Abstract
Background:
Women's mental health is a significant health issue for pregnant women during and after pregnancy. Postpartum depression (PPD) is defined as the presence of depression in the 1st year after delivery. This study reviews the relationship between Vitamin D levels, anxiety and poor sleep quality related to pregnancy.
Materials and Methods:
A systematic search was conducted on documents presented in PubMed, Scopus, ProQuest, Science Direct, Google Scholar and Web of Science databases until March 2020. Three separate search paths were considered for three different disorders with particular keywords. All observational studies that investigate the relationship between Vitamin D levels and PPD, sleep disorders, and anxiety were selected for inclusion in the study.
Results:
Search yielded 14 eligible studies. Quality of most included studies was medium to high. Nine studies reported that Vitamin D deficiency was directly associated to the incidence of PPD and sleep disorders. One study reported an indirect association, and three researches did not observe any association between Vitamin D status, sleep disorders and PPD. One study reported a direct association to anxiety but did not have any relation to PPD.
Conclusions:
PPD and sleep quality during pregnancy associated to Vitamin D deficiency directly. Although studies have several limitations, the importance of sufficient Vitamin D status in pregnant women has been addressed in all studies, especially regarding the prevention of PPD, anxiety, and poor sleep quality during the pregnancy.
Keywords: Anxiety, circadian clock, postpartum depression, sleep disorders, Vitamin D
Introduction
Postpartum depression (PPD) is defined as the presence of depression in the 1st year after delivery.[1] Approximately, 10%–18% of postpartum women in different society are affected by PPD.[2] The reported prevalence of PPD in Iran is 25.3%.[3] A large amount of researches explained adverse effects of PPD on the cognitive and emotional development of infants.[4] Symptoms of PPD are similar to other types of depression including feeling sad, hopeless, nervous, weight loss, and sleep deprivation.[5] Many postpartum women suffer from mental disorders such as anxiety and sleep disorders.[6] While they need sufficient sleep for their energy requirements. Hormonal changes may cause energy loss and alter sleep quality, sleep duration, and eating habits in early weeks of Post partum period.[7] Recommendations for preventing PPD include breast feeding, good asleep, and routine postnatal maternal health care.[8,9] Beside unwanted pregnancy, history of depression, dietary factors such as polyunsaturated fatty acid, Vitamin D, and homocysteine deficiency may have a role in the incidence of PPD.[8] Sleep deprivation can cause pregnancy complications such as gestational hypertension, gestational diabetes, PPD, and cesarean delivery.[10] Vitamin D3 is produced after skin exposed to the sun's ultraviolet rays.[11] Therefore, lack of sun exposure and indoor lifestyle are two major important causes of vitamin D deficiency.[12] Evidence for vitamin D has grown dramatically and a large amount of the present knowledge was published in the last decade.[13] Vitamin D has calcemic and non- calcemic functions.[14] It is a hormone which is needed for normal brain homeostasis and development.[15] In the brain, it promotes neurotransmission, neurogenesis, synaptogenesis, amyloid clearance and the prevent neuronal death.[16] Active form of vitamin D (calcitriol) is associated with production of serotonin by activating synthesis of tryptophan hydroxylase 2(TPH 2) in the brain[17] and also has protective effects against low level of dopamine and serotonin.[18] Insufficient level of vitamin D is also associated with several mental disorders such as anxiety,[19] sleep disorders such as restless leg syndrome (RLS) in pregnant women.[20] On the other hand, supplementation with medium dose of vitamin D (≥800 I. U. daily) had some desirable effects on the treatment of depression, especially when serum level of vitamin D was lower than normal range.[12] Vitamin D supplementation for 2 years was also associated to sleep improvement in 1500 patients with neurologic complaints.[21] Due to the importance of relationship between the levels of vitamin D and some disorders such as PPD, we made a comprehensive conclusion by conducting a systematic review and collecting all related evidence. This study reviews the effect of vitamin D deficiency on PPD, anxiety and poor sleep quality during pregnancy.
Materials and Methods
This systematic review was performed to collect any literatures to assess if vitamin D status is associated to PPD, anxiety and sleep disorders in pregnancy. A systematic search was performed on papers presented in PubMed, Scopus, ProQuest, Science Direct, Google Scholar and Web of Science databases until Oct 2020 without any time or language restriction. To get articles related to each of the disorders, a separate search was conducted by separate keywords. Keywords related to PPD: [postpartum depression OR depression, postpartum OR PPD AND vitamin D OR calciferol OR cholecalciferol OR calcitriol OR 25OHD OR 1,25 OHD]. Keywords related to sleep disorders: [sleep deprivation OR sleep disorders OR sleep quality AND pregnancy OR gestation AND vitamin D OR calciferol OR cholecalciferol OR calcitriol OR 25OHD OR 1,25 OHD]. And keywords related to anxiety: [anxiety AND pregnancy OR gestation AND Vitamin D OR calciferol OR cholecalciferol OR calcitriol OR 25OHD OR 1,25 OHD]. Studies that potentially examined the association between levels of Vitamin D and PPD, anxiety and poor sleep quality were investigated. For every article obtained in the database search, title and abstract was screened. The reference list of relevant articles was also searched. Next, potentially eligible publications also were examined by full text. We excluded duplicates, animal studies, editorials, letters, clinical trials, review articles and studies which assessed other types of depression rather than PPD.
The data extracted from each study included: author and year of publication, design and aim of the study, number of participants, country, and methods used to assess vitamin D status, vitamin D sampling time and method used to depression diagnosis. The data were filled by two independent writers in computer tables, at the end if there were any differences, it was discussed with the third author. The Newcastle-Ottawa Scale was used to assess the quality of the studies. Studies were scaled on the basis of participant selection (4 questions), the comparability of the study and statistical data analysis (2 questions), and the measurement of outcome (3 questions). Maximum number of score which can be given by each study was nine (four stars in selection, three stars in outcome and two stars in comparability section). Table 1 shows the quality of papers entered into the study.
Table 1.
Studies examined association of serum vitamin D and Postpartum depression
| Author (Year) | Study design | Sample size | Country | Method of vitamin D assessment | Vitamin D sampling time | Result | Quality assessment | questionnaires | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Murphy (2010) | Cohort | 97 | South Carolina | Rapid direct radioimmunoassay | First 7 months after delivery | Direct association between vitamin D deficiency and risk of PPD | Fair quality | EPDS | PPD |
| Nielsen (2013) | Case-control | 605 Cases 875 Controls | Denmark | Liquid chromatography-tandem mass spectrometry | At 10-12 and 25 weeks gestation | Indirect association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Gur (2014) | Cohort | 179 | Turkey | ELISA Enzyme-linked immunosorbent assay | At 24 and 28 weeks of gestation | Direct association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Robinson (2014) | Cohort | 796 | Australia | Enzyme immunoassay kit mass spectrometry | At 18 weeks of gestation | Direct association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| GOULD (2015) | Cohort | 1040 | Australia | Mass spectroscopy | As soon as possible after delivery | No association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Fu (2015) | Cohort | 213 | China | E601 modular analyzer | 24-48 hours after delivery | Direct association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Accortt (2016) | Cohort | 91 | USA | Competitive chemiluminescence immunoassay platform | At first trimester | No association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Amini S | cohort | 81 | Iran | ELISA | 1-6 months after delivery | Direct association between vitamin D deficiency and risk of PPD | Good quality | IPAQ, EPDS | PPD |
| Miyake (2016) | Cross-sectional | 1319 | Japan | Diet history questionnaire | 3-4 months after delivery | Direct association between vitamin D deficiency and risk of PPD | Good quality | EPDS | PPD |
| Williams (2016) | cohort | 105 | Michigan | 25-OH-D 125I Radioimmunoassay kit | at 12-20 weeks and 34-36 weeks | Direct association between vitamin D deficiency and risk of antenatal anxiety and depression No association between vitamin D deficiency and risk of PPD | Good quality | BDI, MINI, | Antenatal anxiety, depression and PPD |
| Huang (2014) | Cross sectional | 498 | Caucasian | liquid chromatography-tandem mass spectroscopy (LC-MS/MS) | At 15.4 weeks | Direct association between vitamin D deficiency and risk of PPD | Good quality | DASS-21, PHQ-9 | Antenatal anxiety and depression |
| Cheng (2017) | cohort | 890 | Singapore | Isotope-dilution liquid chromatography- tandem mass spectrometry | At 26-28 weeks gestation | Direct association between vitamin D deficiency and risk of sleep disorders | Good quality | PSQI | Sleep disorders |
| Gunduz (2015) | Cross-sectional | 92 | Turkey | High-performance liquid chromatography | At 36 gestational week | No association between vitamin D deficiency and risk of sleep disorders | Good quality | PSQI | Sleep disorders |
| Almeneessie AS (2020) | cross-sectional case-control | 742 | Arab (Saudi) | Not reported | third trimester | Direct association between vitamin D deficiency and risk of RLS | Good quality | IRLSSG | Sleep disorder-RLS |
PPD: Postpartum depression, RLS: Restless leg syndrome, PSQI: Pittsburgh Sleep Questionnaire, DASS-21: Depression, anxiety, and stress scales, IRLSSG: International RLS Study Group, BDI: Beck depression inventory, MINI: Mini International Neuropsychiatric Interview, PHQ-9: Patient health questionnaire depression module, LC-MS: Liquid chromatography-tandem mass spectroscopy, EPDS: Edinburgh Postnatal Depression Scale, IPAQ: International Physical Activity Questionnaire, ELISA: Enzyme-linked immunosorbent assay
Results
The search strategy and study selection procedure is displayed in Figure 1. A total of 989 articles were yielded by electronic search. After excluding duplicate records, title and abstract of 520 papers were evaluated and 496 articles were excluded. Reasons for excluding were as follows: 279 articles were unrelated, 197 studies assessed other type of depression rather than PPD and 20 publications were excluded because of other reasons. Next, full-text of 14 studies met all inclusion were reviewed. All included studies [Table 1] used a cohort design except for five studies.[22,23,24,25,26] Studies were conducted in different countries. There was heterogeneity in vitaminVitamin D sampling time. All studies were classified as good quality except for one.[27] Nine studies reported that vitamin D deficiency was directly associated to the incidence of PPD and sleep quality.[20,24,25,26,27,28,29,30,31] One study reported an indirect association,[22] three researches did not observe any relationship between vitamin D status, sleep disorders and PPD[23,32,33] and one study reported a direct association to anxiety but did not find any relationship to PPD.[34]
Figure 1.
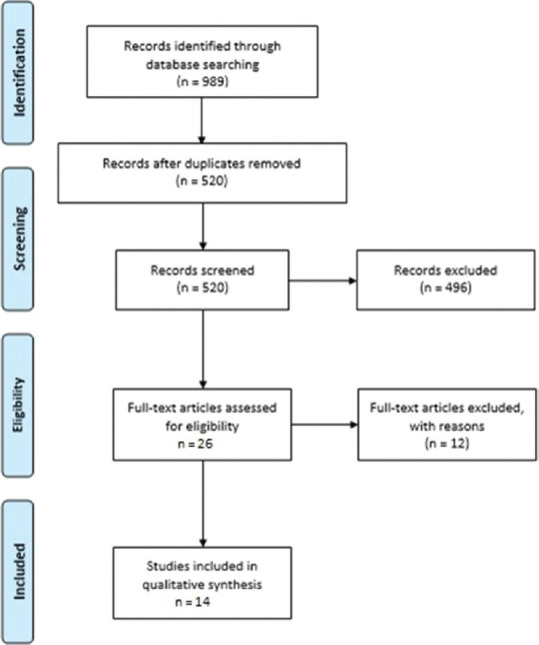
PRISMA flowchart of study selection process in each stage
Discussion
A study conducted on 796 pregnant women in Western Australia measured serum vitamin D concentration at 18 weeks' gestation. PPD was assessed 3 days after delivery with abbreviated form of Edinburgh Postnatal Depression Scale (EPDS). Findings showed that vitamin D had a protective effect on PPD (odds ratio (OR) = 2.19, 95% confidence interval (CI) = 1.26, 3.78).[28] Using a valid method for measuring vitamin D level and assessing vitamin D before the incidence of PPD were two major strengths of the mentioned study. In contrast, limitations were using an abbreviated form of EPDS and unavailable data regarding prenatal mental health.
In another study was carried out in Australia, investigators analyzed 1040 cord blood samples to evaluate vitamin D level in pregnant women. PPD was assessed at 6 weeks and 6 months after delivery with the use of EPDS score. The main aim of this study was to evaluate the effect of omega-3 fatty acid DHA supplementation during pregnancy on PPD. As a secondary result, the study found no significant association between vitamin D concentration and risk of PPD.[32] Some advantages of the mentioned study should be addressed: (1) Large sample size, (2) Using the best method for examining serum vitamin D level, (3) collecting EPDS questionnaire in two phases (early and late after pregnancy) and (4) measuring vitamin D levels before the incidence of PPD. As cord blood has lower level of Vitamin D than maternal blood sample, using cord blood sample was a limitation. Another limitation was assessing the serum level of Vitamin D as the secondary aim of the study, the result may have been confounded with w3 supplementation Moreover, the frequency of severe Vitamin D deficiency (<15 nmol/L) was low and it might affect statistical power of the study.
Accortt et al. focused on the association between Vitamin D, inflammation and risk of PPD.[33] Serum vitamin D concentration was measured in the first trimester. EPDS was used to evaluate PPD. An association between prenatal low level of 25(OH) D3 and the incidence of PPD was marginally significant (β = −0.209, P = 0.058). Observed association was mediated by interleukin-6 and the ratio of interleukin-6 to interleukin-10. The strength of this study was assessing mediatory role of inflammatory markers in the relation between vitamin D and PPD. Missing data during the 6 weeks after giving birth, participants withdrawals, residual confounding factors (e.g. socioeconomic status) were limitations of the mentioned study.
A case control study examined relation between serum vitamin D level and PPD. Women were interviewed by using EPDS at 10–12 and 25 weeks of gestation. Results showed that women with higher serum level of vitamin D (>80 nmol/L) were at greater risk of PPD[22.35] The study recommended that vitamin D binding protein (Gc protein) should be routinely assessed in pregnancy because it plays a key role in bioavailability of vitamin D.[22] One of the limitations of this study is the lack of remeasurement of vitamin D after delivery.
Yoshihiro Miyake et al. assessed 1319 pregnant women for dietary intake of dairy product by diet history questionnaire (DHA). The aim of this study was to assess whether higher intake of dairy products, calcium and vitamin D during pregnancy could decrease risk of PPD. EPDS score was used to assess PPD 3–4 months after delivery. The study showed that dairy-derived vitamin D had no association with PPD. Maybe this is because measurement of vitamin D after pregnancy has not been performed and authors used pregnancy values to evaluate this relationship. Therefore, administering this questionnaire in 3–4 months after delivery was a major limitation of this study. Furthermore, this study measured dietary intake of vitamin D instead of serum level. Moreover, Vitamin D content of dairy products is limited and it does not have important role in total Vitamin D metabolism.[26] In another study, 179 pregnant were examined at gestational weeks 24 and 28. Blood samples were collected in mid-pregnancy. Women were interviewed using EPDS at 1 week, 6 weeks, and 6 months after delivery. The study reported that women with PPD symptoms were more likely to have low level of Vitamin D. The limitations of the study: (1) small samples, (2) effect of season in level of Vitamin D.[30] A cohort study performed at the South Carolina evaluated 97 postpartum women. The study showed that low level of vitamin D was an effective factor for developing PPD. It should be addressed that several confounding factors (e.g. self-esteem, feeling adequate support and experiencing stressful life) were not controlled in mentioned study.[27] Another cohort study was conducted to assess the relation between serum vitamin D level at delivery and PPD in 3 months after delivery. Serum vitamin D was measured in 213 pregnant women in 24–48 h after delivery. Women were followed 3 months after delivery to complete EPDS. The study reported that PPD was most prevalent in women with low level of vitamin D (≤10.2 ng/ml) with an adjusted OR of 0.81 (95% CI 0.70–0.92; P < 0.0001). A major limitation of this study was collecting history of depression by self-report method.[29] Eighty-one Iranian women with a PPD participated in this study. A total of 27 patients were randomly assigned into three groups to receive either 50,000 IU Vitamin D3 fortnightly + 500 mg calcium carbonate daily; or 50,000 IU Vitamin D3 fortnightly + placebo of calcium carbonate daily, or placebo of Vitamin D3 fortnightly + placebo of calcium carbonate daily (placebo group) for 8 weeks. At the baseline and end of the study, the severity score of PPD, levels of 25-hydroxy Vitamin D, calcium. The PPD score had more reduction in the Vitamin D + calcium and Vitamin D + calcium placebo groups than that of the placebo group. The effect of vitamin D on the PPD score was larger when vitamin D was given alone than given together with calcium.[31] A study was performed on American women at high risk of depression to examine the effects of omega-3 supplementation on depressive symptoms in 2016. As the secondary result, the study showed that low level of vitamin D in early pregnancy associate to depressive and anxiety symptoms during pregnancy and not postpartum depression. beck depression inventory (BDI) and mini international neuropsychiatric interview (MINI) were completed at 12–20 weeks, 26–28 weeks, 34–36 weeks, and 6–8 weeks postpartum. Vitamin D levels were measured at 12–20 weeks and 34–36 weeks using radioimmunoassay kit. The major strength of the trial was evaluating vitamin D status and depression symptoms several times. There is a limitation with the study; using BDI instead of EPDS to assess depressive and anxiety symptoms.[34] Y. Huang et al. in a cross-sectional study examined 498 pregnant women at 15.4 weeks. Two questionnaires depression, anxiety, and stress scales (DASS-21) and patient health questionnaire depression module (PHQ-9) were used to determine the correlation between mental health symptoms and serum level of vitamin D. The study reported an inverse association between anxiety symptoms and serum vitamin D concentration, especially in patients who did not report physical activity. Confounders were controlled as the strength of the study includes the history of medicine, diabetes, anxiety, depression, and chronic hypertension.[24] Another cross-sectional study was performed to evaluate even low level of serum vitamin D associate to sleep disturbance in the last trimester of pregnancy. The Pittsburgh Sleep Questionnaire (PSQI) was used to measure sleep quality. Venus blood samples used to determine serum 25-hydroxy vitamin D levels of 92 pregnant women with poor sleep quality. The study did not support any association between low levels of vitamin D and sleep quality.[23]
A cohort study examined 890 pregnant women at 26–28 weeks' gestation in Singapore. PSQI was used as the sleep quality questionnaires. Maternal plasma 25OHD and 24-h dietary recall administered to determine the associations between maternal plasma 25OHD levels, night-time eating and sleep quality. The results in this article showed that vitamin D deficiency (<50 nmol/L) can cause poor sleep quality and night-time eating at mid-pregnancy. The strengths of the study were its large sample size, in addition accounting for confounders include variation in skin tone. Lack of physical activity measurement was one limitation of this study.[20] This cross-sectional case-control study aimed to attending antenatal care clinics. They interviewed pregnant women attending antenatal clinics face-to-face using the International RLS Study Group (IRLSSG) criteria. They assessed the severity of RLS using the IRLSSG severity scale for RLS (IRLS). The study revealed that RLS during pregnancy is linked to low level of vitamin D. Moreover, the physical activity level was evaluated using the short form of the International Physical Activity Questionnaire (IPAQ). A limitations were that only included the mothers who had low levels of serum 25[OH] D (lower than 75 nmol/L) and it is not clear if the same effect would be obtained in mothers with normal serum vitamin D levels. Moreover, the effect of only Ca (without vitamin D) was not studied.[25]
Although studies have several limitations, the importance of sufficient vitamin D status in pregnant women has been addressed in all studies, especially regarding the prevention of PPD. Vitamin D is associated with neuroprotection, neuroplasticity, brain development and regulating of neurotrophic factors. Vitamin D can elevate neurons glutathione metabolism and prevent oxidative degenerative processes by its anti-oxidant activity.[36] Low level of vitamin D alters brain morphology and expression of tyrosine hydroxylase gene which involved in norepinephrine and dopamine synthesis.[36] Normal level of serum vitamin D induces the expression of vitamin D receptors in amygdale, thalamus, hypothalamus, dorsal raphe nucleus and motor neurons.[37] Vitamin D has an important role in the brain glucose transportation by regulating GLUT3.[38] It plays a role in hypothalamic–pituitary– adrenal axis and hormonal and cellular event.[39] Some circadian behavioral and peripheral rhythms disruptions may be due to vitamin D deficiency.[20] 1,25OHD directly or by VDR regulates gene expression,[40] probably including circadian clock genes.[41] Reviewed studies did not control some confounding factors such as seasonal change and physical activity. Level of serum vitamin D alters during a year. The lowest level of vitamin D has been observed in winter.[42] Since all pregnant women did not enroll in studies at the same time, seasonal change could affect our results. Nevertheless, the risk of depression in fall and winter may increase independent of some confounding factors other than vitamin D deficiency.[43] For example, common cold in fall and winter has unfavorable effect on social support and results in incidence of depression. Furthermore, inflammatory markers increased following the common cold may have adverse role in depression.[43,44] In contrast, vacation time in summer can decrease risk of depressive symptoms.[43] Vitamin D binding protein, known as GC protein, is inversely associated with bioavailability of circulating and active form of vitamin D. Although measurement of total 25(OH) D3 (free form + protein-bound form) is common through the studies only free form of 25(OH) D3 can bind to Gc proteins. Gc protein increases during pregnancy and it results in lower level of free 25(OH) D3. It shows that high or normal level of 25(OH) D3 is not necessarily reflect adequate level of free 25(OH) D3 in pregnancy Evidence showed that high level of GC protein could be correlated with deleterious mutation and incidence of depressive symptom.[45] Therefore, it has been suggested that Gc should be measured in pregnancy as a routine test. Some probable reasons for decreased level of 1, 25(OH) D3 during pregnancy are: (1) elevated level of GC protein during pregnancy. It decreases physiologic activity of vitamin D; (2) genetic variations which decrease conversion of 25(OH) D3-1,25(OH) D3; (3) Pretty high level of vitamin D which stimulates 24-hydroxylase enzyme activity and increases conversion of active form 1,25(OH) D3 to inactive metabolite 1,24,25–trihydroxyvitamin D3, (4) Low level of 25(OH) D3 which decreases conversion of 25(OH) D3-1,25(OH) D3. Vitamin D deficiency has been linked to impaired dopaminergic neurotransmission.[46] The role of Vitamin D in the development of RLS was further supported by the higher concentration of Vitamin D binding protein in the cerebrospinal fluid of patients with RLS.[47] However, it is not proven yet whether treatment with Vitamin D can improve RLS symptoms. A recent randomized controlled trial of 35 patients with RLS who received either Vitamin D (50,000 IU caplets) or a placebo revealed that Vitamin D supplementation does not improve RLS symptoms.[48]
Effective and safe dose of vitamin D supplementation during pregnancy is remained controversial. Preeclampsia and IUGR were improved by 400 IU/day of vitamin D3 versus 4000 IU/day during the pregnancy.[49]
Several limitations were common in most included studies. Although most studies assessed PPD in a short period after delivery, it should be expanded to 1 year after delivery.[50] As EPDS can estimate PPD for only previous 7 days, this questionnaire may be useful in short-term investigations. Also, several studies used a self-administered EPDS, PSQI, BDI, MINI, DASS-21, IRLSSG, and PHQ-9 questionnaires and only two studies[26,30] used interviewers to complete them. All studies contained the small sample size. Hence, the sample size should be larger to increase to power of study. Studies did not report any data regarding anti-depressant medication during pregnancy.
Conclusions
Vitamin D deficiency seems to be associated to PPD and sleep disorders. Although studies have several limitations, the importance of sufficient vitamin D status in pregnant and postpartum women has been addressed in all studies, especially regarding the prevention of PPD. Probably, vitamin D supplementation in patients with vitamin D deficiency can help to improve PPD. However supplementing women with normal level of vitamin D cannot have beneficial effect on PPD. However, further studies are needed to determine the effect of vitamin D on PPD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- 1.O'Hara MW, McCabe JE. Postpartum depression: Current status and future directions. Annu Rev Clin Psychol. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo B, Pacheco A, Costa R. Depression during pregnancy and the postpartum period in adolescent and adult Portuguese mothers. Arch Womens Ment Health. 2007;10:103–9. doi: 10.1007/s00737-007-0178-8. [DOI] [PubMed] [Google Scholar]
- 3.Veisani Y, Delpisheh A, Sayehmiri K, Rezaeian S. Trends of postpartum depression in Iran: A systematic review and meta-analysis. Depress Res Treat. 2013;2013:291029. doi: 10.1155/2013/291029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck CT. Postpartum depression: A metasynthesis. Qual Health Res. 2002;12:453–72. doi: 10.1177/104973202129120016. [DOI] [PubMed] [Google Scholar]
- 5.Andrews-Fike C. A review of postpartum depression. Prim Care Companion J Clin Psychiatry. 1999;1:9–14. doi: 10.4088/pcc.v01n0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice SM, Aucote HM, Parker AG, Alvarez-Jimenez M, Filia KM, Amminger GP. Men's perceived barriers to help seeking for depression: Longitudinal findings relative to symptom onset and duration. J Health Psychol. 2017;22:529–36. doi: 10.1177/1359105315605655. [DOI] [PubMed] [Google Scholar]
- 7.Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM. Sleep in normal late pregnancy. Sleep. 1992;15:246–51. doi: 10.1093/sleep/15.3.246. [DOI] [PubMed] [Google Scholar]
- 8.Gould JF, Best K, Makrides M. Perinatal nutrition interventions and post-partum depressive symptoms. J Affect Disord. 2017;224:2–9. doi: 10.1016/j.jad.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Cinisomo S, McKenney K, Di Florio A, Meltzer-Brody S. Associations between postpartum depression, breastfeeding, and oxytocin levels in Latina mothers. Breastfeed Med. 2017;12:436–42. doi: 10.1089/bfm.2016.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191:2041–6. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 11.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spedding S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–18. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaney RP. Does inconclusive evidence for vitamin D supplementation to reduce risk for cardiovascular disease warrant pessimism? Ann Intern Med. 2010;153:208. doi: 10.7326/0003-4819-153-3-201008030-00016. [DOI] [PubMed] [Google Scholar]
- 14.Di Somma C, Scarano E, Barrea L, Zhukouskaya VV, Savastano S, Mele C, et al. Vitamin D and neurological diseases: An endocrine view. Int J Mol Sci. 2017;18:11. doi: 10.3390/ijms18112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Naughton DP. Vitamin D in health and disease: Current perspectives. Nutr J. 2010;9:65. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koduah P, Paul F, Dörr JM. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J. 2017;8:313–25. doi: 10.1007/s13167-017-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28:2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 18.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–71. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 19.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? Faseb J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 20.Cheng TS, Loy SL, Cheung YB, Cai S, Colega MT, Godfrey KM, et al. Plasma Vitamin D deficiency is associated with poor sleep quality and night-time eating at mid-pregnancy in Singapore. Nutrients. 2017;9:1–12. doi: 10.3390/nu9040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–5. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen NO, Strøm M, Boyd HA, Andersen EW, Wohlfahrt J, Lundqvist M, et al. Vitamin D status during pregnancy and the risk of subsequent postpartum depression: A case-control study. PLoS One. 2013;8:e80686. doi: 10.1371/journal.pone.0080686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunduz S, Kosger H, Aldemir S, Akcal B, Tevrizci H, Hizli D, et al. Sleep deprivation in the last trimester of pregnancy and inadequate vitamin D: Is there a relationship? J Chin Med Assoc. 2016;79:34–8. doi: 10.1016/j.jcma.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Huang JY, Arnold D, Qiu CF, Miller RS, Williams MA, Enquobahrie DA. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J Womens Health (Larchmt) 2014;23:588–95. doi: 10.1089/jwh.2013.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeneessie AS, Alyousefi N, Alzahrani M, Alsafi A, Alotaibi R, Olaish AH, et al. Prevalence of restless legs syndrome among pregnant women: A case-control study. Ann Thorac Med. 2020;15:9–14. doi: 10.4103/atm.ATM_206_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyake Y, Tanaka K, Okubo H, Sasaki S, Furukawa S, Arakawa M. Milk intake during pregnancy is inversely associated with the risk of postpartum depressive symptoms in Japan: The Kyushu Okinawa maternal and child health study. Nutr Res. 2016;36:907–13. doi: 10.1016/j.nutres.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Murphy PK, Mueller M, Hulsey TC, Ebeling MD, Wagner CL. An exploratory study of postpartum depression and vitamin d. J Am Psychiatr Nurses Assoc. 2010;16:170–7. doi: 10.1177/1078390310370476. [DOI] [PubMed] [Google Scholar]
- 28.Robinson M, Whitehouse AJ, Newnham JP, Gorman S, Jacoby P, Holt BJ, et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Womens Ment Health. 2014;17:213–9. doi: 10.1007/s00737-014-0422-y. [DOI] [PubMed] [Google Scholar]
- 29.Fu CW, Liu JT, Tu WJ, Yang JQ, Cao Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG. 2015;122:1688–94. doi: 10.1111/1471-0528.13111. [DOI] [PubMed] [Google Scholar]
- 30.Gur EB, Gokduman A, Turan GA, Tatar S, Hepyilmaz I, Zengin EB, et al. Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol. 2014;179:110–6. doi: 10.1016/j.ejogrb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Amini S, Amani R, Jafarirad S, Cheraghian B, Sayyah M, Hemmati AA. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutr Neurosci. 2020:1–11. doi: 10.1080/1028415X.2019.1707396. [DOI] [PubMed] [Google Scholar]
- 32.Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Gibson RA, et al. Association of cord blood vitamin D at delivery with postpartum depression in Australian women. Aust N Z J Obstet Gynaecol. 2015;55:446–52. doi: 10.1111/ajo.12344. [DOI] [PubMed] [Google Scholar]
- 33.Accortt EE, Schetter CD, Peters RM, Cassidy-Bushrow AE. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch Womens Ment Health. 2016;19:373–83. doi: 10.1007/s00737-015-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JA, Romero VC, Clinton CM, Vazquez DM, Marcus SM, Chilimigras JL, et al. Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth. 2016;16:203. doi: 10.1186/s12884-016-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: A nested case-control study. Cancer Causes Control. 2015;26:205–18. doi: 10.1007/s10552-014-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: The Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29. doi: 10.1186/1755-7682-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertone-Johnson ER. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr Rev. 2009;67:481–92. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol. 2004;89-90:557–60. doi: 10.1016/j.jsbmb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 39.Ellsworth-Bowers ER, Corwin EJ. Nutrition and the psychoneuroimmunology of postpartum depression. Nutr Res Rev. 2012;25:180–92. doi: 10.1017/S0954422412000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike JW, Meyer MB. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum Dis Clin North Am. 2012;38:13–27. doi: 10.1016/j.rdc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez-Monreal MA, Cuevas-Diaz Duran R, Moreno-Cuevas JE, Scott SP. A role for 1α,25-dihydroxyvitamin d3 in the expression of circadian genes. J Biol Rhythms. 2014;29:384–8. doi: 10.1177/0748730414549239. [DOI] [PubMed] [Google Scholar]
- 42.Schoenmakers I, Gousias P, Jones KS, Prentice A. Prediction of winter vitamin D status and requirements in the UK population based on 25(OH) vitamin D half-life and dietary intake data. J Steroid Biochem Mol Biol. 2016;164:218–22. doi: 10.1016/j.jsbmb.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Henriksson HE, Sylvén SM, Kallak TK, Papadopoulos FC, Skalkidou A. Seasonal patterns in self-reported peripartum depressive symptoms. Eur Psychiatry. 2017;43:99–108. doi: 10.1016/j.eurpsy.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Herder C, Schmitt A, Budden F, Reimer A, Kulzer B, Roden M, et al. Association between pro- and anti-inflammatory cytokines and depressive symptoms in patients with diabetes-potential differences by diabetes type and depression scores. Transl Psychiatry. 2018;7:1. doi: 10.1038/s41398-017-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glémin S. Surprising fitness consequences of GC-biased gene conversion: I. Mutation load and inbreeding depression. Genetics. 2010;185:939–59. doi: 10.1534/genetics.110.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013;236:77–87. doi: 10.1016/j.neuroscience.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 47.Patton SM, Cho YW, Clardy TW, Allen RP, Earley CJ, Connor JR. Proteomic analysis of the cerebrospinal fluid of patients with restless legs syndrome/Willis-Ekbom disease. Fluids Barriers CNS. 2013;10:20. doi: 10.1186/2045-8118-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wali SO, Abaalkhail B, Alhejaili F, Pandi-Perumal SR. Efficacy of vitamin D replacement therapy in restless legs syndrome: A randomized control trial. Sleep Breath. 2019;23:595–601. doi: 10.1007/s11325-018-1751-2. [DOI] [PubMed] [Google Scholar]
- 49.Ali AM, Alobaid A, Malhis TN, Khattab AF. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia-Randomized controlled trial. Clin Nutr. 2019;38:557–63. doi: 10.1016/j.clnu.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. Am J Obstetrics Gynecol. 1995;173:639–45. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]


