a bioactive compound isolated from Lycoris radiata shows a strong potential against anti-SARS-CoV activity (Li et al., 2005a,b).
6.2. Secondary plant metabolites against COVID-19 virus
6.2.1. Terpenoids
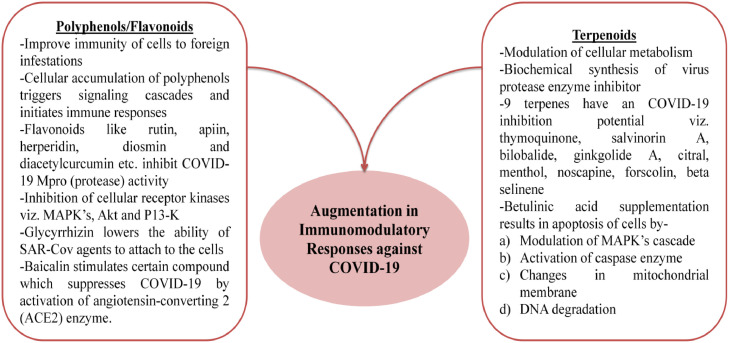
The triterpenes are composed of 6 isoprene units and with squalene as a prototype and are diversely occurrence in plant species (Petr et al., 2006). They play a vital role in modulation of cellular metabolism, specifically the biosynthesis of sterols and have a significant level of antiviral activity (Malinowska et al., 2013). Various triterpenes have been reported to have effect against HSV1 and HSV2 these include dammaradienol, dammarenediol-II, dammarenolic acid, hydroxyhopanone, hydroxydammarenone-I, shoreic acid, ursonic acid, hydroxyoleanonic lactone and eichlerianic acid (Poehland et al., 1987). Structurally coronavirus is composed of single stranded RNA, reported to be one of the longest RNA virus. This RNA strand acts as an RNA messenger, when it transmits into a cell it stimulates the synthesis of two polyproteins which are further composed of a novel replication and transmission complexes that regulate the synthesis of RNA, structural proteins and enhance the activity of protease enzyme. Here protease enzyme has a significant participation in fragmentation of the polyprotein (Cui et al., 2019, John et al., 2015). Apart from designing and chemical synthesizing of protease inhibitor, one of the most recent therapeutic strategies to cure viral infection is to identify the inhibitors of these enzymes in natural products and compounds. Amongst these, terpenoids have a specific significance due to fact of diverse availability in plants and microorganism and low inhibitory concentration i.e. IC50. The terpenoids are the chief secondary metabolites present in more than 36,000 species accounted so far (Augustin et al., 2011). These secondary metabolites have multifaceted therapeutic application viz. anti-cancerous (Topcu et al., 2007), anti-inflammatory and antiviral (Nosrati and Behbahani, 2015), antioxidant (del Cerman et al., 1995) and antibacterial (Angeh et al., 2007).
In a recent report by Shaghaghi (2020) the structure of terpinoid constituents and COVID-19 protease was elucidated from the different databases such as PubChem and Protein Data Bank (PDB). Followed by this, the sophisticated technique of molecular docking was utilized by employing MVD (molegro virtual docker) software. 9 and different terpenes were analyzed for their inhibitory effects. These included thymoquinone extracted from Nigella sativa. Molecular dynamic simulations have also shown that thymoquinone can interact with the attachment of SARS-CoV-2 to the HSPA5 substrate-binding domain b (SBDb) to stress cells and thus reduce the possibility of infection (Elfiky, 2020). Nevertheless, it is potentially time to switch thymoquinone from experiments on the bench to clinical trials for the Covid-19 pandemic (Ahmad et al., 2020a). Salvinorin A derived from Salvia divinorum, Bilobalide and Ginkgolide A extracted from Gingko biloba, citral from Backhousia citriodora, menthol from Mentha, Noscapine extracted from Papaveraceae family, Forscolin from Plectranthus barbatus and Beta Selinene from Apium graveolens. The fallout of this experiment illustrated the strapping interactions of terpenoids in the two enzymatically secluded regions. The fastening of varied amino acids as they were present in the secluded regions of the active site in all the 9 compounds was observed and plays a significant function in enzymatic catalysis. It was further revealed in the study that terpenoids were able to successfully suppress the virus protease enzyme activity. Another report by Chowdhary et al. (2003), suggested antiretroviral and anticancer activities of Betulinic acid a pentacyclic triterpenoid which was extracted from the bark of white Betula alba var. pubescens tree. The betulinic acid was observed to cause apoptosis by modulation of mitogen activated protein kinases (MAPK) cascade and subsequently resulted in activation of caspase enzyme, changes in mitochondrial membrane and DNA fragmentation (Thurnher et al., 2003).
6.2.2. Polyphenols/flavonoids
Amongst many groups of substances polyphenols display a wide range of biological activities. Polyphenols are known to increase the immune to cells to foreign infestations and in response permits cellular accumulation of different types of polyphenols through varied receptors. This subsequently triggers signaling pathways and initiate immune responses (Ding et al., 2018). The natural polyphenols were identified as potent COVID-19 protease (Mpro) inhibitors in an in-silico study conducted by Adem et al. (2020). In this study, efficiency of medicinal plants based on bioactive constituent's viz. flavonoids was analyzed against COVID-19 Mpro and was carried by using molecular docking technique. The COVID-19 virus was docked with 80 different flavonoids and the results indicated that compound such as rutin, apiin, hesperidin, diosmin and diacetylcurcumin etc. had an effective inhibitory effect on protease enzyme and they might have a role in lowering the symptoms of COVID-19 infection.
An imperative class of plant secondary metabolites is flavonoids with antiviral therapeutic potential with more than 5000 different compounds identified and described (Ververidis et al., 2007). These secondary metabolites are considered one of the most profusely present polyphenols in the human diet and are generally found as glycosides and acylglycosides in vegetables and fruits. There are wide array of flavonoids present naturally in plants including quercetin, naringin, hesperetin and catechin and they have been analyzed for their activity against plethora of animal viruses such as HSV-1, respiratory syncytial virus, parainfluenza virus type 3 and polio-virus type 1 (Kaul et al., 1985). The anti-viral activity of flavonoids was reported earlier, against Rous sarcoma, pseudorabies, adenoviruses and Sindbis (Chiang et al., 2003), and also against severe acute respiratory syndrome coronavirus (SARS-CoV) (Yi et al. 2004). The main mechanism by which they suppress the viral infection is by inhibition of cellular receptor kinases including MAPKs, the serine/threonine-specific protein kinase (Akt) and the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3-K) and as consequence interferes with the cellular signal transduction cascades (Villa et al., 2017).
Glycyrrhizin is the chief constituent of Glycyrrhiza glabra root and are rich in flavonoids, β-sitosterol, hydroxyl coumarins and glycyrrhetinic acid. Glycyrrhizin, since the ancient times have been employed in treatment of various ailments including bronchitis, gastritis and jaundice. Furthermore, they have been affirmed to have anti-inflammatory and antioxidant potential that induce the formation of interferons in human body (Ramos-Tovar et al., 2020). It was observed by Pilcher (2003) that Glycyrrhizin lowers the ability of SARS-Cov agents to attach to the cell particularly in the early stages of viral infestation. Similar, observation of imperative anti-SARS-CoV activity as shown by Glycyrrhizin was made by Cinatl et al. (2003). The anti-SARS-CoV activity of Glycyrrhizin was further confirmed by other studies (Yeh et al. 2013). More recently, the in-silico experimentation revealed that Glycyrrhizin has similar behaviour against COVID-19 disease and acts as a potential inhibitor (Mohammadi and Shaghaghi, 2020).
Baicalin, another significant flavones glucuronide extracted from Scutellaria genus has been reported to have antioxidative and anti-apoptotic activity and it has been employed in treatment of diseases like pulmonary atrial hypertension. The anti-SARS-CoV potential of baicalin was initially observed by Chen et al. (2004), who suggested strong inhibitory effect and considerably lower toxic implications on the plant cell lines in-vitro. The in-silico experimentation with baicalin resulted in stimulation of certain compounds that suppressed the COVID-19 infection as a consequence of ACE2 enzyme activation (Liu et al., 2020a). A flavanone glycoside isolated from citrus fruits is Hesperidin, which has been reported to work against COVID-19. Hesperidin rich citrus peel from the waste were used in the study which underwent hydrodynamic cavitation-based speedy expansion and act as antiviral agent against COVID-19 (Meneguzzo et al., 2020). Fig. 2 elucidates prospects of augmentation in immuno-modulatory responses against COVID-19 by polyphneols/flavonoids and terpenoids.
Fig. 2.
Prospects of Augmentation in Immuno-Modulatory Responses against COVID-19 by Polyphenols/Flavonoids and Terpenoids.
6.2.3. Dipeptides
Chymotrypsin a bioactive compound with activity similar to protease is an approved target in novel designing of inhibitors of coronavirus. TCS, India conducted a recent survey on 31 molecules out of a total of 1600000 molecules identified which act as efficient protease inhibitors against COVID-19. One such imperative bioactive dipeptite is Aurantiamide, a plant derivative extracted from Piper aurantiacum, widely distributed in India, Nepal, Thialand, Vietnam and other Asian countries. Aurantiamide acetate is proposed to have diverse bioactivitity and therapeutic potential viz. anti-cancerous and anti-inflammatory (Sengupta, 2019). Co-application of Hydroxychloroquinine and azithromycin has been widely reported to be used as treatment for COVID-19 till present time. Apart from aurantiamide, other potential peptide that has role in combating COVID-19 is peptide EK1 (Lu et al., 2020c).
6.2.4. Sulphated polysaccharides
The polysaccharides are a structurally multifaceted class of biomolecules that have diverse physiochemical characteristics which forms the foundation of its varied application in the field of medicine and pharmaceutical sciences (Olafsdottir and Ingólfsdottir, 2001). The polysaccharides are isolated from plants sources including tree exudates, roots, cell walls and seeds as well as animal sources including chitin, chondroitin sulfate and hyaluronan). In addition to this, several bacterial and fungal sources such as hydrocolloids including xanthan, wellan and gellan are the major sources of natural polysaccharides (Gaikwad et al., 2009). Another significant resource of polysaccharides is algae i.e. mucopolysaccharides and storage polysaccharides have been largely extracted from them. Macroalagae in the form of seed weeds are the major reservoirs of these polysaccharides (Gaikwad et al., 2009).
Hundreds of naturally occurring polysaccharides are presently identified and they bestow richest and most traditional pool of structurally and functionally assorted biopolymers. Apart from its earlier application in the industrial field, recent developments have been made in its employment in the pharmaceutical sector and are considered as pharmacologically active polymers. They have long been recognized for their anti-tumor, anti-coagulant, antiviral and immunomodulatory etc. bioactivities (Martinez et al., 2005). Synthetic and natural polymers of carbohydrate biomolecules have been affirmed to be selective inhibitors or suppressors of enveloped viruses for example HSV, HIV, human cytomegalovirus, respiratory syncytical and influenza virus. Moreover, efforts have been made in advancement in development of polysachharide molecules as specific inhibitors of varied classes of viruses (De Clercq, 1990, 1995). Guduchi or Giloy herb is suggested to contain various diterpene compound and polysaccharides including arabinogalactan polysaccharide (Subhose et al., 2005). These polysaccharides and terpenoids are immunomodulating and adaptogenic in nature. Various studies on Giloy herbal extract revealed that it could cause imperative enhancement in IgG antibodies in the serum and activation of macrophages (Fortunatov, 1952), induction of cell regulated immunity and humoral immunity (Winston and Maimes, 2007). These plants have been widely reported for their potential antiviral activity against H1N1 flu and an immunostimulator.
In, Porphyridium another imperative unicellular microalga, each cell is encapsulated in a sulphated polysaccharides. The capsule outside the cell is dissolved in a growth medium to form exopolysaccharides. These complex sugars are composed of glucuronic acid (10%), galactose (22%), glucose (24%), xylose (38%) and other saccharides such as rhamnose, mannose and arabinose in minute concentration (Geresh et al., 2002). The exopolysaccharides are compatible with the human body and are biodegradable and plays vital role in manufacturing of various medicines, cosmetics and food derivatives. A study carried out by Pujol et al. (2002), revealed identification of various sulfated polysachharides from marine algae and animal resorviors and act as potential inhibitors of human and animal viral infections. A study by Nagle et al. (2020), suggested strong antiviral bioactivity of sulfated polysaccharides isolated from marine red algae. Sulfated polysaccharides are multifarious class of macromolecules and have diverse clinical relevance (Seedevi et al., 2013). They have been found to be broadly distributed in animals, plants and algae found in the saline soil. A report by Karthik et al. (2014), revealed their presence in the marine saline soil is an indication of an adaptation process which might have established during application. The two chief constituents responsible for the antiviral bioactivity of sulfated polysaccharides are chondroitin sulfate and heparin (Ghosh et al., 2009). The exopolysaccharides of Porphyridium have been elucidated to be potent against several virus viz. retrovirus (Talyshinsky et al., 2002), and HSV-1 and HSV-2 (Nagle et al., 2020) etc. Therefore, these biocompatible compounds were suggested to be employed in coating materials of various sanitary items for COVID-19 deterrence.
6.3. Potential role of medicinal plants as antiviral warriors
The naturally occurring products and phytomedicines are coming to the fore all around the world, owing to the orientation of the social fabric to such remedies in different health care centres all across the globe (WHO, 2004). The global outlook for drug formulation is showing a paradigm shift as individuals are facing chronic as well as lifestyle-related disorders, due to which they are turning their stance towards improving life-styles and disease deterrence. However, during ancient period herbal phyto-constituents were the only alternatives for healing the illness as antibiotics were not discovered. Particularly, herbal formulations endow us with toolbox to concoct novel antiviral products. This pragmatically involves the understanding of antiviral mechanisms of natural products and their role in heckling virus life-cycle, replication, assembly and its release. Ever since, the safety and clinical characteristics of traditional medicines have come out, their consumption have been gradually elevating after approval by scientific communities (Mohammadi and Shaghaghi, 2020). Attributable to the side-effects of chemical compounds onto human bodies, it has believed to utilize the herbal therapies with much more effectiveness and nil side-effects. Recently, WHO, has estimated that approximately 80% of the world population has been trusting the herbal therapeutics, yet, a quite a few number of plants have been studied for this noble work (Baell, 2016). Undeniably, traditional drugs are long in the tooth since primordial times and play a significant role to meet global health needs. Usually, Ayurveda, Naturopathy, Siddha, Homopathy, Yoga, Persian medicines, Unani, Romanian, and Chinese conventional medicines have been known since ancient times (Gomathi et al., 2020; Heidary et al., 2020; Yang et al., 2020; Ling, 2020). Usage of these systems in medical field is quite lucrative to suppress viral infections in respiratory systems and modulating inflammatory reactions within immune systems. As depicted earlier, AYUSH has initiated a holistic perspective of medicine to cure presently prevailing anti-viral infections via self remedial processes (AYUSH, 2020).
A wide spectrum of studies is being conducted to design a formulation against coronavirus with the aid of medicinal plants. The pages of history stand testimony to the fact that diversity of medicinal plants such as Indigofera tinctoria, Evolvulus alsinoides, Vitex trifolia, Pergularia daemi, Gymnema sylvestre, Clerodendrumineme, Abutilon indicum, Clitoria tematea, Leucas Aspera, Sphaeranthus indicus, Allium sativum, Cassia alata showed an anti-mice coronavirus activity (SARS-CoV) (Vimalanathan et al., 2009). Amid them, V. trifolia and S. indicus plummeted inflammatory cytokines via NF-κB pathway, insinuated for respiratory discomfort in SARS-CoV (Srivastava et al., 2015). In addition, C. tematea was recognised as MT (Metalloprotease) inhibitor, ADAM17, which is linked with shredding ACE-2 enzyme, often associated with virus replication (Maity et al., 2012). Further, Glycyrrhiza glabra and A. sativum have also been found to impede viral replication against SARS-CoV, while C. ineme was potentially observed to deactivate viral ribosome machinery, revealing its utility against testing SARS-CoV-2-protein translation and protein synthesis (Nourazarian et al., 2016; Keyaerts et al., 2007). Likewise, Strobilanthes cusia also blocked the RNA synthesis of viral species and mediated papain like proteases activities in order to target HCoV (Tsai et al., 2020). In Asia, mountainous zones are lavishly flourished with plants with medicinal value against various respiratory disorders (Amber et al., 2017). For instance, Hyoscyamus niger, Verbascum thapsus, Cynara scolymus, Justicia adhatoda were found to attack the molecules bulging among SARS-CoV-2 and influenza virus and found to inhinit Ca2+ channels (Gilani et al., 2008). Consequently, it could earmark orf3 (Ca2+ channel), stimulating other downstream pathways against viral attack. Notably, various herbal plants showed restraining affects against ACE, for example, Coriandrum sativum, Punica granatum, Boerhaavia diffusa, Cassia occidentalis, Coscinium fenestratum, Embeliaribes etc. (Hussain et al., 2018; Khan and Kumar, 2019). Research is currently being focussed on these plants to scrutinize their effects on COVID-19. Another tropical species Andrographis paniculatain South Asia depicted sturdy competence against viral infections (Yarnell, 2018). More recently, Liu et al. (2020a), found that A. paniculata repressed NOD-like receptor Proteins (NLRP3), IL-1β, C-1 (Caspase-1) molecules, comprehensively associated with SARS-CoV and SARS-CoV-2 pathogenesis. Also, Salacia oblonga reflected inhibitory effects on angiotensin II having direct link with bronchitis and lung damage (He et al., 2011). Strikingly, many herbal plants used to treat HIV proteases can also be a suitable candidate for COVID-19. To expedite, Acacia nilotica, Ocimum sanctum, Eugenia jambolana, Vitex negundo, Euphorbia granulate, Ocimum kikim, Ocimum scharicum, Solanum nigrum etc. were effective against HIV-reverse transcriptase activityand can be potent for SARS-CoV-2 too (Mishra et al., 2014; Rege and Chowdhary, 2014; NAIR, 2012; Thayil Seema and Thyagarajan, 2016). Further, Sambucus ebulus also attack the enveloped viruses, thereby, should be tested to study its target action against this virus (Ganjhu et al., 2015).
Adding to the above mentioned reports, Cheng et al. (2006) investigated the role of triterpene glycosides screened from Heteromorpha, Bupleurum and Scrophularia scordonia against coronavirus 229E by averting the attachment and invasion into the host. Moreover, Artemisia annua, Lindera aggregata, Lycoris radiate, Isatis indigoticahave been known to exhibit anti-SARS-CoV effects (Li et al., 2005a,b). Similarly, naturally occurring repressors have been recognised such as scutellarein, phenolics, myricetin etc. from Isatis indigotica and Torreya nucifera respectively against SARS-CoV enzymes. Another study was reported in Houttuynia cordata against SARS-CoV through inhibition of 3CL-proteases and RNA-polymerases (Yu et al., 2012). Further, Nigella sativa have also been demonstrated to possess multifaceted properties due to the presence of thymoquinone against H9N2 and cytomegaloviral diseases (Tavakkoli et al., 2017). More recently, the role of Piper Betel in combating COVID-19 infections have also been explored (Sengupta, 2019). According to their study, 'Aurantiamide', an active metabolite from Piper aurantiacum has chymotrypsin like-proteases like activities that is a ratified agent for inhibiting coronavirus. In addition to this, it also encompassespiperol, eugenol, catechol, caryophyllene, etragol, chavibetol, betlol, quercetin etc. that also grows body's self-defense mechanism either through reception of viruses or in waning off the viral load within the infected hosts (Sengupta, 2019).Alongside, studies have been depicted the role of herbal drug Gene-Eden-VIR/Novirin against many noxious viruses and this drug is mainly comprised of quercetin, green tea, cinnamon, licorice and selenium (Polansky and Lori, 2020). They found that it disrupts the viral entry, infection, replication, viral proteases, viral quasi-speciesand triggers the immunity, thereby, can be evidently utilised against SARS-CoV-2 respectively (Polansky and Lori, 2020).
Interestingly, the role of tulsi for scientific evidence against COVID-19 has also been elucidated (Goothy et al., 2020). As, it is well known herbal plant for antiviral effects in inhibiting many deadly viruses like vaccinia, dengue, hepatitis, encephalitis etc. by enhancing their survival and defense ability. Moreover, it also reinstate the physiological functions of body through its phenolic and antioxidative property that in turns shields the body from toxic substances (Shivananjappa and Joshi, 2012). The most possible mechanism underlying its immunity boosters lies in triggering humoral and cellular immunity responses (Vaghasiya et al., 2010). Apart from this, modulatory actions of GABA pathway also encompass their multi-modal therapeutic properties, therefore, we can conjecture that it could be efficient in cure of COVID-19. Nevertheless, the role of Persian herbs have also been untangled against COVID-19 via inhibitory action against ACE2 enzyme that enables viral entry into the host cells. Various species like, Allium sativum, Cerasus avium, Berberis integerrima, Alcea digitata, Rubia tinctorum, Peganum harmala etc. were illuminated as ACE2 inhibitors, therefore, could be considered for COVID-19 prevention after proper evaluation (Hiedary et al., 2020).Henceforth, herbal plants could be possibly used to mitigate the emergence of COVID-19 (Table 1 ). Despite the fact that, a lot many medicinal plants have been propagated for this purpose, a lot more research is being carried to formulate the drug specifically for this.
Table 1.
Herbal Formulations as Possible Therapeutics against COVID-19 Infection.
| S.No | Herbal Plant Species | Active Compounds | Mechanism of Action | Therapeutic Property | References |
|---|---|---|---|---|---|
| 1. | Cannabis sativa | Cannabinoid cannabidiol | Anti-inflammatory action by via modulation of gene expression of ACE2 enzyme, serine protease TMPRSS2, protein pre-requisite for SARS-CoV2 invasion into host cells. | Adjunct therapy and utilised as mouthwash and throat gargle products clinically and home use owing to their potential to decrease viral entry via the oral mucosa. | (Wang et al., 2020b) |
| 2. | Glycyrrhiza glabra | Glycyrrhizin, glycyrrhetic acid, liquiritin and isoliquiritin | Counterbalance the activeness of COVID-19 and could be used as an antiviral drug. | Formation of antiviral nano-membrane by licorice processed with PVA solution for potential application as wound dressing materials, musk, gloves and against skin infection by electrospinning. | (Chowdhury et al., 2020) |
| 3. | Citrus sp. | Essential oils, pectins, naringin and hesperidin (flavonoids). | Binds with high affinity to cellular receptors of SARS-CoV-2 that restrain the pro-inflammatory overreaction of the immune system. | Prophylaxis and treatment of COVID-19. | (Meneguzzo et al., 2020) |
| 4. | Porphyridium sp. | Sulfated polysaccharides (carrageenan) | Potent inhibitors of coronaviruses that inhibit the binding or internalization of virus into the host cells. | Biocompatible compounds can be used as a coating material on the sanitary items for COVID-19 prevention. | (Nagle et al., 2020) |
| 5. | Citrus sp. | Hispidin, lepidine E,and folic acid | Inhibition of 3CL hydrolase enzyme known for counteracting the host innate immune response and explain the main interactions in inhibitor-enzyme complex. | Promising therapeutic principle for developing drug candidates as anti-CoViD-19 drug. | (Serseg et al., 2020) |
| 6. | Nilavembu Kudineer | Benzene 123 Triol | Immuno-modulatory activity against ACE2 enzyme receptor, that routes virus entry in the pathogenesis of Novel coronavirus. | Potent anti-viral capacity for drug development. | (Walter et al., 2020) |
| 7. | Curcuma longa, Anogeissus acuminata, and Phyllanthus myrtifolius | Curcumin, Anolignan A, Phyllamyricin B | May act as molecular blockers for the virus or cell surface receptors. | Can be useful alternative potential drug. | (Alabboud and Javadmanesh, 2020) |
| 8. | Nigella sativa | Nigelledine, α- Hederin | Inhibitory action of proteases; CoVs (3CLpro/Mpro) (PDB ID 6LU7 and 2GTB) active sites. | Best potential to act in COVID-19 treatment, testified medicinal use for preventive purpose. | (Bouchentouf and Missoum, 2020) |
| 9. | Camellia sinensis | Polyphenols (Sanguiin, Theaflavin gallate, Theaflavin digallate, Kaempferol, Punicalagin and Protocatechuic acid) | Target COVID-19 main protease (Mpro), key enzyme of coronavirus involved in virus replication and transcription, and impedes viral growth inside the host. | Dietary intake of black tea aids resistance to fight against COVID-19 virus in early stages of human infection. | (Giri et al., 2020) |
| 10. | Zingiber officinale | 6-gingerol | Higher binding affinity at active sites of R7Y COVID-19, main protease essential for replication and reproduction of SARS Cov-2 | Possesses excellent drug likeliness parameters with zero violations. | (Rathinavel et al., 2020) |
| 11. | Citrus sp. | Naringin, Naringenin, Hesperetin and Hesperidin | Inhibited expression of pro-inflammatory cytokines (COX-2, iNOS, IL-1β and IL-6) in macrophage cell line, restrained cytokines via inhibiting HMGB1 expression in a mouse model and impeded binding affinity of ACE 2, receptor of the coronavirus. | Contemplation of potential anti-coronavirus and anti-inflammatory activity of flavonoids, derived phytochemicals are promising in the use of prevention and treatment of 2019-nCoV infection. | (Cheng et al., 2020) |
| 12. | Lawsonia inermis | Fraxetin 1(3H)-isobenzofuranone | Phytochemical, cytotoxicity and anti-inflammatory actions confirmed in fractions of extract as observed as a potent constituents. | Cytotoxic compounds, warrant research to fabricate suitable formulations comprising these constituents. | (Manuja et al., 2020) |
| 13. | Allium sativum, Curcuma longa, Capsicum, Mentha pulegium, Stachysschtschegleevi, Astraglusgossypinus | Diallyl Disulfide, Curcumin, Capsaicin, Limonene, Thymol, Verbascoside, Glucouronic acid | Restrained viral protease enzyme by inhibiting amino acid synthesis. | Compounds investigated especially, Curcumin could be strongest achiever against COVID-19. | (Mohammadi and Shaghaghi, 2020) |
| 14. | Ocimum sanctum, Curcuma longa, Tinospora cordiofolia, Piper nigrum.Zingiber officinale, Syzygium aromaticum, Elettaria cardamomum, Citrus Limon, Withania somnifera | Oleanolic acid, Ursolic acid, Curcumin, Rosmarinic acid, Eugenol, Magnoflorine, Berberine, Piperine, Piperamide, Piperamine 6-gingerol, Quercetin, Hesperetin, Protocatechualdehyde, Caryophyllene,Withaferin | Higher binding affinity with viral and host macromolecular targets and other human pro-inflammatory mediators, SARS-CoV-2 main proteases, spike, human ACE2 and furin proteins. | Regularly consumped in the form of ayurvedic Kadha to boost immunity and dwindle chances of COVID-19 Infection. | (Maurya and Sharma, 2020) |
| 15. | Cnidoscolus aconitifolius | Phenols, Flavonoids, Flavonones and Hydroflavonoles | Highest ACE2 enzyme inhibition, anti-inflammatory activity, modulated α-gene expression for TNF- production in macrophages. | Bioactive compounds could be used for drug formulations. | (Medina and Segura-Campos, 2020) |
| 16. | Scutellaria baicalensis | Baicalein | Anti-SARS-CoV-2 activity via suppressing SARS-CoV-2 3CLpro and replication. | Effective compounds as anti-SARS-CoV-2 inhibitors. | (Liu et al., 2020) |
| 17. | Ginkgo biloba | Ginkgolic acids | Impeded DNA and protein synthesis by binding towards host cell receptors to activate cell-signaling pathways for arresting cell cycle as an inhibitory action. | Sturdy effect of GA on viral infection, to be potentially used to treat coronavirus infections. | (Borenstein et al., 2020) |
| 18. | Allium sativum | Essential oil, Allyl disulfide, Allyl trisulfide | Acted as ACE2 receptor inhibitor for resistance against Coronavirus along with activity against main proteases of SARS-CoV-2. | Essential oil as valuable natural antivirus source, contributing towards preventing the invasion of coronavirus into the human body. | (Thuy et al., 2020) |
| 19. | Curcuma sp., Citrus sp., Alpinia galanga and Caesalpinia sappan | Curcumin, Hesperidin, Galangin and Brazilin | Anti-SARS-CoV-2 through its binding to 3-protein receptors with good affinity performing an inhibitory potential against viral infection and replication. | Consumed in daily life as prophylaxis of COVID-19. | (Utomo and Meiyanto 2020) |
| 20. | Alpinia officinarum, Zingiber officinale | Curcumin and Gingerol | SARS-CoV-2 papain-like protease (PLpro) inhibitors. | Potent drugs to treat corona infections. | (Goswami et al., 2020) |
| 21. | Myrica cerifera, Psorothamnus Arborescens, Phaseolus Vulgaris, Camellia sinensis, Hyptis atrorubens Poit, Amaranthus tricolor, Glycyrrhiza uralensis | Myricitrin, Methyl Rosmarinate, 5,7,3′,4′-Tetrahydroxy-2′-(3,3- dimethylallyl) isoflavone, 3,5,7,3′,4′,5′-hexahydroxy flavanone-3-Obeta-D-glucopyranoside, Myricetin 3 Obeta-D-glucopyranoside, Amaranthin, Licoleafol | Inhibited SARS-CoV-2 3CL pro-activity and virus replication. | Probable inhibitors into clinical drugs for exploring and developing novel natural anti-COVID-19 therapeutic agents in the future. | (ul Qamar et al., 2020) |
| 22. | Nyctanthes arbortristis, Glycyrrhiza glabra, Aloe vera Curcuma longa, Azadirachta indica, Withania sominifera, Cannabis sativa, Ocimum sanctum, Allium cepa,Zingiber officinale | Nictoflorin Astragalin, Lupeol Berberine,Sitosterol, Aloenin, Aloesin, Curcumin, Nimbin, Withanolide, Withaferin A, Shogaol, Quercetin, Ursolic acid, Apigenin, Cannabidiol,Piperine, Gingerol | Potential inhibitors of COVID-19 proteases. | Promising development of herbal medicines. | (Srivastava et al., 2020) |
| 23. | Curcuma longa, Zingiber officinale | Curcumin, Nelfinavir, Lopinavir, Luteolin-7-glucoside, Demethoxycurcumin, Apigenin-7-glucoside, Oleuropein, Catechin, and epicatechin-gallate | Best potential to act as COVID-19 Mpro inhibitors. | Treatment and prophylaxis | (Khaerunnisa et al., 2020) |
| 24. | Ginkgo biloba | Ginkgolide A, Terpenoids | Stronger bond and high affinity with proteases. | Compounds may be considered as effective COVID_19 antiproteases drugs. | (Shaghaghi, 2020) |
| 25. | Camellia sinensis | Epigallocatechin gallate | Targets include main proteases covid-19, post fusion core of 2019-nCoV S2 subunit, prefusion spike glycoproteins and NSP15 endoribonuclease from SARS CoV-2. | Future drug candidate for COVID-19. | (Khan et al., 2020) |
| 26. | Silybum marianum, Withania somnifera, Tinospora cordiofolia and Aloe barbadensis | Silybin, Withaferin A | SARSCoV-2 inhibitors namely, Spike (S) glycoproteins, main protease (Mpro) and RNA-dependent RNA-polymerases (RdRp) | Could be useful traditional medicine for treatment for COVID-19 infection. | (Pandit, 2020) |
| 27. | Citrus, Curcuma longa | Hesperidin, Rutin, Diosmin, Apiin, Diacetyl curcumin | Inhibitory action against SARS-CoV-2 main protease (Mpro). | Medicinal potential to cure COVID-19. | (Adem et al., 2020) |
| 28. | Exocarpium Citri grandis | Flavonoids and Naringin | Antitussive, expectorant, improved lung function, pulmonary fibrosis, and antiviral immune response. | Reference for its clinical application in the prevention and treatment of multiple respiratory diseases, including coronavirus disease. | (Su et al., 2020) |
| 29. | Eucalyptus sp. | Jensenone | COVID-19 Mpro inhibitor | Eucalyptus oil could be use for prevention and cure. | (Sharma and Kaur, 2020) |
| 30. | Betula pubescens | Herbacetin, Isobavachalcone, Quercetin, 3‐β‐d‐glucoside, Helichrysetin and Betulinic acid | Inhibitory compounds against MERS‐CoV 3C‐like proteases (3CLpro). | Flavonoids with these characteristics can be used as templates to develop potent MERS‐CoV 3CLpro inhibitors. | (Jo et al., 2019) |
| 31. | Zingiber Officinale, Piper longum, Syzygium aromaticum, Tragia involucrata, Hygrophila auriculata, Terminalia chebula, Justicia adhatoda,,Saussurea costus,Tinospora sinensis and Premna herbace a | 6-Shogaol, 6-Gingerol, Beta Sitosterol, Piperidine, Apigenin, Piperine, Quercetin, Chlorogenic Acid, Andrographolide, Bharangin, Carvacrol, Cissamine,Costunolide, Cucurbitacin B, Gallic acid, Linoleic acid, Pellitorine, Rutin, Santalic acid, Cynaropicrin, Eugenol, Thymol and Vitexin) | Binding potential with active residues of ACE2 that mediate host viral interface. | Future systematic investigation could validate the efficacy prior to the recommendation. | (Dhanasekaran and Pradeep, 2020) |
7. Ancillary armours to combat COVID-19 viral infection
Coronavirus COVID-19 pandemic is a huge catastrophe that has caused devastating effects on global populations. It has wreaked havoc on populace of the planet and killed enormous human beings worldwide, yet still under ruination. Consequently, this pandemic has led to complete lockdown of different countries around the globe, yet this situation is still prevailing on earth. Since decades, vaccination have been the only means to treat the viral infections. Though, the vaccination for COVID-19 has not been developed yet, owing to which the scenario is worsening. Understanding the gravity of the situation and health crises, it is the responsibility of all the scientific community to look for the alternatives or techniques to develop viral vaccination against COVID-19 infection. The mounting substantiation reveals that having healthy lifestyle, natural food products can boost the immune functions of the body to combat the severity of viral infections. While, improving the immune responses they also provide resistance against pathogenic organisms (Sarfraz et al., 2020).
In the view an argument, early adaptive defense responses might draw a parallel link with superior clinical results, it is pertinent to accentuate that a strong immune system play a pivotal role in the prevention and cure of COVID-19 ailment. The facets for a healthy immune system is to have balanced diet, to be physically fit and ingest vital nutrients, polyphenols and natural antiviral compounds and protect nasal and oropharyngeal mucosal layers along with halting smoking habits (Grant et al., 2020). Considering the ebbs and flow of the current situation, we can robust multi-factorial defense responses against newly surfaced coronavirus.
7.1. Immune system rebooting
The upliftment of immune responses of body is the cutting edge in thwarting the viruses to stay healthy. For this purpose, it is rudimentary to amend the current lifestyle by adding smarter works in 'to do's' list so as to make inner immune/defense competitive against viruses such as COVID-19. This would naturally heal the antiviral effects within the body by dissolution of the avidity of the disease and infection. Majority of these attributes are linked to reboot the functions associated with improved immunity via mediating anti-inflammatory activities, enhancing cell-mediated immune functions, modulated APC (Antigen Presenting Cell) activities and suppressing pro-inflammatory mediators. In addition, they also mediate effective cell-cell communication during innate as well as adaptive immune responses (Sarfraz et al., 2020). Hence, a scientific expedition of escalating immune system through appropriate sleep, judicious exercise, stress-free environment, proper nutritive foods, water intake and consumption of fresh and healthy fruits and vegetables would anticipate the citizenry to cope with coronavirus battle via naturally vaccinating their systems (Jayawardena et al., 2020).
The scientific picturespeculates that different stressors, sleep dispossession and sparkling beverages subdue the immune systemby the release of ACTH (Adrenocorticotrophic Hormones) namely, adrenaline, non-adrenaline and glucocorticoids. This in turn makes a person susceptible to pathogenic infections by weakening the immune system of the body. For instance, immune system mediators like NK (Natural Killer) cells, Helper-T-cells, lymphocytes, antibodies, interleukins (IL4, 5, 10, 13), cytokines are impaired that leads to increased chances of infection along with the reactivation of the latent viruses (Calder et al., 2020). In addition, scarcity of sleep may also hinder the immune pathways within the individual through ACTH-curbed persistent activation of NF-κB that elevates the threat of infections due to plummeted microbial gene expression levels. Meanwhile, the proper sleep balances the hormonal responses within the body to ensure the reorganisation of T-cells towards lymph nodes to boost their immunogenic memory response (Calder et al., 2020). Moreover, consumption of fizzy drinks or alcohol based beverages causes high glucose or CO2 within blood that in turn causes hypercapnia so as to inhibit macrophage activity and pathogen clearance. It also hampers the immuno-modulators, thereby loss of control on invading pathogens. Whilst, proper sleep, active daily routine, exercise etc. augments the immune-surveillance in the form of NK cells, monocytes, neutrophils, T-cells, Immunoglobulin's etc. to counteract adverse health components (Nieman and Wentz, 2019). Apart from this, muti-vitamins intake within human body can wash away the sickness forces through recruiting the immune soldiers (Combs and McClung, 2016). Diet comprising of multi-vitamins wield immune-modulatory possessions on numerous immune cells such as monocytes, neutrophils, lymphocytes, NK cells, dendritic cells that bump up the immunity against pathogens (Lewis et al., 2019; Petric, 2020). Perhaps, these immune cells would trigger the anti-pathogenic responses against coronaviruses via exasperating the levels of Cathelicidin, Defensin β, antimicrobial peptides, phagocytosis, neutrophil migration and chemotaxis, oxidant generation, NK-activation (CD69, CD25), IF-γ, antibodies synthesis, cytokine synthesis and T-cell proliferation respectively.
Inevitably, assorted dietary minerals that are essential immunity mediators should be incorporated into the diet, for example, different fruits, spices and vegetables are abundant with immuno-stimulators that in turn fortifies the innate as well as adaptive immune responses against viral elements (Sarfraz et al., 2020). To elucidate, these foods contain allicin, cucumins, papain, ginsenoside, mangoosteen, chloroquine etc. that has a direct effect on dendritic cells, NK cells, lymphocytes, antibodies in imparting shield against foreign particles invading human body. Taking into account these factors, a healthy balanced diet is quintessential for proper immuno-functioning (Martineau et al., 2017; Petric, 2020). As the diet is a summation of trace elements, mineral supplements, vitamins (A, D, C, E, K), zinc etc, it can fulfil all the repercussions such as hypovitaminosis caused due to viral attack respectively (Gunville et al., 2013; Petric, 2020). Additionally, regular physical activities is adjuvant for boosting immunity and metabolic health specifically, if it's sunny, as ultra-violet and infra-red rays are natural virucidal elements (Lytle and Sagripanti, 2005; Martin et al., 2009). However, the biological activities can also be improved by ingesting high levels of naturally existing polyphenols that not only elicit immunity against viruses but they are also comprised of receptors that identifies and permit their cellular uptake for activating signaling cascade underlying innate immunity (Nieman and Wentz, 2019). To exemplify, curcumin and epigallocatechin gallate causes epigenetic alterations within cells, curb gasto-intestinal immunity and allergic responses (Ding et al., 2018). These compounds predominantly include flavonoids, phenols, stilbenoids that are abundantly present in plants in the form of aglycones or glucose esters (Ma et al., 2014). An in vitro study reported by Lin et al. (2017), depicted the role of resveratrol from grape seeds against coronaviruses. Likewise, other antiviral components like glycyrrhizin from liquorice have been known to show some activity against coronaviruses and SARS, revealing the significance of glycyrrhizin to be used as trial drug in COVID-19 affected patients, in order to develop it as a potent cure drug (Chen et al., 2004; Cinatl et al., 2003; Brush et al., 2006). Nevertheless, other plants like oregano, garlic, ginger, lemon, broccoli, mint, tulsi, fennel, thyme, cinnamon, star anise etc. should be tested in the different forms so as to form an effective drug to fight against COVID-19 (Yasmin et al., 2020). Above all, smoking has a direct impact on immune responses through escalating pathogen immunity and dwindling the defense responses within hosts. Moreover, T-helper cells, CD4, CD25, CD8+ T-cells, memory cells, B and T cells, NK cells, macrophages etc. are also hindered by smoke.Therefore, these steps can reboot the immune system and reinforce the inner forces to battle against severe viral infections COVID-19 respectively.
7.2. Nutrient supplements
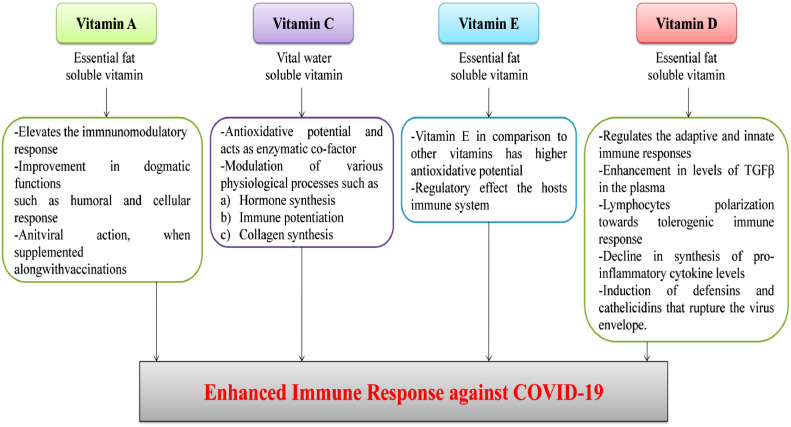
The most momentous weapon against any kind of viral infection is a strong immune system. There are plethora of studies done in past that suggest an imperative role of trace elements and vitamins in normal performance of the immune system (Wintergerst et al., 2007). Furthermore there are reports of elevated resistant to viral infection in response to their supplementation. Vitamin D and A ingestion increased the humoral immunity in association with influenza vaccine in paediatric patients (Patel et al., 2019). Exogenous supplementation of Se has been shown to affirmatively combat influenza infection in alliance with the vaccination (Ivory et al., 2017). Apart from this, wide range of herbals, nutraceuticals and probiotics have been found to be effective against viral infestations and stimulation of immune responses (Kang et al., 2013; Mousa, 2017).
7.2.1. Vitamins
Vitamin A is an essential fat-soluble vitamin which has a major involvement in regulating vision, growth and maturity as well as protection of the mucosal and epithelium integrity of the human body (Huang et al., 2018). It has a crucial participation in elevating the immunomodulatory responses and dogmatic functions in humoral as well as cellular responses. The Vitamin A supplements given to the infants with measles and rabies showed impending improvement (2.1 times) in antibiotic response in association with the vaccinations (Siddiqui et al., 2001). Vitamin D another fat-soluble vitamin and has a vital role in regulating the adaptive and innate immune responses (Rosen et al., 2016). More recently, a study carried out by Aglipay et al. (2017), revealed that supplementation with high dose of vitamin D (2000 IU/day) in contrast to a standard dose (400 IU/day) had no significant effect on the viral infection in the upper respiratory track, however the one third of the population was found to have vitamin D levels less than 30 ng/ml. Similarly, another study suggests vitamin D supplementation with influenza vaccine resulted in enhancement in the levels of TGF-β in the plasma which further indicated that vitamin D directs the lymphocytes polarization towards the tolerogenic immune response (Goncalves-Mendes et al., 2019). Vitamin D has also been affirmed to demonstrate beneficial effect against other viruses including HCV genotype infection (Abu-Mouch et al., 2011) and HCV genotype 2-3 (Nimer and Mouch, 2012). Another report which suggests antiviral capability of Vitamin D was elucidated by Grant et al. (2020). It was further affirmed that Vitamin D was potent in reducing the hazards associated with viral pandemics. The high dose of Vitamin D reduced the hazard of several chronic ailments such as cardiovascular diseases, diabetes mellitus, hypertension, cancers and respiratory tract infection. Grant and co-workers, additional revealed that vitamin D lowers the risk to respiratory tract infection by sustaining tight junction, decline in the synthesis of pro-inflammatory cytokines and killing the envelope of virus via induction of defensins and cathelicidin. Consequently, the risk of cytokine storm is lowered which prevents pneumonia.
Vitamin E is also a fat soluble vital and has an antioxidative potential and regulates the host's immune responses (I Eze et al., 2016). The positive effect of vitamin E against chronic hepatitis was reported by Andreone et al. (2001). They suggested appreciable elevation in normalization of liver enzyme activity and HBV-DNA negativitization. Another vitamin that acts as an important antioxidant and enzymatic co-factor is Vitamin C. It acts as a co-factor in varied physiological processes such as hormone synthesis, immune potentiation and collagen synthesis (Kim et al., 2013). The meta-analysis role of vitamin C in treatment and preventions of common cold was carried out by Hemilä and Chalker (2013). It was concluded in the view of evidences that vitamin C mega dose showed reduction in the frequency of common cold in the community. A study conducted by Utomo and Meiyanto (2020) was aimed at elucidating the potential of Curcuma sp., Citrus sp., Alpinia galanga, and Caesalpinia sappan as an anti SARS-CoV-2 agent via its binding to 3 protein receptors. The protein targets identified were Receptor Binding Domain of spike glycol proteins (RBDS), protease domain of Angiotensin Converting Enzyme-2 PD-ACE2 and SARS-CoV-2 protease by employing molecular docking technique. The result of the study revealed that Citrus sp. had the highest inhibitory potential against SARS-CoV-2, followed by galangal, sappan wood and curcuma species and it was further suggested that they might have antiviral potency against COVID-19. Additionally, different plant based sources of vitamins include, mushrooms, carrots, broccoli, almonds, citrus, guava, amla, avocados etc. Fig. 3 describes the role of Vitamins (A, C, E and D) in combating COVID-19.
Fig. 3.
Role of Vitamins (A, C, E and D) in combating COVID-19.
7.2.2. Minerals
Zinc is one of the indispensible elements which has significant role in modulation of growth and development and the regulation of the immunomodulatory responses (Lindenmayer et al., 2014; Read et al., 2019). Deficiency in the Zn, results in enhanced vulnerability to microbial infections specifically viral. Large number of studies reveal that zinc levels in an individual is a vital factor that regulates the immunity against any kind of viral infection and the Zn-deficient population is found to be more vulnerable to acquiring infections such as HIV and HCV (Read et al., 2019). A study by Acevedo-Murillo et al. (2019), in which 103 children with pneumonia symptoms were observed to show clinical improvement in terms of oxygen saturation, duration of illness and rate of respiration in the Zn supplemented children in comparison to control. They further revealed elevation in the cytokine response in the zinc group.
Another crucial trace element Copper has also been reported to have a key role in growth and differentiation of immune cells (Li et al., 2019). The intracellular concentration of copper modulates the influenza virus life cycle and a thujaplicin-copper chelate suppresses the multiplication of human influenza virus (Rupp et al., 2017; Miyamoto et al., 1998). Another observation made by Turnlund et al. (2004) was that there was an elevation in activity of SOD, plasma ceruloplasmin ad benzykamine oxidase activity in response to higher doses of copper i.e. 7.8 mg/day in comparison to 1.6 mg/day. It was further elucidated that there was a considerable reduction in circulation of neutrophils, antibiotic titers and serum IL-2R against the Beijing strain influenza infestation. Magnesium also has an imperative participation in scheming immune functions in infected cell via affecting immunoglobin biosynthesis, cytolysis dependent upon the antibodies, macrophage response to lymphokines, enhanced adherence of the cells and T and helper cells-B adherence (Liang et al., 2012). There are a few reports which suggest participation of Mg in immunomodulatory responses against viral infestations (Chaigne-Delalande et al., 2013).
In addition to this, another trace element that has been reported to have wide range of pleiotropic effects ranges from anti-inflammatory to antioxidative is Selenium (Se) (Rayman, 2012). Comparatively, the lower concentrations of Se are related to enhancement in the jeopardy of mortality, meager immune responses and cognitive reduction, whereas the higher doses are affirmed to show antiviral activity (Rayman, 2012). Supplementation of Se results in elevation in concentration of Se in the plasma, up-regulation of lymphocyte phospholipid levels and activity of GPOX in the cell, subsequently leading elevation in cellular immune response. The humoral response was shown to be un-affected (Broome et al., 2004). Similar reports of hasty clearance of poliovirus were also reported by Se supplementation (Ivory et al., 2017). Various plants namely, beans, nuts, peanuts, grains, mushrooms, cereals, lentils, garlic, grains, etc. have been wide sources of minerals and must be included in diet.
7.2.3. Nutraceuticals supplements and probiotics
Nutraceuticals are the products which are argued to provide physiological assistance and protection against varied persistent diseases. Wide range of nutraceutical products have been isolated herbal products, dietary supplements, isolated nutrients, genetically engineered foods and processed cereals, beverages and soups (Kalra, 2003). Certain nutraceuticals have been shown earlier to enhance the immune function. McCarty and DiNicolantonio (2020) elucidated that, a few nutraceuticals were able to lower the degree of symptoms and provide respite to the patients infected from coronavirus and influenza. Another significant product is probiotic, they are defined as live micro-organisms which bestow varied health benefits such as improvement in gastrointestinal activity (Sanders, 2008). They also induce certain specific immune responses by augmenting antibody synthesis (Kanauchi et al., 2018). A report by Bunout et al. (2002) suggested the role of probiotics in the improvement in immune response to vaccination in the elderly people. Antibody production against influenza B was elevated significantly against influenza and there was no effect of probiotics on IL-4 and INF secretion by cultured monocytes. Moreover, an observation made by Akatsu et al. (2013) revealed positive effects of supplementation with Bifidobacterium longum on the immunomodulatory responses and intestinal microbiota in elderly people. A significant augmentation in the number of bifidobacteria was recorded in the faecal matter. Furthermore, it was suggested that there was an increase in serum IgA levels. An investigation was carried out to elucidate whether consumption of probiotics such as lactobacilli has an effect on acquired cold symptoms. Reduction in pharyngeal symptoms was significant was observed.
8. Impact of PAK-1 blockers: budding therapeutics against coronavirus
PAKs belong to mammalian kinases family also known as RAC/CDC42-activated kinases that have been came into existence since decades. Amid, these classes PAK1 is most prominent category of "pathogenic kinases" that can cause a plethora of diseases like cancer, inflammation, malaria, dengue, immuno-suppression and many antiviral diseases, if behave abnormally (Maruta, 2020). Strikingly, PAK1-blockers (naturally existing) such as caffeic acid, its esters, bee-product propolis have been observed to inhibit RAC that directly activates PAK1 (Xu et al., 2005). However, previous studies reported that chloroquine a drug active against malaria also suppressed the incidence of SARS/Coronavirus infection, but its mode of action still remains unknown (Vinecent, 2005). Later, it was reported that this drug induced CDK inhibitor (p21) to inhibit PAK1 activity (Maruta, 2014). In line of forging argument, a recent study depicted that tumor-repressed phosphatase PTEN, mainly involved in impeding PAK1 also inhibited the coronavirus-curbed LLC2-linked fibrosis (Lu, 2020). Moreover, LLC2 expression has been observed to correlate with ACE2, a coronavirus receptor and CK2/RAS-PAK1-AP1 signaling cascade (Chen et al., 2010). Henceforth, through all these assumptions we can deduce the PAK1-reliance of corona pathogenesis and a suggestion of using PAK1-blockers for treating pandemic COVID-19 outbreak (Fig. 4 ). Albeit, PAK-1 have a direct link with suppressing the immune system of the hosts, yet, PAK-1 blockers behave as antiviral vaccines in elevating the immune responses (Huynh et al., 2017). Peeping into the previous studies, it was found that rabies vaccine developed in 1885 was active against myriad of viral diseases but it usually took 12-18 months for actual resistance development. Apparently, a fast-track approach of therapeutics in the form of natural PAK-1 blockers are predominantly making its position nowadays to act against prevailing viral diseases like coronavirus.
Fig. 4.
Signalling Pathway Underlying PAK1-Blockers and Viral Infections.
Inevitably, the conventional and recently accepted product propolis, obtained from bees have been known since ancient times. It is formerly obtained from honey-bee extract via honey-bees feeding the buds of poplar and willow trees, thereby, mixing their saliva into it to form hexagonal bee-hives in order to protect their larval species against many pathogenic organisms. This product synthesised is called as propolis and is regarded as herbal medicine with anti-viral properties. In contemporary world, propolis is identified in possessing anti-cancer characteristics due to the presence of an ingredient CAPE (Caffeic Acid Phenyl Ester) involved in down-regulation of RAC for PAK1 inhibition respectively (Xu et al., 2005). Although, the anticancer constituents in propolis varies from product to product according to harvesting property of bee extracts. For instance, the anti-cancer element in Brazilian propolis is Artepillin C (ARC), while in sub-tropical regions of Taiwan and Okinawa, polyphenols namely, Nymphaeols act as direct inhibitors of PAK1 (Nguyen et al., 2017).In view of the fact that, familiar thing among all categories is that they are comprised of PAK1-blockers. Ever since, has been elucidated that, PAK1 tends to cause cancers, viral diseases like HIV, Hepatitis, pappiloma, influenza, ebola, SARS and corona virus along with immune system suppression of hosts, henceforth, propolis would be quintessential in blocking COVID/coronavirus curbed fibrosis in respiratory tract and boosting the immunity of an individual (Maruta, 2014). The effectiveness of propolis also depends on the product, its chemical nature and action. To expedite, CAPE-containing propolis named 'Bio 30′, commercialised by New Zealand is found to be highly active (Maruta, 2014). The optimal doses are 1 ml/10 Kg of the body weight. Regardless of this pre-formed formulation in the market, it could not be used against COVID-19 eruption, due to paucity of its stock in market place. Moreover, the available stocks are being predominantly used for treating patients with deadly cancers and prolonged treatment of genetic brain disorder, NF tumers (Neuro Fibromatosis Type 1, 2) (Maruta and He, 2020). In addition to this, the cell membrane permeability of caffeic acid and ARC is relatively weak, owing to its -COOH moiety. Nonetheless, this feature could be surmounted by triggering the cell membrane permeability via conversion their esters into 1, 2, 3-triazolyl esters that are thousand times more powerful than ARC or caffeine esters (Maruta and Ahn, 2017). Along with this, melatonin hormone (pineal gland hormone) is a serotonin and possess anti-melanogenic activity that is a primitive factor as melanogenesis is directly correlated to PAK1 (Be et al., 2017). Further, it also owns similarity with other anti-PAK1 actions like anti-cancer, antiviral, anti-inflammatory, immuno-boosters etc. Therefore, it is pertinent to mention here that melatonin, a commonly available sleeping pill could be utilized against coronaviral infections. Currently, many investigations are being carried out to high-light the significance of melatonin as another therapeutic resort or COVID-19 adjuvant (Zhang et al., 2020b). Furthermore, glucocorticoid hormone 'ciclesonide' is another choice to cure different inflammatory disorders and it has been widely commercialised under the brand name of Alvesco. It has been officially approved since 1990 and been widely associated with treating adults as well as children. The molecular mechanism forming the basis for anti-inflammatory action is based on the potential of PAK1-blockers that lays the foundation for its pathway. Majorly, it works according to two basic reasons, firstly, inflammation is not possible without PAK1 and PAK1-null mutant in mice depicted no inflammation (Allen et al., 2009). Secondly, ciclesonide inhibited PAK-1 regulated proliferation of lung cancer in A541 cell lines as well as in immuno-deficient mice (Choi et al., 2020). Thus, this hormone was clinically reported to block PAK1 replication and pathogenesis (lung fibrosis) caused by COVID-19 respectively (Sharp and Dange, 2020).
Apart from this, a herbal formulation mainly triterpenes or steroid named 'triptolide' derived from thunder god vine also inhibited RAC followed by blocking PAK1 route (Maruta and Ahn, 2017). This medicine was previously treating the patients infected with dengue virus in restricting the proliferation of virus in lungs by blocking PAK1 signaling cascade (Liou et al., 2008). Conversely, due to its lower water solubility, few years back, its -OH group was phosphorylated to enhance its solubility (Patil et al., 2015). Resultantly, phosphotase-receptive pro-drug of triptolide 'Minnelide' is currently under clinical trials to cure COVID-19 infections as well. Another compound ivermectin has been screened from bacteria but with some lethal effects. Interestingly, these ill-effects were removed later by chemically re-designing the compound. Further, it was pragmatically involved to impede various parasitic infections, cancers etc. eventually causing the impairment of PAK1, which encompasses its anti-cancerous properties (Hashimoto et al., 2009). As a consequence, it could be a good option to counteract coronaviral infection, however, a recent study found the role of Ivermectin to occlude COVID-19 incidence in Vero cell cultures (Caly et al., 2020). Meanwhile, 'Artemisinin', an anti-malarial component originated from plant herb Artemisia annua also showed its active property against malaria and other viral diseases (Sicard et al., 2011). This is however, concerned with the host cells rather than pathogen itself. To illustrate, PAK1 upregulation has been observed on the basis of AM-RAS mediated suppression (PAK1-upstream) and RAF in T-cells (PAK1-RAS downstream) (Maruta, 2014). The dihydro derivatives of AM deactivates the virak infection through upregulation of CKD inhibitor p21 respectively (Maruta, 2014). Additionally, reddish peppercorns (ethanol-water extract) was also found active against PAK1 and cyclin D1 in NF1-deprived negative breast cancer cell line (MDA-MB231). It was also found to suppress MPNST-cell lines where PAK1 was peculiarly initiated (Hirokawa et al., 2006). Concurrently, this extract in the form of tea would enable prevention and cure of COVID-19 incidence owing to its contribution in PAK1 blocking. The basic formulations of these peppercorns in boosting immune system and impeding inflammation during viral infections have also been investigated (Jie et al., 2019). Above all, a peptide namely FK228, identified from soil microbe has been found to restrict the expansion of RAS (pancreatic/colon cancer) carrying oncogenic mutant (K-RAS) respectively (Nakajima et al., 1998). Later, it was also reported to deactivate histone deacetylases and PAK1 in different cancer cell lines. This mainly comprise of breast cancer cell lines as well as NF1-deprived Malignant Peripheral Nerve Sheath Tumor (MPNST) to restrain their proliferation in xenografts of mice (Hirokawa et al., 2005). Therefore, possessing all these features it was approved to cure rare cancer disease cutaneous lymphoma and commercialised as 'Isodax' in market. However, research is being conducted with the belief that it could be most probably utilized for COVID-19 therapy. It is need of an hour to clinically test various PAK1-blockers against blocking virus replication to develop anti-COVID-19 therapy for patients suffering worldwide.
9. Conclusions
Strata of mankind has been marred with the onset of the unexpected pandemics, leading to the atrocious effects on their entire communities. Considering themselves invincible to conquer the world, they have now become the cocoons of their houses against COVID-19 storm. Unfortunately, SARS-CoV-2 has led the global population on their toes due to its highly infectious nature and susceptibility towards humans in causing higher mortalities among them. We can also introspect that about a lot many cases of COVID-19 have been undetected and by looking into the ongoing trends, the cases are going to inflate in the coming future. This would ultimately affect the world economy, specifically within developing and under-developing nations. Besides, WHO has advised the world to take preventive measures, owing to which government has facilitated nationwide shutdowns, self-quarantine, physical distancing to ensure public safety. Thus far, the prevailing drugs and vaccines for antiviral diseases have been used as clinical trials to fight against coronavirus. Furthermore, by studying the gene pool of SARS-CoV-2, researchers would be able to invent the vaccine for this petrified virus. While, the zeal and zest for the discovery of therapeutics have been plummeted in last few years, yet the dominance of plants and their products are still gaining light in this darkness. Our review projected the possible role of herbal plants and their sources in the treatment of COVID-19 infections due to its bioactive ingredients acting as substantial warriors in this battle. Intriguingly, pro-active devotions in this research would definitely enable us to develop vaccines or drugs to cure COVID-19, for which it is pre-requisite to unlock this treasure of information pertaining to traditional medicines. However, there is an urgent need to invest time on these studies for acquiring about appropriate doses and formulations for discovering drugs or vaccines from these formulations. For this purpose, scientific evidences and comprehensive pharmaco-dynamic knowledge related to medicinal plants should be made available to scientists to design clinical trials. Integration of this concept would certainly develop the drug therapy in the near future. All what we can do is wait for the hope of radiance at the terminating point of the tunnel, in solitude.
10. Future perspectives and conclusions
Due to the ongoing disaster of COVID-19, there is a pressing need for the discovery of alternative remedial measures. There is a wide scope of herbal medicines that have been used since traditional times. They have been considered as potent clinical agents against wide array of viral diseases due to their anti-viral properties. These natural products of Ayurveda are being tested for treating COVID-19. Basically, these formulations are comprised of huge number of phytochemicals such as terpenoids, alkaloids, flavonoids, phenols, tannins, polyphenols, saponins, polysaccharides, proteins, lipids and peptides that possess myriad of functions against viral invasion, penetration, replication, expression, assembly and release. Moreover, medicinal plants and their natural ingredients proved to be the most promising alternatives to prevent or cure the infection and spread of this disease since its outbreak. However, they are still being tested under in vitro conditions to get the direct evidence on the positive health of COVID-19 patients. In the present review, we have elaborated the potential role of herbal formulations to treat COVID-19 through summarising the ongoing trials of plants and their products against this deadly virus. Although, the investigations for evaluation of different plant genera as anti SARS-CoV-2 agents, are insufficient. But research is in the pipeline to untangle their potential positive aspect and we expect these studies to be conducted in a more elaborated manner in future. Yet, many scientists are still engaged in these studies to develop a suitable antidode for this virus. The future possibilities upholds the fact that by combining these studies with effective technology and testing we can demonstrate their role in blocking the life-cycle of this virus, protein denaturation of receptor proteins and role of certain proteases enzymes could be studied.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
RB, PA, KK: Conceptualization; SKK, RK AB, VB Data curation; AS, AA, KK, RK: Methodology; KK, SKK, AS, AB, PO: Resources; PO, AS, AB: Software; RB, PA, KK: Supervision; RB, KK, PA, VB, PO, AS, Roles/Writing - original draft; RB, PA, AA, KK: Writing - review & editing.
Declaration of Competing of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influencethe work reported in this paper.
Acknowledgments
None.
References
- Abu-Mouch S., Fireman Z., Jarchovsky J., Zeina A.R., Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J. Gastroenterol. 2011;17(47):1590–5184. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Murillo J.A., Garcia-Leon M.L., Firo-Reyes V., Santiago Cordova J.L., Gonzalez-Rodriguez A.P., Wong-Chew R.M. Zinc supplementation promotes a Th1 response and improves clinical symptoms in less hours in children with pneumonia younger than 5 years old. A randomized controlled clinical trial. Front. Pedia. 2019;7:431. doi: 10.3389/fped.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya K.P. Resource poor countries ought to focus on early detection and containment of novel corona virus at the point of entry. Clin. Epidemiol. Glob. Health. 2020 doi: 10.1016/j.cegh.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem, S., Eyupoglu, V., Sarfraz, I., Rasul, A., Ali, M., 2020. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in silico strategy unveils a hope against CORONA. DOI. 10.20944/preprints202003.0333.v1. [DOI] [PMC free article] [PubMed]
- Aglipay M., Birken C.S., Parkin P.C., Loeb M.B., Thorpe K., Chen Y., Laupacis A., Mamdani M., Macarthur C., Hoch J.S., Mazzulli T. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Rehman M.U., Alkharfy K.M. An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. Eur. Rev. Med. Pharm. Sci. 2020;24(7):4030–4034. doi: 10.26355/eurrev_202004_20873. [DOI] [PubMed] [Google Scholar]
- Akatsu H., Iwabuchi N., Xiao J.Z., Matsuyama Z., Kurihara R., Okuda K., Yamamoto T., Maruyama M. Clinical effects of probiotic bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J. Parenter. Enter. Nutr. 2013;37(5):631–640. doi: 10.1177/0148607112467819. [DOI] [PubMed] [Google Scholar]
- Akram M., Tahir I.M., Shah S.M.A., Mahmood Z., Altaf A., Ahmad K., Munir N., Daniyal M., Nasir S., Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother. Res. 2018;32(5):811–822. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- Alabboud M., Javadmanesh A. In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS-CoV-2 main protease. DYSONA -Life Sci. 2020;1:44–63. [Google Scholar]
- Allen J.D., Jaffer Z.M., Park S.J., Burgin S., Hofmann C., Sells M.A., Chen S., Derr-Yellin E., Michels E.G., McDaniel A., Bessler W.K. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood J. Am. Soc. Hematol. 2009;113(12):2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amber R., Adnan M., Tariq A., Mussarat S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017;69(2):109–122. doi: 10.1111/jphp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone P., Fiorino S., Cursaro C., Gramenzi A., Margotti M., Di Giammarino L., Biselli M., Miniero R., Gasbarrini G., Bernardi M. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antivir.Res. 2001;49(2):75–81. doi: 10.1016/s0166-3542(00)00141-8. [DOI] [PubMed] [Google Scholar]
- Angeh J.E., Huang X., Swan G.E., Mollman U., Sattler I., Eloff J.N. Novel antibacterial triterpenoid from Combretum padoides [Combretaceae] Arkat USA. 2007;11:113–120. [Google Scholar]
- Augustin J.M., Kuzina V., Andersen S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochem. 2011;72(6):435–457. doi: 10.1016/j.phytochem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Baell J.B. Feeling nature's PAINS: natural products, natural product drugs, and pan assay interference compounds (PAINS) J. Nat. Prod. 2016;79(3):616–628. doi: 10.1021/acs.jnatprod.5b00947. [DOI] [PubMed] [Google Scholar]
- Balachandar V., Mahalaxmi I., Kaavya J., Vivekanandhan G., Ajithkumar S., Arul N., Singaravelu G., Kumar N.S., Devi S.M. COVID-19: emerging protective measures. Eur. Rev. Med. Pharm. Sci. 2020;24(6):3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- Be P.T., Nguyen B.C., Tawata S., Yun C.Y., Kim E.G., Maruta H. The serum/PDGF-dependent" melanogenic" role of the minute level of the oncogenic kinase PAK1 in melanoma cells proven by the highly sensitive kinase assay. Drug Discov. Ther. 2017;10(6):314–322. doi: 10.5582/ddt.2016.01062. [DOI] [PubMed] [Google Scholar]
- Borenstein R., Hanson B.A., Markosyan R.M., Gallo E.S., Narasipura S.D., Bhutta M., Shechter O., Lurain N.S., Cohen F.S., Al-Harthi L., Nicholson D.A. Ginkgolic acid inhibits fusion of enveloped viruses. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-61700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchentouf S., Missoum N. Identification of compounds from Nigella Sativa as new potential inhibitors of 2019 novel coronasvirus (Covid-19): molecular docking study. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12055716.v1. Preprint. [DOI] [Google Scholar]
- Broome C.S., McArdle F., Kyle J.A., Andrews F., Lowe N.M., Hart C.A., Arthur J.R., Jackson M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004;80(1):154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- Brush J., Mendenhall E., Guggenheim A., Chan T., Connelly E., Soumyanath A., Buresh R., Barrett R., Zwickey H. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD69 expression and immune cell activation in humans. Phytotherapy Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Natl. Prod. Derivatives. 2006;20(8):687–695. doi: 10.1002/ptr.1938. [DOI] [PubMed] [Google Scholar]
- Bui T.T., Fan Y., Piao C.H., Van Nguyen T., Shin D.U., Jung S.Y., Hyeon E., Song C.H., Lee S.Y., Shin H.S., Chai O.H. Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell. Immunol. 2019;351 doi: 10.1016/j.cellimm.2019.104035. [DOI] [PubMed] [Google Scholar]
- Bunout D., Hirsch S., de la Maza M.P., Munoz C., Haschke F., Steenhout P., Klassen P., Barrera G., Gattas V., Petermann M. Effects of prebiotics on the immune response to vaccination in the elderly. J. Paren. Ent. Nutr. 2002;26(6):372–376. doi: 10.1177/0148607102026006372. [DOI] [PubMed] [Google Scholar]
- Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., et al. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco F.R., Schmidt G., Romero A.L., Sartoretto J.L., Caparroz‐Assef S.M., Bersani‐Amado C.A., Cuman R.K.N. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: evidence for humor‐and cell‐mediated responses. J. Pharma Pharmacol. 2009;61(7):961–967. doi: 10.1211/jpp/61.07.0017. [DOI] [PubMed] [Google Scholar]
- Chaigne-Delalande B., Li F.Y., O'Connor G.M., Lukacs M.J., Jiang P., Zheng L., Shatzer A., Biancalana M., Pittaluga S., Matthews H.F., Jancel T.J. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Ojha D., Mondal S., Goswami D. In: Evidence-Based Validation of Herbal Medicine. Muherjee P.K., editor. Elsevier, Public Health Care Collection; 2015. Validation of antiviral potential of herbal ethnomedicine; pp. 175–200. [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Hung I.F.N., Lee T.S.W., Guan Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.Y., Chang S.C., Wu H.Y. Upregulation of the chemokine (C-C motif) ligand 2 372 (CCL2) via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling path- 373 way. J. Virol. 2010;84:7703–7712. doi: 10.1128/JVI.02560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L., Zheng, W., Li, M., Huang, J., Bao, S., Xu, Q., Ma, Z., 2020. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2.Preprints2020, 2020020313. [DOI] [PMC free article] [PubMed]
- Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharm. Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikezie P.C., Ibegbulem C.O., Mbagwu F.N. Bioactive principles from medicinal plants. Res. J. Phytochem. 2015;9(3):88–115. [Google Scholar]
- Chiru T., Fursenco C., Ciobanu N., Dinu M., Popescu E., Ancuceanu R., Volmer D., Raal A. Use of medicinal plants in complementary treatment of the common cold and influenza–perception of pharmacy customers in Moldova and Romania. J. Herb. Med. 2020;2020 doi: 10.1016/j.hermed.2020.100346. [DOI] [Google Scholar]
- Choi H.S., Kim S.L., Kim J.H., Lee D.S. The FDA-approved anti-asthma medicine ciclesonide inhibits lung cancer stem cells through hedgehog signaling-mediated SOX2 regulation. Int. J. Mol. Sci. 2020;21(3):1014. doi: 10.3390/ijms21031014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M.A., Shahid M.A., Kashem M.A. Scope of natural plant extract to deactivate COVID-19. Eur. PMC. 2020 doi: 10.21203/rs.3.rs-19240/v1. [DOI] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs G.F., Jr, McClung J.P. In: Combs G.F., McClung J.M, editors. Academic press; 2016. pp. 1–593. [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nature Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Selective virus inhibitors. Microbiol. 1990;13(2):165–178. [PubMed] [Google Scholar]
- De Clercq E. Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. J. Med. Chem. 1995;38(14):2491–2517. doi: 10.1021/jm00014a001. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran S., Pradeep P.S. Scope of phytotherapeutics in targeting ACE2 mediated Host-Viral Interface of SARS‐CoV2 that causes COVID-19. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12089730.v1. Preprint. [DOI] [Google Scholar]
- Ding S., Jiang H., Fang J. Regulation of immune function by polyphenols. J. Immunol. Res. 2018 doi: 10.1155/2018/1264074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Zhao K., Shi Z.L., Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunatov M.N. Experimental use of phytoncides for therapeutic and prophylactic purpose. Vopr. Pedia. Okhrany Materia. 1952;20(2):55–58. [PubMed] [Google Scholar]
- Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.F, Duan Q, Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.041. S0163-4453(20)30170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad M.S., Meshram B.G., Chaugule B.B. On occurrence of the genus Porphyridium Nageli: new to India. J. Algal Biomass Util. 2009;1:102–106. [Google Scholar]
- Ganjhu R.K., Mudgal P.P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. Virus Dis. 2015;26(4):225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geresh S., Adin I., Yarmolinsky E., Karpasas M. Characterization of the extracellular polysaccharide of Porphyridium sp.: molecular weight determination and rheological properties. Carbo. Polym. 2002;50(2):183–189. [Google Scholar]
- Ghosh T., Chattopadhyay K., Marschall M., Karmakar P., Mandal P., Ray B. Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiol. 2009;19(1):2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- Gilani A.H., Khan A.U., Raoof M., Ghayur M.N., Siddiqui B.S., Vohra W., Begum S. Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fundam. Clin. Pharmacol. 2008;22(1):87–99. doi: 10.1111/j.1472-8206.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Gilca M., Tiplica G.S., Salavastru C.M. Traditional and ethnobotanical dermatology practices in Romania and other eastern European countries. Clin. Dermatol. 2018;36(3):338–352. doi: 10.1016/j.clindermatol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Giri S., Lal A.F., Singh S. Battle against Coronavirus: Repurposing old friends (Food borne polyphenols) for new enemy (COVID-19) ChemRxiv. 2020 doi: 10.26434/chemrxiv.12108546.v1. Preprint. [DOI] [Google Scholar]
- Gomathi M., Padmapriya S., Balachandar V. Drug studies on rett syndrome: from bench to bedside. J. Autism Dev. Disord. 2020:1–25. doi: 10.1007/s10803-020-04381-y. [DOI] [PubMed] [Google Scholar]
- Goncalves-Mendes N., Talvas J., Dualé C., Guttmann A., Corbin V., Marceau G., Sapin V., Brachet P., Evrard B., Laurichesse H., Vasson M.P. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goothy S.S.K., Goothy S., Choudhary A., Potey G.G., Chakraborty H., Kumar A.H., Mahadik V.K. Ayurveda's holistic lifestyle approach for the management of coronavirus disease (COVID-19): possible role of tulsi. Int. J. Res. Pharm. Sci. 2020;11(SPL1):16–18. [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Kumar M., Ghosh S.K., Das A. Natural product compounds in alpinia officinarum and ginger are potent SARS-CoV-2 papain-like protease inhibitors. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12071997.v1. Preprint. [DOI] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S., Du B. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunville C., Mourani P., Ginde A. The role of vitamin D in prevention and treatment of infection. Inflamma. Allergy-Drug Targets (Formerly Curr. Drug Targets-Inflamm. Allergy) 2013;12(4):239–245. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.R., Cao Q.D., Hong Z.S, Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- an update on the status. Mil. Med. Res. 2020;7(1):1–11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.C., Prasad S., Tyagi A.K., Kunnumakkara A.B., Aggarwal B.B. Neem (Azadirachta indica): an indian traditional panacea with modern molecular basis. Phytomed. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Hamilton A.C. Medicinal plants, conservation and livelihoods. Biodiver. Conserv. 2004;13(8):1477–1517. [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Lin Q., Ni Z., You L. Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respi. Viruses. 2020 doi: 10.1111/irv.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Messerli S.M., Sudo T., Maruta H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov. Ther. 2009;3(6):243–246. [PubMed] [Google Scholar]
- He L., Qi Y., Rong X., Jiang J., Yang Q., Yamahara J., Murray M., Li Y. The ayurvedic medicine salacia oblonga attenuates diabetic renal fibrosis in rats: suppression of angiotensin II/AT1 signaling. Evid. Based Complement. Alternat. Med. 2011;2011 doi: 10.1093/ecam/nep095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary F., Varnaseri M., Gharebaghi R. The potential use of Persian herbal medicines against COVID-19 through angiotensin-converting enzyme 2. Arch. Clin. Infect. Dis. 2020;15(COVID-19) [Google Scholar]
- Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson J. COVID-19: faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020;17(5):259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa Y., Arnold M., Nakajima H., Zalcberg J., Maruta H. Signal therapy of breast cancers by the HDAC inhibitor FK228 that blocks the activation of PAK1 and abrogates the tamoxifen-resistance. Cancer Biol. Ther. 2005;4(9):956–960. doi: 10.4161/cbt.4.9.1911. [DOI] [PubMed] [Google Scholar]
- Hirokawa Y., Nheu T., Grimm K., Mautner V., Maeda S., Yoshida M., Komiyama K., Kluwe L., Maruta H. Sichuan pepper extracts block the PAK1/cyclin D1 pathway and the growth of NF1-deficient cancer xenograft in mice. Cancer Biol. Ther. 2006;5(3):305–309. doi: 10.4161/cbt.5.3.2404. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain F., Jahan N., Rahman K.U., Sultana B., Jamil S. Identification of hypotensive biofunctional compounds of coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/4643736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh N., Wang K., Yim M., Dumesny C.J., Sandrin M.S., Baldwin G.S., Nikfarjam M., He H. Depletion of p21-activated kinase 1 up-regulates the immune system of APC∆ 14/+ mice and inhibits intestinal tumorigenesis. BMC Cancer. 2017;17(1):431. doi: 10.1186/s12885-017-3432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I Eze J., Ajanwachukwu N., C, Animoke P., O, Onoja S., N, Anosa G., U, Eze U. Immune response, anaemia and oxidative stress in Trypanosoma brucei infected rats fed vitamin E supplemented diet. Anti-Infect. Agents. 2016;14(1):28–37. [Google Scholar]
- Ivory K., Prieto E., Spinks C., Armah C.N., Goldson A.J., Dainty J.R., Nicoletti C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2017;36(2):407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr: Clinical Res. Rev. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Kanda T., Nakamoto S., Saito K., Nakamura M., Wu S., Haga Y., Sasaki R., Sakamoto N., Shirasawa H., Okamoto H. The JAK2 inhibitor AZD1480 inhibits hepatitis A virus replication in Huh7 cells. Biochem. Biophys. Res. Comm. 2015;458(4):908–912. doi: 10.1016/j.bbrc.2015.02.058. [DOI] [PubMed] [Google Scholar]
- Jie Y., Li S., Ho C.T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus) Food Sci. Hum. Well. 2019;8(2):115–125. [Google Scholar]
- Jo S., Kim H., Kim S., Shin D.H., Kim M.S. Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chem. Biol. Drug Des. 2019;94(6):2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S.E.S., Tomar S., Stauffer S.R., Mesecar A.D. Targeting zoonotic viruses: Structure-based inhibition of the 3C-like protease from bat coronavirus HKU4—the likely reservoir host to the human coronavirus that causes Middle East Respiratory Syndrome (MERS) Bioorgan. Med. Chem. 2015;23(17):6036–6048. doi: 10.1016/j.bmc.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra E.K. Nutraceutical-definition and introduction. Aaps. Pharmsci. 2003;5(3):27–28. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr. Pharmaceu. Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E.J., Kim S.Y., Hwang I.H., Ji Y.J. The effect of probiotics on prevention of common cold: a meta-analysis of randomized controlled trial studies. Kor. J. Family Med. 2013;34(1):2. doi: 10.4082/kjfm.2013.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik L., Kumar G., Keswani T., Bhattacharyya A., Chandar S.S., Rao K.B. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul T.N., Middleton E., Jr, Ogra P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15(1):71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Prepr. 2020:1–14. doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- Khan, M.F., Khan, M.A., Khan, Z.A., Ahamad, T., Ansari, W.A., 2020. Identification of dietary molecules as therapeutic agents to combat COVID-19 using molecular docking studies.
- Khan M.Y., Kumar V. Mechanism & inhibition kinetics of bioassay-guided fractions of Indian medicinal plants and foods as ACE inhibitors. J. Tradit. Complement. Med. 2019;9(1):73–84. doi: 10.1016/j.jtcme.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee Y.C. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin‐induced asthma model. J. Pharma. Pharmacol. 2009;61(3):353–359. doi: 10.1211/jpp/61.03.0010. [DOI] [PubMed] [Google Scholar]
- Kim S.O., Kundu J.K., Shin Y.K., Park J.H., Cho M.H., Kim T.Y., Surh Y.J. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-κ B in phorbol ester-stimulated mouse skin. Oncogene. 2005;24(15):2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim H., Bae S., Choi J., Lim S.Y., Lee N., Kong J.M., Hwang Y.I., Kang J.S., Lee W.J. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Net. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiça T.F., Alves S.H., Weiblen R., Lovato L.T. In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014;45(1):209–214. doi: 10.1590/S1517-83822014005000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.S., Navaratnam V. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac. J. Trop. Biomed. 2013;3(7):505–514. doi: 10.1016/S2221-1691(13)60105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuhara T., Iwai Y., Takahashi H., Hatakeyama D., Echigo N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. Plos Curr. 2009;1 doi: 10.1371/currents.RRN1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem F.F., Opook F., Herng D.L.J., Na C.S., Lawson F.P., Tyng C.F. Molecular mechanism of action of repurposed drugs and traditional Chinese medicine used for the treatment of patients infected with COVID-19: a systematic review. medRxiv. 2020 doi: 10.1101/2020.04.10.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E.D., Meydani S.N., Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71(4):487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li Y., Ding C. The role of copper homeostasis at the host-pathogen axis: from bacteria to fungi. Int. J. Mol. Sci. 2019;20(1):175. doi: 10.3390/ijms20010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Sun N., Zhao J.X., Sun Y.G., Huang J.G., Lei H.M., Guo J.H., Hu Y.L., Wang W.K., Li H.Q. In vitro evaluation of antiviral activity of tea seed saponins against porcine reproductive and respiratory syndrome virus. Antivir. Ther. 2015;20:743–752. doi: 10.3851/IMP2937. [DOI] [PubMed] [Google Scholar]
- Li, G., De Clercq, E., 2020. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). 10.1038/d41573-020-00016-0. [DOI] [PubMed]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., But P.P., Ooi V.E. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antivir. Res. 2005;68(1):1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Liang R.Y., Wu W., Huang J., Jiang S.P., Lin Y. Magnesium affects the cytokine secretion of CD4+ T lymphocytes in acute asthma. J. Asthma. 2012;49(10):1012–1015. doi: 10.3109/02770903.2012.739240. [DOI] [PubMed] [Google Scholar]
- Lin L.L., Shan J.J., Xie T., Xu J.Y., Shen C.S., Di L.Q., Chen J.B., Wang S.C. Application of traditional Chinese medical herbs in prevention and treatment of respiratory syncytial virus. Evid.-Based Complementary Altern. Med. 2016;6082729:1–14. doi: 10.1155/2016/6082729. Article ID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer G.W., Stoltzfus R.J., Prendergast A.J. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv. Nutr. 2014;5(1):1–6. doi: 10.3945/an.113.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C.Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) J. Integr. Med. 2020 doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J.T., Chen Z.Y., Ho L.J., Yang S.P., Chang D.M., Liang C.C., Lai J.H. Differential effects of triptolide and tetrandrine on activation of COX-2, NF-κB, and AP-1 and virus production in dengue virus-infected human lung cells. Eur. J. Pharmacol. 2008;589(1-3):288–298. doi: 10.1016/j.ejphar.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Tren. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Lu, X., Xiang, Y., Du, H., Wing‐Kin Wong, G., 2020a. SARS-CoV-2 infection in children - understanding the immune responses and controlling the pandemic. Pediatr. Allergy Immunol. 10.1111/pai.13267. [DOI] [PubMed]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Strand K.A., Mutryn M.F., Tucker R.M., Jolly A.J., Furgeson S.B., Moulton K.S., Nemenoff R.A., Weiser-Evans M.C. PTEN (phosphatase and tensin homolog) protects against Ang II (Angiotensin II)-induced pathological vascular fibrosis and remodeling—brief report. Arterioscl. Throm. Vas. Biol. 2020;40(2):394–403. doi: 10.1161/ATVBAHA.119.313757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese J. Integrat. Med. 2020:1–8. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C.D., Sagripanti J.L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005;79(22):14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Kosińska-Cagnazzo A., Kerr W.L., Amarowicz R., Swanson R.B., Pegg R.B. Separation and characterization of soluble esterified and glycoside-bound phenolic compounds in dry-blanched peanut skins by liquid chromatography–electrospray ionization mass spectrometry. J. Agric. Food Chem. 2014;62(47):11488–11504. doi: 10.1021/jf503836n. [DOI] [PubMed] [Google Scholar]
- Mackenzie J.S., Smith D.W. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don't. Microbiol Aust. 2020;MA20013 doi: 10.1071/MA20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity N., Nema N.K., Sarkar B.K., Mukherjee P.K. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Ind. J. Pharm. 2012;44(5):584. doi: 10.4103/0253-7613.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska M., Sikora E., Ogonowski J. Production of triterpenoids with cell and tissue cultures. Acta Biochim. Polonica. 2013;60(4) [PubMed] [Google Scholar]
- Mamidi P., Gupta K. Septic Shock and delirium. Int. J. Complemen. Alter. Med. 2017;8(2):00257. [Google Scholar]
- Manuja A., Rathore N., Chaudhary S., Kumar B. Phytochemical screening, cytotoxicity and anti-inflammatory activities of the leaf extracts from Lawsonia inermis of Indian origin to explore its potential for medicinal uses. Med. Chem. 2020 doi: 10.2174/1573406416666200221101953. (Shariqah (United Arab Emirates)) [DOI] [PubMed] [Google Scholar]
- Markotić A., Kuzman I. The third coronavirus epidemic in the third millennium: what's next? Croat. Med. J. 2020;61(1):1. doi: 10.3325/cmj.2020.61.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exercise Sport Sci. Rev. 2009;37(4):157. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.J.A., Del Olmo L.M.B., Benito P.B. In: Atta-ur-Rayman, editor. Vol. 30. Elsevier; USA: 2005. Antiviral activities of polysaccharides from natural sources; pp. 393–418. (Studies in Natural Products Chemistry). [Google Scholar]
- Maruta H. Tackling the coronaviral infection: blocking either the “pathogenic” kinase PAK1 or RNA-dependent RNA polymerase (RdRP) J. Infect. Dis. Therpy. 2020;8(2):357–418. [Google Scholar]
- Maruta H. Herbal therapeutics that block the oncogenic kinase PAK1: a practical approach towards PAK1‐dependent diseases and longevity. Phytother. Res. 2014;28(5):656–672. doi: 10.1002/ptr.5054. [DOI] [PubMed] [Google Scholar]
- Maruta H., Ahn M.R. From bench (laboratory) to bed (hospital/home): how to explore effective natural and synthetic PAK1-blockers/longevity-promoters for cancer therapy. Eur. J. Med. Chem. 2017;142:229–243. doi: 10.1016/j.ejmech.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Maruta H., He H. PAK1-blockers: potential therapeutics against COVID-19. Med. Drug Discov. 2020 doi: 10.1016/j.medidd.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya D., Sharma D. Evaluation of traditional ayurvedic preparation for prevention and management of the novel Coronavirus (SARS-CoV-2) using molecular docking approach. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12110214.v1. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty, M.F., DiNicolantonio, J.J., 2020. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. DOI.10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed]
- Medina U., Segura-Campos M.R. In vitro antioxidant and anti-inflammatory activity of chaya extracts (Cnidoscolus aconitifolius (Mill.) IM Johnst) Nutr. Hosp. 2020;37(1):46–55. doi: 10.20960/nh.02752. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzo, F., Ciriminna, R., Zabini, F., Pagliaro, M., 2020. Hydrodynamic cavitation-based rapid expansion of hesperidin-rich products from waste citrus peel as a potential tool against COVID-19. Preprints 2020, doi:10.20944/preprints202004.0152.v1.
- Ministry of Ayush, Government of India, 2020. Homeopathy for prevention of coronavirus infections.
- Mishra S., Aeri V., Gaur P.K., Jachak S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/808302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto D., Kusagaya Y., Endo N., Sometani A., Takeo S., Suzuki T., Arima Y., Nakajima K., Suzuki Y. Thujaplicin–copper chelates inhibit replication of human influenza viruses. Antivir. Res. 1998;39(2):89–100. doi: 10.1016/s0166-3542(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Mohammadi N., Shaghaghi N. Inhibitory effect of eight secondary metabolites from conventional medicinal plants on COVID_19 virus protease by molecular docking analysis. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11987475.v1. Preprint. [DOI] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa H.A.L. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J. Evid.-Based Complementary Altern. Med. 2017;22(1):166–174. doi: 10.1177/2156587216641831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murariu D., Strajeru S., Milica C., Radu S. Report of a Working Group on Medicinal and Aromatic Plants. 2002. Status of the Romanian medicinal and aromatic plant collection; p. 109. [Google Scholar]
- Nagle, V., Gaikwad, M., Pawar, Y., Dasgupta, S., 2020. Marine red alga porphyridium sp. as a source of sulfated polysaccharides (SPs) for combating against COVID-19. Preprints 2020, 2020040168.
- Nair R. HIV-1 reverse transcriptase inhibition by Vitex negundo L. leaf extract and quantification of flavonoids in relation to anti-HIV activity. J. Cell Mol. Biol. 2012;10(2):53–59. [Google Scholar]
- Nakajima H., Kim Y.B., Terano H., Yoshida M., Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res. 1998;241(1):126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- Nguyen B.C.Q., Yoshimura K., Kumazawa S., Tawata S., Maruta H. Frondoside A from sea cucumber and nymphaeols from Okinawa propolis: natural anti-cancer agents that selectively inhibit PAK1 in vitro. Drug Discov. Ther. 2017;11(2):110–114. doi: 10.5582/ddt.2017.01011. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. J. Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikhat S., Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci. Tot. Env. 2020 doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer A., Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. 2012;18(8):800. doi: 10.3748/wjg.v18.i8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrati M., Behbahani M. Molecular docking study of HIV-1 protease with triterpenoides compounds from plants and mushroom. Arak. Uni. Med. Sci. J. 2015;18(3):67–79. [Google Scholar]
- Nourazarian S.M., Nourazarian A., Majidinia M., Roshaniasl E. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pacific J. Cancer Prev. 2016;16(18):8563–8566. doi: 10.7314/apjcp.2015.16.18.8563. [DOI] [PubMed] [Google Scholar]
- Olafsdottir E.S., Ingólfsdottir K. Polysaccharides from lichens: structural characteristics and biological activity. Planta Med. 2001;67(03):199–208. doi: 10.1055/s-2001-12012. [DOI] [PubMed] [Google Scholar]
- Omara E., Kam A., Alqahtania A., Li K., Razmovski-Naumovski V., Nammi S., Chan K., Roufogalis B., Li G. Herbal medicines and nutraceuticals for diabetic vascular complications: mechanisms of action and bioactive phytochemicals. Curr. Pharm. Des. 2010;16(34):3776–3807. doi: 10.2174/138161210794455076. [DOI] [PubMed] [Google Scholar]
- Panda A.K. Current status of infectious diseases in Ayurveda. Ayursurabhi. 2005;2005:66–67. [Google Scholar]
- Panda A.K., Dixit A.K., Rout S., Mishra B., Purad U.V., Kar S. Ayurveda practitioners consensus to develop strategies for prevention and treatment of corona virus disease (COVID-19) J-AIM. 2020;5(1):98–106. [Google Scholar]
- Papp N., Bartha S., Boris G., Balogh L. Traditional uses of medicinal plants for respiratory diseases in Transylvania. Nat. Prod. Comm. 2011;6(10) [PubMed] [Google Scholar]
- Papp N., Birkás-Frendl K., Bencsik T., Stranczinger S., Czégényi D. Survey of traditional beliefs in the Hungarian Csángó and Székely ethnomedicine in Transylvania. Romania. Rev. Bras. Farmacogn. 2014;24(2):141–152. [Google Scholar]
- Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Pharm. Rev. 2014;8(16):73. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Penkert R.R., Jones B.G., Sealy R.E., Surman S.L., Sun Y., Tang L., DeBeauchamp J., Webb A., Richardson J., Heine R. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11(10):907. doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S., Lis L.G., Schumacher R.J., Norris B.J., Morgan M.L., Cuellar R.A., Blazar B.R., Suryanarayanan R., Gurvich V.J., Georg G.I. Phosphonooxymethyl prodrug of triptolide: synthesis, physicochemical characterization, and efficacy in human colon adenocarcinoma and ovarian cancer xenografts. J. Med. Chem. 2015;58(23):9334–9344. doi: 10.1021/acs.jmedchem.5b01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr D., Marian H., David V., Alica H., Miroslav K., David B., Lenka M., Milan U., Jan S. Anti-inflammatory and antitumorpromoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Nat. Prod. Rep. 2006;23:394–411. [Google Scholar]
- Petric, D. 2020. Immune system and COVID-19. 5e767524299bf1892, 1-5.
- Pieroni A., Nedelcheva A., Dogan Y. Local knowledge of medicinal plants and wild food plants among Tatars and Romanians in Dobruja (South-East Romania) GeneT. Resour. Crop Ev. 2015;62(4):605–620. [Google Scholar]
- Pilcher H. Liquorice may tackle SARS. Nature. 2003 doi: 10.1038/news030609-16. [DOI] [Google Scholar]
- Poehland B.L., Carte B.K., Francis T.A., Hyland L.J., Allaudeen H.S., Troupe N. In vitro antiviral activity of dammar resin triterpenoids. J. Nat. Prod. 1987;50(4):706–713. doi: 10.1021/np50052a022. [DOI] [PubMed] [Google Scholar]
- Polansky H., Lori G. Coronavirus (COVID-19), first indication of efficacy of gene-eden-VIR/novirin in SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Pujol C.A., Estevez J.M., Carlucci M.J., Ciancia M., Cerezo A.S., Damonte E.B. Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir. Chem. Chemother. 2002;13(2):83–89. doi: 10.1177/095632020201300202. [DOI] [PubMed] [Google Scholar]
- Pundarikakshudu K., Kanaki N.S. Analysis and regulation of traditional Indian medicines (TIM) J. AOAC Int. 2019;102(4):977–978. doi: 10.5740/jaoacint.18-0376. [DOI] [PubMed] [Google Scholar]
- Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., Xie, C., Ma, K., Shang, K., Wang, W., Tian, D.S., 2020. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. pii, ciaa248. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed]
- Ramos-Tovar E., Muriel P. In: Dietary Intervention in Liver Diseases. Watson R.R., Preedy V.R., editors. Acaddemic Press; 2020. Phytotherapy for the liver; pp. 101–121. [Google Scholar]
- Rathinavel T., Palanisamy M., Palanisamy S., Subramanian A., Thangaswamy S. Phytochemical 6-gingerol–a promising drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020;6(4):1482–1489. [Google Scholar]
- Ravishankar B., Shukla V.J. Indian systems of medicine: a brief profile. Afr. J. Tradit. Complement. Altern. Med. 2007;4(3):319–337. doi: 10.4314/ajtcam.v4i3.31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege A.A., Chowdhary A.S. Evaluation of Ocimum sanctum and Tinospora cordifolia as probable HIV protease inhibitors. Int. J Pharm. Sci. Rev. Res. 2014;25(1):315–318. [Google Scholar]
- Rosen Y., Daich J., Soliman I., Brathwaite E., Shoenfeld Y. Vitamin D and autoimmunity. Scand. J. Rheumatol. 2016;45(6):439–447. doi: 10.3109/03009742.2016.1151072. [DOI] [PubMed] [Google Scholar]
- Rupp J.C., Locatelli M., Grieser A., Ramos A., Campbell P.J., Yi H., Steel J., Burkhead J.L., Bortz E. Host cell copper transporters CTR1 and ATP7A are important for influenza A virus replication. Virol. J. 2017;14(1):11. doi: 10.1186/s12985-016-0671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salciccioli J.D., Marshall D.C., Pimentel M.A., Santos M.D., Pollard T., Celi L.A., Shalhoub J. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit. Care. 2015;19(1):13. doi: 10.1186/s13054-014-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.E. Probiotics: definition, sources, selection, and uses. Clin. Infect. Dis. 2008;46(Supplement_2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- Sarfraz, I., Rasul, A., Hussain, G., Adem, S., Ali, M., 2020. Natural immune boosters as first-line armours to combat viral infection-COVID19: myth or science? Preprints 2020, 2020030427 (doi: 10.20944/preprints202003.0427.v1).
- Seedevi P., Sudharsan S., Kumar S.V., Srinivasan A., Vairamani S., Shanmugan A. Isolation and characterization of sulphated polysaccharides from Codium tomentosum (J. Stackhouse, 1797) collected from southeast coast of India. Adv. Appl. Sci. Res. 2013;4(5):72–77. [Google Scholar]
- Sengupta, P.S., 2019. Use of piper betel to combat COVID19. PREPARE@ U-Preprint Archive. 10.36375/prepare_u.a92.
- Serseg, T., Benarous, K., Yousfi, M., 2020. Hispidin and lepidine E: two natural compounds and folic acid as potential inhibitors of 2019-novel coronavirus main protease (2019-nCoVMpro), molecular docking and SAR study. arXiv preprint arXiv: 2004.08920. [DOI] [PubMed]
- Shaghaghi N. Molecular docking study of novel COVID-19 protease with low risk terpenoides compounds of plants. ChemRxiv. 2020 DOI, 10. [Google Scholar]
- Shah A., Krishnamurthy R. Swine flu and its herbal remedies. Int. J. Eng. Sci. 2013;2(2):68–78. [Google Scholar]
- Sharma, A.D., Kaur, I., 2020. Molecular docking studies on Jensenone from eucalyptus essential oil as a potential inhibitor of COVID 19 corona virus infection. arXiv preprint arXiv:2004.00217.
- Sharp K., Dange D. Computational drug simulation: a step to the possible cure of COVID-19. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12129129.v2. Preprint. [DOI] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivananjappa M., Joshi M. Aqueous extract of tulsi (Ocimum sanctum) enhances endogenous antioxidant defenses of human hepatoma cell line (HepG2) J. Herbs. Spices Med. Plants. 2012;18(4):331–348. [Google Scholar]
- Shukla S., Bajpai V.K., Kim M. Plants as potential sources of natural immunomodulators. Rev. Environ. Sci. Bio. 2014;13(1):17–33. [Google Scholar]
- Sicard A., Semblat J.P., Doerig C., Hamelin R., Moniatte M., Dorin‐Semblat D., Spicer J.A., Srivastava A., Retzlaff S., Heussler V., Waters A.P. Activation of a PAK‐MEK signalling pathway in malaria parasite‐infected erythrocytes. Cell. Microbiol. 2011;13(6):836–845. doi: 10.1111/j.1462-5822.2011.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F.Q., Ahmad M.M., Kakar F., Akhtar S. The role of vitamin A in enhancing humoral immunity produced by antirabies vaccine. East Mediterr. Health J. 2001;7(4-5):799–804. [PubMed] [Google Scholar]
- Song J.M., Lee K.H., Seong B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005;68(2):66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo P.P., Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo. 2015;29(1):1–4. [PubMed] [Google Scholar]
- Srivastava, A.K., Kumar, A., Misra, N., 2020. on the inhibition of COVID-19 protease by indian herbal plants: an in silico investigation. ArXiv preprint arXiv: 2004.03411.
- Srivastava R.A.K., Mistry S., Sharma S. A novel anti-inflammatory natural product from Sphaeranthus indicus inhibits expression of VCAM1 and ICAM1, and slows atherosclerosis progression independent of lipid changes. Nutr. Metab. 2015;12(1):20. doi: 10.1186/s12986-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W.W., Wang Y.G., Li P.B., Wu H., Zeng X., Shi R., Zheng Y.Y., Li P.L., Peng W. The potential application of the traditional Chinese herb Exocarpium Citri grandis in the prevention and treatment of COVID-19. Tradit. Med. Res. 2020;5(3):60–166. [Google Scholar]
- Subhose V., Srinivas P., Narayana A. Basic principles of pharmaceutical science in Ayurvĕda. Bull. Indian Inst. Hist. Med. 2005;35(2):83–92. [PubMed] [Google Scholar]
- Sumithira P., Mangala S.D., Sophie A.M., Latha C.P. Antiviral and antioxidant activities of two medicinal plants. Int. J. Curr. Sci. 2012;256:261. [Google Scholar]
- Tabuti J.R., Lye K.A., Dhillion S.S. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J. Ethnopharmacol. 2003;88(1):19–44. doi: 10.1016/s0378-8741(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Talyshinsky M.M., Souprun Y.Y., Huleihel M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002;2(1):8. doi: 10.1186/1475-2867-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli A., Ahmadi A., Razavi B.M., Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran. J. Pharm. Res. 2017;16:2. [PMC free article] [PubMed] [Google Scholar]
- Thayil Seema M., Thyagarajan S.P. Methanol and aqueous extracts of Ocimum kilimandscharicum (Karpuratulasi) inhibits HIV-1 reverse transcriptase in vitro. Int. J. Pharmacogn. Phytochem. Res. 2016;8:1099–1103. [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y.C., Lewin S.R., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher D., Turhani D., Pelzmann M., Wannemacher B., Knerer B., Formanek M., Wacheck V., Selzer E. Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck-J. Sci. Spec. 2003;25(9):732–740. doi: 10.1002/hed.10231. [DOI] [PubMed] [Google Scholar]
- Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Thi Phuong Loan H., Triet N.T., Anh T.T.V., Quy P.T., Tat P.V., Hue N.V. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toots M., Yoon J.J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G., Shean R.C. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11(515):eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu G., Ertas A., Kolak U., Ozturk M., Ulubelen A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. Arkivoc. 2007;7:195–208. [Google Scholar]
- Tsai Y.C., Lee C.L., Yen H.R., Chang Y.S., Lin Y.P., Huang S.H., Lin C.W. Antiviral action of tryptanthrin isolated from strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10(3):366. doi: 10.3390/biom10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnlund J.R., Jacob R.A., Keen C.L., Strain J.J., Kelley D.S., Domek J.M., Keyes W.R., Ensunsa J.L., Lykkesfeldt J., Coulter J. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am. J. Clin. Nutr. 2004;79(6):1037–1044. doi: 10.1093/ajcn/79.6.1037. [DOI] [PubMed] [Google Scholar]
- ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharmaceut. Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo, R.Y., Meiyanto, E., 2020. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints 2020, 2020030214 (doi: 10.20944/preprints202003.0214.v1).
- Vaghasiya J., Datani M., Nandkumar K., Malaviya S., Jivani N. Comparative evaluation of alcoholic and aqueous extracts of Ocimum sanctum for immunomodulatory activity. Int. J. Pharm. Biol. Res. 2010;1(1):25e9. [Google Scholar]
- Varshney P., Dash S.K., Bhatia A.K. In vitro and in vivo antiviral potential of hot aqueous extract of ocimum sanctum and argemone mexicana leaves. Med. Plant Res. 2013;3(11) doi: 10.5376/mpr.2013.03.0011. [DOI] [Google Scholar]
- Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P.K.S.M., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ververidis F., Trantas E., Douglas C., Vollmer G., Kretzschmar G., Panopoulos N. Biotechnology of flavonoids and other phenylpropanoid‐derived natural products. Part I: chemical diversity, impacts on plant biology and human health. Biotechnol. J.: Healthcare Nutr. Technol. 2007;2(10):1214–1234. doi: 10.1002/biot.200700084. [DOI] [PubMed] [Google Scholar]
- Villa T.G., Feijoo-Siota L., Rama J.L.R., Ageitos J.M. Antivirals against animal viruses. Biochem. Pharmacol. 2017;133:97–116. doi: 10.1016/j.bcp.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalanathan S., Ignacimuthu S., Hudson J.B. Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharm. Biol. 2009;47(5):422–429. [Google Scholar]
- Walter T.M., Justinraj C.S., Nandini V.S. Effect of Nilavembu kudineer in the Prevention and Management of COVID–19 by inhibiting ACE2 Receptor. Siddha Papers. 2020;15(2) [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 doi: 10.1172/jci.insight.137799. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Kovalchuk, A., Li, D., Ilnytskyy, Y., Kovalchuk, I., Kovalchuk, O., 2020b. In search of preventative strategies: novel anti-inflammatory high-CBD cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Preprints 2020, 2020040315 (doi: 10.20944/preprints202004.0315.v1). [DOI] [PMC free article] [PubMed]
- Wang X., Jia W., Zhao A., Wang X. Anti‐influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Winston D., Maimes S. Adaptogens: herbs for strength. Stam. Stress Rel. 2007:226–227. [Google Scholar]
- Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metabol. 2007;51(4):301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. S0016-5085(20)30282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.W., Ikeda K., Kobayakawa A., Ikami T., Kayano Y., Mitani T., Yamori Y. Downregulation of Rac1 activation by caffeic acid in aortic smooth muscle cells. Life Sci. 2005;76(24):2861–2872. doi: 10.1016/j.lfs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Zhang H. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell E. Herbs for viral respiratory infections. Altern. Complement. Ther. 2018;24(1):35–43. [Google Scholar]
- Yasmin A.R., Chia S.L., Looi Q.H., Omar A.R., Noordin M.M., Ideris A. In Feed Additives. Academic Press; 2020. Herbal extracts as antiviral agents; pp. 115–132. [Google Scholar]
- Yeh C.F., Wang K.C., Chiang L.C., Shieh D.E., Yen M.H., San Chang J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013;148(2):466–473. doi: 10.1016/j.jep.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.S., Lee J., Lee J.M., Kim Y., Chin Y.W., Jee J.G., Keum Y.S., Jeong Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;117583 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30(7):1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]