Abstract
Purpose of Review
To summarize the last 10 years of literature regarding the effects of whole-body vibration (WBV) on bone in children, and if WBV results in increased bone acquisition.
Recent Findings
WBV intervention appears to be a safe intervention with beneficial effects on bone mass in some diseases and syndromes, but there is still low evidence for WBV in clinical practice. The positive effects on muscle strength, balance, and walking speed are more conclusive. One of the takeaways of this review is that well-trained individuals may not further improve bone mass with WBV; thus, interventions are more beneficial in pediatric individuals with Down syndrome or severe motor disabilities with low bone mass and reduced activity levels.
Summary
WBV appears to be a safe non-pharmacological anabolic approach to increase bone mass in some pediatric populations; however, longer (> 6 months) and larger prospective studies are needed to elucidate the efficacy of WBV on bone health in young individuals.
Keywords: Bone mineral density, Pediatric, Mechanical oscillation, Rehabilitation, Physical activity, Skeleton
Introduction
Bone mass increases gradually under healthy conditions during childhood and reaches a plateau (a.k.a. peak bone mass) in early adulthood, which serves as a “bone bank” for the remainder of life. Longitudinal growth and bone modeling during childhood is a complex process of both resorption and formation that is necessary for skeletal growth and it has been shown by a number of studies that physical activity increases bone formation and bone acquisition [1, 2].
Whole-body vibration (WBV) was initially developed in the 1970s to prevent loss of muscle and bone mass in cosmonauts during prolonged spaceflights [3]. The underlying mechanism of concept that WBV could increase bone mass relates to the mechanostat theory; that is, bone adapts its strength to mechanical forces that are mostly imposed by muscle [4••, 5]. An early study in sheep by Rubin et al. [6] showed that low-level mechanical stimulation resulted in a strong anabolic response through increased bone formation in trabecular bone after 1 year. These results were further strengthened by experimental studies in rats where the anabolic activity on bone, suppressed by disuse, was normalized by mechanical stimulation [7]. As a development of these positive results in animal models with anabolic effects on bone, WBV has been developed for humans as an anabolic option to improve bone mass. WBV could be an alternative to replace and/or complement regular physical activity. Intervention including WBV has also shown a number of metabolic effects [8, 9] and, in addition, WBV increases muscle power and muscle strength [10]. The mechanical stimulation from WBV affects bone cells, such as osteocytes, which results in altered expression of Wnt-signaling proteins, e.g., sclerostin, resulting in increased bone mass [11••, 12••].
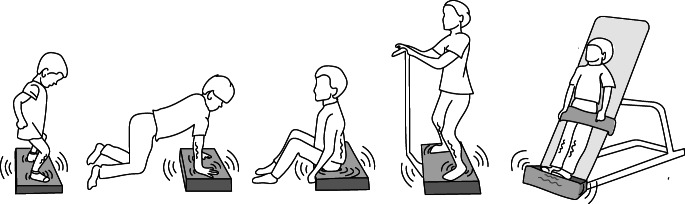
WBV has, therefore, received increasing attention as a treatment option including pediatric patient populations with individuals in the phase of bone acquisition. Young individuals with a broad variety of diseases which leads to poor bone health could be considered for WBV, hopefully without side effects, as a non-pharmacological anabolic approach to increase balance, neuromuscular function, and bone mass [13••]. The literature presents various WBV platforms with vibration strategies and as scientists should be able to reproduce the study and data, there was an early need for an international consensus on how to report data and how to describe the vibration intervention by the International Society of Musculoskeletal and Neuronal Interactions [14••]. Vibration platforms do not only differ with respect to vibration parameters such as frequency, amplitude, and acceleration, but also in the type of mode they vibrate, that is, side-alternating by oscillation around a horizontal anteroposterior central axis, or synchronous vibration with uniform acceleration and peak-to-peak displacement for the entire surface. Figure 1 demonstrates different uses and positions of WBV platforms. For the included studies in this review, the different settings of vibration parameters and intervention details are presented in Table 1.
Fig. 1.
Schematic examples of different uses of WBV platforms
Table 1.
Summary information of pediatric WBV studies (during the last 10 years) with major bone outcomes
| Reference | Study design | N | Age (years) Gender |
Type of WBV | Intervention details | Major bone outcomes |
|---|---|---|---|---|---|---|
| Healthy children and adolescents | ||||||
| Binkley et al. [15] (2014) | Randomized controlled trial | 20 |
6–10 M + F |
WBV on upper limbs HMMS 14 Hz, 0.75 g; LMMS 29 Hz, 0.30 g |
HMMS 2 min and LMMS 5 min 3 days/week for 12 weeks |
Increased trabecular BMD in the radius |
| Harrison et al. [16] (2015) | Randomized controlled trial | 36 |
9–12 M |
Two WBV standing platforms, high or low magnitude, high > 2 g or low < 1 g | On either 1, 3 or 5 successive days | Bone turnover markers PINP and CTX increased after 8 days |
| Gomez-Bruton et al. [17] (2018) | Randomized controlled trial | 51 |
14 ± 2 M + F |
WBV standing platform 38 Hz, 4 mm, 12 g |
15 min, 3 days/week for 6 months | No effect on bone strength or bone structure |
| Muscle degenerative disorders | ||||||
| Bianchi et al. [18] (2013) | Randomized controlled trial | 21 |
9 ± 4 M |
Low-magnitude high-frequency | 10 min/day for 1 year | Increased BMD |
| Söderpalm et al. [19] (2013) | Observational prospective trial | 6 |
6–12 M |
WBV standing platform 16–24 Hz, 4 mm, 2.1–4.6 g |
2–3 times/week for 3 months | No effect on bone mass |
| Petryk et al. [20] (2017) | Observational prospective trial | 5 |
6–22 M |
WBV standing platform Low-magnitude high-frequency, 30–90 Hz |
10 min/day for 6 months | Uncertain effects on cortical and trabecular parameters |
| Severe motor disabilities | ||||||
| Ruck et al. [21] (2010) | Randomized controlled trial | 20 |
6–12 M + F |
WBV standing platform 12–18 Hz, 4 mm, 2.6 g |
9 min/school day for 6 months | No effect on bone mass |
| Afzal et al. [22] (2014) | Crossover pilot study | 14 |
3–20 M + F |
WBV standing platform Low-magnitude, 30 Hz, 0.3 g |
20 min/day, 5 days/week for 12 months | Positive effects on bone mass, especially in spine BMD Z-score |
| Gusso et al. [23] (2016) | Clinical trial without control group | 40 |
11–21 M + F |
WBV standing platform 12–20 Hz |
9 min, 4 days/week for 20 weeks | Positive effects on bone mass (total body, lumbar spine and lower limbs) |
| Kilebrant et al. [24] (2015) | Observational, prospective | 19 |
5–16 M + F |
WBV standing platform 40–42 Hz, 0.2 mm |
5–15 min twice/week for 6 months | Positive effects on bone mass for total body BMD |
| Reyes et al. [25] (2011) | Randomized controlled trial | 65 |
6–9 M + F |
WBV device on elbows and knees Low-magnitude high-frequency, 60–90 Hz, 0.1 mm, 0.3 g |
5 min/day, 7 days/week for 6 months | Positive effects on regional bone mass, ultradistal radius |
| Stark et al. [26] (2010) | Retrospective, home-based WBV and other training | 78 |
Mean 10 M + F |
WBV standing platform 5–25 Hz, 0–3.9 mm |
6-month home-based training | Positive effects on bone mass |
| Wren et al. [27] (2010) | Randomized controlled trial | 31 |
6–12 M + F |
WBV standing platform 30 Hz, 0.3 g |
10 min/day for 6 months | Positive effects on bone mass, especially in cortical bone |
| Osteogenesis imperfecta | ||||||
| Hoyer-Kuhn et al. [28] (2014) | Retrospective, home-based WBV, and other training | 53 |
2–25 M + F |
WBV standing platform 15–20 Hz, 0–7.8 mm, 3.5–6.3 g |
Twice daily (3 × 3 min) for 6 months | Positive effects on bone mass. Total body without head BMD increased. |
| Högler et al. [29] (2017) | Randomized controlled trial | 24 |
5–16 M + F |
WBV standing platform 20–25 Hz, 2–6 mm |
Twice daily (3 × 3 min) for 5 months | No effect on bone mass |
| Other groups | ||||||
|
Down syndrome Matute-Llorente et al. [30] (2016) |
Randomized controlled trial | 25 |
12–18 M + F |
WBV standing platform 25–30 Hz, 2 mm, 2.5–3.6 g |
3 times per week with 10 repetitions of 10–20 s, for 20 weeks | Positive effects on bone mass |
|
Obesity Tubic et al. [12••] (2019) |
Randomized controlled trial | 30 |
7–17 M + F |
WBV standing platform 16–24 Hz, 4 mm, 2.1–4.6 g |
7–10 min, 3 days/week for 3 months | No effect on bone mass, but effects for serum sclerostin |
|
Overweight Erceg et al. [31] (2015) |
Randomized controlled trial | 20 |
8–10 M |
WBV standing platform 30–40 Hz, low-high, 1.9–6.2 g |
3 times per week with 10 repetitions of 30–60 s, for 10 weeks | No effect on bone mass |
|
Anorexia nervosa DiVasta et al. [32] (2017) |
Randomized double-blind trial | 41 |
13–21 F |
WBV standing platform LMMS 32–37 Hz, 0.3 g |
10 min/day during hospitalization of 5 days | Prevents a decline in bone turnover during bed rest |
|
Idiopathic scoliosis Lam et al. [33] (2013) |
Randomized controlled trial | 149 |
15–25 F |
WBV standing platform LMMS 32–37 Hz, 0.3 g |
20 min/day, 5 days/week for 12 months | Positive effects on bone mass |
|
Thalassemia Fung et al. [34] (2012) |
Longitudinal crossover pilot trial | 18 |
10–18 M + F |
WBV standing platform LMMS 30 Hz, 0.3 g |
20 min/day for 6 months | Positive effects on bone mass |
|
Hemophilia El-Shamy [35] (2017) |
Randomized controlled trial | 30 |
9–13 M |
WBV standing platform 30–40 Hz, 2–4 mm |
15 min/day, 3 days/week for 12 weeks | Positive effects on bone mass |
|
Thermally injured Edionwe et al. [36] (2016) |
Randomized controlled trial | 19 |
11–13 M + F |
WBV standing platform 30–40 Hz, 2–4 mm |
12–15 min/day, 5 days/week for 6 weeks | Stabilizing the decline in bone mass |
|
Crohn’s disease Leonard et al. [37] (2016) |
Randomized controlled trial | 138 |
8–21 M + F |
WBV standing platform LMMS 30 Hz, 0.3 g |
10 min/day for 12 months | Increased vertebral trabecular BMD, but inconsistent effects on axial and appendicular trabecular volumetric BMD |
F, female; HMMS, high-magnitude mechanical stimulation; LMMS, low-magnitude mechanical stimulation; M, male
Some studies have been reported about the effects of WBV on bone mass in children and adolescents; however, the potential effects and protocols with optimal vibration parameters are still uncertain. This review aimed to assess the literature during the last 10 years regarding the effects of vibration treatment on bone in pediatric populations. Research publications were identified by searching PubMed with the applied search string (filter 10 years): vibration AND (bone OR skeleton OR BMD OR osteoporosis) AND (children OR adolescents OR pediatric) AND human, until February 2020 without language restrictions. A total of 156 publications were found with this search strategy. Table 1 summarizes the selected original articles during the last 10 years regarding the effects of WBV intervention protocols in pediatric populations.
Safety of WBV
Most studies covered by this review did not report serious adverse events of WBV, which is in conjunction with other reviews on this topic [38, 39, 40••, 41••]. Söderpalm et al. [19] studied WBV exercise (2 to 3 times a week, 3 months) in patients with Duchenne muscular dystrophy (DMD). The circulating levels of creatine kinase did not change over the study period, thus indicating that WBV exercise, at this magnitude, was well tolerated and did not induce further skeletal muscle damage. No serious adverse events were reported in the meta-analysis by Saquetto et al. [40••] comprising 176 patients with cerebral palsy (CP) from 6 studies, and WBV was considered well tolerated in these cohorts although that potential long-term risks require more research. In a study with adult women, lower leg itching and erythema were reported [42]. Another study in children with CP reported that 80% of the participants experienced redness of the feet after the first treatment session [21]. As reported by the review by Bell et al. [41••], many studies do not provide information on adverse events; however, we would like to highlight the importance of reporting adverse events and all negative side effects in future clinical WBV studies, since this has to be taken into account in future clinical practice guidelines.
Effect of WBV on Healthy Children and Adolescents
There are only few studies concerning WBV intervention and the effect on bone mass in healthy young individuals. A randomized controlled trial in healthy pre-pubertal children with high and low mechanical stimulation vibration for 12 weeks increased trabecular bone mineral density (BMD) in the forearm [15]. Rapid effects of WBV on bone remodeling have been studied in healthy pre-pubertal boys by using biomarkers of bone turnover. After 5 consecutive days of WBV training (applying two platforms with high and low-magnitude vibration), it was demonstrated that the bone formation marker PINP (i.e., type I procollagen intact amino-terminal propeptide) increased by 25% and the bone resorption marker CTX (i.e., carboxy-terminal cross-linking telopeptide of type I collagen) by 10%; however, no effect was found for serum osteocalcin, osteoprotegerin, or sclerostin [16]. The authors suggested that irrespectively of the magnitude of vibration, the healthy growing bone tissue does have the capacity to respond quickly to WBV training. The review by Marin-Puyalto et al. [43••] concluded that interventions with WBV appears to be more effective in increasing bone mass in young individuals with compromised bone mass in comparison with postmenopausal women. No effect was found on bone strength or structure in a study with healthy adolescent swimmers who performed swimming training and WBV intervention three times a week during a 6-month study period. These authors suggested that WBV intervention was not intense enough to achieve positive effects on skeletal strength [17].
Muscle Degenerative Disorders
Both Duchenne and Becker muscular dystrophies are X-linked progressive neuromuscular disorders caused by loss-of-function mutations in the gene DMD coding for the protein dystrophin. Affected patients with DMD have their first signs of muscle weakness during childhood. Becker muscular dystrophy is usually milder and more varied. Poor bone health is common in patients with DMD, and long-term corticosteroid treatment further increases the risk for osteoporosis and fragility fractures [41••, 44].
Bianchi et al. [18] showed in a small pilot study that BMD increased in spine, total body, and femoral neck in patients with DMD, which is in contrast to another small study in which no effects were found on bone mass, muscle strength, or biomarkers of bone turnover [19]. In another small study, Petryk et al. [20] observed uncertain effects on cortical and trabecular parameters.
Most WBV studies in patients with DMD are small observational investigations, which makes it difficult to draw any significant conclusions regarding the efficacy of WBV in patients with muscle degenerative disorders. However, WBV interventions appear to be well tolerated in patients with muscular dystrophies; hence, larger controlled trials are needed to establish potential benefits of WBV before any clinical implications can be made.
Severe Motor Disabilities
Fragility fractures, as a consequence of reduced BMD, are common complications in children with severe motor disabilities such as CP and Rett syndrome [45]. The prevalence rate for fragility fractures is nearly 20% in non-ambulatory children and young adults with CP [46]. There is, therefore, an increasing interest in WBV as a non-pharmacological anabolic approach in children with severe motor disabilities to increase neuromuscular function, balance, and bone mass. For this review, we found 8 intervention studies reported in PubMed (during the last 10 years) about WBV therapy in children with severe motor disabilities.
In a study with 16 patients with CP, aged 9 years, spasticity was reduced and ambulatory function improved after 8 weeks of WBV intervention; however, bone parameters were not investigated [47]. A randomized controlled pilot study with WBV treatment in 20 children with CP detected improved mobility function but did not detect any positive effect on bone tissue after 6 months of treatment [21]. However, positive effects on cortical and trabecular bone have been demonstrated in a number of studies on patients with CP and Rett syndrome [22–27]. Saquetto et al. [40••] published a systematic review with meta-analysis on 6 studies with 176 children with CP demonstrating increased femur BMD after WBV intervention. The efficacy of WBV as a bone anabolic therapy in children with severe motor disabilities appears to be mostly beneficial. However, despite the favorable data reported, there is still not enough evidence to support WBV in clinical practice in children and adolescents with disabilities, which also is in agreement with a recent systematic review [48••].
Osteogenesis Imperfecta
Osteogenesis imperfecta (OI) is a rare hereditary disease, which can result in extreme bone fragility, limited mobility, and substantial growth deficiency [49]. The majority of patients with OI have a loss-of-function mutation in one of the two genes coding for collagen type I alpha chains, COL1A1 or COL1A2; however, there are also at least 18 other genes that have been associated with OI phenotypes [50]. Pharmacologic treatment regimens with bisphosphonates have successfully been implemented as clinical routine for children with OI to reduce bone resorption, to maximize linear growth, and to reduce the burden of fractures and pain [51]. Bisphosphonates have an approximately decade-long half-life in bone and potential adverse events are still not fully elucidated. Despite treatment, the newly remodeled bone would still comprise defective collagen type I in the classical OI types. WBV has gained some interest as a non-pharmacological anabolic approach for children with OI.
As in WBV intervention studies in children with severe motor disabilities, increased motor function and walking distance have been found as well as an increase in total body BMD (less head) [28]. In contrast, Högler et al. [29] found no significant changes in bone mass. A recent review, in which only 3 eligible studies were found, concluded that WBV intervention could be an alternative option in the management for improving mobility and functional parameters [52].
Effect of WBV Intervention in Other Groups
There is a large clinical need for further interventional studies about the effects of WBV on bone tissue and bone acquisition in a number of pediatric conditions and syndromes. Positive effects of WBV were demonstrated on all bone mineral content (BMC) and BMD parameters in a randomized controlled trial in individuals with Down syndrome [30]. These findings were supported by a recent review on WBV training, comprising 5 studies including 171 individuals with Down syndrome, which stated that WBV has positive effects on BMD, body composition, and balance [53].
There is an increasing prevalence worldwide of obesity and overweight. WBV intervention has been studied in overweight children; however, there are only two studies regarding the effects of WBV on bone during the last 10 years. One recent randomized study found decreased serum levels of sclerostin after a 12-week WBV intervention in children with obesity, which implies that WBV has direct effects on bone mechanotransduction [12••]. The other study on overweight subjects completed a 10-week WBV intervention, which showed increased BMC and BMD measurements [31]. On the other spectrum of weight disorders, anorexia nervosa is a disease with highly negative effects on bone tissue. One study in females with anorexia nervosa, aged 16 years, showed that daily low-magnitude mechanical stimulation prevented a reduction in bone turnover during bed rest; however, bone mass was not investigated in this study [32].
During the last 10 years, some studies have been published in other disorders or diseases but only as isolated publications with small patients groups, which makes it challenging to summarize the effects of WBV for each disease. In a study including young females with idiopathic scoliosis, the participants used WBV and it proved effective in improving areal BMD at femoral neck and lumbar spine [33]. Single studies exist in hematological diseases such as thalassemia and hemophilia. Fung et al. [34] found that WBV increased total body BMC and areal BMD in a pilot study with adolescent and adult patients with thalassemia. Beneficial effect of WBV training, in terms of increased BMD and quadriceps strength, was also demonstrated in a study on patients with hemophilia [35]. WBV training has also been studied in children recovering from burns who performed regular exercise in conjunction with WBV, which improved leg strength but with reportedly small decreases in some BMC and BMD measurements [36]. Leonard et al. [37] conducted a large WBV intervention in a pediatric cohort of Crohn’s disease and found increased vertebral trabecular BMD, but inconsistent effects on axial and appendicular trabecular volumetric BMD. More and larger clinical studies are clearly needed to draw significant conclusions about the effects of WBV in the described disease groups and other not yet studied populations.
Perspectives and Concluding Remarks
From this overview of pediatric studies focusing on the last 10 years, it appears that WBV is a safe intervention with few adverse events. WBV, using vibrating platforms of various brands and vibration parameters, has demonstrated beneficial effects on bone mass in some diseases and syndromes in pediatric populations, but definitely not unequivocally in all reported clinical trials. The reported positive effects on muscle strength, balance, and walking speed are more conclusive, in accordance with the mechanostat theory, which in turn could contribute to increased amounts of regular physical activity leading to favorable effects on bone mass and possibly reduced number of fractures. It should be noted that pediatric bone tissue may respond differently in comparison with adult bone since bone tissue is undergoing both modeling and remodeling during longitudinal growth. The response to mechanical stimulation might be different in pediatric bone in contrast to adult bone due to differences in microstructure and mineral-to-collagen ratio.
One of the takeaways of this review is that healthy well-trained children and adolescents, who already perform high amounts of physical activity, may not benefit from WBV training since the additive effect does not appear to be further beneficial or intense enough to achieve additional positive effects on skeletal strength. In general, WBV seems to be more beneficial in children and adolescents with low bone mass and reduced activity levels in children with Down syndrome or severe motor disabilities such as CP. The duration for most of the reported WBV studies on bone mass has been rather short (< 6 months), and possibly too short, reflecting the bone modeling/remodeling cycle and to significantly measure a positive net gain in bone mass to access the full potential of WBV. This could partly be explained by practical reasons since WBV interventions are usually quite time-consuming and staff demanding.
The number of reported randomized controlled studies in pediatric populations is clearly inadequate to develop and implement clinical practice guidelines, both in healthy individuals and in most groups of diseases and syndromes. Further and larger prospective studies, longer than 6 months, are still needed to assess the efficacy of WBV on bone mass and bone health in pediatric populations. From a clinical point of view regarding bone health, and in order to make the most of WBV interventions, we also recommend that future research on WBV should focus on exploring optimal vibration parameters (i.e., duration, treatment time, vibration frequency, and peak-to-peak displacements), since reported protocols for these parameters are highly variable. We conclude, from this pediatric review on the last 10 years, that WBV is a safe non-pharmacological anabolic approach to increase bone mass in some pediatric populations.
Acknowledgments
The authors thank Ingibjörg Sigurdardóttir for the illustration.
Funding Information
Open access funding provided by University of Gothenburg. This work was supported by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-716831 and 678871), and ALF grants from Region Östergötland.
Compliance with Ethical Standards
Conflict of Interest
Diana Swolin-Eide and Per Magnusson declare no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Footnotes
This article is part of the Topical Collection on Pediatrics
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. Br J Sports Med. 2002;36(4):250–257. doi: 10.1136/bjsm.36.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Detter FT, Rosengren BE, Dencker M, Nilsson JA, Karlsson MK. A 5-year exercise program in pre- and peripubertal children improves bone mass and bone size without affecting fracture risk. Calcif Tissue Int. 2013;92(4):385–393. doi: 10.1007/s00223-012-9691-5. [DOI] [PubMed] [Google Scholar]
- 3.Gojanovic B, Feihl F, Liaudet L, Gremion G, Waeber B. Whole-body vibration training elevates creatine kinase levels in sedentary subjects. Swiss Med Wkly. 2011;141:w13222. doi: 10.4414/smw.2011.13222. [DOI] [PubMed] [Google Scholar]
- 4••.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2(2):73–85. [PubMed] [Google Scholar]
- 5.Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcif Tissue Int. 2002;70(5):405–407. doi: 10.1007/s00223-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 6.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 7.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15(12):2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 8.Bellia A, Salli M, Lombardo M, D’Adamo M, Guglielmi V, Tirabasso C, et al. Effects of whole body vibration plus diet on insulin-resistance in middle-aged obese subjects. Int J Sports Med. 2014;35(6):511–516. doi: 10.1055/s-0033-1354358. [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231(4):305–314. doi: 10.1620/tjem.231.305. [DOI] [PubMed] [Google Scholar]
- 10.Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35(6):1033–1041. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- 11••.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34(5):658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Tubic B, Zeijlon R, Wennergren G, Obermayer-Pietsch B, Mårild S, Dahlgren J, et al. Randomised study of children with obesity showed that whole body vibration reduced sclerostin. Acta Paediatr. 2019;108(3):502–513. doi: 10.1111/apa.14531. [DOI] [PubMed] [Google Scholar]
- 13••.Ward LM, Rauch F. Anabolic therapy for the treatment of osteoporosis in childhood. Curr Osteoporos Rep. 2018;16(3):269–276. doi: 10.1007/s11914-018-0434-z. [DOI] [PubMed] [Google Scholar]
- 14••.Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 15.Binkley TL, Parupsky EC, Kleinsasser BA, Weidauer LA, Speckerr BL. Feasibility, compliance, and efficacy of a randomized controlled trial using vibration in pre-pubertal children. J Musculoskelet Neuronal Interact. 2014;14(3):294–302. [PubMed] [Google Scholar]
- 16.Harrison R, Ward K, Lee E, Razaghi H, Horne C, Bishop NJ. Acute bone response to whole body vibration in healthy pre-pubertal boys. J Musculoskelet Neuronal Interact. 2015;15(2):112–122. [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Bruton A, Gonzalez-Aguero A, Matute-Llorente A, Julian C, Lozano-Berges G, Gomez-Cabello A, et al. Effects of whole body vibration on tibia strength and structure of competitive adolescent swimmers: a randomized controlled trial. PM R. 2018;10(9):889–897. doi: 10.1016/j.pmrj.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi ML, Vai S, Morandi L, Baranello G, Pasanisi B, Rubin C. Effects of low-magnitude high-frequency vibration on bone density, bone resorption and muscular strength in ambulant children affected by Duchenne muscular dystrophy. J Bone Miner Res. 2013;28(Suppl. 1):S341. [Google Scholar]
- 19.Söderpalm AC, Kroksmark AK, Magnusson P, Karlsson J, Tulinius M, Swolin-Eide D. Whole body vibration therapy in patients with Duchenne muscular dystrophy--a prospective observational study. J Musculoskelet Neuronal Interact. 2013;13(1):13–18. [PubMed] [Google Scholar]
- 20.Petryk A, Polgreen LE, Grames M, Lowe DA, Hodges JS, Karachunski P. Feasibility and tolerability of whole-body, low-intensity vibration and its effects on muscle function and bone in patients with dystrophinopathies: a pilot study. Muscle Nerve. 2017;55(6):875–883. doi: 10.1002/mus.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: a randomized controlled pilot study. J Musculoskelet Neuronal Interact. 2010;10(1):77–83. [PubMed] [Google Scholar]
- 22.Afzal SY, Wender AR, Jones MD, Fung EB, Pico EL. The effect of low magnitude mechanical stimulation (LMMS) on bone density in patients with Rett syndrome: a pilot and feasibility study. J Pediatr Rehabil Med. 2014;7(2):167–178. doi: 10.3233/prm-140286. [DOI] [PubMed] [Google Scholar]
- 23.Gusso S, Munns CF, Colle P, Derraik JG, Biggs JB, Cutfield WS, et al. Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci Rep. 2016;6:22518. doi: 10.1038/srep22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilebrant S, Braathen G, Emilsson R, Glansen U, Söderpalm AC, Zetterlund B, et al. Whole-body vibration therapy in children with severe motor disabilities. J Rehabil Med. 2015;47(3):223–228. doi: 10.2340/16501977-1921. [DOI] [PubMed] [Google Scholar]
- 25.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res. 2011;26(8):1759–1766. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 26.Stark C, Nikopoulou-Smyrni P, Stabrey A, Semler O, Schoenau E. Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. J Musculoskelet Neuronal Interact. 2010;10(2):151–158. [PubMed] [Google Scholar]
- 27.Wren TA, Lee DC, Hara R, Rethlefsen SA, Kay RM, Dorey FJ, et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J Pediatr Orthop. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer-Kuhn H, Semler O, Stark C, Struebing N, Goebel O, Schoenau E. A specialized rehabilitation approach improves mobility in children with osteogenesis imperfecta. J Musculoskelet Neuronal Interact. 2014;14(4):445–453. [PubMed] [Google Scholar]
- 29.Högler W, Scott J, Bishop N, Arundel P, Nightingale P, Mughal MZ, Padidela R, Shaw N, Crabtree N. The effect of whole body vibration training on bone and muscle function in children with osteogenesis imperfecta. J Clin Endocrinol Metab. 2017;102(8):2734–2743. doi: 10.1210/jc.2017-00275. [DOI] [PubMed] [Google Scholar]
- 30.Matute-Llorente A, Gonzalez-Aguero A, Gomez-Cabello A, Tous-Fajardo J, Vicente-Rodriguez G, Casajus JA. Effect of whole-body vibration training on bone mass in adolescents with and without Down syndrome: a randomized controlled trial. Osteoporos Int. 2016;27(1):181–191. doi: 10.1007/s00198-015-3232-9. [DOI] [PubMed] [Google Scholar]
- 31.Erceg DN, Anderson LJ, Nickles CM, Lane CJ, Weigensberg MJ, Schroeder ET. Changes in bone biomarkers, BMC, and insulin resistance following a 10-week whole body vibration exercise program in overweight Latino boys. Int J Med Sci. 2015;12(6):494–501. doi: 10.7150/ijms.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiVasta AD, Feldman HA, Rubin CT, Gallagher JS, Stokes N, Kiel DP, et al. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporos Int. 2017;28(4):1255–1263. doi: 10.1007/s00198-016-3851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–1636. doi: 10.1007/s00198-012-2144-1. [DOI] [PubMed] [Google Scholar]
- 34.Fung EB, Gariepy CA, Sawyer AJ, Higa A, Vichinsky EP. The effect of whole body vibration therapy on bone density in patients with thalassemia: a pilot study. Am J Hematol. 2012;87(10):E76–E79. doi: 10.1002/ajh.23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Shamy S. Effect of whole body vibration training on quadriceps strength, bone mineral density, and functional capacity in children with hemophilia: a randomized clinical trial. J Musculoskelet Neuronal Interact. 2017;17(2):19–26. [PMC free article] [PubMed] [Google Scholar]
- 36.Edionwe J, Hess C, Fernandez-Rio J, Herndon DN, Andersen CR, Klein GL, Suman OE, Amonette WE. Effects of whole-body vibration exercise on bone mineral content and density in thermally injured children. Burns. 2016;42(3):605–613. doi: 10.1016/j.burns.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, Zemel BS, Mahboubi S, Howard Whitehead K, Herskovitz R, Lee D, Rausch J, Rubin CT. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric Crohn’s disease: a randomized placebo-controlled trial. J Bone Miner Res. 2016;31(6):1177–1118. doi: 10.1002/jbmr.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010;21(12):1969–1980. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matute-Llorente A, Gonzalez-Aguero A, Gomez-Cabello A, Vicente-Rodriguez G, Casajus Mallen JA. Effect of whole-body vibration therapy on health-related physical fitness in children and adolescents with disabilities: a systematic review. J Adolesc Health. 2014;54(4):385–396. doi: 10.1016/j.jadohealth.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 40••.Saquetto M, Carvalho V, Silva C, Conceicao C, Gomes-Neto M. The effects of whole body vibration on mobility and balance in children with cerebral palsy: a systematic review with meta-analysis. J Musculoskelet Neuronal Interact. 2015;15(2):137–144. [PMC free article] [PubMed] [Google Scholar]
- 41••.Bell JM, Shields MD, Watters J, Hamilton A, Beringer T, Elliott M, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2017;1:Cd010899. doi: 10.1002/14651858.CD010899.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo CR, Lauretani F, Bandinelli S, Bartali B, Cavazzini C, Guralnik JM, Ferrucci L. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil. 2003;84(12):1854–1857. doi: 10.1016/s0003-9993(03)00357-5. [DOI] [PubMed] [Google Scholar]
- 43••.Marin-Puyalto J, Gomez-Cabello A, Gonzalez-Aguero A, Gomez-Bruton A, Matute-Llorente A, Casajus JA, et al. Is vibration training good for your bones? An overview of systematic reviews. Biomed Res Int. 2018;2018:5178284. doi: 10.1155/2018/5178284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Söderpalm AC, Magnusson P, Åhlander AC, Karlsson J, Kroksmark AK, Tulinius M, Swolin-Eide D. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord. 2007;17(11–12):919–928. doi: 10.1016/j.nmd.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Fehlings D, Switzer L, Agarwal P, Wong C, Sochett E, Stevenson R, et al. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: a systematic review. Dev Med Child Neurol. 2012;54(2):106–116. doi: 10.1111/j.1469-8749.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 46.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110(1 Pt 1):e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 47.Cheng HY, Yu YC, Wong AM, Tsai YS, Ju YY. Effects of an eight-week whole body vibration on lower extremity muscle tone and function in children with cerebral palsy. Res Dev Disabil. 2015;38:256–261. doi: 10.1016/j.ridd.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 48••.Leite HR, Camargos ACR, Mendonca VA, Lacerda ACR, Soares BA, Oliveira VC. Current evidence does not support whole body vibration in clinical practice in children and adolescents with disabilities: a systematic review of randomized controlled trial. Braz J Phys Ther. 2019;23(3):196–211. doi: 10.1016/j.bjpt.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tauer JT, Robinson ME, Rauch F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR Plus. 2019;3(8):e10174. doi: 10.1002/jbm4.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 52.Sa-Caputo DC, Dionello CDF, Frederico E, Paineiras-Domingos LL, Sousa-Goncalves CR, Morel DS, et al. Whole-body vibration exercise improves functional parameters in patients with osteogenesis imperfecta: a systematic review with a suitable approach. Afr J Tradit Complement Altern Med. 2017;14(3):199–208. doi: 10.21010/ajtcam.v14i3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saquetto MB, Pereira FF, Queiroz RS, da Silva CM, Conceicao CS, Gomes NM. Effects of whole-body vibration on muscle strength, bone mineral content and density, and balance and body composition of children and adolescents with Down syndrome: a systematic review. Osteoporos Int. 2018;29(3):527–533. doi: 10.1007/s00198-017-4360-1. [DOI] [PubMed] [Google Scholar]