Abstract
The gut microbiota is symbiotic with the human host and has been extensively studied in recent years resulting in increasing awareness of the effects of the gut microbiota on human health. In this review, we summarize the current evidence for the effects of gut microbes on the integrity of the cerebral blood–brain barrier (BBB), focusing on the pathogenic impact of gut microbiota disorders. Based on our description and summarization of the effects of the gut microbiota and its metabolites on the nervous, endocrine, and immune systems and related signaling pathways and the resulting destruction of the BBB, we suggest that regulating and supplementing the intestinal microbiota as well as targeting immune cells and inflammatory mediators are required to protect the BBB.
Keywords: microbiota, metabolites, blood–brain barrier
Introduction
Under physiological conditions, the intestinal microbiota and the human body are in a mutually beneficial relationship. The human body provides a protected environment and nutrients for the microbiome,1 while the microbes participate in the metabolism, digestion and absorption of substances. The gut microbiota is constantly changing but maintained in a relatively stable state, participating in various physiological processes. Gut microbes dysbiosis can cause dysfunction of many organs, including the brain.2 The blood–brain barrier (BBB) is important for protection of the brain and preventing microbes and toxins from entering the central nervous system (CNS). Many studies have shown that gut microbiota disorders are related to the destruction of the BBB,3–5 although the mechanism remains to be clarified.
The Composition and Function of the Blood–Brain Barrier
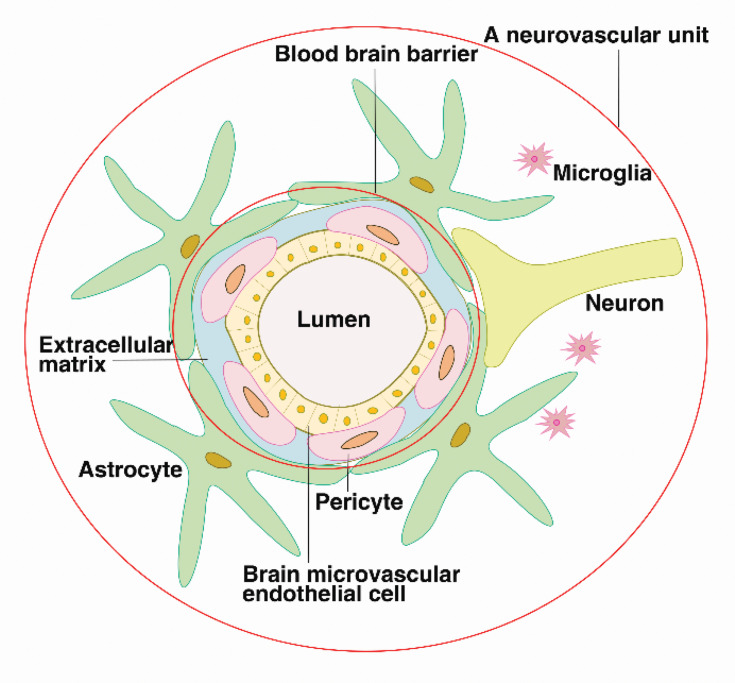
The BBB refers to the barrier between the plasma and brain cells formed by the brain capillaries and glial cells and the barrier between the plasma and cerebrospinal fluid formed by the choroid plexus. The BBB consists of brain microvascular endothelial cells (BMEC), the perivascular foot of astrocytes, a basement membrane (BM) and pericytes (PCs). In July 2001, the National Institute of Neurology and Stroke (NINDS) proposed the concept of a neurovascular unit (NVU) to emphasize the dynamic interaction between the BBB, neurons, the extracellular matrix (ECM), and microglia. The components of this unit work together to regulate the structure and function of the BBB.6,7
BMECs are held together by tight junction (TJ) proteins to form an endothelial cell barrier, which includes claudin, occludin, and zonula occludens (ZO) and junctional adhesion molecules (JAMs) linked to the cytoskeleton.8,9 BMECs overlap and occlude adjacent vascular endothelial cells (ECs) to form a high-resistance barrier to molecules and metal ions that strictly controls the paracellular transport of solutes and liquids.10,11 The EC barrier effectively prevents the passage of macromolecular substances through the EC junctions and maintain the ion homeostasis in the brain.12 TJs are particularly important for BBB function, and their loss can greatly increase its permeability.13,14 Cerebrovascular ECs have a very low transcytosis rate due to the lack of plasma membrane vesicles.15–17 PCs protrude from the surface of ECs, spanning multiple EC bodies, and wrapping around the capillary endothelium.18 The contractile proteins in PCs regulate the diameter of capillaries,19 regulating transcytosis and immune cell transport across the BBB.
ECs are also surrounded by a continuous BM, which is composed of collagen, laminin, nestin, heparin sulfate proteoglycan and other glycoproteins. The ECM is secreted by EC, PCs and astrocytes. The BM provides binding sites for signal transduction of many cytokines (VEGF, Wnt, etc.) and forms the second part of the BBB, which can be destroyed by matrix metalloproteinases (MMPs).20
Astrocytes are the most abundant glial cell type and play an important role in neurovascular regulation.21 The perivascular foot of astrocytes surrounds approximately 85% of the surface of the brain capillaries outside the BM, forming another physical, transport, and metabolic barrier. It also communicates with neurons to establish endothelial neuronal connections,22 which transmit neural signals to the local vasculature, affecting barrier physiology by altering arteriolar expansion and blood flow.23,24 (see Figure 1)
Figure 1.
The blood–brain barrier is composed of brain microvascular endothelial cells, pericytes, the continuous basement membrane and the perivascular feet of astrocytes, preventing the entry of harmful substances into the brain tissue. The blood–brain barrier interacts with extracellular matrices, neurons, and microglia, forming neurovascular units, which regulate the structure and function of the blood–brain barrier.
This multilayer membrane structure of brain capillaries constitutes a protective barrier for brain tissue. The BBB serves as a key regulator of the entry of nutrients from blood sources and compounds required for brain health. It also prevents the entry of potentially harmful molecules and cells into the brain, and maintains brain homeostasis and a suitable microenvironment for neuronal growth.1,25,26
The Effects of Blood–Brain Barrier Disruption on the Brain
Destruction of the BBB is involved in a variety of acute and chronic CNS diseases and neuropsychiatric disorders, such as encephalomyelitis, multiple sclerosis (MS), Alzheimer’s disease and schizophrenia. High levels of superoxide,27 activation of MMPs,28 and upregulation of inflammatory mediators in the CNS can cause damage to the BBB. Degradation of the BBB leads to increased permeability and leakage, resulting in the recruitment of immune cells to the CNS29 and the occurrence of neuroinflammation.30
The Impact of Gut Microbiota Disorders on the BBB
Gut Microbiota Disorders Associated with the BBB
The human body contains about 100 trillion microbes from approximately 1000 species, of which the intestinal microbiota accounts for more than 90%, mainly comprising Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria.31 These microbes play important roles in many physiological functions, such as metabolism,32,33 nutrient absorption in the gut, synthesis of beneficial bioactive molecules,34 regulation of neurotransmitters, maintenance of the integrity and function of barriers,35,36 and the immune system.37 Under normal circumstances, intestinal microbes are combined in a certain proportion to form an ecological balance. When the internal and external environment changes, this balance is broken, resulting in gut microbiota disorders. Diet, infection and oral antibiotics can change the structure of intestinal microflora.38–40 The disorder of intestinal microbes may promote the occurrence of some diseases. Studies have confirmed that intestinal microbes disorders are associated with many CNS diseases, including Alzheimer’s disease (AD),41 Parkinson’s disease (PD),42 and amyotrophic lateral sclerosis.43 Thus, intestinal microbes disorders are is considered to be the important cause of dementia.44,45
A growing number of studies suggest that gut microbes have an important influence on BBB integrity. In 2014, Braniste et al found that different regions of the brain in germ-free (GF) mice (including the frontal cortex, hippocampus and striatum) displayed increased BBB permeability compared to pathogen-free (PF) mice. The increased BBB permeability was associated with decreased expression of occludin and claudin-5. After fecal transplantation from PF mice or administration of short-chain fatty acid (SCFA)-producing bacteria to GF mice, the expression of TJs increased, and the integrity of the BBB was restored. This indicated the establishment of communication between the gut microbiota and the BBB during embryonic development, which persists throughout life.3 Fröhlich et al reported that induced intestinal dysbiosis induced in mice through the administration of antibiotics resulted in reduced expression of TJ proteins in the hippocampus, without a reduction in the prefrontal cortex and hypothalamus.46
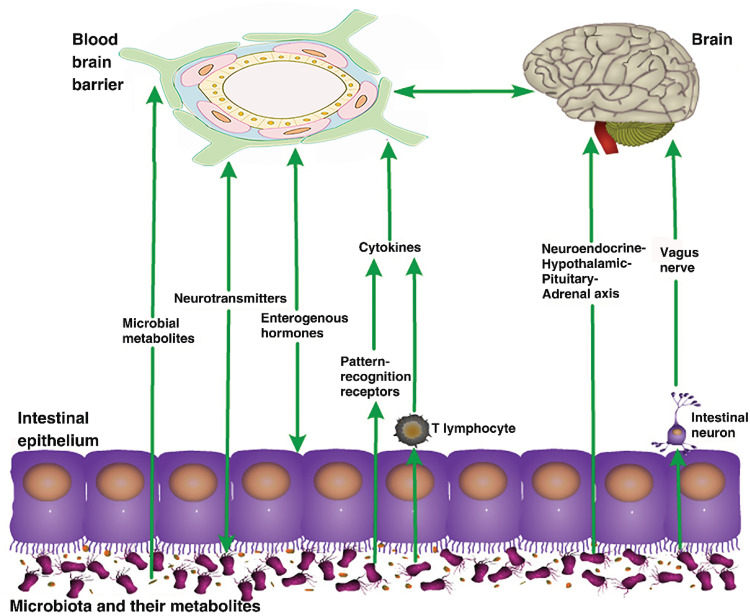
These findings indicate the importance of the gut microbiota in the BBB, although the mechanism remains unclear. Current research suggests that the gut microbiota regulate the BBB through a variety of pathways, including the vagus and sympathetic nerves,47 immune48 and endocrine systems,49 and intestinal microbial metabolites such as SCFAs and lipopolysaccharides (LPS).50,51 (see Figure 2)
Figure 2.
Pathways of the effects of gut microbiota on the blood–brain barrier. The gut microbiota can affect the structure and function of the blood–brain barrier through various pathways, such as their metabolites, and the nervous, endocrine, and immune systems.
Microorganisms and Their Metabolites
Microorganisms in the gut communicate with the brain via mechanical stimulation of intestinal mucosal cells, causing local inflammation by producing toxins, receptor-mediated signaling, and increasing intestinal permeability.52,53 When the intestinal flora is in disorder, some pathogenic bacteria in the gastrointestinal tract can directly stimulate intestinal chromaffin cells to release serotonin, which can activate endogenous afferents and cause nerve reflex, thereby enhancing the release of chloride and water to the intestinal cavity, thus stimulating intestinal motility.54 The gut contains numerous bacteria, which produce LPS, and convert dietary components into a series of metabolites such as SCFAs, trimethylamine and serotonin. These metabolites regulate homeostasis, maintain BBB integrity and affect brain function.55 The gut-vascular barrier (GVB) prevents the entry of bacteria into the bloodstream from the intestines. Following destruction of the GVB, bacteria and their toxic metabolites enter the bloodstream, causing an inflammatory response.
Lipopolysaccharide
LPS is the main component of the cell wall of Gram-negative bacteria, which is lysed and shed after bacterial death. Gut microbiota disorders can lead to increased LPS release, increasing intestinal permeability, and affecting intestinal and general health.56,57 LPS activates gastrointestinal immune cells to release inflammatory cytokines from the gut. In vitro experiments, Kacimi et al studied the model of endothelial cell death only in the presence of microglia and found that LPS induced the death of microglia rather than EC. However, when microglia were co-cultured with EC, LPS increased EC death. Furthermore, inhibiting microglial activation can prevent injury to the EC. Thus, it has been proposed that LPS disrupts the BBB by activating microglia to damage EC.58 Singh et al demonstrated the interaction of EC with LPS and the main component of the cell wall of Gram-positive bacteria, lipoteichoic acid (LTA) in vitro. They found that the toxins did not cross the endothelial barrier, but the mRNA levels of ZO-1, occludin and JAM were suppressed, while mRNA levels of tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1 β) were increased. These findings indicated that disruption of the BBB and release of pro-inflammatory cytokines induce neuroinflammation.59 In addition, LPS affects adhesion proteins and membrane transporters, as well as the basal lamina, and extracellular matrix.60 LPS also activates Toll-like receptor 4 on microglia to release inflammatory cytokines and chemokines in the CNS,61 and enhances neuronal apoptosis,62,63 thereby affecting the BBB and CNS.
Short-Chain Fatty Acids
SCFAs, which are produced mainly by digestion of dietary fiber by the beneficial intestinal microbes, are biologically active molecules that can pass the BBB.64,65
SCFAs act as signaling molecules by binding to G protein-coupled receptors (GPCRs).66 SCFAs stimulate GPCRs, the free fatty acid receptors (FFAR2 or FFAR3), on intestinal epithelial cells and brain EC,67–71 protecting the BBB from oxidative stress.67 SCFAs can enter the interior of cells and inhibit histone deacetylase (HDAC), thus activating gene expression.72 HDAC blocks the transcription of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF).73 In addition, SCFAs stimulate synapse and outer membrane vesicles (OMV) for exocytosis.74 OMVs, which encapsulate some bioactive molecules secreted by bacteria, enter the systemic circulation and cross the BBB to induce an inflammatory response.75 Erny et al observed that both GF and temporary or partial eradication of the host microbiota resulted in microglial defects that partially restored microglial features by recolonization with a complex microbiota. It was also found that mice lacking the SCFA receptor FFAR2 mirrored the microglial defects found under GF conditions, suggesting that SCFAs regulate microglial homeostasis.76
Neural Pathway
The neural pathway is mediated by the interaction of the gut microbiota with the central, autonomic, and enteric nervous systems,45 and participates in normal neurological functions, such as the development and formation of neurons and myelin,77 the development of the neurotransmitter system,78 neural signaling and BBB integrity.
Microbial populations can regulate the CNS development and affect the expression and signaling of amygdala transcriptional genes.79 Significant amygdalar and hippocampal expansion was observed in GF mice compared to that in the conventional colonization mice. The basolateral amygdala pyramidal neurons of GF mice had more stubby and mushroom spines, while the ventral hippocampal pyramidal neurons were shorter and less branched,80 with decreased BDNF expression in the hippocampus.81
Metabolites produced by the gut microbes act locally on the intestinal neurons that innervate the gut and are transmitted to the brain via neural signals. Metabolites also activate Toll-like receptors present in intestinal epithelial cells,82,83 producing intestinal inflammatory responses and increasing apoptosis and loss of intestinal neurons,84 thus affecting the function of the enteric and central nervous systems.
Vagus Nerve
The vagus nerve is an important pathway by which the intestinal microbiota communicates with the brain. The gut microbiota can act on the intestinal neurons and communicate with the brain by altering the vagus signals to stimulate anti-inflammatory reflexes, release mediators such as acetylcholine, and interact with immune cells to reduce inflammation.47 Stimulation of the vagus nerve reduces the co-localization of neutrophils and endothelial cell adhesion molecule (ICAM)-1 induced by LPS stimulation, as well as decreasing gene expression of hypothalamic inflammatory mediators (IL-6, CXCL-1, ICAM-1) and attenuating inflammatory responses in brain areas.85 In the rat model of ischemic stroke, non-invasive vagus nerve stimulation was also observed to reduce BBB leakage in the lesion area, improve TJ levels, and reduce MMP-2/9 expression to protection the integrity of the BBB.86
Neurotransmitters
Neurotransmitters are neurochemicals that transmit neural signals between synapses. Intestinal microbes are symbiotic with the human body to secrete neurotransmitters (GABA, 5-HT, catecholamine, and histamine).87 Elevated enteric neurotransmitters may be involved in the pathological process of abnormal excitation of intestinal nerve cells. While being transported to the brain through the blood circulation and neural channels, they may also activate the vagal nerve chemoreceptor by paracrine, and finally stimulate anti-inflammatory reflex.47,88 By controlling the function of the BBB, neurotransmitters regulate the transmission of information between the periphery and the brain.89,90 Gut dysbiosis changes the production of 5-HT, which is also called serotonin. Changes in the local concentration of 5-HT are transmitted to the brain along the gut-brain axis, affecting CNS signaling.91 The attenuation of pro-inflammatory factors and the elevation of tryptophan and the serotonergic precursor was observed in rats following treatment with bifidobacteria.92 The gut microbes can convert histidine to histamine. Histamine affects both anti-apoptotic proteins and autophagy proteins through the heat shock response pathway, and induces microglia anti-inflammation to reduce neuroinflammation as well as motor neuron death.93
Endocrine Pathway
Neuroendocrine-Hypothalamic-Pituitary-Adrenal Axis
The endocrine pathway allows the transfer of humoral factors to mediate bidirectional activity between the gut microbiota and the brain.94,95 Changes in the structure of the gut microbiota drive the pro-inflammatory state, which leads to increased permeability of the intestinal barrier.96 LPS crosses the intestinal epithelial barrier and activates the neuroendocrine-hypothalamic-pituitary-adrenal axis.97 As a result, mast cells are activated and corticotropin-releasing hormone (CRH) is released, resulting in increased permeability of the blood–brain barrier. CRH and adrenocorticotropic hormone (ACTH) can also directly activate microglia to release neuroinflammatory mediators and promote neuroinflammation in the brain.98 Exposure of neonates to LPS results in increased ACTH and corticosterone production in response to stress and decreased brain glucocorticoid receptor (GR) density.99 Bifidobacterium administration increases the diversity of intestinal microbes in maternal separation mice, which not only downregulates intestinal inflammation, but also weakens the excessive stress response of the hypothalamus-pituitary-adrenal axis, thereby affecting brain biochemistry and behavior.100
Enterogenous Hormones
Intestinal microbes regulate the number and activity of enteroendocrine cells and the synthesis and secretion of biological hormones through local stimulation and production of metabolites.101,102 Hormones such as leptin, ghrelin, and glucagon-like peptide 1 (GLP-1), which are synthesized in the intestines, regulate energy homeostasis and have protective effects on neurotoxicity induced by toxic microbial metabolites.103–105 In response to SCFAs, enteroendocrine cells release the neuropeptides GLP-1 and PYY to enhance satiety via the neuroendocrine pathway,106,107 while the expression is reduced in GF mice.108 GLP-1 is secreted by intestinal L cells and participates in the regulation of a variety of central nervous system functions.109 Changes in GLP-1 content are related to changes in gut microbes. After traumatic brain injury (TBI), the number of bacterial species in the faecal microbes of mice decreased significantly.110 Clostridium butyricum (Cb) can produce a large amount of SCFA butyrate in the intestinal tract to stimulate the production of gastrointestinal hormone in the colon.111 After supplement of Cb, reduction of inflammatory reaction and intestinal permeability, improvement of neurological dysfunction and BBB injury are observed in TBI mice, which is considered to be related to increased GLP-1 secretion.112
Immune Pathway
Under normal circumstances, the intestinal microbiota and the host are in a symbiotic state. Following disruption of the intestinal flora, the microorganisms and their metabolites interaction with the host immune system may occur.91,113 The changes in the composition of the gut microbiota lead to increased intestinal permeability and stimulation of an immune response by the bacteria and their toxic metabolites. The activated immune cells and the secreted immune signal molecules then reach the BBB via the blood circulation. Systemic inflammation and elevated levels of circulating cytokines upregulate adhesion molecules, chemokines, and MMPs in the BBB and the brain,114,115 and downregulate TJs to increase the permeability of the BMEC layer.116 The disrupted BBB allows the entry of fibrin, which is deposited as insoluble fibrin and activates an immune response.117 Solutes and toxins enter the brain causing increased inflammation and recruitment of leukocytes and macrophages,114 which stimulate the inflammatory signaling of the NVU.118 Thus, intracerebral inflammation and neurodegeneration are exacerbated via a vicious cycle.
T Lymphocytes
Atarashi et al isolated bacterial strains that induced the proliferation of regulatory T cells (Tregs) from human stool samples, suggesting that the intestinal flora can activate Tregs.119 SCFAs also induce the production and differentiation of Tregs by inhibiting HDAC activity.120,121 In multiple sclerosis, the species abundance of intestinal microbes decreased, with the depletion of Clostridia XIVa and IV Clusters.122 Intestinal dysbiosis leads to reduced the content of propionic acid in serum and feces,123 decreased Treg cells and increased helper T cells (Th1 and Th17) in peripheral blood.124 Activated T lymphocytes migrate from the periphery to the CNS, secreting cell adhesion molecules and chemokines, and leading to infiltration of the CNS by monocytes and macrophages. In this way, the blood–brain barrier is destroyed.125
Microglia
Microglia are innate immune cells of the CNS and play an role in immunological surveillance in the brain by participating in information transfer and clearing cell debris.125 The gut microbiota plays an important role in regulating the maturation and function of microglia.76 GF mice showed immature gene expression profiles and morphological differences, such as increased microglial cell volume and branching. After colonization with diverse microbial communities, GF mice showed a mature microglial phenotype similar to that of SPF mice. After treatment with antibiotics, microglia isolated from SPF mice showed a cell morphology similar to that in GF mice.76 Following destruction of the BBB, microglia are activated by inflammatory substances and oxidative stress. The activated microglia exhibit an amoebic phenotype and mediate phagocytosis from a branching state.126 The microglia upregulate a variety of active proteins, including major histocompatibility complex (MHC) I, MHC II, and secrete multiple cytokines and chemokines. Activated microglia can be divided into pro-inflammatory (M1 type) and anti-inflammatory (M2 type) subtypes, which interact with infiltrating T lymphocytes to generate nociceptive or neuroprotective outcomes.127
Inflammatory Cytokines
An imbalance in the intestinal flora can lead to increased intestinal permeability. Bacterial cell wall antigens are recognized as patterns and combined to produce pro-inflammatory cytokines. Pro-inflammatory cytokines can be transported to tissues including the brain to initiate inflammatory processes,128,129 leading to extravasation of leukocytes, upregulation of vascular cell adhesion protein 1 (VCAM-1), ICAM-1 and MMPs,130 and disruption of the BBB integrity. Increased BBB permeability allows the entry of pathogens and toxins into the brain. Astrocytes adopt a pro-inflammatory phenotype, releasing IL-1β, IL-6, TNF-α and prostaglandins,131 which influence crossing of the paracellular and transcellular barrier. In addition, the inflammatory cytokines TNF-α and IL-1β can also induce the expression of CXCL1 and CCL2,132 which participate in the recruitment of immune cells to the brain and further promote the inflammatory response.
Signaling Pathways
Wnt/β-Catenin Signaling Pathway
The Wnt signaling pathway is activated by binding of the Wnt protein to the N-terminal cysteine rich domain of the Frizzled (FZD) protein family receptors. The FZD protein family forms part of the seven-transmembrane GPCRs family,133 and is responsible for immobilizing Wnt proteins on the cell surface. The signal is transmitted to the Disheveled (DVL) protein in the cell via the C-terminus of the FZD protein.134–137 DVL binds to the Axin/GSK3/APC complex, thereby inhibiting the degradation of β-catenin (β-cat) in the cytoplasm. Following the increase in cytoplasmic levels, β-cat is transferred into the nucleus and acts as a transcription factor subunit to induce transcription of the target gene, resulting in subsequent cellular responses. Activation of the canonical Wnt/β-catenin pathway has been reported to control BBB differentiation and maturation,138,139 and play a positive role in the development of BBB by regulating TJ protein expression.138 Inactivation of β-cat leads to a significant downregulation of claudin3, upregulation of plasma membrane vesicle-associated proteins and decomposition of the BBB.140
Nuclear Factor Kappa-B (NF-κB) Signaling Pathway
The NF-κB pathway is a proteinase-dependent receptor signaling pathway that is activated by microbial pathogens, LPS, cytokines, heat shock protein 90 (HSP90) and high mobility group proteins (HMGB1) in the blood. Normally, the NF-κB dimer binds to the Inhibitor of kappa-B (IκB) protein and remains in the cytoplasm. Stimulation of the upstream signals leads to activation of the IκB kinase (IKK) complex and IκB protein phosphorylation. Following ubiquitination, the IκB protein is then targeted for proteasome-dependent degradation and NF-κB is released into the nucleus to activate downstream gene transcription. NF-κB regulates the expression of numerous genes such as cytokines (IL-1β, IL-6, TNF-α, GM-CSF), inflammatory chemokines (RANTES, MCP-1), and adhesion molecules (VCAM-1, ICAM-1, E-selectin), and plays important roles in various aspects of inflammatory and innate immune responses.141,142 Activation of the NF-κB signaling pathway promotes glial cell activation143 and expression of ICAM-1, VCAM-1, IL-6, IL-8 and monocyte chemoattractant protein 1 (MCP-1), which contribute to the destruction of the BBB.144
c-Jun N-Terminal Kinase (JNK) Signaling Pathway
The c-Jun N-terminal kinase, also known as stress-activated protein kinase, is a member of the mitogen-activated protein kinase (MAPK) family. The JNK signaling pathway is activated by various factors such as cytokines (IL-1, TNF-α), growth factors (EGF, PDGF), GPCRs, and stress, and is involved in cell proliferation, differentiation and apoptosis and other biological processes.145 The JNK protein is serine/threonine protein kinase encoded by three genes, Jnk1, Jnk2 and Jnk3, and located mainly in the cytoplasm.146 After stimulation, JNKK1/MKK4/SEK1 or JNKK2/MKK7 mediates JNK activation by phosphorylation of Thr183 and Thr185.147 The activated JNK is then transported into the nucleus, where it phosphorylates c-Jun and activates the apoptotic signaling pathway. Studies have shown that JNK inhibitors reduce MMP-9 expression, and increase the expression of TJ proteins (ZO-1, claudin-5, occludin), thereby preventing BBB destruction.148–150
Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT) Signaling Pathway
JAK is a non-receptor tyrosine protein kinase (PTK) the substrate of which is STAT. JAK is rapidly recruited and activated by cell surface receptors (eg, interferons, interleukins, and growth factors). Activated JAK phosphorylates the tyrosine residue of the receptor, providing a binding site for proteins containing an SH2 domain. STAT binds to the receptor and then is also phosphorylated by activated JAK. Activated STATs form dimers and enter the nucleus to induce transcription of the target gene. This signaling pathway is known as the JAK-STAT pathway. In an animal model of ischemia/reperfusion injury, Gong et al found that inhibition of JAK/STAT signaling activation increased TJ levels and reduced BBB permeability.151 Chaudhuri et al also demonstrated that activated STAT1 induces IL-6 expression and reduces the expression of claudin-5, ZO-1 and ZO-2 in BMEC. The STAT1 inhibitor fludarabine attenuates this downregulation of claudin-5 and ZO-1 and blocks the migration of monocytes across the BBB.152
Toll-Like Receptors (TLRs) Signaling Pathway
TLRs, which were the first pattern-recognition receptors (PRRs) to be discovered, recognize pathogen-associated molecular patterns (PAMPs) on the surface of pathogenic microbes to initiate an innate immune response. TLRs are a type I transmembrane protein receptor composed of an intracellular segment, a transmembrane region, and an extracellular segment. The extracellular domain directly recognizes and binds to pathogens or their products, activating signaling pathways, and inducing expression of certain immune effector molecules. The TLR signaling pathway is divided into two main pathways: the myeloid differentiation factor 88 (MyD88)-dependent pathway and the MyD88-independent pathway. The MyD88-dependent pathway mediates NF-κB activation and produces cytokines, while the MyD88-independent pathway induces the production of type I interferon (IFN).153 When the intestinal epithelium is damaged, LPS enters the blood, causing peripheral immune activation. TLR4, which is expressed in various cell types in the CNS,154 induces immune activation and neuroinflammation in the brain. The mutual interaction between TLR2 and TLR4 affects brain health.155 Mayerhofer et al demonstrated that the TLR2 agonist LTA increases the circulating levels of cytokines (TNF-α, IL-6, IFN-γ, etc.) and cytokine mRNA expression in the brain in mice, and is also involved in transcriptional downregulation of TJ proteins (claudin 5, occludin) in the brain.155 In a model that mimics the human BBB, Paradis et al showed that TLR4 increases monocyte migration and stimulates the migration of monocytes across the BBB in response to CCL19.156
Nucleotide-Binding Oligomerization Domain-Like Receptor (NLR) Signaling Pathway
NLRs belong to the family of intracellular PRRs. The NLR proteins have a central oligomerization domain, NOD,157 which plays an important role in self-oligomerization and activation. The C-terminus of the leucine-rich repeat is responsible for sensing upstream ligand signals and modulating NLR activity.158–160 The N-terminus of the effector domain activates the downstream signaling pathway by interacting with downstream proteins.161 NLRs participate in the activation of multiple signaling pathways involved in processes such as autophagy, signal transduction, and inflammatory body formation.162 This results in activation of the NF-κB signaling pathway, the production and release of pro-inflammatory cytokines (interleukins, chemokines), and initiation of innate and adaptive immune responses. Using LPS and muramyl dipeptide (MDP) to induce the expression of inflammasomes (NOD2, NLRP3 and caspase-1) and cytokines in human cerebral ECs, Nagyőszi et al showed that NLRs and inflammasomes can be activated in brain ECs.163 Ge et al found that caspase-1 inhibitors prevent the apoptosis of damaged BMECs by inhibiting the expression of caspase-1 and pro-inflammatory cytokines, thereby reducing BBB damage after TBI.164
Potential Therapeutic Tools for Promotion and Restoration of BBB Integrity
Current studies have indicated that the gut microbiota and brain perform complex bidirectional activities via the gut-brain-axis. However, the mechanisms by which the intestinal microbes affects the BBB and brain health of the host remain to be clarified. Supplementation of probiotics, prebiotics, synbiotics, and transplantation of fecal microbes may reduce the entry of harmful metabolites into the systemic circulation, contributing to the integrity of the gut and BBB.165 Lactobacillus plantarum MTCC 9510 supplementation improved the intestinal and BBB integrity and reduced the abundance of Enterobacteriaceae.166 Patients receiving probiotic treatment exhibited reduced serum C-reactive protein levels, improved insulin metabolism, and increased in Mini-Mental State Examination (MMSE) scores.167,168 In addition, further studies of therapies targeting immune cells and peripheral cytokines that may also reduce inflammation-induced BBB hyperpermeability and prevent the entry of harmful substances into the brain are warranted.
Conclusions
The gut microbiota affects the activity of the brain through a variety of different mechanisms. The brain is influenced by the interaction of the gut microbiota with intestinal cells, metabolite production by microbes, secretion of gut hormones, and changes in neural and immune signaling and blood circulation to the BBB. Further studies of the correlation of the specific changes in the number and type of species in the gut microbiota and signaling via the gut-brain axis at the molecular level are required to fully elucidate the mechanism by which the microbes influence the brain. Therefore, corresponding strategies aimed at controlling the gut microbiota are implicated in the prevention and treatment of certain neurological and psychological illnesses.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81873034), the Natural Science Foundation Project of CQ CSTC (cstc2014jcyjA10083 & cstc2018jcyjAX0158), the Medical Research Project of Chongqing Health Commission (2015MSXM014 & 2016MSXM198).
Disclosure
The authors have declared that they do not have competing interests for this work.
References
- 1.Wiley NC, Dinan TG, Ross RP, et al. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: implications for human and animal health. J Anim Sci. 2017;95(7):3225–3246. doi: 10.2527/jas.2016.1256 [DOI] [PubMed] [Google Scholar]
- 2.Jones L, Kumar J, Mistry A, et al. The transformative possibilities of the microbiota and mycobiota for health, disease, aging, and technological innovation. Biomedicines. 2019;7(2):24. doi: 10.3390/biomedicines7020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263. doi: 10.2174/1381612811319280009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leclercq S, Mian FM, Stanisz AM, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. doi: 10.1038/ncomms15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SL, Kirk RD, DaSilva NA, et al. Polyphenol microbial metabolites exhibit gut and blood-brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites. 2019;9(4):78. doi: 10.3390/metabo9040078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins B, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- 8.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 9.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res. 2014;355(3):657–673. doi: 10.1007/s00441-014-1821-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daneman R, Rescigno M. The gut immune barrier and the blood-brain barrier: are they so different? Immunity. 2009;31(5):722–735. doi: 10.1016/j.immuni.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain Res Brain Res Rev. 2003;42(3):221–242. doi: 10.1016/s0165-0173(03)00177-2 [DOI] [PubMed] [Google Scholar]
- 13.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4):493–506. doi: 10.1083/jcb.201412147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreone BJ, Chow BW, Tata A, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94(3):581–594 e585. doi: 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow BW, Gu C. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron. 2017;93(6):1325–1333 e1323. doi: 10.1016/j.neuron.2017.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rustenhoven J, Jansson D, Smyth LC, et al. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci. 2017;38(3):291–304. doi: 10.1016/j.tips.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37(10):3300–3317. doi: 10.1177/0271678X17722436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci. 2018;12:930. doi: 10.3389/fnins.2018.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott NJ, Rönnbäck L, H E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 23.Moura RP, Almeida A, Sarmento B. The role of non-endothelial cells on the penetration of nanoparticles through the blood brain barrier. Prog Neurobiol. 2017;159:39–49. doi: 10.1016/j.pneurobio.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 24.Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311–336. doi: 10.1007/s00401-018-1815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington M. For lack of gut microbes, the blood-brain barrier ‘leaks’. Lab Anim. 2015;44(1):6. doi: 10.1038/laban.682 [DOI] [PubMed] [Google Scholar]
- 27.Goes AVD, Wouters D, Pol SMAVD, et al. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15(10):1852–1854. doi: 10.1096/fj.00-0881fje [DOI] [PubMed] [Google Scholar]
- 28.Al-Dasooqi N, Gibson RJ, Bowen JM, et al. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp Biol Med. 2010;235(10):1244–1256. doi: 10.1258/ebm.2010.010082 [DOI] [PubMed] [Google Scholar]
- 29.Dopkins N, Nagarkatti PS, Nagarkatti M. The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology. 2018;154(2):178–185. doi: 10.1111/imm.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair AT, Ramachandran V, Joghee NM, et al. Gut microbiota dysfunction as reliable non-invasive early diagnostic biomarkers in the pathophysiology of parkinson’s disease: a critical review. J Neurogastroenterol Motil. 2018;24(1):30–42. doi: 10.5056/jnm17105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeke KA, Boesmans L, Boets E. Modulating the microbiota in inflammatory bowel diseases: prebiotics, probiotics or faecal transplantation? Proc Nutr Soc. 2014;73(4):490–497. doi: 10.1017/S0029665114000639 [DOI] [PubMed] [Google Scholar]
- 32.Velagapudi VR, Hezaveh R, Reigstad CS, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51(5):1101–1112. doi: 10.1194/jlr.M002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- 34.Alkasir R, Li J, Li X, et al. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90–102. doi: 10.1007/s13238-016-0338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12(3):129–134. doi: 10.1016/j.tim.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 37.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz S, Mazel-Sanchez B, Kandasamy M, et al. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome. 2018;6(1):9. doi: 10.1186/s40168-017-0386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nettles R, Ricks KD, Koide RT. The dynamics of interacting bacterial and fungal communities of the mouse colon following antibiotics. Microb Ecol. 2020;3. doi: 10.1007/s00248-020-01525-6. [DOI] [PubMed] [Google Scholar]
- 41.Wu SC, Cao ZS, Chang KM, et al. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat Commun. 2017;8(1):24. doi: 10.1038/s41467-017-00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 43.Zhai CD, Zheng JJ, An BC, et al. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: establishment of bacterial and archaeal communities analyses. Chin Med J. 2019;132(15):1815–1822. doi: 10.1097/CM9.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanguinetti E, Collado MC, Marrachelli VG, et al. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep. 2018;8(1):4907. doi: 10.1038/s41598-018-23261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welcome MO. Gut microbiota disorder, gut epithelial and blood-brain barrier dysfunctions in etiopathogenesis of dementia: molecular mechanisms and signaling pathways. Neuromolecular Med. 2019;21(3):205–226. doi: 10.1007/s12017-019-08547-5 [DOI] [PubMed] [Google Scholar]
- 46.Frohlich EE, Farzi A, Mayerhofer R, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014;817(5):115–133. doi: 10.1007/978-1-4939-0897-4_5 [DOI] [PubMed] [Google Scholar]
- 48.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14(3):143–159. doi: 10.1038/nrgastro.2016.191 [DOI] [PubMed] [Google Scholar]
- 49.Cani PD, Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab. 2016;5(9):743–752. doi: 10.1016/j.molmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- 51.Fessler EB, Chibane FL, Wang Z, et al. Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Curr Pharm Des. 2013;19(28):5105–5120. doi: 10.2174/1381612811319280009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726. doi: 10.1371/journal.ppat.1003726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Mahony SM, Hyland NP, Dinan TG, et al. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214(1):71–88. doi: 10.1007/s00213-010-2010-9 [DOI] [PubMed] [Google Scholar]
- 54.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2019;1–23. doi: 10.1080/19490976.2019.1638722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson E, Cryan JF, Fitzgerald GF, et al. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 2014;73(4):477–489. doi: 10.1017/S0029665114001426 [DOI] [PubMed] [Google Scholar]
- 57.Min L, Wang B, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kacimi R, Giffard RG, Yenari MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm. 2011;8:7. doi: 10.1186/1476-9255-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh AK, Yin Jiang GS. Effects of bacterial toxins on endothelial tight junction in vitro: a mechanism-based investigation. Toxicol Mech Methods. 2007;17(6):331–347. doi: 10.1080/15376510601077029 [DOI] [PubMed] [Google Scholar]
- 60.Erdo F, Denes L, Lange ED. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab. 2017;37(1):4–24. doi: 10.1177/0271678X16679420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139(3):313–326. doi: 10.1016/j.pharmthera.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S, Kim JH, Kim JH, et al. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011;286(51):43855–43870. doi: 10.1074/jbc.M111.299248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S, Lee WH, Lee MS, et al. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res. 2012;90(3):540–550. doi: 10.1002/jnr.22779 [DOI] [PubMed] [Google Scholar]
- 64.Makki K, Deehan EC, Walter J, et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 65.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 67.Hoyles L, Snelling T, Umlai UK, et al. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6(1):55. doi: 10.1186/s40168-018-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68 Suppl 22(2):S102–111. doi: 10.1111/j.1753-4887.2010.00345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho L, Ono K, Tsuji M, et al. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18(1):83–90. doi: 10.1080/14737175.2018.1400909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, Ho L, Faith J, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease beta-amyloid oligomerization. Mol Nutr Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.201400544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt J, Smith NJ, Christiansen E, et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J Biol Chem. 2011;286(12):10628–10640. doi: 10.1074/jbc.M110.210872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cenit MC, Sanz Y, Codoner-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23(30):5486–5498. doi: 10.3748/wjg.v23.i30.5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X, Chen PS, Dallas S, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11(8):1123–1134. doi: 10.1017/S1461145708009024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39(3):424–429. doi: 10.1038/ijo.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muraca M, PutIgnani L, FIerabraccI A, et al. Gut microbiota-derived outer membrane vesicles- under-recognized major players in health and disease. Discov Med. 2015;19(106):343–348. [PubMed] [Google Scholar]
- 76.Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6(4):e774. doi: 10.1038/tp.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- 79.Stilling RM, Ryan FJ, Hoban AE, et al. Microbes & neurodevelopment–absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220. doi: 10.1016/j.bbi.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 80.Luczynski P, Whelan SO, O’Sullivan C, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016;44(9):2654–2666. doi: 10.1111/ejn.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- 82.Barajon I, Serrao G, Arnaboldi F, et al. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57(11):1013–1023. doi: 10.1369/jhc.2009.953539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brun P, Giron MC, Qesari M, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145(6):1323–1333. doi: 10.1053/j.gastro.2013.08.047 [DOI] [PubMed] [Google Scholar]
- 84.Reichardt F, Chassaing B, Nezami BG, et al. Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J Physiol. 2017;595(5):1831–1846. doi: 10.1113/JP273269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schweighofer H, Rummel C, Roth J, et al. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Med Exp. 2016;4(1):19. doi: 10.1186/s40635-016-0091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Yang LY, Orban L, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 2018;11(4):689–698. doi: 10.1016/j.brs.2018.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roshchina VV. New Trends and Perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv Exp Med Biol. 2016;874:25–77. doi: 10.1007/978-3-319-20215-0_2 [DOI] [PubMed] [Google Scholar]
- 88.Zhang S, Cheng S, Jiang X, et al. Gut-brain communication in hyperfunction of 5-hydroxytryptamine induced by oral zinc oxide nanoparticles exposure in young mice. Food Chem Toxicol. 2020;135:110906. doi: 10.1016/j.fct.2019.110906 [DOI] [PubMed] [Google Scholar]
- 89.Holzer P. Neuropeptides, microbiota, and behavior. Int Rev Neurobiol. 2016;131:67–89. doi: 10.1016/bs.irn.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 90.Wall R, Cryan J F, Rose P R, et al. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10 [DOI] [PubMed] [Google Scholar]
- 91.O’Mahony SM, Clarke G, Borre YE, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 92.Desbonnet L, Garrett L, Clarke G, et al. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 93.Apolloni S, Caputi F, Pignataro A, et al. Histamine is an inducer of the heat shock response in SOD1-G93A models of ALS. Int J Mol Sci. 2019;20(15):3793. doi: 10.3390/ijms20153793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13(1):43. doi: 10.1186/s12970-016-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farzi A, Frohlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018;15(1):5–22. doi: 10.1007/s13311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esposito P, Chandler N, Kandere K, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303(3):1061–1066. doi: 10.1124/jpet.102.038497 [DOI] [PubMed] [Google Scholar]
- 97.de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223. doi: 10.3389/fimmu.2015.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12(10):947–953. doi: 10.1016/j.autrev.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 99.Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15(1):376–384. doi: 10.0000/PMID7823142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moya-Perez A, Perez-Villalba A, Benitez-Paez A, et al. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56. doi: 10.1016/j.bbi.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 101.Wichmann A, Allahyar A, Greiner TU, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14(5):582–590. doi: 10.1016/j.chom.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 102.Christiansen CB, Gabe MBN, Svendsen B, et al. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017 [DOI] [PubMed] [Google Scholar]
- 103.Bayliss JA, Lemus M, Santos VV, et al. Acylated but not des-acyl ghrelin is neuroprotective in an MPTP mouse model of Parkinson’s disease. J Neurochem. 2016;137(3):460–471. doi: 10.1111/jnc.13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattson MP, Barger SW, Cheng B, et al. Beta-amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer’s disease. Trends Neurosci. 1993;16(10):409–414. doi: 10.1016/0166-2236(93)90009-b [DOI] [PubMed] [Google Scholar]
- 105.Shen XL, Song N, Du XX, et al. Nesfatin-1 protects dopaminergic neurons against MPP+/MPTP- induced neurotoxicity through the C-Raf-ERK1/2-dependent anti-apoptotic pathway. Sci Rep. 2017;7:40961. doi: 10.1038/srep40961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silva AD, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10–20. doi: 10.5009/gnl.2012.6.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brooks L, Viardot A, Tsakmaki A, et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab. 2017;6(1):48–60. doi: 10.1016/j.molmet.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duca FA, Swartz TD, Sakar Y, et al. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS One. 2012;7(6):e39748. doi: 10.1371/journal.pone.0039748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salcedo I, Tweedie D, Li Y, et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Treangen TJ, Wagner J, Burns MP, et al. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757. doi: 10.3389/fimmu.2018.02757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tolhurst G, Heffron H, Lam YS, et al. Short- chain fatty acids stimulate glucagon- like peptide- 1 secretion via the G- protein- coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li H, Sun J, Du J, et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. 2018;30(5):e13260. doi: 10.1111/nmo.13260 [DOI] [PubMed] [Google Scholar]
- 113.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi: 10.1113/JP273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thomsen MS, Birkelund S, Burkhart A, et al. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J Neurochem. 2017;140(5):741–754. doi: 10.1111/jnc.13747 [DOI] [PubMed] [Google Scholar]
- 115.Skelly DT, Hennessy E, Dansereau MA, et al. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1Β, TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8(7):e69123. doi: 10.1371/journal.pone.0069123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 1999;64(21):1941–1953. doi: 10.1016/S0024-3205(99)00139-3 [DOI] [PubMed] [Google Scholar]
- 117.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. doi: 10.1007/s00281-011-0290-8 [DOI] [PubMed] [Google Scholar]
- 118.Perry VH. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun. 2007;21(1):45–46. doi: 10.1016/j.bbi.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 119.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331 [DOI] [PubMed] [Google Scholar]
- 120.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 122.Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429. doi: 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067–1080. doi: 10.1016/j.cell.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 124.Liu J, Mori M, Sugimoto K, et al. Peripheral blood helper T cell profiles and their clinical relevance in MOG-IgG-associated and AQP4-IgG-associated disorders and MS. J Neurol Neurosurg Psychiatry. 2020;91(2):132–139. doi: 10.1136/jnnp-2019-321988 [DOI] [PubMed] [Google Scholar]
- 125.Legroux L, Arbour N. Multiple sclerosis and T lymphocytes: an entangled story. J Neuroimmune Pharmacol. 2015;10(4):528–546. doi: 10.1007/s11481-015-9614-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strachan-Whaley M, Rivest S, Yong VW. Interactions between microglia and T cells in multiple sclerosis pathobiology. J Interferon Cytokine Res. 2014;34(8):615–622. doi: 10.1089/jir.2014.0019 [DOI] [PubMed] [Google Scholar]
- 128.Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chai J, Song Q. Quantitative and multiplexed study of endothelial cell inflammation. Cell Biochem Biophys. 2014;70(3):1783–1790. doi: 10.1007/s12013-014-0129-8 [DOI] [PubMed] [Google Scholar]
- 131.Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hennessy E, Griffin EW, Cunningham C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1beta and TNF-alpha. J Neurosci. 2015;35(22):8411–8422. doi: 10.1523/JNEUROSCI.2745-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koval A, Purvanov V, Egger-Adam D, et al. Yellow submarine of the Wnt/Frizzled signaling: submerging from the G protein harbor to the targets. Biochem Pharmacol. 2011;82(10):1311–1319. doi: 10.1016/j.bcp.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 134.Tauriello DV, Jordens I, Kirchner K, et al. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci U S A. 2012;109(14):E812–820. doi: 10.1073/pnas.1114802109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang H-C, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5(7):234. doi: 10.1186/gb-2004-5-7-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Punchihewa C, Ferreira AM, Cassell R, et al. Sequence requirement and subtype specificity in the high-affinity interaction between human frizzled and dishevelled proteins. Protein Sci. 2009;18(5):994–1002. doi: 10.1002/pro.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Strutt D, Madder D, Chaudhary V, et al. Structure-function dissection of the frizzled receptor in drosophila melanogaster suggests different mechanisms of action in planar polarity and canonical Wnt signaling. Genetics. 2012;192(4):1295–1313. doi: 10.1534/genetics.112.144592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Polakis P. Formation of the blood-brain barrier: wnt signaling seals the deal. J Cell Biol. 2008;183(3):371–373. doi: 10.1083/jcb.200810040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood–brain barrier. Cell Tissue Res. 2014;355(3):687–699. doi: 10.1007/s00441-014-1811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183(3):409–417. doi: 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ranjan Sen BD. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. cell. 1986;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6 [DOI] [PubMed] [Google Scholar]
- 142.Atchison ML, Perry RP. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. cell. 1987;48(1):121–128. doi: 10.1016/0092-8674(87)90362-X [DOI] [PubMed] [Google Scholar]
- 143.Jin X, Wang T, Liao Y, et al. Neuroinflammatory reactions in the brain of 1,2-DCE-intoxicated mice during brain edema. Cells. 2019;8(9):987. doi: 10.3390/cells8090987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.MMJ A-O, Desa MNM. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38(7):1349–1368. doi: 10.1007/s10571-018-0609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 146.Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15(11):2760–2770. doi: 10.1002/j.1460-2075.1996.tb00636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Plotnikov A, Zehorai E, Procaccia S, et al. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813(9):1619–1633. doi: 10.1016/j.bbamcr.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 148.Li X, Wang X, Xie J, et al. Suppression of angiotensin-(1-7) on the disruption of blood-brain barrier in rat of brain glioma. Pathol Oncol Res. 2019;25(1):429–435. doi: 10.1007/s12253-018-0471-z [DOI] [PubMed] [Google Scholar]
- 149.Lu L, Wang M, Wei X, et al. 20-HETE inhibition by HET0016 decreases the blood-brain barrier permeability and brain edema after traumatic brain injury. Front Aging Neurosci. 2018;10:207. doi: 10.3389/fnagi.2018.00207 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.Zhu H, Dai R, Zhou Y, et al. TLR2 ligand pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-kappaB signaling pathways in primary brain microvascular endothelial cells. Neurochem Res. 2018;43(10):1897–1904. doi: 10.1007/s11064-018-2607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gong P, Zhang Z, Zou Y, et al. Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur J Pharmacol. 2019;854:289–297. doi: 10.1016/j.ejphar.2019.04.028 [DOI] [PubMed] [Google Scholar]
- 152.Chaudhuri A, Yang B, Gendelman HE, et al. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood. 2008;111(4):2062–2072. doi: 10.1182/blood-2007-05-091207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bourgeois C, Majer O, Frohner IE, et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol. 2011;186(5):3104–3112. doi: 10.4049/jimmunol.1002599 [DOI] [PubMed] [Google Scholar]
- 154.Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2017;12(2):193–196. doi: 10.4103/1673-5374.200795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mayerhofer R, Frohlich EE, Reichmann F, et al. Diverse action of lipoteichoic acid and lipopolysaccharide on neuroinflammation, blood-brain barrier disruption, and anxiety in mice. Brain Behav Immun. 2017;60:174–187. doi: 10.1016/j.bbi.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paradis A, Bernier S, Dumais N. TLR4 induces CCR7-dependent monocytes transmigration through the blood-brain barrier. J Neuroimmunol. 2016;295–296:12–17. doi: 10.1016/j.jneuroim.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 157.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3(5):371–382. doi: 10.1038/nri1086 [DOI] [PubMed] [Google Scholar]
- 158.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83(1):13–30. doi: 10.1189/jlb.0607402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gay N, Proell M, Riedl SJ, et al. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One. 2008;3(4):e2119. doi: 10.1371/journal.pone.0002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonardi V, Dangl JL. How complex are intracellular immune receptor signaling complexes? Front Plant Sci. 2012;3:237. doi: 10.3389/fpls.2012.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tuncer S, Fiorillo MT, Sorrentino R. The multifaceted nature of NLRP12. J Leukoc Biol. 2014;96(6):991–1000. doi: 10.1189/jlb.3RU0514-265RR [DOI] [PubMed] [Google Scholar]
- 162.Kim YK, Shin JS, Nahm MH. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J. 2016;57(1):5–14. doi: 10.3349/ymj.2016.57.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagyoszi P, Nyul-Toth A, Fazakas C, et al. Regulation of NOD-like receptors and inflammasome activation in cerebral endothelial cells. J Neurochem. 2015;135(3):551–564. doi: 10.1111/jnc.13197 [DOI] [PubMed] [Google Scholar]
- 164.Ge X, Li W, Huang S, et al. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res. 2018;1697:10–20. doi: 10.1016/j.brainres.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 165.Bhattarai Y. Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. 2018;30(6):e13366. doi: 10.1111/nmo.13366 [DOI] [PubMed] [Google Scholar]
- 166.Dhaliwal J, Singh DP, Singh S, et al. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J Appl Microbiol. 2018;125(1):257–269. doi: 10.1111/jam.13765 [DOI] [PubMed] [Google Scholar]
- 167.Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in aheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38(6):2569–2575. doi: 10.1016/j.clnu.2018.11.034 [DOI] [PubMed] [Google Scholar]