Abstract
Many migratory species have shifted their geographic distribution in response to climate change, yet the underlying mechanisms are poorly understood, particularly for mammals. We hypothesized that generational shifts are underlying the observed colonization of hibernation sites further north in a migratory bat, the common noctule (Nyctalus noctula). To evaluate our hypothesis, we collected long-term data on the migratory status and demography of common noctules in a recently colonized hibernation area. Based on isotopic data of 413 individuals, we observed a significant decline in the proportion of long-distance migrants from 2004 to 2015 for both sexes and across all age groups. Demographic data collected between 2007 and 2016 from 3394 individuals demonstrated that subadult males were more abundant during the early colonization stage, followed by a gradual shift to a more balanced age and sex composition. Our results suggest that the colonization of hibernacula at higher latitudes is promoted by generational shifts, involving mostly first-year males. Generational shifts seem to be a likely mechanism for distribution changes in other bats and potentially also in other mammals.
Keywords: migratory behaviour, climate change, demography, hibernacula, stable isotope analysis, wintering area
1. Introduction
The current geological epoch of the Anthropocene is characterized by unprecedented rates of human-driven environmental changes [1]. These rapid changes force animals to respond adequately to survive and reproduce [2]. Migratory species are uniquely vulnerable to anthropogenic threats such as climate change [3,4]. The most commonly observed response of migratory species to climate change is an altered departure and arrival phenology. Yet, migratory animals may also change the geographic distribution of their wintering and summering areas. Such changes present key conservation challenges because altered phenology may cause mismatches between migratory events and resource availability. Also, newly established areas may be poorly protected [5]. Besides understanding these specific changes, it is equally important to investigate the underlying mechanisms, preferably based on long-term data that are sensitive to the dynamics of climate change. This might improve our ability to predict how responsive migratory species are to climate change.
Recently, Gill et al. [6] evaluated two possible mechanisms driving changes in the phenology and geographic distribution of black-tailed godwits. The first postulated mechanism was based on individual plasticity, i.e. individuals decide on destinations using environmental or social cues and thus migratory strategies may vary throughout their lifetimes. The second mechanism is based on migratory strategies changing from generation to generation, assuming that juveniles are key in colonizing new areas because of their dispersal capacity, whereas adults may not be able to respond adequately because they lack the flexibility in migration strategies. This mechanism may lead to a shift in the geographic distribution over several generations when individuals with successful migration strategies survive and reproduce. For black-tailed godwits, the authors confirmed the importance of a generational shift [6]. Yet, it is unknown if this mechanism is also relevant for other migratory taxa, such as mammals.
Bats are a diverse mammal taxon, with many species known to migrate over long distances [7]. Bats are particularly sensitive to adverse environmental conditions because the powered flight is energetically demanding [8], forcing individuals to feed almost constantly when airborne [9]. Accordingly, insectivorous bats strongly depend on the availability of insects throughout their active season. During winter, temperate-zone bats enter hibernation, a prolonged state of rest with lowered body temperature and metabolic rates. Bat survival during hibernation depends on an appropriate balance of fat stores, an adequate timing of hibernation and suitable hibernacula. Hence, many temperate-zone bats migrate to hibernate, particularly tree-dwelling species such as common noctules (Nyctalus noctula).
In common noctules, females migrate to hibernacula over longer distances than males [10,11], because in summer, females reach higher latitudes to give birth to their young. Males remain at lower latitudes where they mate with females in autumn close to hibernacula [10]. After weaning, young males are more likely to disperse than young females, and males do so in random directions [12]. Yet, most young males from northern breeding grounds seem to establish in areas south of their natal region [13], where they also occupy hibernacula at the northern border of the wintering range [14]. Survival of bats in these hibernacula depends on local temperatures and the length of the winter period [15], making the survival in these hibernacula highly dependent on climate change.
Shifts in hibernation latitude have already been documented for common noctules [16]. Over the last three decades, this species has colonized new wintering sites further north by around 4° latitude compared to previous wintering sites in Eastern Europe. The recent change in the distribution has led to a partial overlap of hibernation and breeding areas [14,17] (electronic supplementary material, S1), resulting in populations consisting of both migrating and non-migratory individuals [18]. Presumably, these changes in migratory behaviour may be driven by milder winters and longer plant growth periods [16,19,20]. However, the exact mechanisms mediating the observed changes in wintering sites remain unknown for bats. A mechanism based on behavioural plasticity would enable bats to respond quickly to new environmental conditions, whereas a generational shift may require more time since it depends on the recruitment of subadults with appropriate migration strategies. Our previous work demonstrated an individual consistency of migratory strategies in common noctules [11]. This argues against the individual plasticity hypothesis. Therefore, we hypothesized that changes in the northern limit of winter distribution of common noctules bats may be mediated by generational shifts.
Here, we used long-term observation data of common noctules in a region with newly occupied hibernacula in Eastern Europe to evaluate how migratory strategies changed within local wintering populations over time. Specifically, we used hydrogen stable isotope ratios in fur keratin to identify the summer origin of bats observed during 12 years in a newly colonized hibernation area. In the remainder of our paper, we refer to regional bats as those that remained within the range of similar isotopic composition, so-called isoclines, which encompasses usually 300–500 km in Europe, whereas long-distance migrants move beyond one or several isoclines [11]. Additionally, we document the age (subadults/adults) and sex composition in hibernacula of common noctules throughout this period. In line with the generational shift hypothesis and the male-biased dispersal documented for this species, we predict young males to be more present during the early colonization than other individuals. Due to climate change, young males may survive better at higher latitudes than before, which may account for the observed shift further north in the species' winter distribution, even when young males disperse in random directions [12,21].
2. Material and methods
(a). Study site
We conducted our fieldwork between 2004 and 2017 in the Kharkiv region (49.9935°N, 36.2304°E; Ukraine), where the local climate is strongly seasonal, with mostly dry-cold winters (−7.1°C in January) and hot summers (+20.5°C in July). Although common noctules are known to breed there since the onset of monitoring in the early twentieth century, the first hibernating common noctule was documented in 1986 [22].
(b). Collection of fur samples and demographic parameters
Fur samples were obtained from carcasses (n = 137) in the repository of the Bat Rehabilitation Center (BRC) of Feldman Ecopark and from individuals (n = 276) that were encountered in hibernacula in the Kharkiv area (https://datadryad.org/stash/share/mV6qK-JyQwbj4StU3h1On0Z6Ip3Ph38ULw45A8bes0w). Fur samples were taken with scissors from the interscapular region (electronic supplementary material, S4, for a more detailed description of moulting in common noctules). Between 2007 and 2016, we conducted surveys of 10 hibernacula and noted the sex and age (subadult (sad), i.e. individuals younger than 1 year, adult (ad)) of bats based on tooth wear and reproductive organs [23] (electronic supplementary material, S4).
(c). Stable isotope analysis
Stable hydrogen isotope ratios of bat fur were analysed at the Leibniz Institute for Zoo and Wildlife Research (IZW) in Berlin as described before [24]. Samples were dried in silver capsules over several days in a drying oven and then transferred to an autosampler. We report stable isotope ratios of non-exchangeable hydrogen in fur keratin in the delta notation (δ) as parts per thousand (‰) deviations from Vienna Standard Mean Ocean Water (V-SMOW). The precision of measurements was always better than 3‰ (one standard deviation) for repeated measurements of internal keratin standards.
(d). Statistics
We performed statistical analyses in the R 3.6.0 environment [25]. First, we used the ‘prepsource' function from the ‘IsoriX' package [26] to aggregate data from the Global Network of Isotopes in Precipitation (GNIP) for years (from 1960 to present time) (electronic supplementary material, S2) [27]. Next, we used the ‘IsoriX' package to assign the geographic origin of bats. We classified all bats to either regional or long-distant migrants based on a published transfer function [11]. We categorized bats as regional if δ2Hf values were inside of the 95% confidence interval (CI) of the expected δ2Hf values for the specific site and as long-distance migrants if individual δ2Hf values fell outside the 95% CI. To estimate the probability of being a long-distance migrant, we used binomial generalized linear model (GLM) via the ‘glm' function from R, with the binary migratory category (regional versus long-distance migrant) as a response variable and year, sex, age and interaction of year with sex and age as explanatory variables. To trace changes in demographic structure, we used a multinomial model from the package ‘nnet' [28] function ‘multinom' with year factor as the explanatory variable and the sex-age category (subadult male, subadult female, adult male or adult female) as a response. To test if migratory status was influenced by the proportion of subadult males, we used the ‘glm' function with migratory category as a response variable and subadult male versus all other sex-age categories as a binary explanatory variable.
3. Results
(a). Temporal changes in the geographic origin of hibernating bats
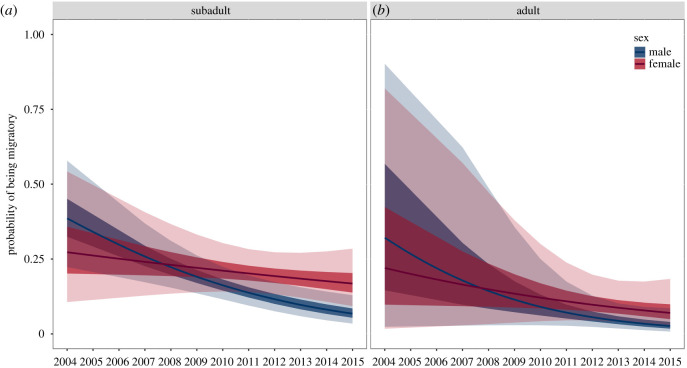
For hibernating common noctules of our study area, we did not observe an effect of the interaction of sex or age with time on the inferred migratory status, which suggests that both sexes and all age groups responded over time in a similar way (table 1). The probability of hibernating common noctules to be categorized as long-distance migrants decreased throughout the 12-year period, irrespective of sex and age (figure 1). Additionally, we observed an effect of sex on the probability of assignment as a long-distance migrant, with females being 2.8 times more likely to be long-distance migrants than males.
Table 1.
Odds ratio, 95% confidence interval (CI) and p-values for the binomial GLM fit of the migratory status data. Significant effects are highlighted in italics.
| predictors | migratory status |
||
|---|---|---|---|
| odds ratios | CI | p | |
| (intercept, male sad) | 0.07 | 0.04–0.15 | <0.001 |
| year | 0.82 | 0.73–0.92 | 0.001 |
| female | 2.78 | 1.09–7.08 | 0.032 |
| ad | 0.37 | 0.11–1.22 | 0.104 |
| year : female | 1.15 | 0.96–1.38 | 0.126 |
| year : ad | 0.94 | 0.68–1.30 | 0.703 |
| observations | 413 | ||
| AIC | 328.406 | ||
Figure 1.
Probability for hibernating common noctule bats (Nyctalus noctula) of getting assigned as a long-distance migrant over the 12-year study period in the Kharkiv area.
(b). Temporal changes in the demography of hibernating bats
Between 2007 and 2016, our hibernacula surveys comprised 3,394 individuals (https://datadryad.org/stash/share/XmsW1SVv9mebDMjjQOW_tLOAnuZ-Yfbm1qsy1hlm5Zc). We observed a decrease in the proportion of subadult males from 60% to 23% and an increase in the proportion of adult males from 7% to 35% and adult females from 12% to 25% in the winter population (figure 2; table 2). The probability of being a subadult male did not reliably predict the probability of being migratory (electronic supplementary material, S3). Thus, changes in the proportions of demographic groups alone did not explain the decrease in long-distance migrants.
Figure 2.
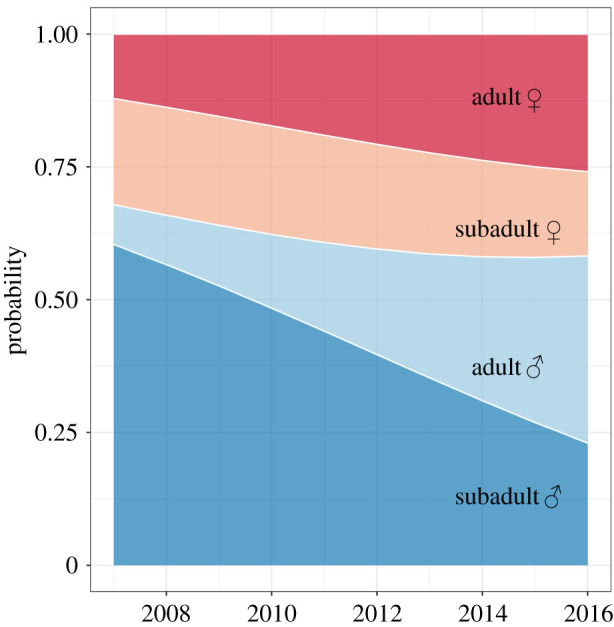
Probability for hibernating common noctules (Nyctalus noctula) of being in one out of four sex-age categories during the 10-year study period.
Table 2.
Odds ratio, 95% confidence interval (CI) and p-values and categorical response for the multinomial model fit of the sex and age classes during the 12-year study period. Significant effects are highlighted in italics.
| predictors | Sex-age group |
|||
|---|---|---|---|---|
| odds ratios | CI | p | response | |
| (intercept) | 0.09 | 0.07–0.13 | <0.001 | male ad |
| year | 1.32 | 1.26–1.38 | <0.001 | male ad |
| (intercept) | 0.31 | 0.23–0.41 | <0.001 | female sad |
| year | 1.08 | 1.04–1.13 | <0.001 | female sad |
| (intercept) | 0.17 | 0.12–0.23 | <0.001 | female ad |
| year | 1.21 | 1.16–1.26 | <0.001 | female ad |
| observations | 3394 | |||
| AIC | 8997.969 | |||
4. Discussion
We studied the mechanism underlying the colonization of a new wintering area in common noctule bats. We observed that regional bats dominated the hibernacula while the proportion of migrants decreased over time. Furthermore, first-year males were more abundant during the early colonization and likely also the first to colonize the new wintering area. Our findings are consistent with a generational shift mechanism for the colonization of the new wintering area at higher latitude [6]. This is the first study showing the relevance of generational shifts underlying a changing geographic distribution of a mammal, most likely driven by climate change.
We showed that winter aggregations of common noctules in the recently occupied sites were predominantly formed by regional individuals. During our study period, we noted fewer long-distance migrants with a northern summer origin and thus a lower variability in migratory behaviour, yet females tended to migrate over longer distances than males [11]. The decrease in the proportion of long-distance migrants in the hibernacula might be explained by two, not mutually exclusive, processes. On the one hand, the absolute number of long-distance migrants may have decreased over time as a result of a change in migration behaviour. On the other hand, the number of non-migratory individuals may have increased over time as a result of an increasing local population. Since we lack data on the sizes of source populations, we can only speculate about these scenarios but both seem likely for our study area. Indeed, long-distance migrants may become less abundant in the study region as northern areas become more suitable for wintering. This would be consistent with the occurrence of milder winter allowing for shorter hibernation periods [16]. The disappearance of long-distance migrants from the wintering population might simultaneously be driven by old long-distance migrants not being replaced by conspecifics with a similar migration strategy. Notably, common noctule bats are relatively short-lived (2–3 year lifespan [29,30]), which could explain the fast demographic changes observed in our study. Since reproductive rates of common noctules are relatively high for a bat [31], it is likely that favourable climatic conditions could cause rapid growth of the local populations, when breeding and hibernating individuals benefit from an extended plant growth period.
We observed higher recruitment of subadult males than of subadult females in the newly colonized wintering area. This pattern contrasts with those observed in black-tailed godwits where juveniles of both sexes mediate the change in distribution [6,32]. For common noctules, we expected this sex-difference because young males are more likely to disperse than young females [12,21]. Despite males being known to disperse randomly, we observed that young males from northern breeding grounds dispersed most likely to the south where they established in an area close to the wintering site. By doing so, some young males may establish successfully at higher latitudes than previous generations because climate change may have turned wintering conditions suitable. This could move the northern border of the wintering area to high latitudes over time. We consider it likely that the summer range in common noctules is also undergoing a similar northward shift like the one documented for the wintering range, yet we acknowledge that we lack data on this. Male-biased dispersal is commonly observed in other mammals as well [33], including other bats [34,35]. Therefore, sex differences in the early establishment of wintering areas may occur more broadly across migratory bats and other mammals.
The relevance of generational shifts for mediating changes in the geographic distribution of animals implies that populations may respond slowly to global climate change. The capacity to respond adequately on the population level may be defined by the level of intra-specific variation in migratory strategies, the reproductive rate and the rate at which young individuals are able to settle at new, suitable wintering areas, which is largely defined by the dispersal capacity in case of bats. We envisage that common noctule bats may respond relatively quickly to changing climatic conditions, owing to their relatively short lifespan, high reproductive rate and high dispersal capacity. We anticipate that species with a lower intra-specific variation of migration strategies, lower reproductive rates and a lower dispersal capacity may require more time to adjust to a changing climate. In the extreme, these species may not be able to respond adequately to a rapidly changing climate. The conservation of mammal species may require the development of suitable measures that take into account the susceptibility of a species to climate change which seems to be largely defined by how species-specific traits influence the speed at which generational shifts can compensate the effect of a changing climate.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Anja Luckner and Yvonne Klaar for analysing stable isotope ratios, and Dr Yuliia Kuznetsova, Dr Alyona Prylutska, Victor Kovalov, Vitaliy Hukov, Olena Rodenko and all members of the Bat Rehabilitation Center of Feldman Ecopark Team for help with sample collection. We thank Dr Shannon Currie for valuable comments on an earlier version of the manuscript. We also thank the handling editor and reviewers for their thoughtful comments which helped improve the manuscript.
Ethics
Fieldwork was carried out under license numbers Kh_KU_1006 8212 260. The Bat Rehabilitation Center works under the permission of Kharkiv Oblast Authority of Ecology and Natural Resources, and Ethical Commission of V.N. Karazin Kharkiv National University.
Data accessibility
Data from the study have been deposit in the electronic supplementary materials (table S3 and table S4) and is available from the Dryad Digital Repository Table S3: https://datadryad.org/stash/share/mV6qK-JyQwbj4StU3h1On0Z6Ip3Ph38ULw45A8bes0w Table S4: https://datadryad.org/stash/share/XmsW1SVv9mebDMjjQOW_tLOAnuZ-Yfbm1qsy1hlm5Zc.
Authors' contributions
K.A.K., A.V. and C.C.V. designed the study; C.C.V. and A.C. advised the students; K.A.K. and A.V. carried out the fieldwork; K.A.K., A.C. and L.S.L. performed the analyses. K.A.K., C.C.V. and A.C. wrote the manuscript. All authors offered comments, agreed to be held accountable for the content herein and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
K.A.K. was supported by a DAAD doctoral scholarship. A.V. and the Bat Rehabilitation Center of Feldman Ecopark have been funded by the International Charitable Foundation ‘Oleksandr Feldman Foundation'.
References
- 1.Waters CN, et al. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, aad2622 ( 10.1126/science.aad2622) [DOI] [PubMed] [Google Scholar]
- 2.Radchuk V, et al. 2019. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 10, 3109 ( 10.1038/s41467-019-10924-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilroy JJ, Gill JA, Butchart SHM, Jones VR, Franco AMA. 2016. Migratory diversity predicts population declines in birds. Ecol. Lett. 19, 308–317. ( 10.1111/ele.12569) [DOI] [PubMed] [Google Scholar]
- 4.Studds C, Marra P. 2007. Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim. Res. 35, 115–122. ( 10.3354/cr00718) [DOI] [Google Scholar]
- 5.Runge CA, Martin TG, Possingham HP, Willis SG, Fuller RA. 2014. Conserving mobile species. Front. Ecol. Environ. 12, 395–402. ( 10.1890/130237) [DOI] [Google Scholar]
- 6.Gill JA, Alves JA, Gunnarsson TG. 2019. Mechanisms driving phenological and range change in migratory species. Phil. Trans. R. Soc. B 374, 20180047 ( 10.1098/rstb.2018.0047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming TH, Eby P. 2003. Ecology of bat migration. In Bat ecology (eds Kunz TH, Fenton MB), pp. 156–208. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Speakman J, Thomas D. 2003. Physiological ecology and energetics of bats. In Bat ecology (eds Kunz T, Fenton M), pp. 430–490. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 9.Voigt CC, Sörgel K, Šuba J, Keišs O, Pētersons G. 2012. The insectivorous bat Pipistrellus nathusii uses a mixed-fuel strategy to power autumn migration. Proc. R. Soc. B 279, 3772–3778. ( 10.1098/rspb.2012.0902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strelkov PP. 1969. Migratory and stationary bats (Chiroptera) of the European part of the Soviet Union. Acta Zool. Cracoviensia 14, 393–436. [Google Scholar]
- 11.Lehnert LS, et al. 2018. Variability and repeatability of noctule bat migration in Central Europe: evidence for partial and differential migration. Proc. R. Soc. B 285, 20182174 ( 10.1098/rspb.2018.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petit E, Balloux F, Goudet J. 2001. Sex-biased dispersal in a migratory bat: a characterization using sex-specific deographic parameters. Evolution 55, 635–640. ( 10.1111/j.0014-3820.2001.tb00797.x) [DOI] [PubMed] [Google Scholar]
- 13.Sluiter JW, Van Heerdt PF.. 1964. Seasonal habits of the noctule bat (Nyctalus noctula). Arch. Neerl. Zool. 16, 423–439. ( 10.1007/s13398-014-0173-7.2) [DOI] [Google Scholar]
- 14.Strelkov PP. 2002. Materials on wintering of migratory bat species (Chiroptera) on the territory of the former USSR and adjacent regions. Part 2. Nyctalus noctula. Plecotus al, 35–56.
- 15.Humphries MM, Thomas DW, Speakman JR. 2002. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418, 313–316. ( 10.1038/nature00828) [DOI] [PubMed] [Google Scholar]
- 16.Godlevska LV. 2015. Northward expansion of the winter range of Nyctalus noctula (Chiroptera: Vespertilionidae) in Eastern Europe. Mammalia 79, 315–324. ( 10.1515/mammalia-2013-0178) [DOI] [Google Scholar]
- 17.Strelkov PP. 1997. Breeding area and its position in range of migratory bats species (Chiroptera, Vespertilionidae) in East Europe and adjacent territories. Communication 1. Zool. ZHURNAL 76, 1073–1082. [Google Scholar]
- 18.Vlaschenko A, Prylutska A, Kravchenko K, Rodenko O, Hukov V, Timofieieva O, Holovchenko O, Moiseienko M, Kovalov V. 2020. Regional recaptures of bats (Chiroptera, Vespertilionidae) ringed in Eastern Ukraine. Zoodiversity 54, 53 ( 10.15407/zoo2020.01.053) [DOI] [Google Scholar]
- 19.Rebelo H, Tarroso P, Jones G. 2010. Predicted impact of climate change on European bats in relation to their biogeographic patterns. Glob. Chang. Biol. 16, 561–576. ( 10.1111/j.1365-2486.2009.02021.x) [DOI] [Google Scholar]
- 20.Jones G, Rebelo H. 2013. Responses of bats to climate change: learning from the past and predicting the future. In Bat evolution, ecology, and conservation, pp. 457–478. New York, NY: Springer. [Google Scholar]
- 21.Petit E, Mayer F. 1999. Male dispersal in the noctule bat (Nyctalus noctula): where are the limits? Proc. R. Soc. Lond. B 266, 1717–1722. ( 10.1098/rspb.1999.0837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlaschenko AS. 2011. Research history and list of records of bats (Chiroptera) in the Kharkov Region in the XIX and XX centuries. Plecotus al 14, 26–54. [Google Scholar]
- 23.Kravchenko K, Vlaschenko A, Pprylutska A, Rodenko O, Hukov V, Shuvaev V. 2017. Year-round monitoring of bat records in an urban area: Kharkiv (NE Ukraine), 2013, as a case study. Turkish J. Zool. 41, 530–548. ( 10.3906/zoo-1602-51) [DOI] [Google Scholar]
- 24.Kravchenko KA, Lehnert LS, Vlaschenko AS, Voigt CC. 2019. Multiple isotope tracers in fur keratin discriminate between mothers and offspring. Rapid Commun. Mass Spectrom. 33, 907–913. ( 10.1002/rcm.8417) [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 26.Courtiol A, Rousset F, Rohwäder M-S, Soto DX, Lehnert LS, Voigt CC, Hobson KA, Wassenaar LI, Kramer-Schadt S.. 2019. Isoscape computation and inference of spatial origins with mixed models using the R package IsoriX. In Tracking animal migration with stable isotopes (eds Hobson KA, Wassenaar LI), pp. 207–236. Amsterdam, The Netherlands: Academic Press; ( 10.1016/b978-0-12-814723-8.00009-x) [DOI] [Google Scholar]
- 27.2020. IAEA/WMO. Global network of isotopes in precipitation. The GNIP Database See https://www.iaea.org/services/networks/gnip.
- 28.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York: NY: Springer; See http://books.google.com/books?id=X_KQcDpSB8MC&pgis=1. [Google Scholar]
- 29.Heise G, Blohm T. 2003. Zur AItersstruktur weiblicher Abendsegler (Nyctalus noctula) in der Uckermark. Nyctalus 9, 3–13. [Google Scholar]
- 30.Steffens R, Zöphel U, Brockmann D. 2004. 40 Jahre Fledermausmarkierungszentrale Dresden: methodische Hinweise und Ergebnisübersicht.
- 31.Kleiman DG. 1969. Maternal care, growth rate, and development in the noctule (Nyctalus noctula), pipistrelle (Pipistrellus pipistrellus), and serotine (Eptesicus serotinus) bats. J. Zool. 157, 187–211. ( 10.1111/j.1469-7998.1969.tb01697.x) [DOI] [Google Scholar]
- 32.Verhoeven MA, Loonstra AHJ, Hooijmeijer JCEW, Masero JA, Piersma T, Senner NR. 2018. Generational shift in spring staging site use by a long-distance migratory bird. Biol. Lett. 14, 20170663 ( 10.1098/rsbl.2017.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenseth N, Lidicker JW, Lidicker W. 1992. Animal dispersal: small mammals as a model. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 34.Heise G. 1982. Zu Vorkommen, Biologie und Ökologie der Rauhhautfledermaus (Pipistrellus nathusii) in der Umgebung von Prenzlau (Uckermark), Bezirk. Nyctalus 1, 281–300. [Google Scholar]
- 35.Lundberg K, Gerell R. 1986. Territorial advertisement and mate attraction in the bat Pipistrellus pipistrellus. Ethology 71, 115–124. ( 10.1111/j.1439-0310.1986.tb00577.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the study have been deposit in the electronic supplementary materials (table S3 and table S4) and is available from the Dryad Digital Repository Table S3: https://datadryad.org/stash/share/mV6qK-JyQwbj4StU3h1On0Z6Ip3Ph38ULw45A8bes0w Table S4: https://datadryad.org/stash/share/XmsW1SVv9mebDMjjQOW_tLOAnuZ-Yfbm1qsy1hlm5Zc.