Abstract
Background
Much evidence unveils the significance of long non-coding RNAs (lncRNAs) in diverse cancers. This study was designed to clarify the function and mechanism of lncRNA GATA6 antisense RNA 1 (GATA6-AS1) in the progression of non-small cell lung cancer (NSCLC).
Methods
GATA6-AS1, miR-543 and Raf kinase inhibitor protein (RKIP) mRNA expressions were detected by qRT-PCR. Chi-square test was adopted to analyze the relationship between GATA6-AS1 expression and the clinicopathological parameters of NSCLC patients. NSCLC cells H1299 and H460 cells were used as overexpression or knockdown models, respectively, and cell proliferation and metastasis were determined by CCK-8 and Transwell assays. RKIP, E-cadherin, N-cadherin, STAT3, p-STAT3 expressions in NSCLC cells were detected by Western blot. The targeting relationship between GATA6-AS1 and miR-543 was confirmed by dual-luciferase reporter assay.
Results
GATA6-AS1 was significantly lowly expressed in NSCLC tissues and cell lines, and its low expression level was significantly correlated with larger tumor size and positive lymph node metastasis. GATA6-AS1 overexpression inhibited the proliferation, migration, invasion and epithelial–mesenchymal transition of NSCLC cells, while GATA6-AS1 knockdown caused the opposite effects. Mechanistically, it was confirmed that GATA6-AS1 impeded NSCLC cell proliferation and metastasis by adsorbing miR-543 and up-regulating the expression of RKIP.
Conclusions
As a tumor suppressor, GATA6-AS1 participates in suppressing the progression of NSCLC by modulating the miR-543/RKIP axis.
Keywords: NSCLC, GATA6-AS1, miR-543, RKIP, proliferation, metastasis
Introduction
Non-small cell lung cancer (NSCLC), the most common pathological type of lung cancer, is with the highest morbidity and mortality among cancers, which seriously threatens human health.1–3 In the early stage of NSCLC, there is a lack of obvious clinical manifestations; when diagnosed, for many patients, lymphatic or distant metastasis has occurred, so the prognosis is poor, and the 5-year survival rate of NSCLC is less than 20%.4,5 Clarifying the molecular mechanism of the development of NSCLC is very important for developing novel treatment strategies, especially for those with advanced diseases.
Long non-coding RNA (lncRNA) is a hot research topic in recent years. Its length is more than 200 bp, and it modulates gene transcription or translation mainly through epigenetic regulation, RNA splicing, chromatin remodeling, sponging microRNA (miRNA).6,7 LncRNA is closely related to tumorigenesis and cancer progression. For example, lncRNA MEG3 suppresses the proliferation and metastasis of gastric cancer through regulating p53 signaling pathway.8 Knockdown of lncRNA PANDAR inhibits breast cancer cell proliferation, invasion and epithelial–mesenchymal transition (EMT).9 It is reported that GATA6 antisense RNA 1 (GATA6-AS1) is down-regulated in lung squamous cell carcinoma, and its abnormal expression is associated with poor prognosis of patients with lung cancer.10,11 However, the detailed function and mechanism of GATA6-AS1 in regulating the malignant biological behaviors of NSCLC cells remain unclear.
MiRNA pairs with the 3ʹ-UTR of mRNA to modulate gene expression at post-transcriptional level, resulting in translation inhibition or mRNA degradation.12,13 A lot of miRNAs are crucial players in NSCLC progression. For example, miR-362 promotes the metastasis of NSCLC cells by down-regulating Sema3A;14 miR-330-5p overexpression suppresses the growth of NSCLC by inhibiting NOB1.15 A previous study reports that miR-543 is highly expressed in NSCLC and exerts tumor-promoting effects via repressing PTEN.16 However, the mechanism of abnormal expression of miR-543 in NSCLC is not clear.
Raf kinase inhibitor protein (RKIP) is a tumor metastasis inhibitor and belongs to the phosphoethanolamine binding protein (PEBP) family.17 RKIP can not only interfere with the Raf-1-MEK1/2-ERK1/2 signaling pathway, but also inhibit signal transduction of NF-κB and G protein-coupled receptor kinases, thereby reducing cancer cell viability and metastasis in multiple cancers including NSCLC.18–22 RKIP expression was significantly reduced in NSCLC tissues, its defect will activate JAK/STAT3 signaling pathway to promote cancer progression.22 However, the upstream mechanisms that cause RKIP dysregulation in NSCLC have not yet been elucidated.
This study aimed to explore the clinical significance, function and molecular mechanism of GATA6-AS1 in NSCLC. It was confirmed that GAT6A-AS1 was down-regulated in NSCLC, and its low expression was associated with unfavorable pathological parameters of NSCLC patients. Furthermore, it was confirmed that GATA6-AS1 repressed the proliferation and metastasis of NSCLC cells through regulating miR-543/RKIP axis.
Materials and Methods
Tissue Samples
This study was under the approval and guidance of the Ethics Committee of Huaian Hospital and strictly complied with the Declaration of Helsinki. The cancerous tissues of 50 patients with NSCLC and the corresponding adjacent tissues were obtained during surgery, and immediately frozen and stored in liquid nitrogen for next analysis. The pathological diagnosis of these tissues was independently confirmed by two experienced pathologists. All patients involved in this work provided written informed consents.
Cell Culture
Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) was the provider of human normal lung epithelial cells (BEAS-2B) and human NSCLC cell lines (A549, 95-D, H1299, H292, and H460 cells). All cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma, St. Louis, MO, USA), and cultured at 37°C in 5% CO2 with saturated humidity. When the cells reached about 85% confluence, passage was performed at the ratio of 1:3.
Cell Transfection
NSCLC cells in logarithmic growth phase were used for transfection. Short hairpin RNA (shRNA) against GATA6-AS1 (sh-GATA6-AS1), GATA6-AS1 overexpression plasmid (pcDNA3.1-GATA6-AS1), shRNA against RKIP, RKIP overexpression plasmid, miR-543 mimics, miR-543 inhibitors and corresponding negative controls were purchased from GenePharma (Shanghai, China). When cells reached 50% to 70% confluence, NSCLC cells were transfected with Lipofectamine® 3000 (Invitrogen, ThermoFisherScientific, Inc, Carlsbad, CA, USA) in accordance with the supplier’s protocols. The transfection efficiency was examined by quantitative real-time polymerase chain reaction (qRT-PCR) 24 h after transfection.
RNA Extraction and qRT-PCR
Total RNA was extracted from NSCLC tissues or cells with TRIzol reagent (Invitrogen, Shanghai, China) following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using a PrimeScript RT Reagent Kit and gDNA Eraser (Takara). The expressions of GATA6-AS1, miR-543, and RKIP were analyzed with SYBR Green Master Mix (Takara, Dalian, China). Real-time PCR was carried out on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers used were as follows: GATA6-AS1, 5′-ACCACAACCACTACCTTATGGCGTA-3′ (forward) and 5′-TGCCATCTGGACTGCTGGACAATA-3′ (reverse); miR-543, 5′-CCAGCTACACTGGGCAGCAGCAATTCATGTTT-3′ (forward) and 5′-CTCAACTGGTGTCGTGGA-3′ (reverse); RKIP, 5′-AAGAATAGACCCACCAGCAT-3′ (forward) and 5′-AACCAGCCAGACATAGCG-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); and GAPDH, 5′-TGTCCGTCGTGGATCTGA-3′ (forward) and 5′-TTGCTGTTGAAGTCGCAGGAG-3′ (reverse); β-actin, 5′-ACCGAGCGTGGCTACAGCTTCACC-3′ (forward) and 5′-AGCACCCGTGGCCATCTCTTTCTCG-3′ (reverse). The relative expression levels of GATA6-AS1, miR-543, and RKIP were calculated using the 2−ΔΔCt method with U6 or GAPDH as the internal control (Supplementary Figure 1).
Cell Counting Kit-8 (CCK-8) Assay
NSCLC cells in the logarithmic growth phase were inoculated in 96-well plates (1 × 103 cells/well), each well contained 100 μL medium, and three duplicate wells were set in each group. The viability of cells was detected at 24 h, 48 h, 72 h and 96 h after inoculation, respectively. At each time point, 10 μL CCK-8 solution (Beyotime Biotechnology, Shanghai, China) was dripped into each well, and the culture was continued for 1 h. After that, the optical density (OD) value of the wells at 450nm was measured by a microplate reader. 4 d later, the proliferation curve was plotted based on the OD values.
Transwell Migration and Invasion Experiments
Transfected NSCLC cells were harvested, and resuspended, and the concentration of cell suspension was adjusted to 5 × 105 cells/mL with serum-free medium. 200 μL cell suspension was added into the upper chamber of the Transwell system (8 μL pore size, Corning, Beijing, China); 500 μL RPMI 1640 complete medium with 10% FBS was dripped into the lower chamber, with 3 duplicate wells in each group. The cells were cultured at 37°C and in 5% CO2 for 48 h. After that, the migrated cells were fixed with anhydrous alcohol for 15 min and stained with crystal violet solution for 20 min. Following that, the chamber was placed under a microscope, and five fields of view were randomly selected, and the average number of migrated cells was calculated. In invasion assay, Matrigel® (Millipore, Bedford, MA, USA) was used to cover the membrane of the Transwell system before the cells were inoculated, and the other procedures of the experiment were as the same as migration experiment.
Western Blot
NSCLC cells transfected for 48 h were harvested, immersed twice with pre-chilled PBS, centrifuged at 3500 r/min for 5 min, after that, the supernatant was discarded, and RIPA lysate (Beyotime biotechnology, Shanghai, China) was loaded and the cells were placed on ice for 30 min to extract proteins, which were qualified by BCA method. Then, loading buffer was added and the mixture was denatured in boiling water. Denatured protein was taken for SDS-PAGE, and then transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). After that, the membrane was blocked with 5% skim milk for 2 h. Next, primary antibodies anti-RKIP (abcam, ab76582, 1:1000), anti-N-cadherin (abcam, ab202030, 1:1000), anti-E-cadherin (abcam, ab40772, 1:1000), anti-STAT3 (abcam, ab68153, 1:1000), anti-phosphorylated STAT3 (p-STAT3) (abcam, ab76315, 1:500), and anti-GAPDH (abcam, ab181602, 1:1000) were loaded and the PVDF membrane was incubated at 4°C overnight. Afterwards, the membrane was immersed and washed in TBST for 10 min for 3 times, and horseradish peroxidase-labeled secondary antibody (abcam, ab205718, 1:2000) was loaded and the PVDF membrane was incubated at room temperature for 1 h. After the membrane was rinsed with TBST for 10 min for 3 times again, the protein bands were then developed using the Immobilon™ Western chemiluminescent HRP substrate (Millipore, Bedford, MA, USA). After that, the GeneGnome XRQ chemiluminescence imaging system was used to take pictures.
Luciferase Reporter Assay
Wild type GATA6-AS1 and mutant GATA6-AS1 fragments containing the predicted binding sites were constructed and integrated into the pmiRGLO dual-luciferase miRNA target expression reporter (Promega, Madison, WI, USA) to construct GATA6-AS1 wild type (GATA6-AS1-WT) reporter and GATA6-AS1-mutant (GATA6-AS1-MUT) reporter vectors. GATA6-AS1-WT reporter or GATA6-AS1-MUT reporter, together with miR-543 mimics or negative control miRNA, was transfected into H1299 and H460 cells. 48 h after transfection, the luciferase activity of each group was determined according to the instructions of dual-luciferase reporter assay system (Promega, Madison, WI, USA), and the firefly luciferase activity was normalized to renilla luciferase activity.
Xenograft Model
The animal experiments were approved by the Animal Care and Use Committee of Huaian Hospital. Female BALB/c nude mice (5 weeks old) were purchased from Topbiotech Biotechnology Co., Ltd. (Shenzhen, China). Mice were randomly divided into Vector group and GATA6-AS1 group with 10 mice in each group. In the lung metastasis study, 1×107 H1299 cells were injected into the caudal vein of each mouse, respectively. Two weeks later, mice were sacrificed and lung metastasis was evaluated by hematoxylin-eosin (HE) staining.
Statistical Analysis
Statistical analysis was performed by SPSS22.0 software (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± standard deviation. The comparison of the data between the two groups was conducted by the student’s t-test. Chi-square test was used to analyze the correlation between GATA6-AS1 and pathological parameters. Differences of P < 0.05 were considered statistically meaningful.
Results
The Expression and Clinical Significance of GATA6-AS1 in NSCLC
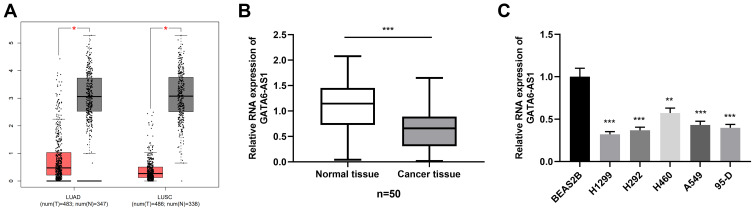
To explore GATA6-AS1 expression in NSCLC tissues, we searched it in GEPIA database, and it showed that GATA6-AS1 was significantly down-regulated in both lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) tissues (Figure 1A). Subsequently, qRT-PCR was employed to measure GATA6-AS1 expression in 50 pairs of NSCLC tissues and normal tissues adjacent to the cancer. Consistently, the results showed that GATA6-AS1 expression in NSCLC tissues was significantly down-regulated compared with normal tissues adjacent to cancer (Figure 1B). Moreover, GATA6-AS1 expression was markedly down-regulated in five NSCLC cells (A549, 95-D, H1299, H292, and H460 cells) in comparison to normal lung epithelial cell lines (BEAS2B cells) (Figure 1C). These data hints that GATA6-AS1 was a tumor suppressor in NSCLC. Next, the 50 cases of NSCLC tissues were divided into GATA6-AS1 high expression group and low expression group according to the median expression of GATA6-AS1; then chi-square test suggested that the low expression level of GATA6-AS1 was significantly correlated with larger tumor size and positive lymph node metastasis (Table 1).
Figure 1.
GATA6-AS1 was down-regulated in NSCLC tissues and cells. (A) The expression of GATA6-AS1 in NSCLC tissues and normal lung tissues was analyzed with TCGA data using GEPIA. (B) The expression of GATA6-AS1 in 50 cases of NSCLC and adjacent normal tissues was detected by qRT-PCR. (C) The expression of GATA6-AS1 in normal lung epithelial cell lines (BEAS2B) and NSCLC cell lines (A549, H1299, H292, 95-D, and H460 cells) was detected by qRT-PCR. *P<0.05, **P < 0.01, ***P < 0.001.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time PCR.
Table 1.
The Association Between GATA6-AS1 Expression and the Clinicopathological Characteristics of NSCLC Patients
| Characteristics | Number (n=50) | GATA6-AS1 Expression | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Gender | |||||
| Male | 29 | 14 | 15 | 0.0821 | 0.7745 |
| Female | 21 | 11 | 10 | ||
| Age (years) | |||||
| <55 | 28 | 13 | 15 | 0.3247 | 0.5688 |
| ≥55 | 22 | 12 | 10 | ||
| Lymphatic metastasis | |||||
| Negative | 17 | 5 | 12 | 4.3672 | 0.0366 |
| Positive | 33 | 20 | 13 | ||
| Histology | |||||
| Squamous cell carcinoma | 20 | 11 | 9 | 0.3333 | 0.5637 |
| Adenocarcinoma | 30 | 14 | 16 | ||
| Tumor size | |||||
| <3cm | 13 | 3 | 10 | 5.0936 | 0.0240 |
| ≥3cm | 37 | 22 | 15 | ||
GATA6-AS1 Inhibited NSCLC Cell Proliferation, Migration and Invasion
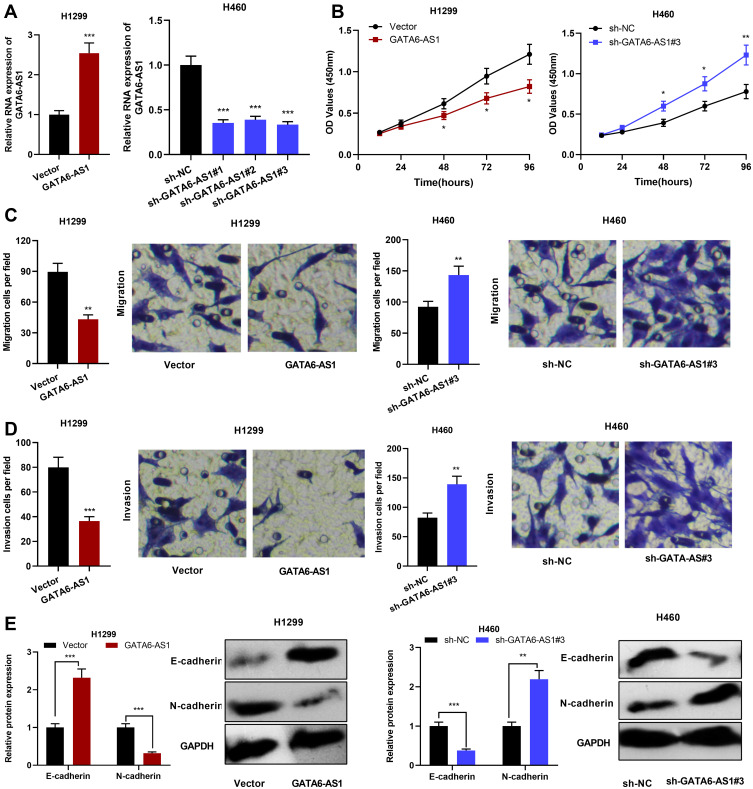
In order to explore the biological function of GATA6-AS1, GATA6-AS1 overexpression plasmid was transfected into H1299 cells, and shRNA against GATA6-AS1 was employed to knock down the expression of GATA6-AS1 in H460 cells (Figure 2A). Subsequently, CCK-8 was adopted to detect cell proliferation, the results of which showed that in comparison with the control group, the up-regulation of GATA6-AS1 markedly inhibited the proliferation of H1299 cells, while knocking down GATA6-AS1 facilitated the proliferation of H460 cells (Figure 2B). In addition, Transwell assay was employed to detect cell migration and invasion, and the results suggested that GATA6-AS1 overexpression inhibited cell migration and invasion, and knocking down GATA6-AS1 effected oppositely (Figure 2C and D). Western blot was employed to examine EMT indicators including E-cadherin and N-cadherin, and the results indicated that GATA6-AS1 overexpression remarkably increased E-cadherin expression and decreased N-cadherin, and the knockdown of GATA6-AS1 promoted the EMT process (Figure 2E). Additionally, in A549 cells, GATA6-AS1 overexpression significantly suppressed the proliferation, migration, invasion and EMT; while its knockdown had opposite effects (Supplementary Figure 2). To further investigate the effect of GATA6-AS1 on NSCLC progression in vivo, H1299 cells with GATA6-AS1 overexpression and control cells were transplanted into the lateral caudal vein of nude mice, respectively. Two weeks later, mice were killed and lung metastases were examined by HE stain. As shown, the number and size of tumor nodules in lung tissues in GATA6-AS1 overexpression group were decreased significantly compared with the control group (Supplementary Figure 3). The above results indicated that GATA6-AS1 was involved in inhibiting the malignant biological behaviors of NSCLC cells.
Figure 2.
GATA6-AS1 inhibited NSCLC cell proliferation, migration, invasion and EMT. (A) qRT-PCR was used to detect GATA6-AS1 expression in H1299 cells transfected with GATA6-AS1 overexpression plasmid and H460 cells transfected with GATA6-AS1 shRNA. (B) CCK-8 method was used to detect the proliferation of NSCLC cells after overexpression or knockdown of GATA6-AS1. (C and D) Transwell assay was used to detect the migration and invasion of NSCLC cells. (E) Western blot was employed to detect the expressions of EMT indicators (E-cadherin and N-cadherin).*P < 0.05, **P < 0.01, ***P < 0.001.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time PCR; EMT, epithelial–mesenchymal transformation; CCK-8, cell counting kit-8.
GATA6-AS1 Targeted miR-543
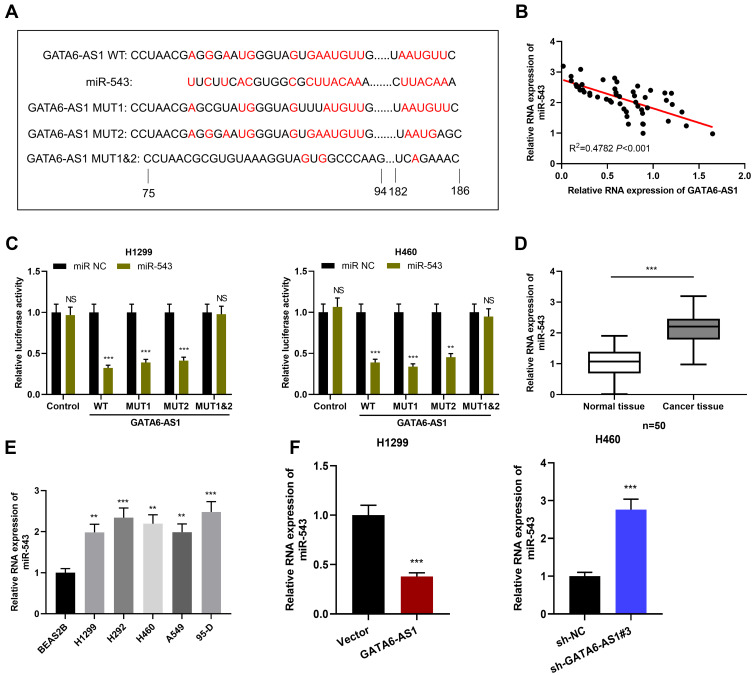
In order to figure out the downstream mechanism of GATA6-AS1 in NSCLC, we predicted the downstream target of GATA6-AS1 through LncBase Predicted v2 database and found that miR-543 was one of its potential targets (Figure 3A). Importantly, there was a negative correlation between GATA6-AS1 expression and miR-543 expression in NSCLC tissues (Figure 3B). Furthermore, dual-luciferase reporter assay proved that miR-543 mimics could reduce the luciferase activity of GATA6-AS1-WT reporter, GATA6-AS1-MUT1 reporter and GATA6-AS1-MUT2 reporter, but had no significant effect on that of GATA6-AS1-MUT1&2 reporter (Figure 3C). In addition, qRT-PCR results displayed that miR-543 expression in NSCLC tissues and their cell lines was significantly up-regulated (Figure 3D and E), which was consistent with previous report.16 In addition, it was observed that GATA6-AS1 overexpression could down-regulate miR-543 expression in H1299 cells, while knocking down GATA6-AS1 could lead to opposite result in H460 cells (Figure 3F). These results suggested that GATA6-AS1 targeted miR-543 to negatively regulate its expression.
Figure 3.
GATA6-AS1 targeted miR-543. (A) The bioinformatics database LncBase Predicted v2 predicted that GATA6-AS1 contained potential binding site for miR-543. (B) qRT-PCR was adopted to detect the correlation between GATA6-AS1 and miR-543 expression in NSCLC tissues. (C) The targeting relationship between GATA6-AS1 and miR-543 was confirmed by dual-luciferase reporter gene experiments. (D and E) The expression of miR-543 in NSCLC tissues and cells was detected by qRT-PCR. (F) qRT-PCR was used to detect the expression of miR-543 in NSCLC cells with GATA6-AS1 overexpression or knockdown. **P < 0.01, ***P < 0.001, NS: P > 0.05.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; miR543, microRNA-543; qRT-PCR, quantitative real-time PCR.
GATA6-AS1 Participated in Regulating NSCLC Cell Proliferation and Metastasis by Regulating miR-543
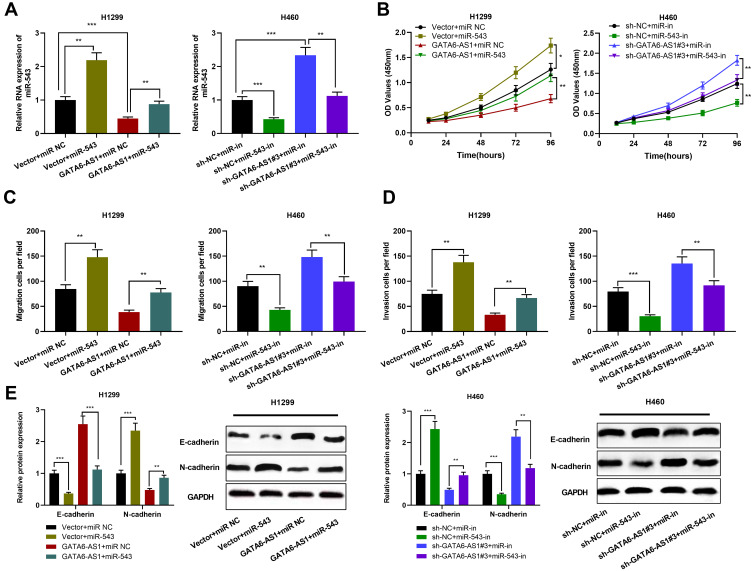
In order to explore the function of GATA6-AS1/miR-543 axis in NSCLC, we transfected miR-543 mimics into H1299 cells overexpressing GATA6-AS1, and miR-543 inhibitors into H1299 cells with GATA6-AS1 knocked down (Figure 4A). The role of GATA6-AS1/miR-543 axis in the proliferation and metastasis of NSCLC cells was detected by CCK-8, Transwell and Western blot assays. As shown, miR-543 mimics significantly promoted the proliferation, migration, invasion and EMT of H1299 cells; while miR-543 inhibitors repressed these malignant biological behaviors of H460 cells (Figure 4B–E). Additionally, it was found that miR-543 overexpression attenuated the inhibitory effects on H1299 cell proliferation, migration, invasion, and EMT induced by overexpression of GATA6-AS1 (Figure 4B–E). Besides, miR-543 inhibitors partially reversed the effects of knocking down GATA6-AS1 on the proliferation, migration, invasion and EMT of H460 cells (Figure 5B–D). These results indicated that GATA6-AS1 was involved in regulating NSCLC cell proliferation and metastasis by regulating miR-543.
Figure 4.
MiR-543 partially reversed the inhibitory effect of GATA6-AS1 on NSCLC cells. (A) MiR-543 mimics was transfected into H1299 cells with GATA6-AS1 overexpression; miR-543 inhibitors was used to transfect H460 cells with GATA6-AS1 knockdown. The expression of miR-543 in NSCLC cells was detected by qRT-PCR. (B–E) Up-regulation of miR-543 attenuated the inhibitory effects of GATA6-AS1 overexpression on H1299 cell proliferation, migration, invasion and EMT; inhibiting miR-543 reversed the effects of knockdown of GATA6-AS1 on cell proliferation, migration, invasion, and EMT in H460 cells. *P < 0.05, **P < 0.01, ***P < 0.001.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; miR543, microRNA-543; qRT-PCR, quantitative real-time PCR; EMT, epithelial–mesenchymal transformation.
Figure 5.
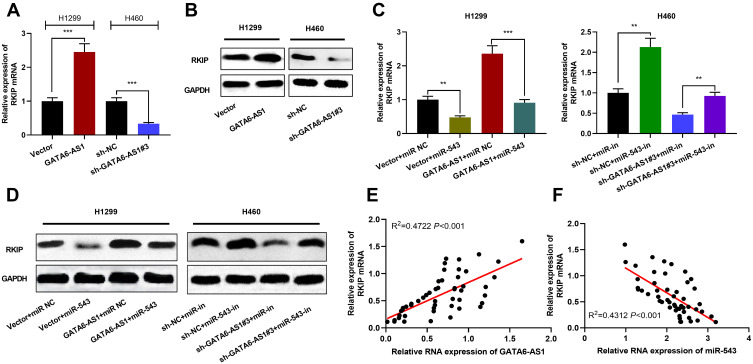
GATA6-AS1/miR-543 axis was involved in the regulation of RKIP expression. (A) qRT-PCR was used to detect the expression of RKIP mRNA in NSCLC cells overexpressing or knocking down GATA6-AS1. (B) Western blot was used to detect the expression of RKIP protein in NSCLC cells with GATA6-AS1 overexpression or knockdown. (C and D) Overexpression of miR-543 inhibited the role of GATA6-AS1 overexpression in promoting RKIP mRNA and protein; miR-543 inhibitors reversed the inhibitory effects of knockdown GATA6-AS1 on RKIP mRNA and protein. (E and F) qRT-PCR was adopted to detect the correlation between RKIP mRNA and GATA6-AS1 or miR-543 expression in NSCLC tissues. **P < 0.01, ***P < 0.001.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; miR-543, microRNA-543; qRT-PCR, quantitative real-time PCR; RKIP, Raf kinase inhibitor protein.
GATA6-AS1/miR-543 Axis Regulated RKIP Expression
After confirming that GATA6-AS1 can regulate miR-543 expression, we tried to explore the downstream targets of miR-543. Previous studies have shown that RKIP participates in the progression of NSCLC,22 and miR-543 can target RKIP in prostate cancer.23 In this work, the role of GATA6-AS1/miR-543 axis in regulating RKIP was explored. As shown, GATA6-AS1 overexpression could significantly increase RKIP mRNA and protein levels, and knocking down GATA6-AS1 could inhibit RKIP mRNA and protein levels in NSCLC cells (Figure 5A and B). In addition, miR-543 overexpression reduced the up-regulation of RKIP induced by GATA6-AS1, and miR-543 inhibition offset the inhibitory effect of GATA6-AS1 knockdown on RKIP expression (Figure 5C and D). It was also demonstrated that that RKIP mRNA was positively correlated with GATA6-AS1 expression in NSCLC tissues and negatively with the expression of miR-543 (Figure 5E and F). These results indicated that GATA6-AS1 can regulate RKIP expression in NSCLC cells by regulating miR-543.
The Restoration of RKIP Expression Attenuated the Inhibitory Effect of GATA6-AS1 on the Progression of NSCLC
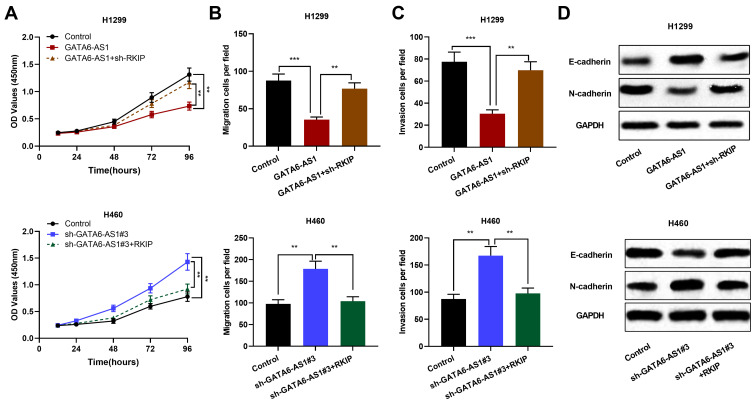
To investigate whether GATA6-AS1 was involved in regulating proliferation, migration, invasion and EMT of NSCLC cells by regulating RKIP, we transfected sh-RKIP into H1299 cells with GATA6-AS1 overexpression and RKIP overexpression plasmid into H460 cells with GATA6-AS1 knockdown, respectively. Subsequently, cell proliferation, migration, invasion and EMT were detected. The results suggested that knocking down the expression of RKIP weakened the inhibitory effect of overexpressing GATA6-AS1 on the proliferation, migration, invasion and EMT of NSCLC. Conversely, the promotion of lung cancer cell proliferation, migration, invasion and EMT induced by knocking down GATA6-AS1 was partially reversed by RKIP overexpression of (Figure 6A–D). It is reported that the tumor-suppressive function of RKIP in NSCLC is mediated by its regulatory function on STAT3 phosphorylation and activation.22 In this work, Western blot showed that compared with the control group, overexpression of GATA6-AS1 significantly inhibited the expression level of phosphorylated STAT3 (p-STAT3), knockdown of GATA6-AS1 enhanced the p-STAT3 expression, and knockdown or overexpression of RKIP can reverse the effects of GATA6-AS1 on STAT3 activation (Supplementary Figure 4). These results indicated that GATA6-AS1 could inhibit the progression of NSCLC by up-regulating RKIP and inhibiting the STAT3 signaling pathway.
Figure 6.
The recovery of RKIP expression in NSCLC cells partially reversed the tumor-suppressive effect of GATA6-AS1. (A–D) RKIP knockdown reversed the inhibitory effects of GATA6-AS1 overexpression on H1299 cell proliferation, migration, invasion, and EMT; overexpression of RKIP reversed the promoting effect of knocking down GATA6-AS1 on the malignant biological behaviors of H460 cells. **P < 0.01, ***P < 0.001.
Abbreviations: GATA6-AS1, lncRNA GATA6-AS1; NSCLC, non-small cell lung cancer; EMT, epithelial–mesenchymal transformation; RKIP, Raf kinase inhibitor protein.
Discussion
In recent years, more and more studies indicated that lncRNA is crucial in the development of NSCLC, affecting tumor cell proliferation, metastasis and apoptosis. For example, lncRNA MIR4435-2HG accelerates NSCLC progression by activating β-catenin signaling;24 lncRNA GASL1 suppresses the growth of NSCLC cells by down-regulating TGF-β1, and its low expression level indicates adverse prognosis of the patients;25 lncRNA NBAT-1 is down-regulated in NSCLC tissues, and overexpression of NBAT-1 impedes cancer cell and promotes apoptosis.26 In this research, it is confirmed that GATA6-AS1 was reduced in NSCLC tissues and cells, and its low expression level was associated with larger tumor size and lymphatic metastasis of NSCLC patients. The following functional experiments showed that overexpression of GATA6-AS1 significantly suppressed proliferation, migration, invasion and EMT of NSCLC cells, while knockdown of GATA6-AS1 promoted these malignant biological behaviors of NSCLC. The present study not only validated the previous reports on the expression pattern and prognostic value of GATA6-AS1 in NSCLC,10,11 but also reported its biological function in NSCLC for the first time. Our data suggested that GATA6-AS1 could be a promising diagnostic biomarker and therapeutic target for NSCLC.
MiRNAs are non-coding small molecule RNAs that are evolutionarily highly conserved and ubiquitous in human body. Dysregulation of miRNAs may lead to malignant transformation of cells, and they also participate in regulating the malignancy of cancer cells.27–31 miR-543 has been reported to be crucial regulator in tumors. For example, miR-543 enhances the migration, invasion, and EMT of esophageal cancer cells via targeting phospholipase A2 group IVA;32 miR-543 is overexpressed in colorectal cancer tissues and cell lines, and high expression of miR-543 is associated with shorter overall survival time and disease-free survival time of the patients, and it promotes the proliferation and metastasis of cancer cells by targeting KLF4.33 It has been found that lncRNA can function as competitive endogenous RNA (ceRNA) to adsorb miRNA, thereby participating in regulating gene expression and affecting tumor progression. It has been reported that LINC-PINT is involved in suppressing the proliferation of esophageal cancer cells by adsorbing miR-543 and miR-576-5p;34 lncRNA H19 accelerates the proliferation and metastasis of NSCLC cells by inhibiting the expression of miR-200a.35 In this study, it was found that there were two binding sites between GATA6-AS1 and miR-543; GATA6-AS1 up-regulation caused a decrease in miR-543 expression in NSCLC cells, while miR-460 expression increased in H460 cells with GATA6-AS1 knockdown. Additionally, it was found that the inhibitory effect of GATA6-AS1 overexpression on NSCLC cell proliferation and metastasis was partially offset by miR-543 mimics. These data indicate that GATA6-AS1 inhibits the proliferation, migration, invasion and EMT process of NSCLC cells by repressing miR-543.
RKIP is a highly conserved and widely expressed cytosolic protein that regulates many important physiological functions, such as cardiac and neural functions, spermatogenesis and reproduction.36 Previous studies have shown that RKIP plays a tumor-suppressive role in a variety of tumors. For example, RKIP overexpression inhibits the proliferation, migration, and invasion of prostate cancer cells, and overexpression of RKIP leads to the inhibition of the NF-kB signaling pathway;21 RKIP impedes the migration and invasion of breast cancer by modulating the expression of CCL5 and regulating the infiltration of tumor-associated macrophages.37 Moreover, RKIP can block the activation of STAT3.38 STAT3 plays an important role in cancer progression, which can regulate cancer cell proliferation, metastasis, immunosuppression, angiogenesis and other malignant phenotypes.39 Currently, several lncRNAs have been documented to affect the activation of STAT3. For example, lncRNA FEZF1-AS1, lncRNA DILC and lncRNA TSLNC8.40–42 In this work, it was proved that in NSCLC, RKIP was negatively regulated by miR-543, and positively regulated by GATA6-AS1. We found that increased expression of miR-543 attenuated the promotion of GATA6-AS1 on RKIP expression. In addition, the decrease in RKIP expression in NSCLC cells attenuated the inhibitory effects of GATA6-AS1 overexpression on cell proliferation, migration, invasion, and EMT, while the increase in RKIP expression partially reversed the cancer-promoting effect caused by knocking down GATA6-AS1. Additionally, GATA6-AS1 could inhibit STAT3 signaling pathways, which was mediated by RKIP. The above data indicate that GATA6-AS1 participates in suppressing the malignant progression of NSCLC cells by adsorbing miR-543 and up-regulating the expression of RKIP.
In summary, this study confirms that GATA6-AS1 is a tumor suppressor in NSCLC. Mechanistically, GATA6-AS1 inhibits NSCLC cell proliferation, migration, invasion, and EMT through the miR-543/RKIP axis. This work expands our knowledge on NSCLC progression, and it provides clues for clinical diagnosis and treatment of NSCLC.
Disclosure
The authors declare that they have no competing interests for this work.
References
- 1.Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer. 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Elgizouli M, Schöttker B, Holleczek B, Nieters A, Brenner H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clin Epigenetics. 2016;25(8):127. doi: 10.1186/s13148-016-0292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu S, Yang X, Li X, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–148. doi: 10.1016/j.canlet.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Scarola M, Comisso E, Pascolo R, et al. Epigenetic silencing of Oct4 by a complex containing SUV39H1 and Oct4 pseudogene lncRNA. Nat Commun. 2015;6(1):7631. doi: 10.1038/ncomms8631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan TN. (6)-Methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–564. doi: 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(17):3850–3856. [PubMed] [Google Scholar]
- 9.Li Y, Su X, Pan H. Inhibition of lncRNA PANDAR reduces cell proliferation, cell invasion and suppresses EMT pathway in breast cancer. Cancer Biomark. 2019;25(2):185–192. doi: 10.3233/CBM-182251 [DOI] [PubMed] [Google Scholar]
- 10.Chen WJ, Tang RX, He RQ, et al. Clinical roles of the aberrantly expressed lncRNAs in lung squamous cell carcinoma: a study based on RNA-sequencing and microarray data mining. Oncotarget. 2017;8(37):61282–61304. doi: 10.18632/oncotarget.18058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Y, Zhang X, Lin Z, et al. SFTA1P, LINC00968, GATA6-AS1, TBX5-AS1, and FEZF1-AS1 are crucial long non-coding RNAs associated with the prognosis of lung squamous cell carcinoma. Oncol Lett. 2019;18(4):3985–3993. doi: 10.3892/ol.2019.10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 14.Luo D, Zhang Z, Zhang Z, et al. Aberrant expression of miR-362 promotes lung cancer metastasis through downregulation of sema3A. J Immunol Res. 2018;2018:1687097. doi: 10.1155/2018/1687097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong R, Liu W, Guo Y, et al. Inhibition of NOB1 by microRNA-330-5p overexpression represses cell growth of non-small cell lung cancer. Oncol Rep. 2017;38(4):2572–2580. doi: 10.3892/or.2017.5927 [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Jiang W, Zhang X, et al. LINC-PINT alleviates lung cancer progression via sponging miR-543 and inducing PTEN. Cancer Med. 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odabaei G, Chatterjee D, Jazirehi AR, Goodglick L, Yeung K, Bonavida B. Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis. Adv Cancer Res. 2004;91:169–200. [DOI] [PubMed] [Google Scholar]
- 18.Beshir AB, Ren G, Magpusao AN, Barone LM, Yeung KC, Fenteany G. Raf kinase inhibitor protein suppresses nuclear factor-κB-dependent cancer cell invasion through negative regulation of matrix metalloproteinase expression. Cancer Lett. 2010;299(2):137–149. doi: 10.1016/j.canlet.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng L, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin Ther Targets. 2008;12(10):1275–1287. doi: 10.1517/14728222.12.10.1275 [DOI] [PubMed] [Google Scholar]
- 20.Datar I, Feng J, Qiu X, et al. RKIP inhibits local breast cancer invasion by antagonizing the transcriptional activation of MMP13. PLoS One. 2015;10(8):e0134494. doi: 10.1371/journal.pone.0134494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu CX, Li WZ, Guo YL, et al. Tumor suppressor RKIP inhibits prostate cancer cell metastasis and sensitizes prostate cancer cells to docetaxel treatment. Neoplasma. 2018;65(2):228–233. doi: 10.4149/neo_2018_170203N72 [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Duan G, Zhao C, et al. Reduced RKIP expression levels are associated with frequent non-small cell lung cancer metastasis and STAT3 phosphorylation and activation. Oncol Lett. 2017;13(5):3039–3045. doi: 10.3892/ol.2017.5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y, Liu XH, Zhu HC, Wang L, Ning JZ, Xiao CC. MiR-543 promotes proliferation and epithelial-mesenchymal transition in prostate cancer via targeting RKIP. Cell Physiol Biochem. 2017;41(3):1135–1146. doi: 10.1159/000464120 [DOI] [PubMed] [Google Scholar]
- 24.Qian H, Chen L, Huang J, et al. The lncRNA MIR4435-2HG promotes lung cancer progression by activating β-catenin signalling. J Mol Med (Berl). 2018;96(8):753–764. doi: 10.1007/s00109-018-1654-5 [DOI] [PubMed] [Google Scholar]
- 25.Su WZ, Yuan X. LncRNA GASL1 inhibits tumor growth of non-small cell lung cancer by inactivating TGF-β pathway. Eur Rev Med Pharmacol Sci. 2018;22(21):7282–7288. [DOI] [PubMed] [Google Scholar]
- 26.Lei T, Lv ZY, Fu JF, Wang Z, Fan Z, Wang Y. LncRNA NBAT-1 is down-regulated in lung cancer and influences cell proliferation, apoptosis and cell cycle. Eur Rev Med Pharmacol Sci. 2018;22(7):1958–1962. doi: 10.26355/eurrev_201804_14721 [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67(1):129–139. doi: 10.1007/s13105-010-0050-6 [DOI] [PubMed] [Google Scholar]
- 28.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost. 2012;107(4):605–610. doi: 10.1160/TH11-12-0836 [DOI] [PubMed] [Google Scholar]
- 29.Qi R, Wang DT, Xing LF, Wu ZJ. miRNA-21 promotes gastric cancer growth by adjusting prostaglandin E2. Eur Rev Med Pharmacol Sci. 2018;22(7):1929–1936. doi: 10.26355/eurrev_201804_14717 [DOI] [PubMed] [Google Scholar]
- 30.Fu H, Song W, Chen X, et al. MiRNA-200a induce cell apoptosis in renal cell carcinoma by directly targeting SIRT1. Mol Cell Biochem. 2018;437(1–2):143–152. doi: 10.1007/s11010-017-3102-1 [DOI] [PubMed] [Google Scholar]
- 31.Wen DY, Pan DH, Lin P, et al. Downregulation of miR‑486‑5p in papillary thyroid carcinoma tissue: a study based on microarray and miRNA sequencing. Mol Med Rep. 2018;18(3):2631–2642. doi: 10.3892/mmr.2018.9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Diao C, Wang X, et al. MiR-543 promotes migration, invasion and epithelial-mesenchymal transition of esophageal cancer cells by targeting phospholipase A2 group IVA. Cell Physiol Biochem. 2018;48(4):1595–1604. doi: 10.1159/000492281 [DOI] [PubMed] [Google Scholar]
- 33.Zhai F, Cao C, Zhang L, Zhang J. miR-543 promotes colorectal cancer proliferation and metastasis by targeting KLF4. Oncotarget. 2017;8(35):59246–59256. doi: 10.18632/oncotarget.19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Chen J, Wang L, et al. Linc-PINT acted as a tumor suppressor by sponging miR-543 and miR-576-5p in esophageal cancer. J Cell Biochem. 2019;120(12):19345–19357. doi: 10.1002/jcb.28699 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Feng C, Li Y, Ma Y, Cai R. LncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol Cell Biochem. 2019;460(1–2):1–8. doi: 10.1007/s11010-019-03564-1 [DOI] [PubMed] [Google Scholar]
- 36.Keller ET, Fu Z, Brennan M. The role of Raf kinase inhibitor protein (RKIP) in health and disease. Biochem Pharmacol. 2004;68(6):1049–1053. doi: 10.1016/j.bcp.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 37.Datar I, Qiu X, Ma HZ, et al. RKIP regulates CCL5 expression to inhibit breast cancer invasion and metastasis by controlling macrophage infiltration. Oncotarget. 2015;6(36):39050–39061. doi: 10.18632/oncotarget.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yousuf S, Duan M, Moen EL, et al. Raf kinase inhibitor protein (RKIP) blocks signal transducer and activator of transcription 3 (STAT3) activation in breast and prostate cancer. PLoS One. 2014;9(3):e92478. doi: 10.1371/journal.pone.0092478 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- 40.Bian Z, Zhang J, Li M, et al. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24(19):4808–4819. doi: 10.1158/1078-0432.CCR-17-2967 [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Sun W, Shen W, et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol. 2016;64(6):1283–1294. doi: 10.1016/j.jhep.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Li Z, Liu L, et al. Long noncoding RNA TSLNC8 is a tumor suppressor that inactivates the interleukin-6/STAT3 signaling pathway. Hepatology. 2018;67(1):171–187. doi: 10.1002/hep.29405 [DOI] [PubMed] [Google Scholar]