Abstract
Gastrointestinal stromal tumors (GISTs) are rare. Although most commonly found in the stomach, GISTs in the jejunum are among the rarest subtypes. A 46-year-old woman presented to the surgical department with proximal jejunal mass found in the examinations after abdominal pain and melena. Computed tomography imaging showed a 2.3 cm sized well-defined heterogenous enhancing mass in the proximal jejunum, and GIST was strongly suspected. The jejunal mass was identified 5 cm below the ligament of Treitz intraoperatively. She underwent robotic-assisted jejunal resection with intracorporeal robot-sewn anastomosis. The patient’s postoperative course was uneventful, and she was discharged on the seventh postoperative day. A robotic approach for GIST in the proximal jejunum is a safe and feasible procedure with good surgical outcomes.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs), occurring in smooth muscle interstitial cells of Cajal, are the most common mesenchymal tumors of the gastrointestinal tract. They can occur throughout the gastrointestinal tract, and the small intestine is the second-most frequent location after the stomach. The proximal jejunal GISTs are the rarest subtype in the small intestinal groups. Most GISTs are asymptomatic and diagnosed incidentally during an abdominal radiological investigation or a surgery for another aetiology [1]. Surgical resection is the primary treatment. Recently, robotic surgery for GISTs has been frequently reported. We report our first experience of a robotic-assisted resection and anastomosis for the proximal jejunal GIST with successful surgical outcome.
CASE REPORT
A 46-year-old single woman presented to the surgical department with intermittent abdominal pain and melena for 1 month. She had no significant medical history. The physical examination and all routine laboratory investigations were unremarkable. Abdominopelvic computed tomography (CT) showed a 2.3 cm sized well-defined heterogenous enhancing mass in the proximal jejunum and GIST was strongly suspected (Fig. 1).
Figure 1.
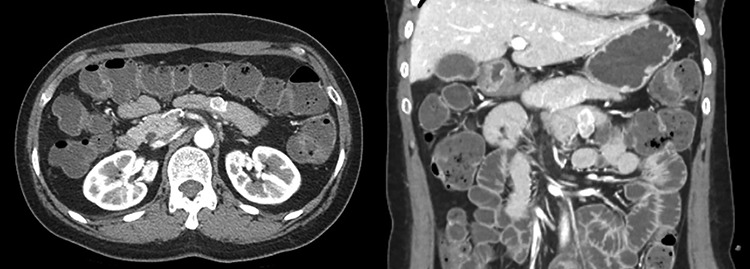
Abdomen CT showed a 2.3 cm sized well-defined heterogenous enhancing mass in the proximal jejunum and GIST was strongly suspected.
We planned robot-assisted resection for the proximal jejunal GIST because the patient wanted less pain and scar. The patient was placed in the supine position with arms laid along the body. After carrying out a pneumoperitoneum induction through Veress needle, an 8 mm port was placed just below the umbilicus for a 30 degree optics, and three other 8 mm robotic trocars were positioned (Fig. 2). After the insertion of the ports, the patient was placed in a reverse Trendelenburg position, inclined 15 degree to the right. The tumor was found in the proximal jejunum below 5 cm from the ligament of Treitz (Fig. 3). The jejunal segment was resected taking a 1-cm margin from both sides. When resecting the jejunum, 12 mm robotic trocar and robotic staplers were used. End-to-end robotic sewn anastomosis was performed with V-Loc 3-0 suture (Fig. 4). The specimen was extracted with rapped in the endo bag via 12 mm trocar site. The operative time was 150 minutes. The patient’s postoperative course was uneventful, and she was discharged on the seventh postoperative day. Histopathology confirmed a 2.1 × 1.2 × 2.0 cm sized spindle-cell GIST with strong positive staining for c-kit, DOG-1, CD34, smooth muscle actin and clear resection margin. Mitotic count was <5 for 50 high-power field, and the proliferative index evaluated by Ki67 was <10%.
Figure 2.
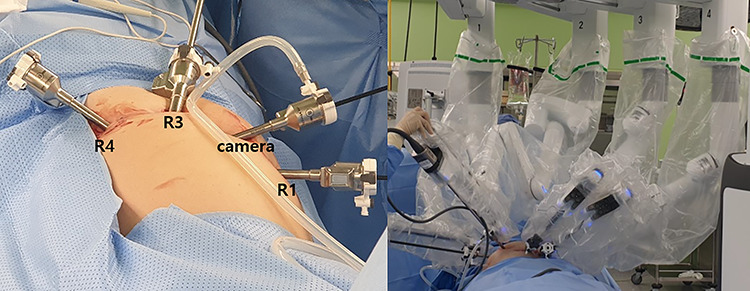
Trocars position with da Vinci Xi system.
Figure 3.
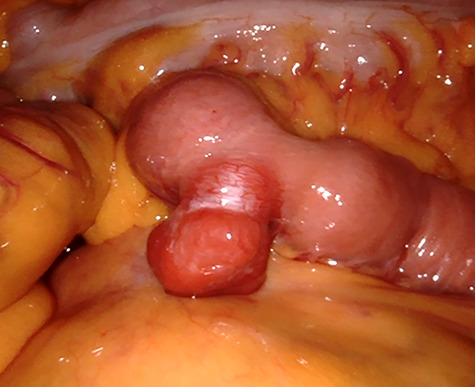
GIST in the jejunum as exophytic lesion.
Figure 4.
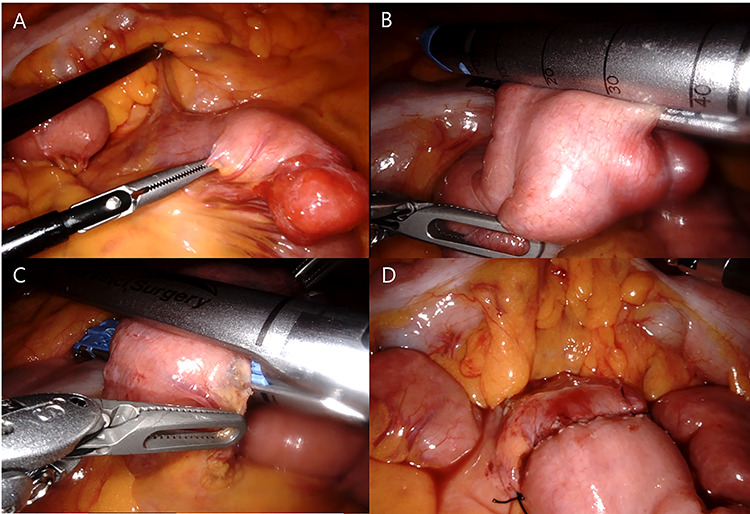
Robotic jejunal resection and anastomosis: identification (A), distal transaction (B), proximal transaction (C) and after anastomosis (D).
DISCUSSION
GISTs are the most common mesenchymal neoplasms of the gastrointestinal tract. They can occur throughout the gastrointestinal tract, but mostly in the stomach (60%) and the small intestine (30%), and rarely in the esophagus, colon, rectum or appendix [2]. The symptoms of GISTs are nonspecific and depend on the size and location. GISTs of the small intestine are typically asymptomatic when they are small and may be diagnosed incidentally from CT, endoscopy or during surgery. As tumors grow larger, various symptoms appear. The most common symptom is a gastrointestinal bleeding that presents in 50% of the patients, followed by abdominal pain (20–50%) and gastrointestinal obstruction (10–30%) [3]. Miettinen et al. [4] reported the median size of GISTs of the small intestine being as large as 7 cm at the time of diagnosis.
Surgical resection is the primary treatment for patients with localized or potentially resectable GIST lesions. The aim of surgery is complete resection with macroscopic and microscopic negative margins to prevent local recurrence. Lymph nodes metastasis is very rare and lymphadenectomy is not necessary. For small intestinal GISTs, partial small bowel resection with the tumor is the standard procedure. Gentle handling is necessary because the injury of the pseudocapsule may cause the dissemination of the tumor cells and may lead to poor patient prognosis [5]. Surgical resection can be performed with open, laparoscopic and robotic method depending on the location and shape of the tumor. The role for laparoscopy in the resections of GISTs continues to expand. Laparoscopic surgery is a safe and effective approach to remove GISTs with similar oncologic outcomes compared to open surgery [6].
Laparoscopic and robotic surgery has the advantage of faster postoperative recovery, less pain and has better cosmetic results. The Asian consensus guidelines recommend laparoscopic resection for GIST up to 5 cm size when it is located in a favorable location [7]. However, laparoscopic surgery has a high risk of injury to the pseudocapsule because GISTs tend to be very friable. There are also significant disadvantages to a laparoscopic surgery as limitations in instrument movement andrjaa301 amplification of hand tremors. Robotic surgery was introduced to overcome these problems of laparoscopic surgery and has been actively used recently. The robotic surgery provides an increased precision of instrument movement because of the joint structure of robotic arms and 3D-amplified view. This benefit helps in intracorporeal resection and anastomosis of bowel surgery. However, the matter of high cost of robotic surgery is a remaining issue.
In conclusion, a robotic approach for GIST in the proximal jejunum is a safe and feasible procedure with good surgical outcomes. However, further studies are necessary in order to investigate the effective role of robotic surgery in the treatment of GISTs.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
Contributor Information
Myeong Hun Oh, Department of Surgery, Pusan National University Hospital, Busan, Korea.
Byoung Chul Lee, Department of Surgery, Pusan National University Hospital, Busan, Korea.
References
- 1. Sankey RE, Maatouk M, Mahmood A, Raja M. Case report: Jejunal gastrointestinal stromal tumour, a rare tumour, with a challenging diagnosis and a successful treatment. J Surg Case Rep 2015;2015:rjv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer 2005;104:1781–8. [DOI] [PubMed] [Google Scholar]
- 3. Marcella C, Shi RH, Sarwar S. Clinical overview of GIST and its latest management by endoscopic resection in upper GI: a literature review. Gastroenterol Res Pract 2018;2018:6864256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477–89. [DOI] [PubMed] [Google Scholar]
- 5. Lai EC, Lau SH, Lau WY. Current management of gastrointestinal stromal tumors--a comprehensive review. Int J Surg 2012;10:334–40. [DOI] [PubMed] [Google Scholar]
- 6. Kim JJ, Lim JY, Nguyen SQ. Laparoscopic resection of gastrointestinal stromal tumors: does laparoscopic surgery provide an adequate oncologic resection? World J Gastrointest Endosc 2017;9:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishida T. Asian consensus guidelines for gastrointestinal stromal tumor: what is the same and what is different from global guidelines. Transl Gastroenterol Hepatol 2018;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


