Abstract
Efficient therapeutic strategies are needed to counter the COVID-19 pandemic, caused by the SARS-CoV-2 virus. In a context where specific vaccines are not yet available, the containment of the pandemic would be facilitated with efficient prophylaxis. We screened several clinical trials repositories and platforms in search of the prophylactic strategies being investigated against COVID-19 in July 2020. Up to July 5, 2020, only one clinical trial result was published, although we found 112 clinical trial protocols targeting medical workers (n = 70, 63%), patients relatives (n = 20, 18%) or individuals at risk of severe COVID-19 (n = 14, 13%). (Hydroxy)chloroquine was the most frequently evaluated treatment (n = 69, 62%), before BCG vaccine (n = 12, 11%), this followed by numerous antivirals and immune enhancers. Ninety-eight (88%) clinical trials were randomized with a median of planned inclusions of 530 (IQR 258–1299). Both pre- and post-exposure prophylaxes are investigated.
Keywords: Prophylaxis, COVID-19, Repurposed chemotherapy, SARS-CoV-2
1. Introduction
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is an emerging human coronavirus discovered in Wuhan, China, in December 2019. It causes COVID-19 (coronavirus-induced disease of 2019), which developed into a pandemic in early 2020: in July 5, 2020, more than 11.2 million persons had been infected worldwide and more than 530,000 died [1]. In the past six months, more than 12,400 articles have been published and scientific data collected from thousands of patients has been released. This impressive research and clinical work made better understanding of the disease and its different phases possible. Numerous clinical trials are currently investigating multiple therapeutic candidates and strategies [2], including prophylaxis.
Prophylaxis refers to measures taken to prevent the onset of the disease. There are two main categories of prophylaxis: pre-exposure prophylaxis (PrEP), where individuals are treated before encountering the pathogen, and post-exposure prophylaxis (PEP), which targets individuals who may have been infected (for example through contact with patients) although not yet developed symptoms. Both strategies have been extensively studied and efficiently used in public health policies against several pathogens such as HIV [3].
Prophylaxis is an interesting strategy for COVID-19 since it could both contain the spread of the SARS-CoV-2 and prevent the development of COVID-19, especially in patients at risk of severe forms. Indeed, we are lacking antiviral drugs that could decrease the mortality of patients with COVID-19 and only a few candidate vaccines against SARS-CoV-2 have reached the step of phase 1 or 2 clinical trials. As a consequence, most of the investigations on COVID-19 prophylaxis have used repurposed drugs which are already available in large quantities and whose safety has already been tested. In this review, we conducted a systematic census of clinical trial protocols on repurposing approaches for COVID-19 prophylaxis. We then discuss the rationale supporting the identified prophylaxis strategies and the therapeutic perspectives they raise. SARS-CoV-2 specific vaccines, non-pharmaceutical health measures and treatments to confirmed COVID-19 patients fall out of the scope of this review.
2. Methods
An extensive review of currently registered clinical trials was performed to identify relevant studies. A search for the official registration files and protocols of clinical trials was conducted on July 5, 2020 on the clinicaltrials.gov repository [4], the EudraCT repository [5], the anticovid platform [6], the covid-nma platform [7] and the covid-trials platform [8], using the keywords “prophylaxis”, “PrEP” and “prevention”, except in the covid-nma platform where the keywords “healthy” and “exposed” were also searched in the data file. A search in PubMed was conducted on July 5, 2020 with the default parameters and the key words “COVID-19 prophylaxis”, “COVID-19 prophylax*” and “COVID-19 vacc*”, in order to identify published results of the clinical trials identified on the above detailed platforms.
The eligibility criteria were developed using the Patient Intervention Comparison Outcomes Study type (PICOS) framework [9].
Inclusion criteria were:
-
•
population: persons without known infection with SARS-CoV-2;
-
•
intervention/comparator: any antiviral agent or drug already approved for clinical use against other conditions than COVID-19. Report selection and data extraction were performed manually by the authors. We excluded trials evaluating therapeutic strategies whose description was not sufficient to identify a specific drug;
-
•
outcomes: any outcome evaluating the infection with SARS-CoV-2;
-
•
study type: interventional clinical trial.
Data was manually curated. We extracted for each clinical trial the data corresponding to the variables “drug”, “mode of administration”, “treatment dose”, “duration”, “number of recipients”, “country”, “official date of beginning”, “nature of the control group”, “trial randomization”, “targeted population” and “trial phase”, then compiled them in Online material Table S1.
3. Results
3.1. Number of studies
We found 507 studies concerning COVID-19 prophylaxis on the clinicaltrials.gov repository [4], 53 on the EudraCT repository [5], 611 on the anticovid platform [6], 69 on the covid-nma platform [7] and 101 on the covid-trials platform [8]. The search on PubMed for scientific articles revealed only one published clinical trial result [10] corresponding to the clinical trials included in our study, out of 1944 total results of the query.
After eliminating the duplicates and the studies that were not testing prophylaxis (n = 1229), 112 relevant clinical trials were identified (Fig. 1 ), summarized in Table 1 . Ninety-eight (88%) clinical trials were randomized with a median of planned inclusions of 530 (IQR 258–1299).
Fig. 1.
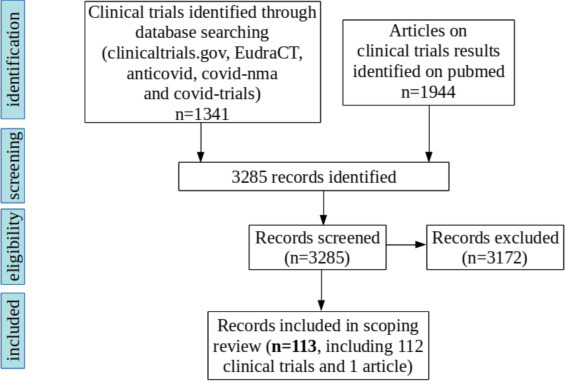
Flow chart of scientific literature search for clinical trials on prophylaxis for COVID-19.
Table 1.
Description of the clinical trials registered for the prophylaxis of COVID-19.
| n = 112 (%) | |
|---|---|
| Treatment | |
| Anti-malarial drugs | |
| (Hydroxy)chloroquinea | 69 (62) |
| Mefloquine | |
| Vaccines | 1 (1) |
| BCG vaccine | 12 (11) |
| Mycobacterium w | 1 (1) |
| Mycobacterium s manresensis | 1 (1) |
| Measles vaccines | 1 (1) |
| Antiviral drugs | |
| Lopinavir/Ritonavir | 3 (3) |
| Nitazoxanide | 3 (3) |
| Interferonb | 2 (2) |
| Nitric oxide | 2 (2) |
| Tenofovir + emtricitabine | 2 (2) |
| Bromhexine | 2 (2) |
| Arbidol | 2 (2) |
| Favipiravir | 1 (1) |
| Antibiotics and antiseptics | |
| Azithromycin | 3 (3) |
| Ivermectin | 2 (2) |
| Povidone-iodine | 2 (2) |
| Probiotics | |
| Immune modulators | |
| Lactobacillus coryniformis K8 | 1 (1) |
| Convalescent serum or purified immunoglobulins | 3 (3) |
| PUL-042 inhalation | 1 (1) |
| Quercetin | 1 (1) |
| QuadraMune | 1 (1) |
| Lactoferrin | 1 (1) |
| Levamisole + isoprinosine | 1 (1) |
| Others | |
| Thiazide + calcium blocker | 1 (1) |
| Mesenchymal stem cells | 1 (1) |
| Melatonin | 1 (1) |
| Targeted populationc | |
| Medical workers | 70 (63) |
| Patients relatives | 20 (18) |
| At-risk individuals | 14 (13) |
| Others | 16 (14) |
| Administration moded | |
| Oral | 83 (78) |
| Intradermal | 14 (13) |
| Inhaled/spray | 6 (6) |
| Intravenous | 2 (2) |
| Others | 2 (2) |
| Unspecified | 6 (6) |
| Study design | |
| Randomized | 98 (88) |
| Non-randomized | 13 (12) |
| Unspecified | 1 (1) |
| Total number of planned inclusions | |
| 200 and less | 26 (23) |
| 201–999 | 47 (42) |
| 1000 and more | 38 (34) |
| Unspecified | 1 (1) |
9 trials included an association of hydroxychloroquine with other drugs: hydroxychloroquine + azithromycin (two trials), hydroxychloroquine + arbidol (two trials), hydroxychloroquine + bromhexine, hydroxychloroquine + tenofovir + emtricitabine, hydroxychloroquine + lopinavir/ritonavir (two trials), hydroxychloroquine + ivermectin + zinc + vitamin C + povidone-iodine.
Interferons are simultaneously immune modulators and antivirals.
8 trials included several categories.
1 trial included both inhaled and oral treatments.
The complete list of trials analyzed for this review can be found in Online material Table S1.
3.2. Clinical trials protocols and results
Most trials focused on hydroxychloroquine (n = 69, 62%), followed by BCG vaccine (n = 12, 11%). The most frequently evaluated routes of administration were oral (n = 83, 78%), intradermal for non-specific vaccines (n = 14, 13%) and inhaled (n = 6, 6%). Both PrEP and PEP were investigated, with a substantial number of trials on PrEP for exposed medical workers (Online material Table S1). We noticed that the proportion of trials targeting at-risk categories increased over time (from 7% until late April to 20% during the last two months), while those targeting patients’ relatives decreased (from 24% until late April to 9% during the last two months). PrEP trials investigating hydroxychloroquine were conducted over 60 (IQR = 56–90) days in median, while the median duration of PEP studies on hydroxychloroquine was 5 (IQR = 5–14) days after exposure.
4. Discussion
In this section, we will first detail the rationale supporting the use of the main prophylactic treatments (used in at least two clinical trials). Then we will identify the main unsolved questions about COVID-19 prophylaxis and draw perspectives for further clinical developments.
4.1. (Hydroxy)chloroquine
Chloroquine derivatives, most notably hydroxychloroquine sulfate, inhibit coronavirus membrane fusion through an increase in endosomal pH and disrupt the glycosylation of their glycoproteins, as evidenced with SARS-CoV-1 [11]. They were suggested to be efficient against SARS-CoV-2 in vitro [12], [13], [14] and allegedly improved the disease in COVID-19 patients through a decrease in disease duration, viral charge and pneumonia symptoms [15], [16], although these reports are widely questioned and side effects are suspected [17], [18]. An investigation of hydroxychloquine as PrEP and PEP in macaques infected with SARS-CoV-2 revealed no improvement compared to untreated and infected controls [19]. These early results and the ease with which hydroxychloroquine can be produced and administered in high quantities may explain why it is the most investigated prophylaxis against COVID-19 despite very low evidence, with 62% (69/112) of all clinical trials analyzed in this review, involving more than 180,000 subjects in total (Table 2 ). The proportion of trials investigating hydroxychloroquine decreased after the publication of studies attributing side effects to this treatment (68% of trials before May against 52% in May and June); the relatively high remaining proportion of trials on hydroxychloroquine can partially but not completely be attributed to trials which were designed before a scientific consensus against hydroxychloroquine began to emerged, but started only recently.
Table 2.
Drugs investigated in more than two clinical trials, doses used and extent of the studies.
| Drug | Number of trials | Dose | Duration (days) | Number of participants |
|---|---|---|---|---|
| (Hydroxy)chloroquine | 69 | 400 mg/week to 600 mg/day (loading dose 200–1200 mg) | Median: 60 (IQR 22–84) | 180377 |
| BCG vaccine | 12 | 0.2–3 million CFU | 1 | 18970 |
| Lopinavir/ritonavir | 3 | Lopinavir: 400–800 mg/day Ritonavir: 100–200 mg/day |
5–60 | 2840 |
| Nitazoxanide | 3 | 1200–1800 mg/day | 7–42 | 1600 |
| Interferons | 2 | 2 × 180 μg/week | 14 | 614 |
| Nitric oxide | 2 | Inhalation of 160 ppm NO for 30 minutes per day | 14 | 670 |
| Tenofovir + emtricitabine | 2 | Emtricitabine: 200 mg/day Tenofovir: 25–245 mg/day | 84 | 5378 |
| Bromhexine | 2 | 24 mg/day | 14–60 | 540 |
| Umifenovir | 2 | ? | ? | 1000 |
| Azithromycine | 3 | 500 mg/week to 250 mg/day | 40–112 | 1300 |
| Ivermectin | 2 | 12 mg/day | 42 | 5266 |
| Povidone/iodine | 2 | 3 doses/day | 21–42 | 5250 |
| Convalescent serum and derivates | 3 | Variable | 1 | 2170 |
4.2. Tuberculosis or measles vaccines
The BCG tuberculosis vaccine is known to have non-specific protective effects against respiratory infections. Moreover, the geographical distribution of BCG vaccination is negatively correlated with the prevalence and mortality of COVID-19 [20], although the significance of this correlation is debated [21]. Twelve clinical trials of BCG vaccination are being conducted on medical workers exposed to COVID-19 or persons belonging to categories at risk of developing severe forms of COVID-19.
Similarly, Mycobacterium w and Mycobacterium s. manresensis, two other tuberculosis vaccines, are tested as anti COVID-19 prophylaxis (both PrEP and PEP) respectively in the trials NCT04353518 and NCT04452773.
An in silico comparison of SARS-CoV-2 proteins with those of the measles, mumps and rubella viruses suggested that the antigens of the MMR vaccine may immunize patients against SARS-CoV-2 epitopes [22]. Although this hypothesis has not yet been tested in vitro or in vivo, it prompted the launch of the NCT04357028 trial, where the MMR vaccine is used as PrEP for medical workers.
4.3. Lopinavir/ritonavir
Lopinavir/ritonavir [LPV/RTV), a protease inhibitor, was reported to improve SARS [23], although this study was criticized due to biases in patients’ assignment [24]. Its safety profile is ascertained by its widespread use against HIV [25]. LPV/RTV is investigated as COVID-19 PEP in the CORIPREV-LR trial (NCT04321174) and the COPEP trial (NCT04364022), and as PrEP in the COVIDAXIS trial (NCT04328285).
4.4. Nitazoxanide
Nitazoxanide is a broad-spectrum antiviral that amplifies cytoplasmic RNA sensing and type 1 IFN signaling. It inhibits SARS-CoV-2 replication in vitro [12] and is tested as PrEP for 600 elderly people in special care institutions in the trial NCT04343248, 200 patient relatives in the trial NCT04435314, and for 800 medical workers in the trial NCT04359680.
4.5. Interferons
Type 1 interferons (IFN) are cytokines with pro-inflammatory and unspecific antiviral properties. Although they are produced by the organism when an infection occurs, treating COVID-19 patients with additional IFN is thought to be protective in the early phases of infection, when acceleration in the recruitment of adaptive immunity can facilitate viral clearance [26]. Therefore, type 1 interferons appear suited to prophylaxis or early disease treatment, and to immunocompromized patients [27]. In late stages of the disease, an excessive immune response could be deleterious and the role of interferons is more debated. In macaques, prophylactic pegylated IFNα2b administered intramuscularly one on two days at 3 mg/kg decreased SARS-CoV-1 replication and lung damage [28]. As a therapy, IFNα2b has been reported to reduce SARS-CoV-2 infection duration [29] in a small-scale, non-randomized clinical trial. IFNα1b was used as a prophylaxis on hundreds of health care workers in China, many of whom were directly exposed to COVID-19 patients, and administered by nasal drops, in combination or not with thymosin-α1 (a putative enhancer of cellular innate immunity) [30]. No COVID-19 case was reported in the individuals who received the prophylaxis. Although very promising, this result must be further confirmed, since it stems from a non-randomized clinical trial. Different modes of IFN administration are studied, notably subcutaneous pegylated IFNλ1a in a phase 2 clinical trial (NCT04344600).
4.6. Nitric oxide
Nitric oxide, a signaling molecule and unspecific antimicrobial, inhibits SARS-CoV-1 replication in vitro [31]. It is investigated as PrEP for medical workers in contact with COVID-19 patients in the trial NCT04312243, where it is administered through gas. In the NCT04337918 trial, it is used both as PrEP for medical workers and as PEP. Several modes of administration are investigated in this trial: gargling, nasopharyngeal irrigation and nasal spray.
4.7. Nucleosides
Tenofovir disoproxil and emtricitabine are nucleoside inhibitors of HIV reverse transcriptase. Their use against COVID-19 in two clinical trials was probably prompted by the discovery that tenofovir binds SARS-CoV-2 RdRp [32], suggesting an antiviral effect.
4.8. Bromhexine
Bromhexine is a potent and specific inhibitor of the TMPRSS2 protease involved in SARS-CoV-2 spike protein maturation [33]. It is investigated in an early phase 1 clinical trial (NCT04340349).
4.9. Umifenovir (arbidol)
Umifenovir is a broad-spectrum antiviral approved in Russia and China which impairs viral membrane fusion [34] and displays anti SARS-CoV-2 effects. It was correlated with improvements in COVID-19 in a small-scale (16 patients in the arbidol group), non-randomized clinical trial [35]. It is investigated as PEP in the trials ChiCTR2000029803 and ChiCTR2000029592, combined with hydroxychloroquine.
4.10. Azithromycine
Azithromycine is an antibiotic and antiviral reported to synergize with hydroxychloroquine against COVID-19 in the controversial report of Gautret et al. [16]. Since then, the combination of azithromycin with hydroxychloroquine was shown to increase the risk of heart failure and cardiovascular mortality in a meta-analysis study [36], and did not improve viral loads in SARS-CoV-2 infected macaques, to which it was administered as PEP [19]. Azithromicin is nevertheless compared with hydroxychloroquine as PrEP for medical workers in the trials NCT04344379 and NCT04354597, and is also investigated alone in the NCT04369365 trial.
4.11. Ivermectin
Ivermectin is an FDA-approved broad spectrum anti-parasitic and anti-viral agent. It was shown to inhibit SARS-CoV-2 replication in vitro [37]. However, the concentrations necessary to achieve inhibition in this study cannot be reached in patients’ blood without serious side effects [38]. Ivermectin is however investigated in the phase 3 clinical trials NCT04446104 and 2020-001994-66 at doses of 12 mg/day, which is significantly higher than the doses used against parasites (of the order of 100 mg every few months).
4.12. Povidone-iodine
Povidone-iodine is an antiseptic administered as nasal spray or mouthwash. It demonstrated efficient inactivation of SARS-CoV-2 with durations of contact and concentrations seemingly suited for clinical use [39]. Its effects as PrEP administered as throat spray are tested in the trials NCT04364802 and NCT04446104.
4.13. Passive immunotherapy
Convalescent serum intravenous administration has been proposed as a passive antibody prophylaxis or therapy against COVID-19 [40] following the hypothesis that antibodies developed by the donor, who had been infected by SARS-CoV-2 and recovered, could protect the recipient against potential infection. Convalescent serum has already been used as a therapy against MERS-CoV [41], SARS-CoV-1 [42], [43] and SARS-CoV-2 [44], and resulted in improved prognosis, but has not yet been tested as a prophylaxis. The number of recovered patients is already very high and is expected to grow further; thus, if the pool of potential donors is efficiently harnessed, large quantities of convalescent serum could be produced and convalescent plasma may become a good candidate for prophylaxis. Therefore, convalescent serum has been included in the guidelines of the Infectious Diseases Society of America [45] for both PrEP and PEP. This treatment is tested as PEP, with 150 individuals belonging to categories highly susceptible to develop a severe disease, in a phase 2 clinical trial (NCT04323800). In the NCT04383548 trial, hyperimmune immunoglobulins purified from convalescent serum will be administered to 20 individuals belonging to at-risk categories.
An alternative to convalescent serum prophylaxis is the use of monoclonal antibodies (mAbs) against the spike protein of SARS-CoV-2. A cocktail of 2 neutralizing mAbs binding to non-overlapping sites on SARS-CoV-2 spikes [46] is investigated in the NCT04452318 trial as PEP.
However, the most relevant dose of convalescent serum has yet to be determined, and convalescent serum or mAbs treatments raise the risk of antibody-dependent enhancement of infection (ADE), a process observed in a few coronaviruses [47]. Consequently, investigations aiming to determine if convalescent antibodies for SARS-CoV-2 could induce ADE are warranted.
4.14. Perspectives
Most of the trial protocols studied here are randomized and include a large number of patients. Prophylaxis research efforts are mainly concentrated on (hydroxy)chloroquine, a fact which explains the high proportion of trials on orally administered drugs, and have already been reported for clinical therapeutic trials [2]. This trend goes against accumulating clinical evidence suggesting that chloroquine derivatives are inefficient or even harmful against SARS-CoV-2 infections [17], [18]. Numerous other antivirals potentially active on SARS-CoV-2 are investigated, but in a limited number of studies. Two thirds of the trials on immune enhancers are testing vaccines against tuberculosis or measles, notably the BCG vaccine. Numerous trials on hydroxychloroquine or BCG vaccine are redundant because they follow identical or very similar protocols.
Both PrEP and PEP are investigated. PrEP strategies target at-risk individuals (such as elderly or with chronic medical conditions such as obesity [48]) or, in most cases, medical workers highly exposed to infectious patients, on the protection of whom special emphasis is placed in order to keep health systems functional through the pandemic. We propose that the progressive increase in the proportion of trials targeting at-risk individuals, as new trials on patients’ relatives became rare simultaneously, reflects the transition from a situation of low SARS-CoV-2 prevalence, during which the pandemic may have been efficiently contained through ring prophylaxis, to a situation of high viral prevalence in which the priority is to protect vulnerable populations.
An important challenge with prophylactic treatments is that they must be pursued or repeated until the recipient is immunized or falls out of the priority categories since the protective effects are short-lived: from a few hours with interferon nasal drops [30] to a few weeks with convalescent serum [40]. The long duration of treatments and the fact that they are targeted on healthy individuals make proposing easily administered treatments with an excellent tolerance on an outpatient basis essential (which gives a further explanation for the prevalence of oral treatments in this study). Compared with therapeutic treatments, more risks are taken and less advantages are expected, which may lead to exclude treatments such as hydroxychloroquine for which side effects have been reported. Conversely, prophylactic doses may be lowered compared to therapeutic doses since fewer pathogens must be eradicated in order to prevent an infection than to eliminate an established pathology. Thus, certain drugs unsuited for therapy may be usable for prophylaxis in relevant doses.
Naturally, the prophylactic strategies evaluated are centered on the early antiviral action of drugs or the stimulation of the immune system, e.g. with interferons or convalescent antibodies. The anti-inflammatory strategies described elsewhere are reserved for patients with a severe disease and an excessive immune response to the virus [49].
Passive immunotherapy emerges as a potent prophylaxis strategy due to the current lack of a vaccine against SARS-CoV-2. The use of convalescent serum is well tolerated and can be easily upscaled thanks to the already high and still increasing number of immunized individuals, and can be safely practiced even in developing countries with limited access to pharmaceutical drugs [50]. Purified anti-SARS-CoV-2 immunoglobulines can be purified from convalescent serum, but at the price of complex manufacturing. The US Food and Drug Administration issued guidelines recommending the use of convalescent serum samples only with neutralizing titers superior to 1/160 [51].
Numerous mAbs able to neutralize SARS-CoV-2 have already been purified. Although their production is challenging to upscale; a cocktail of two broad spectrum neutralizing mAbs is already investigated in the clinical trial NCT04452318 and other trials are expected to follow suit as the manufacturing capacities improve. The use of cocktails of mAbs with non-overlapping binding sites on viral proteins is recommended in order to avoid the selection of viral resistance [52].
Only one clinical trial (NCT04308668) had its results published at the time of this study [10]. This randomized trial assessed hydroxychloroquine vs. placebo with 821 participants as PEP. The treatment consisted of a loading dose of 800 mg hydroxychloroquine, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days. Hydroxychloroquine yielded no significant effect of protection against disease development, and caused mild side effects in 40% of treated participants, confirming the accumulating evidence of hydroxychloroquine toxicity. New clinical trials results are expected to be published in the coming weeks.
We only included trials that were registered up to July 5, 2020 in this review, but new approaches could be tested in future trials, notably antivirals that demonstrated prophylactic efficiency against coronaviruses, such as EIDD-2801 [53].
5. Conclusion
Numerous strategies of prophylaxis against COVID-19 are currently investigated, and target different steps of the virus life cycle or the patient immune system. (Hydroxy)chloroquine is being evaluated in 62% of the registered clinical trials we found, while numerous prophylactic strategies were investigated in a small number of trials. This discrepancy highlights the need to increase the number of trials investigating antiviral drugs and immunity enhancers, in order to achieve an extensive cover of all promising candidate treatments against COVID-19.
Ethical Approval
All procedures performed in studies involving human partic-pants were in accordance with the 1964 Helsinki declaration and its later amendments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contribution of authors
Erwan Sallard: data curation; formal analysis; investigation; methodology; visualization; roles/writing – original draft.
Drifa Belhadi: validation; writing – review & editing.
François-Xavier Lescure: writing – review & editing.
Yazdan Yazdanpanah: writing – review & editing.
Nathan Peiffer-Smadja: conceptualization; methodology; project administration; supervision; writing – review & editing.
Disclosure of interest
The authors declare that they have no competing interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.medmal.2020.09.013.
Online material. Supplementary data
References
- 1.2020. John Hopkins University coronavirus resource center. [Cited 2020 Jul 5. Available from: https://coronavirus.jhu.edu/map.html] [Google Scholar]
- 2.Fragkou P.C., Belhadi D., Peiffer-Smadja N., Moschopoulos C.D., Lescure F.-X., Janocha H., et al. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26(8):988–998. doi: 10.1016/j.cmi.2020.05.019. [S1198743X20302962] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krakower D.S., Jain S., Mayer K.H. Antiretrovirals for primary HIV prevention: the current status of pre- and post-exposure prophylaxis. Curr HIV/AIDS Rep. 2015;12(1):127–138. doi: 10.1007/s11904-014-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ClinicalTrials.gov; 2020. Home. [Cited 2020 Apr 28. Available from: https://www.clinicaltrials.gov/] [Google Scholar]
- 5.2020. EU clinical trials register. [Cited 2020 Apr 27. Available from: https://www.clinicaltrialsregister.eu] [Google Scholar]
- 6.Peiffer-Smadja N., Lescure F.-X., Sallard E., Ravaud P., Vegreville B., Zeitoun J.-D. Anticovid, a comprehensive open-access real-time platform of registered clinical studies for COVID-19. J Antimicrob Chemother. 2020;75(9):2708–2710. doi: 10.1093/jac/dkaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.elise. Covid-19 - Living NMA [Internet]. Covid-19 - Living NMA. [cited 2020 Apr 28]. Available from: https://covid-nma.com/.
- 8.Thorlund K., Dron L., Park J., Hsu G., Forrest J.I., Mills E.J. A real-time dashboard of clinical trials for COVID-19. Lancet Digit Health. 2020;2(6):e286–e287. doi: 10.1016/S2589-7500(20)30086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [NEJMoa2016638] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14(1):72–77. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 16.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen L.S., Dolladille C., Drici M.-D., Fenioux C., Alexandre J., Mira J.-P., et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization pharmacovigilance database. Circulation. 2020;142(3):303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [Cited 2020 Jul 27; available from: http://www.nature.com/articles/s41586-020-2558-4] [DOI] [PubMed] [Google Scholar]
- 20.Shet A., Ray D., Malavige N., Santosham M., Bar-Zeev N. Differential COVID-19-attributable mortality and BCG vaccine use in countries. MedRxiv. 2020 doi: 10.1101/2020.04.01.20049478. [Cited 2020 Jun 25] [DOI] [Google Scholar]
- 21.Hensel J., McGrail D.J., McAndrews K.M., Dowlatshahi D., LeBleu V.S., Kalluri R. Exercising caution in correlating COVID-19 incidence and mortality rates with BCG vaccination policies due to variable rates of SARS CoV-2 testing. MedRxiv. 2020 doi: 10.1101/2020.04.08.20056051. [Cited 2020 Jun 25] [DOI] [Google Scholar]
- 22.Franklin R., Young A., Neumann B., Fernandez R., Joannides A., Reyahi A., et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. MedRxiv. 2020 doi: 10.1101/2020.04.10.20053207. [Cited 2020 Jun 25]; [DOI] [Google Scholar]
- 23.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. Low D, editor. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks C., King M., Gulick R., White C., Eron J., Kessler H. Long-term safety and durable antiretroviral activity of lopinavir/ritonavir in treatment-naive patients: 4 year follow-up study. AIDS. 2004;18:775–779. doi: 10.1097/00002030-200403260-00008. [DOI] [PubMed] [Google Scholar]
- 26.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H., et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.04.19.20068015. [Cited 2020 Jun 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A.M., Rimmelzwaan G.F., van Amerongen G., et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Chen V., et al. Interferon-a2b treatment for COVID-19. MedRxiv. 2020 doi: 10.1101/2020.04.06.20042580. [Cited 2020 Jun 25] [DOI] [Google Scholar]
- 30.Meng Z., Wang T., Li C., Chen X., Li L., Qin X., et al. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. MedRxiv. 2020 doi: 10.1101/2020.04.11.20061473. [Cited 2020 Jun 25] [DOI] [PubMed] [Google Scholar]
- 31.Åkerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist Å., Mirazimi A. Nitric oxide inhibits the replication cycle of Severe Acute Respiratory Syndrome Coronavirus. JVI. 2005;79(3):1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas J., Heinlein C., Kim T., Hernandez S., Malik M., True L. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villalaín J. Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J Phys Chem B. 2010;114(25):8544–8554. doi: 10.1021/jp102619w. [DOI] [PubMed] [Google Scholar]
- 35.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., et al. Arbidol combined with LPV/r versus LPV/r alone against coronavirus disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane J.C.E., Weaver J., Kostka K., Duarte-Salles T., Abrahao M.T.F., Alghoul H., et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. MedRxiv. 2020 doi: 10.1101/2020.04.08.20054551. [Cited 2020 Jul 5] [DOI] [Google Scholar]
- 37.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momekov G., Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnology & Biotechnological Equipment. 2020;34(1):469–474. [Google Scholar]
- 39.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in-vitro inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;29:529–533. doi: 10.1111/jopr.13209. [jopr.13209] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casadevall A., Pirofski L. The convalescent sera option for containing COVID-19. J Clin Investig. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko J.-H., Seok H., Cho S.Y., Eun Ha Y., Baek J.Y., Kim S.H., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh K.-M., Chiueh T.-S., Siu L.K., Lin J.-C., Chan P.K.S., Peng M.-Y., et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen C., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.IDSA COVID-19 guidelines. [Internet]. Available from: https://www.idsociety.org/practice- guideline/covid-19-guideline-treatment-and-management.
- 46.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1011. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. Gallagher T, editor. J Virol. 2019;94(5):e02015–e02019. doi: 10.1128/JVI.02015-19. [/jvi/94/5/JVI.02015-19.atom] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynants L., Van Calster B., Bonten M.M.J., Collins G.S., Debray T.P.A., De Vos M., et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders J., et al. Pharmacologic treatments for COVID-19: a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [Available from: https://jamanetwork.com/journals/jama/fullarticle/2764727] [DOI] [PubMed] [Google Scholar]
- 50.Lanza F., Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID-19 infected patients. Br J Haematol. 2020;190(1) doi: 10.1111/bjh.16814. [Cited 2020 Jul 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.2020. Recommendations for investigational COVID-19 convalescent plasma. [Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma] [Google Scholar]
- 52.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


