Abstract
Objective
Traditional Chinese medicine plays a significant role in the treatment of the pandemic of coronavirus disease 2019 (COVID-19). Tanreqing Capsule (TRQC) was used in the treatment of COVID-19 patients in the Shanghai Public Health Clinical Center. This study aimed to investigate the clinical efficacy of TRQC in the treatment of COVID-19.
Methods
A retrospective cohort study was conducted on 82 patients who had laboratory-confirmed mild and moderate COVID-19; patients were treated with TRQC in one designated hospital. The treatment and control groups consisted of 25 and 57 cases, respectively. The treatment group was given TRQC orally three times a day, three pills each time, in addition to conventional Western medicine treatments which were also administered to the control group. The clinical efficacy indicators, such as the negative conversion time of pharyngeal swab nucleic acid, the negative conversion time of fecal nucleic acid, the duration of negative conversion of pharyngeal-fecal nucleic acid, and the improvement in the level of immune indicators such as T-cell subsets (CD3, CD4 and CD45) were monitored.
Results
COVID-19 patients in the treatment group, compared to the control group, had a shorter negative conversion time of fecal nucleic acid (4 vs. 9 days, P = 0.047) and a shorter interval of negative conversion of pharyngeal-fecal nucleic acid (0 vs. 2 days, P = 0.042). The level of CD3+ T cells increased in the treatment group compared to the control group ([317.09 ± 274.39] vs. [175.02 ± 239.95] counts/μL, P = 0.030). No statistically significant differences were detected in the median improvement in levels of CD4+ T cells (173 vs. 107 counts/μL, P = 0.208) and CD45+ T cells (366 vs. 141 counts/μL, P = 0.117) between the treatment and control groups.
Conclusion
Significant reductions in the negative conversion time of fecal nucleic acid and the duration of negative conversion of pharyngeal-fecal nucleic acid were identified in the treatment group as compared to the control group, illustrating the potential therapeutic benefits of using TRQC as a complement to conventional medicine in patients with mild and moderate COVID-19. The underlying mechanism may be related to the improved levels of the immune indicator CD3+ T cells.
Keywords: Tanreqing Capsule, Coronavirus disease 2019, COVID-19 diagnostic testing, Negative conversion rate
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in widespread infection and severe consequences [1], leading to extreme hardships for many countries [2], [3]. Through the rapid global spread of COVID-19, the pandemic has had a profound impact on low- and middle-income countries [4]. However, drugs that may prevent or cure the disease are still under development, and their safety remains controversial. Traditional Chinese medicine (TCM) can contribute as an alternative measure. TCM practitioners have played a clinical role in several countries and regions; this may speed up the experimental research and clinical use of TCM herbs worldwide [5].
The National Health Commission of the People’s Republic of China has issued seven editions of the Protocol for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia [6]. In the Shanghai Traditional Chinese Medicine Protocol for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia (trial 2nd edition) [7], Tanreqing Capsule (TRQC) is recommended for treating patients with mild COVID-19. TRQC, a listed Chinese patent drug, is composed of five ingredients, including Scutellariae Radix, bear bile powder, goat horn, Lonicerae Japonicae Flos and Forsythiae Fructus, which has the effects of heat-clearing, phlegm dissipation and detoxification. It is used for the treatment of wind-warmth lung-heat disease, which is triggered by wind-heat attacking the lung. The symptoms include fever, aversion to wind, cough, phlegm or pharyngalgia, running nose and dry mouth.
This retrospective cohort study evaluated the efficacy of TRQC in patients with moderate and mild COVID-19 symptoms, through analyses of the negative conversion time of fecal nucleic acid, the duration of negative conversion of pharyngeal-fecal nucleic acid, the negative conversion time of pharyngeal swab nucleic acid and the length of hospital stay. Because the count of T-cell subsets is correlated to the severity and prognosis of COVID-19, we investigated the effects of TRQC on immune indicators, such as the improvement in the level of T-cell subsets (CD3, CD4 and CD45), as a proxy for treatment efficacy.
2. Methods
2.1. Study design and patients
For this single-center retrospective study, 315 hospitalized patients with laboratory-confirmed COVID-19 who had been admitted to the Shanghai Public Health Clinical Center between January 26 and April 15, 2020 were screened using the following inclusion and exclusion criteria.
The inclusion criteria were as follows: (1) positive nucleic acid test (NAT) of SARS-CoV-2 in pharyngeal swabs; (2) mild and moderate COVID-19 according to the Protocol for Diagnosis and Treatment of 2019 Novel Coronarvirus Pneumonia [6]; 3) patients with positive result of NAT in pharyngeal swabs or fecal samples during hospitalization; 4) patients aged 16–85 years, male or female.
The exclusion criteria were as follows: (1) death during hospitalization or readmissions; (2) respiratory tract bacterial infections due to primary or secondary immunodeficiency, congenital respiratory malformation, congenital heart disease, gastroesophageal reflux or lung malformation; (3) asthma or other chronic airway diseases needing maintenance therapy, acute respiratory tract bacterial infection (i.e., bronchiectasis, tonsillitis, bronchitis, rhinosinusitis, and otitis media) and severe pulmonary interstitial diseases; (4) severe systemic diseases (i.e., malignancy, autoimmune diseases, and liver or renal diseases) or surgeries (splenectomy, and organ transplantation) that might affect the assessment of efficacy; (5) pregnant or lactating women; (6) having participated in a clinical trial within three months; (7) known allergies to the study treatment medications; (8) other conditions as judged by the investigators.
The protocol has been registered with the China Clinical Trial Registry (website: www.chictr.org.cn; No. ChiCTR2000033320). The Medical Ethics Committee of Shanghai Public Health Clinical Center approved the study with ethics approval No. YJ-2020-S065-02.
2.2. Treatment
The clinicians treated the patients according to the Protocol for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia [6]. Both groups were given oxygen therapy, antiviral medications, antibiotics and other conventional treatments from Western medicine. In addition to the conventional Western medicine, the treatment group was given TRQC orally, three times a day, three pills each time, from the day of admission to the day before discharge.
2.3. Clinical characteristics and indicators
Data were collected retrospectively from electronic medical records. Baseline characteristics, including demographic features, clinical classification, fever on admission, Western medicine treatments (antiviral medications, antibiotics and oxygen therapy) and underlying diseases, were collected for each subject.
All patients in the treatment and control groups received daily pharyngeal swab NATs (at a 24-hour sampling interval). In the early stage of the COVID-19 outbreak, the fecal nucleic acid was not used for every patient in the Shanghai Public Health Clinical Center. When the fecal NAT gained widespread use in the Shanghai Public Health Clinical Center, nearly all patients began receiving them. Thus, once fecal NAT was in use, patients in the two groups were also subjected to fecal NAT once a day, with a 24-hour sample interval.
The elapsed time from the first positive NAT to the time which two consecutive (at the 24-hour sample interval) reverse transcriptase-polymerase chain reaction (RT-PCR) results for viral RNA in pharyngeal swabs were negative, was used to calculate “the negative conversion time of NAT.” Similarly, the first time that fecal NAT turned negative was used to calculate “the negative conversion time of fecal nucleic acid,” but without requiring two consecutive negative results. The interval between when the NAT of pharyngeal swabs and the NAT of fecal samples turned negative was called “the duration of negative conversion of pharyngeal-fecal nucleic acid.” The length of hospital stay for all patients was also recorded.
CD3, CD4, and CD45+ T cells were detected in both groups on the day of admission and the day before discharge. Fasting blood was collected in heparin tubes in the early morning and the expression of CD3, CD4 and CD45+ T cells was analyzed using a flow cytometer (Beckman Coulter, China). The difference between the admission and discharge tests for each index was used as the corresponding improvement value (dCD3, dCD4, and dCD45).
The NATs and T cell tests were conducted by the Department of Clinical Laboratory of Shanghai Public Health Clinical Center.
2.4. Diagnostic and clinical classification criteria
The diagnostic and clinical classification criteria for COVID-19 were based on the Protocol for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia [6].
The diagnostic criteria for confirming COVID-19 cases are as follows: suspected cases simultaneously having the following etiological or serological results: 1) positive result of real-time fluorescent RT-PCR assay for oral pharyngeal swab specimen; 2) the sequence of the viral genome being highly homologous to that of the known novel coronavirus; 3) serum-specific antibodies for SARS-CoV-2, IgM and IgG, are positive; serum specific IgG antibody for SARS-CoV-2 changes from negative to positive, or the level of IgG during the recovery period is 4-fold higher than that during the acute period.
The classification criteria for clinical COVID-19 are as follows.
-
(1)
Mild type: clinical symptoms are mild, and no pneumonia features are detected in radiological imaging.
-
(2)
moderate type: fever, respiratory or other symptoms, and pneumonia features detected in radiological imaging.
2.5. Statistical analysis
SPSS 25.0 (IBM Corporation) statistical software was utilized for most data analyses; however the comparisons of negative conversion time of nucleic acid and length of hospital stay between treatment and control groups were analyzed by using GraphPad Prism 8 software (GraphPad Software, LLC). The categorical variables are presented as counts and percentages and were compared using chi-square tests. Continuous variables are reported as mean ± standard deviation, or as median, lower quartile and upper quartile. For continuous data that met the criteria of the normality test, an independent t-test was used to make comparisons between groups. Otherwise, the Wilcoxon rank-sum test was used. The threshold for significance of statistical tests was set at P < 0.05.
3. Results
3.1. Patients
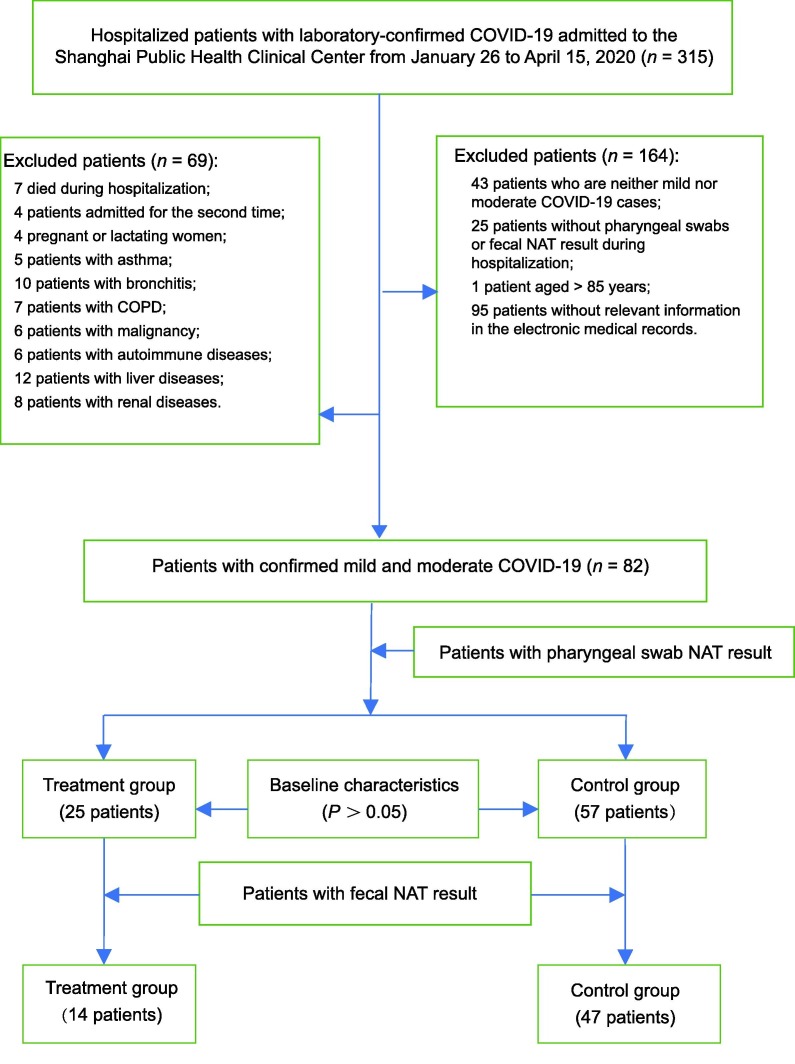
Initially, 315 patients admitted to the Shanghai Public Health Clinical Center between January 26 and April 15, 2020 were included in the study. Subsequently, we excluded the following patients: 43 patients who had neither mild nor moderate COVID-19 cases, 25 patients without pharyngeal swabs or fecal NAT result during hospitalization, 1 patient aged > 85 years, 95 patients without relevant information in their electronic medical records, 7 who died during hospitalization, 4 patients admitted for the second time, 4 pregnant or lactating women, 5 patients with asthma, 10 patients with bronchitis, 7 patients with chronic obstructive pulmonary disease, 6 patients with malignancy, 6 patients with autoimmune diseases, 12 patients with liver diseases, 8 patients with renal diseases. Therefore, data from the 82 patients that met the inclusion and exclusion criteria, with 25 in the treatment group and 57 in the control group, were used in this retrospective analysis (Fig. 1 ).
Fig. 1.
Study flow chart. COVID-19: coronavirus disease 2019; COPD: chronic obstructive pulmonary disease; NAT: nucleic acid test.
3.2. Baseline information
Among the 25 cases in the treatment group, 21 were moderate and 4 were mild; the cohort was comprised of 11 males and 14 females. Among the 57 cases in the control group, 50 were moderate and 7 were mild; 23 were males and 34 were females. The median ages of the treatment and control groups was 33 and 38 years, respectively, and were not statistically different (P = 0.290). The median intervals between the onset and the admission of the two groups were 4 days for each, and were also not statistically different (P = 0.955). The treatment and control groups were also similar in terms of fever (11 and 28, respectively; P = 0.669), medications antiviral (12 and 20, respectively; P = 0.270), antibiotics (6 and 13, respectively; P = 0.906) and oxygen therapy (20 and 49, respectively; P = 0.496). Seven cases from the treatment group and 16 from the control group were identified as having underlying diseases, which was not statistically different (P = 0.837). Both groups were comparable in terms of their demographic characteristics, clinical classification, fever on admission, Western medicine treatments and underlying diseases (Table 1 ).
Table 1.
Basic information of 82 patients with COVID-19.
| Baseline characteristics | Treatment group (n = 25) | Control group (n = 57) | Z/χ2 value | P value |
|---|---|---|---|---|
| Moderate type (n [%]) | 21 (84.00) | 50 (87.72) | 0.207 | 0.649 |
| Male (n [%]) | 11 (44.00) | 23 (40.35) | 0.095 | 0.757 |
| Age (year, median [lower quartile, upper quartile]) | 33 (23–53) | 38 (29–58) | –1.058 | 0.290 |
| Time interval between onset to admission (d, median [lower quartile, upper quartile]) | 4 (2–7) | 4 (2–7) | –0.056 | 0.955 |
| Fever (n [%]) | 11 (44.00) | 28 (49.12) | 0.183 | 0.669 |
| Medications antiviral (n [%]) | 12 (48.00) | 20 (35.09) | 1.218 | 0.270 |
| Antibiotics (n [%]) | 6 (24.00) | 13 (22.81) | 0.014 | 0.906 |
| Oxygen therapy (n [%]) | 20 (80.00) | 49 (85.96) | 0.463 | 0.496 |
| Underlying diseases (n [%]) | 7 (28.00) | 16 (28.07) | 0.042 | 0.837 |
Statistical methods: Chi-square test and Wilcoxon rank-sum test. COVID-19: coronavirus disease 2019.
3.3. Nucleic acid negative conversion, condition and length of hospital stay
In the early days of the COVID-19 outbreak, the fecal NST was not used in the Shanghai Public Health Clinical Center; thus, fecal NST results were not obtained for all patients. There were 14 cases in the treatment group and 47 cases in the control group that had NST results for both pharyngeal swabs and fecal samples (Fig. 1).
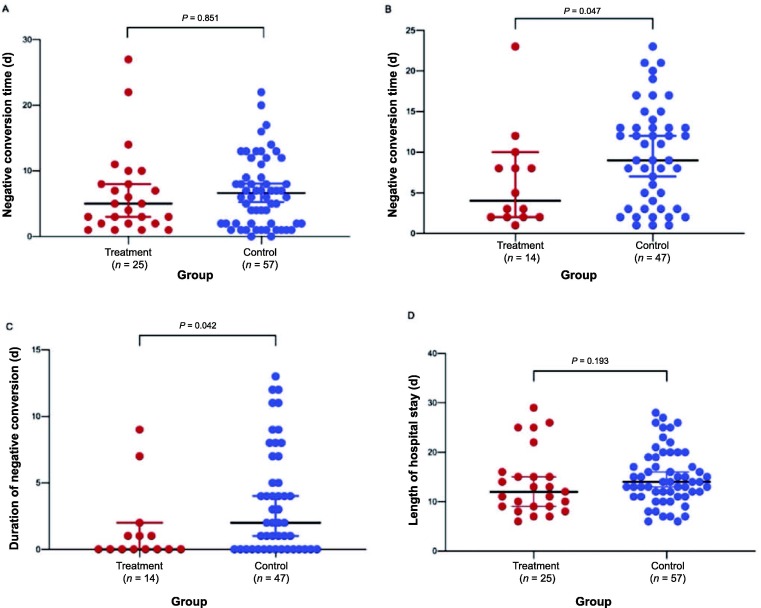
The negative conversion time of fecal nucleic acid was shorter in the treatment group, compared to the control group (4 vs. 9 days; P = 0.047). The negative conversion of pharyngeal-fecal nucleic acid was also shorter in the treatment group than in the control group (0 vs. 2 days; P = 0.042) (Fig. 2 ).
Fig. 2.
Comparison of the negative conversion time of nucleic acid and length of hospital stay between treatment and control groups. A: negative conversion time of pharyngeal swab nucleic acid; B: negative conversion time of fecal nucleic acid; C: duration of negative conversion of pharyngeal-fecal nucleic acid; D: length of hospital stay. Data were analyzed by independent t-test and Wilcoxon rank-sum test, and shown as the median value and 95% confidence intervals.
3.4. Improvement in the level of immune indicators
There was no significant difference in the levels of CD3+, CD4+ and CD45+ T cells before and after treatment in either of the two groups (P > 0.05; Table 2 ). However, the level of CD3+ T cells (dCD3) increased in the treatment group relative to the control group (P = 0.030), while there were no statistically significant differences between the treatment and control groups with regard to the median improvement of CD4+ T cells (P = 0.208) and CD45+ T cells (P = 0.117).
Table 2.
Comparison of improvement in the level of immune indicators between treatment and control groups.
| Immune indicator (counts/μL) | Treatment group |
Control group |
t/Z value | P value | ||
|---|---|---|---|---|---|---|
| n | Mean ± SD/median (lower quartile, upper quartile) | n | Mean ± SD/median (lower quartile, upper quartile) | |||
| CD3+ T cells | ||||||
| Before treatment | 23 | 1013.57 ± 474.24 | 47 | 1112.37 ± 538.48 | –0.767 | 0.445 |
| After treatment | 23 | 1382.79 ± 480.82 | 47 | 1235.04 ± 513.41 | 1.171 | 0.246 |
| Difference | 23 | 317.09 ± 274.39 | 47 | 175.02 ± 239.95 | 2.219 | 0.030 |
| CD4+ T cells | ||||||
| Before treatment | 24 | 583.5 (450.25–774.75) | 47 | 590 (446–783) | –0.233 | 0.816 |
| After treatment | 24 | 740 (620–965 | 47 | 684 (570.5–932) | –1.070 | 0.284 |
| Difference | 24 | 173 (62–293.75) | 47 | 107 (24.5–221) | –1.258 | 0.208 |
| CD45+ T cells | ||||||
| Before treatment | 23 | 1431.57 ± 619.41 | 47 | 1543.21 ± 744.98 | –0.635 | 0.527 |
| After treatment | 23 | 1907.83 ± 566.75 | 47 | 1732.79 ± 774.72 | 0.980 | 0.331 |
| Difference | 23 | 366 (105–820) | 47 | 141 (14–419) | –1.569 | 0.117 |
T-cell subsets are not detected on all the 82 patients involved. Data were analyzed by independent t-test and Wilcoxon rank-sum. SD: standard deviation.
3.5. Safety
The most common adverse event was diarrhea, which was experienced by four patients in the treatment group and three in the control group. There was no statistically significant difference in the recurrence rate of adverse events between the two groups (P = 0.241). Other adverse events were not observed. No serious adverse events were reported.
4. Discussion
Although the sample size was limited, statistical differences was detected in the negative conversion time for fecal nucleic acid (P = 0.047) and the duration of negative conversion time for pharyngeal-fecal nucleic acid (P = 0.042) between the treatment and control groups. This result shows the potential therapeutic effects of using TRQC along-side conventional medicine in mild and moderate COVID-19 patients.
TCM and biological agents are frequently used in registered COVID-19 clinical trials in China [8]. We have found that treatments that combine Chinese and Western medicine provide clues for managing COVID-19 patients in the Shanghai Public Health Clinical Center [9]. Improved patients’ outcomes have been noted when TCM and Western medicine are used together to treat COVID-19 [10]. Furthermore, a pilot randomized controlled trial has shown that TCM has a beneficial effect for the treatment of COVID-19; the death rate was lower in patients receiving Chinese herbal medicine plus standard care than patients receiving standard care alone [11]. Moreover, a core outcome set (COS) for clinical trials of COVID-19 has been published and the COS included SARS-CoV-2 NATs and lymphocytes [12].
The positive rate of the COVID-19 NAT of nasopharyngeal swab is 38.13%, the positive rate of 2019-nCoV sputum NAT is 48.68%, and the positive rate of 2019-nCoV fecal NAT is 9.83%. The positive rate of nasopharyngeal swab nucleic acid detection is not high. Additionally, SARS-CoV-2 can be detected in the digestive tract using fecal/anal swabs. The use of multiple-site NATs can improve diagnostic accuracy and reduce the false-negative rate, thus could guide the clinical treatment and evaluate the therapeutic effect [13]. Current evidence shows that the gastrointestinal infection caused by the virus can persist for a long time, even after the respiratory virus is no longer detectable. Therefore, the fecal NST for SARS-CoV-2 in COVID-19 patients should be routinely performed [14]. Currently, the fecal NAT is a primary method for diagnosis, efficacy evaluation, and isolation release of COVID-19 patients and is widely used in the Shanghai Public Health Clinical Center. Furthermore, this center has adopted stringent requirements for the discharge standard of patients with COVID-19, i.e., the pharyngeal swab NAT must be negative in two consecutive tests (24-hour sampling interval), and the fecal NAT must simultaneously be negative at least once.
The results of this study also showed that TRQC is related to the improvement in the level of the immune indicator T-cell subset CD3 among COVID-19 patients. Recent studies have found that the total number of lymphocytes and CD3 T-lymphocytes decreased significantly in severe patients, suggesting that COVID-19 is detrimental to the level of lymphocytes in humans, which would aggravate the patient’s condition [15]. The count of T-cell subset CD3 is correlated to the severity and prognosis of COVID-19. CD3 T-cells in the peripheral blood of COVID-19 patients decrease significantly compared to healthy individuals [16], [17]. The reduced levels of T-lymphocyte subsets may be a bio-marker for the early diagnosis of COVID-19 [18], and the regulation of changes in the immune factors may be important for the treatment of COVID-19.
Moreover, it has been found that use of TRQC in the clinical treatment of COVID-19 and other lung diseases can cause diarrhea in some patients. Hence, the effects of TRQC on the human digestive system are receiving extra attention. According to TCM theory, the lung and the large intestine are closely related in human physiology and pathology, i.e., “the lung stands in interior-exterior correlation with the large intestine.” A previous study showed that stimulating the intestine with purgative drugs (Mangxiao or Dahuang) specifically regulated the secretion of substance P, vasoactive intestinal peptide and their receptors in lung tissues [19]. This confirms the specific link between the lung and the large intestine and establishes the correlation between TRQC and the shortening of the negative conversion time of fecal nucleic acid. The mechanism might be related to improvements in the immune system of the patients; this phenomenon could also be explained by TCM theory.
Therefore, based on the important TCM concept of “the lung stands in interior-exterior correlation with the large intestine,” we focused on studying the side effects of TRQC, such as diarrhea. This purgative effect might be combined with other methods to treat COVID-19 and other lung diseases, and the underlying mechanism might be related to immune-mediated lung injury.
5. Limitations
This was a single medical center study, the sample size was limited, and thus, the findings may not be representative of the general population. This was an observational study, the level of evidence of which was relatively lower than a randomized controlled trial.
6. Conclusions
Although the sample size was limited, statistical differences were found in the reduction of the negative conversion time of fecal nucleic acid and the duration of negative conversion of pharyngeal-fecal nucleic acid between the treatment and control groups. This showed the potential effects of using TRQC as a complement to conventional medicine in mild and moderate COVID-19 patients. A correlation was established between TRQC and the reduction in the negative conversion time of fecal nucleic acid and the duration of negative conversion of pharyngeal-fecal nucleic acid in COVID-19 patients; the underlying mechanism may be related to the improved immune indicator CD3+ T cells.
Funding
This study was supported by Emergency Scientific Research Project of Shanghai Health Committee and Shanghai Administration of Traditional Chinese Medicine (No. 2020YJ01), Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (No. 14DZ2273200) and Shanghai Key Clinical Specialty (No. SHSLCZDZK05101).
Authors’ contribution
ZW and CQ contributed to the research conception and design. ZX and XY were responsible for data collection and analyses. ZX, XY, CX, WJM, SZJ, SM, LLJ, ZYB, ZYL, XGH, SMY, SXM, LYF and CXR participated in data interpretation and manuscript review and writing. All authors contributed to the scientific discussion of the data and of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Prof. Hua Lyu, Cheng-hai Liu, Wei-an Yuan and Ren-jie Chen, for their kind and professional assistance in data statistics and manuscript writing during our revision.
References
- 1.Omary M.B., Eswaraka J., Kimball S.D., Moghe P.V., Panettieri R.A., Scotto K.W. The COVID-19 pandemic and research shutdown: staying safe and productive. J Clin Invest. 2020;130(6):2745–2748. doi: 10.1172/JCI138646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sani G., Janiri D., Di Nicola M., Janiri L., Ferretti S., Chieffo D. Mental health during and after the COVID-19 emergency in Italy. Psychiatry Clin Neurosci. 2020;74(6):372. doi: 10.1111/pcn.13004. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T.H.D., Vu D.C. Summary of the COVID-19 outbreak in Vietnam – Lessons and suggestions. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bong C.L., Brasher C., Chikumba E., McDougall R., Mellin-Olsen J., Enright A. The COVID-19 pandemic: effects on low and middle-income countries. Anesth Analg. 2020;131(1):86–92. doi: 10.1213/ANE.0000000000004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling C.-Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) J Integr Med. 2020;18(2):87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Commission & State Administration of Traditional Chinese Medicine of the People’s Republic of China. Protocol for diagnosis and treatment of 2019 novel coronavirus pneumonia. (2020-03-03) [2020-06-20]. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml [Chinese].
- 7.Shanghai Municipal Health Commission & Shanghai Municipal Administration of Traditional Chinese Medicine. Shanghai traditional Chinese medicine protocol for diagnosis and treatment of 2019 novel coronavirus pneumonia. (2020-02-24) [2020-06-20]. http://wsjkw.sh.gov.cn/zyygz2/20200224/a1f1aab9745e4490867cb4aaf40eaad0.html [Chinese].
- 8.Lu L., Li F., Wen H., Ge S., Zeng J., Luo W. An evidence mapping and analysis of registered COVID-19 clinical trials in China. BMC Med. 2020;18(1):167. doi: 10.1186/s12916-020-01612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. BST. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 10.Cui H.T., Li Y.T., Guo L.Y., Liu X.G., Wang L.S., Jia J.W. Traditional Chinese medicine for treatment of coronavirus disease 2019: a review. Trad Med Res. 2020;5(2):65–73. [Google Scholar]
- 11.Ya Ye, G-CHAMPS Collaborative Group Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): evidence from China. Front Med (Lausanne) 2020;7:256. doi: 10.3389/fmed.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu R.J., Zhao C., Liang T.X., Hao X.Z., Huang Y., Zhang X.Y. Core outcome set for clinical trials of COVID-19 based on traditional Chinese and Western medicine. Front Pharmacol. 2020;11:781. doi: 10.3389/fphar.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Liu J., Li S., Peng Z., Xiao Z., Wang X. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J., Sun J., Hu Y. Enteric involvement of SARS-CoV-2: Implications for the COVID-19 management, transmission, and infection control. Virulence. 2020;11(1):941–944. doi: 10.1080/21505594.2020.1794410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z., Zheng Z., Wu K.e., Junhua Z. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging. 2020;12(9):7639–7651. doi: 10.18632/aging.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang M., Guo Y., Luo Q., Huang Z.K., Zhao R., Liu S.Y. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J Infect Dis. 2020;222(2):198–202. doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He R., Lu Z., Zhang L., Fan T., Xiong R., Shen X. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Fan C.Y., Wang A.l., Zou Y.L., Yu Y.H., He C. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong X.G., Zheng F.J., Li Y.H., Xu H., Wang Q., Liu Y.C. Specific link between lung and large intestine: a new perspective on neuropeptide secretion in lung with herbal laxative stimulation. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/547837. [DOI] [PMC free article] [PubMed] [Google Scholar]