Abstract
Application of nanocompounds and plant growth promoting rhizobacteria (PGPR) plays an important role in improving plant growth and soil health. In the present study, response of two PGPR (PS2-KX650178 and PS10-KX650179) along with nanozeolite and nanochitosan was studied on Fenugreek (Trigonella foenum-graecum), on the basis of physiological and biochemical parameters of soil and plant in pot experiment for 45 days. A significant increase (1.5–2 folds) in plant height, leaf number, leaf area and fresh weight over control was observed in Fenugreek plants when treated with nanocompounds and PGPR. Combined treatment also showed the highest level of total chlorophyll (3.27 mg g−1), sugar (6.14 μg mg−1 dry wt), soluble leaf protein (295.37 mg g−1 fresh weight) and catalase activity (23.84 U g−1 tissue) in Fenugreek plants. GC–MS analysis of plant metabolites revealed the abundance of phenols which are known to improve biotic/abiotic stresses in plants. Activity of Fluorescein Diacetate hydrolase enzyme was 2.5 times higher in the combined treatment of nanozeolite with PS10 than in control. An increase of 11% in alkaline phosphatase activity was observed in the same treatment with respect to control. The results obtained from the pot experiment clearly indicate that nanocompounds along with PGPR improved the growth of plants and soil health which suggest their benefits in agriculture practices to increase crop production.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02448-2) contains supplementary material, which is available to authorized users.
Keywords: Fenugreek, Nanozeolite, Nanochitosan, Bacillus sp., PGPR
Introduction
Several limiting factors like irrigation facilities, nutrient management and shortfall of extension facilities are usually related to low agricultural production in India (India Country overview 2008). Management of agricultural practices using new technologies is found to be economical and environment friendly. Application of nanocompounds in agriculture are reported to improve crop production (Rastogi et al. 2019; Elsheery et al. 2020b). It is presumed that nanocompounds used in agriculture practices may affect rhizospheric microbiota, nature of root exudates and soil health. Since plant health depends on the accessibility of essential micro/macro nutrients fixed, solubilized, or recycled through soil microorganisms; hence, a slight change in microbial population can make a significant effect on plant/soil health (Burke et al. 2015). Most of the soil enzymes are secreted extracellularly by microbial population residing in the rhizospheric regions of the plants and help in recycling of nutrients which support microbial growth in the soil and enhance crop production (Elliott et al. 1993). Introduction of several chemicals, including pesticides, sludge, fertilizers etc. in the soil system may affect soil health drastically. Disturbance in enzymatic activities of soil can be related to microbiological state of a soil (Chai et al. 2015; Khalifa et al. 2017). However, according to Khati et al. (2019b), Kukreti et al. (2020) application of nanocompounds at recommended concentrations can improve the microbiological state of the soil and help in maintenance of soil health.
Zeolites are low-density aluminosilicate compounds and possess micropores of 0.3–2.0 nm dimensions (Szostak et al. 1992). Properties that make zeolites exceptionally desirable for improving the physicochemical nature of the soil are its uniform particle size distribution and large internal porosity which result in high water retention and high CEC (Cation Exchange Capacity) to hold available nutrients for long (Ok et al. 2003). Micron-sized crystals of nanozeolites are responsible for the performance of the compound. Decreased crystal size of nanozeolite is found beneficial which broadens the application of alumino silicates for different purposes (Mintova et al. 2016). Application of nanozeolite @ 30% in soil with alfalfa straw enhanced the fungal and bacterial population (Aminiyan et al. 2018) and also improved soil health under maize cultivation when applied @ 50 mg L−1 (Khati et al. 2018).
Chitosan has outstanding biocompatibility, film forming capacity, mechanical strength and antifungal property (Yang et al. 2010). Nanochitosan has multifold antifungal property in comparison to its bulk form hence can be used as a potent and safe antifungal agent in agriculture practices (Yien et al. 2012). Application of nanochitosan on Camellia sinensis leaves enhanced the defence response and accumulation of defence enzymes (Chandra et al. 2015). Application of nanochitosan on pearl millet showed enhanced percent seed germination as compared to control (Siddaiah et al. 2018). Parul (2019) also reported that nanochitosan and nanozeolite along with PGPR improved maize yield under field condition as compared to control.
PGPR constitute about 2–5% of the entire rhizospheric microbiota. Main bacterial genera belonging to PGPR are Azospirillum, Acetobacter, Azotobacter, Pseudomonas, Burkholderia, Paenibacillus and Bacillus etc. (Lugtenberg et al. 2001; Helaly et al. 2017). Generally, PGPR promote plant growth directly by facilitating nutrient resource acquisition or modulating the level of plant hormone and indirectly by inhibiting the plant pathogens as biocontrol agents (Glick 2012; Naser et al. 2016). Chaudhary and Sharma (2019) reported that nanogypsum @ 50 mg L−1 supports the growth of PGPR in laboratory condition. Similarly, Khati et al. (2019a) also reported that supplementation of nanozeolite @ 50 ppm in medium supported the growth of Bacillus sp. Very few studies are available on the association of nanocompounds and PGPR and their impact on plants and soil health. Therefore, the objective of this study was to analyse the effect of nanocompounds (nanozeolite and nanochitosan) and PGPR on the health of Fenugreek plants as well as on the microbial activities of the soil under pot experiment.
Materials and methods
Bacterial strains and growth condition
Two bacterial inoculants (PS2 and PS10), used for the present study were recovered from the rhizospheric soil of an agriculture field located at Crop Research Centre of the University. Both the bacterial cultures were gram positive and showed PGPR activities like indole acetic acid production, phosphate solubilization, siderophore and ammonia production (Khati et al. 2019a). Nanocompounds used in this study were purchased from Intelligent Materials Pvt. Ltd. India with stock numbers NS6130-09-905 and NS6130-09-918, respectively. The size of nanozeolite was < 80 nm, pH 7–8, refractive index 1.47, purity 99.9% and bulk density 0.6–0.8 g cm−3 (Khati et al. 2019b). The size of nanochitosan was < 80 nm, pH 6–7 and purity 99%. To prepare bacterial inoculums for seed bacterization, nutrient broth (with and without nanocompounds) was prepared, sterilized, and inoculated with respective bacterial culture(s). Inoculated medium was incubated at 30 °C for 24 h till the optical density reached 0.6 (105–106 cfu ml−1) at 600 nm. The concentration of nanocompounds used in the experiment was 50 mg L−1. Optical density was measured using UV–Vis spectrophotometer (Shimadzu Corporation, Japan).
Pot trial and seed sterilization
A pot experiment was conducted to study the response of Nz and Nch on soil and Fenugreek health in the departmental net house of the University. A high yielding variety of Fenugreek (Pusa early bunching) was obtained from Vegetable Research Centre, Govind Ballabh University of Agriculture and Technology, Pantnagar. This variety of Fenugreek takes about 125 days for seed formation and is resistant to downy mildew and rots. Twenty-seven pots (5.0 kg capacity) were filled with 3.0 kg of soil. After washing with running water, healthy seeds were surface sterilized for 5 min in 0.2% HgCl2 solution. Sterilized seeds were rinsed 2–3 times with sterilized distilled water.
Seed bacterization
Sterilized seeds were dried and treated with actively growing bacterial cultures/culture(s) supplemented with nanocompound(s) individually under laminar air flow. Details of different treatments are given in Table 1. Carboxy methyl cellulose (1%) was added in the culture broth to help proper adherence of bacterial culture to the seeds. Eight seeds were sown per pot at the depth of 2.5 cm in the soil with the help of sterilized forceps. Pots were regularly watered as per the requirement (depending upon the soil moisture content).
Table 1.
Details of nanocompounds and PGPR treatments
| Treatments | Abbreviations used |
|---|---|
| Absolute control | Control |
| Nanozeolite | Nz |
| Nanochitosan | Nch |
| Bacterial culture PS2 | PS2 |
| Bacterial culture PS2 with nanozeolite | PS2 + Nz |
| Bacterial culture PS2 with nanochitosan | PS2 + Nch |
| Bacterial culture PS10 | PS10 |
| Bacterial culture with nanochitosan | PS10 + Nz |
| Bacterial culture with nanochitosan | PS10 + Nch |
Sampling of plants and soil
Seed germination percentage was calculated using the following formula:
Agronomical parameters
Fenugreek plants were harvested after 45 days of the experiment. A number of leaves per plant, leaf area, plant height and fresh weight were recorded. The method described by Yoshida et al. (1972) was used to calculate leaf area.
Estimation of chlorophyll
After adding the fresh 50 mg chopped leaves in DMSO (10 ml), tubes were incubated for 3 h at 60 °C in a water bath. Chlorophyll was measured according to Hiscox and Israelstam (1979).
Calculations:
Estimation of carotenoid
Absorbance of the same extract (used for chlorophyll) was recorded at 480 nm using spectrophotometer to estimate carotenoid content in the plants (Kirk and Allen 1965).
Estimation of total sugar
Fenugreek leaves were dried in hot air oven at 80 °C for 48 h to estimate total sugar according to DuBois et al. (1956). Dried leaves were powdered and added in 10 ml ethyl alcohol (80%) in a test tube. Homogenate was centrifuged for 15 min at 1000 rpm then 1 ml of supernatant was added in 4 ml ice cold Anthrone reagent. Mixture was shaken properly and boiled for 10 min in water bath. Absorbance was recorded at 620 nm. The amount of total sugar was calculated by using glucose as standard.
Estimation of reducing and nonreducing sugars
The method of Nelson-Somogyi (1944) was adopted to estimate reducing sugar. For this 1 ml of copper reagent was added to ethanolic extract (1 ml) of plant leaves. Mixture was heated for 20 min and then cooled. 1 ml of arsenomolybdate reagent was added in mixture and incubated at 37 °C for 15 min. Resulting solution was diluted with distilled water and absorbance was recorded at 520 nm. Nonreducing sugar was estimated according to the formula given by Loomis and Shull (1937).
Protein estimation
The protein content of fresh leaves was estimated after 45 days following the method of Bradford (1976). Fresh leaves were crushed by adding 5 ml of 0.2 M Tris Cl (pH 8.0) to make fine slurry. Slurry was centrifuged at 10,000 rpm at 4 °C for 20 min and obtained supernatant (0.1 ml) was transferred into fresh tubes and stored at 4 °C till further use. 20 μl of protein extract was taken and the volume was made 300 μl using double-distilled water. 3 ml Bradford dye was added to the extract and incubated for 5 min at 37 ºC. Absorbance was recorded at 595 nm using spectrophotometer.
Catalase test
The method of Luck (1963), as mentioned in Sadasivam and Manikam (1992) was adopted to measure the catalase activity in Fenugreek plants. 20% (w/v) homogenate of plant leaves was prepared in H2O2 phosphate buffer and centrifuged at 1000 rpm. 100 µl of enzyme extract was added in 3 ml of phosphate buffer in a cuvette and mixed properly. Decrease in the absorbance was recorded at 240 nm.
Gas chromatography–mass spectroscopy analysis
Shade-dried Fenugreek leaves of 45 days old plants were extracted in methanol. GC–MS analysis of plant extract was performed using silica column (30 m 90.25 mm) with a gas flow of 1 ml min−1 at 8 °C using Shimadzu GC–MS QP Ver. 2010. Temperature was maintained at 240 °C. The organic compounds present in different samples were identified by comparing with the standards or the mass spectrum matched with inbuilt library (Wiley 9). GC–MS analysis was out sourced from Central Instrumentation Facility, JNU, New Delhi.
Soil analysis
Soil pH was determined by pH meter using 1:2.5 ratios of soil and water. NH3–N, NO3–N, P2O5 and total organic carbon (TOC) of the test soil were determined using soil testing kit (Hi media).
Soil enzyme activity
Fluorescein diacetate (FDA) hydrolysis
A 1 g fresh soil was incubated with 50 ml of 60 mM sodium phosphate buffer (pH 7.6) and 0.5 ml of FDA solution as a substrate. Suspension was incubated for 1 h at 24 °C with continuous shaking. Aliquots (6 ml) were withdrawn at different time intervals. 2 ml acetone was added to terminate the reaction. Soil suspension was centrifuged at 8000 rpm for 5 min followed by filtration to obtain clear supernatant. Absorbance was taken at 490 nm to quantify the end product fluorescein (Schuner and Rosswall 1982).
Dehydrogenase activity
Dehydrogenase activity of the test soil was estimated according to the method given by Casida et al. (1964) using TTC (Triphenyl tetrazolium chloride) solution. 5 g soil was added in 5 ml of TTC (2 g in 100 ml, 0.1 M Tris buffer, pH 7.4). Acetone solvent (25 ml) was added to extract triphenyl formazan (TPF). Reaction mixture was centrifuged at 4500 rpm for 10 min at 4 °C. Obtained supernatant was filtered through Whatman filter paper No. 1. Absorbance was recorded at 485 nm. Dehydrogenase activity was expressed as µg TPF 5 g−1 dry soil 8 h−1.
Alkaline phosphatase activity
To determine alkaline phosphatase activity, 1 g of soil was added to a test tube containing 250 µl toluene. 4 ml of modified universal buffer (100 mM, pH11) and 1 ml p-nitro phenyl triphenyl formazan (TPF) were added. Samples were incubated at 37 °C for 1 h followed with addition of 1 ml of CaCl2. After incubation 4 ml of Tris buffer (0.1 M, pH 12) was added to stop the reaction. Mixture reaction was filtered using Whatmann filter no. 2. Enzyme activity was determined by measuring the absorbance of product formation (i.e., pNP) at 400 nm and expressed as µg pNP g−1 dry soil 2 h−1 (Tabatabai and Bremner 1969).
Statistical data treatment
Completely randomize design (CRD) was used to conduct the pot experiment. The statistical analysis of plant and soil parameters was carried out using an one-way analysis of variance (ANOVA) with the help of SPSS, ver. 16.0 software. Significant differences among means were tested with Duncan’s Multiple Range Test (DMRT) at P < 0.05. The data represented in the tables and figures are expressed as means of three replicates ± standard deviation (SD).
Results
Germination percentage
Treated seeds showed enhanced percent seed germination than the control. Maximum germination (87.5%) was observed in PS10 + Nch and PS10 + Nz followed by PS2 + Nch and Nz (79.16%), PS2 + Nz and PS2 (75%), Nch (70.83%), PS10 (66.68%) treatments and lowest was observed in control (66.66%) (Fig. 1).
Fig. 1.

Germination of Fenugreek under different treatments. Mean values with standard deviation bars
Agronomical parameters
According to Table 2, maximum plant height (28.63 cm), was observed in the soil treated with PS2 + Nch followed by PS10 (27.4 cm), PS10 + Nch (26.00 cm), PS2 (25.63), PS2 + Nz (25.4 cm), Nch (24.53 cm), Nz (23.9 cm), PS10 + Nz (22.93 cm) and lowest was observed in control (19.67 cm). Difference in the plant height of control and other treatments was significant. Maximum leaf area (1.85 cm2) and number of leaves (94.66) were found in PS2 + Nz treatment. Maximum (3.13 g) and minimum (1.63 g) plant fresh weight was observed in PS2 + Nch and PS10 + Nch treatments, respectively.
Table 2.
Effect of nanocompounds and PGPR on plant health parameters of Fenugreek plants in the pot experiment
| Treatments | Plant height (cm) | Leaf area (cm2) | No. of leaves | Fresh wt (g) |
|---|---|---|---|---|
| Control | 19.67 ± 1.36a | 1.05 ± 0.18a | 57.33 ± 2.09a | 1.39 ± 0.14a |
| Nch | 24.53 ± 1.19bc | 1.41 ± 0.24ab | 64.00 ± 3.21ab | 1.89 ± 0.49abc |
| Nz | 23.90 ± 3.25bc | 1.51 ± 0.10bc | 62.00 ± 1.00ab | 1.77 ± 0.16abc |
| PS10 | 27.40 ± 1.90cd | 1.45 ± 0.05abc | 80.33 ± 3.31bc | 2.36 ± 0.47bcd |
| PS10 + Nch | 26.00 ± 1.21bc | 1.59 ± 0.19bc | 69.00 ± 3.54ab | 1.63 ± 0.17ab |
| PS10 + Nz | 22.93 ± 2.36ab | 1.53 ± 0.03bc | 78.00 ± 1.40abc | 2.48 ± 0.44cde |
| PS2 | 25.63 ± 2.36bc | 1.43 ± 0.15abc | 77.66 ± 4.72abc | 2.33 ± 0.39bcd |
| PS2 + Nch | 28.63 ± 3.59d | 1.53 ± 0.39bc | 93.00 ± 1.91c | 3.13 ± 0.68e |
| PS2 + Nz | 25.40 ± 0.80bc | 1.85 ± 0.32c | 94.66 ± 1.93c | 2.94 ± 0.12de |
Values within a column followed by single letters (a, b, c, d, e) show significant varietal difference by Duncan’s test. Mean ± SD (standard deviation)
Chlorophyll and carotenoid content in plants
Total chlorophyll, chlorophyll a, chlorophyll b and carotenoid content were maximum in PS2 + Nz treatment. Whereas in other treatments percent increase in total chlorophyll was 11.40% (PS2 + Nch) and 10.64% (PS2) as compared to control. Maximum carotenoid content was observed in PS2 + Nz and PS2 treated plants with 15.85% and 10.97% increase, respectively over control (Table 3).
Table 3.
Effect of nanocompounds and PGPR on total chlorophyll, Chl. a, Chl. b and carotenoid content in Fenugreek plants
| Treatments | Total chl (mg g−1) |
Chl. a (mg g−1) |
Chl. b (mg g−1) |
Carotenoid (mg g−1) |
|---|---|---|---|---|
| Control | 2.63 ± 0.11b | 1.90 ± 0.02c | 0.75 ± 0.03c | 0.164 ± 0.003b |
| Nch | 2.35 ± 0.02a | 1.70 ± 0.07a | 0.65 ± 0.03a | 0.150 ± 0.006a |
| Nz | 2.50 ± 0.07b | 1.78 ± 0.07ab | 0.72 ± 0.02bc | 0.162 ± 0.002b |
| PS10 | 2.61 ± 0.04b | 1.90 ± 0.02c | 0.72 ± 0.03bc | 0.162 ± 0.002b |
| PS10 + Nch | 2.58 ± 0.04b | 1.86 ± 0.01bc | 0.73 ± 0.02c | 0.164 ± 0.005b |
| PS10 + Nz | 2.50 ± 0.07b | 1.82 ± 0.02bc | 0.68 ± 0.03ab | 0.152 ± 0.005a |
| PS2 | 2.91 ± 0.05c | 2.11 ± 0.08d | 0.82 ± 0.01d | 0.182 ± 0.007c |
| PS2 + Nch | 2.93 ± 0.10c | 2.08 ± 0.07d | 0.87 ± 0.01d | 0.180 ± 0.002c |
| PS2 + Nz | 3.27 ± 0.04d | 2.31 ± 0.06e | 0.96 ± 0.02e | 0.190 ± 0.005d |
Values within a column followed by single letters (a, b, c, d, e) show significant varietal difference by Duncan’s test. Mean ± SD (standard deviation)
Sugar and protein content of Fenugreek leaves
Maximum level of total sugar (6.14 μg mg−1 dry wt) was observed in the plants treated with PS10 + Nz and minimum (5.29 μg mg−1 dry wt) in nanozeolite treated plants. Results of pot experiment revealed that maximum (1.26 μg mg−1 dry wt) and minimum (0.95 μg mg−1 dry wt) levels of reducing sugar were observed in the plants treated with PS2 + Nch and Nz, respectively. Effect of the treatments on nonreducing sugar was insignificant (Table 4). Maximum (295.37 μg mg−1 fresh wt) and minimum (222.78 μg mg−1 fresh wt) levels of protein were reported in the plants treated with PS2 + Nch and Nz, respectively. Significant increase in the protein content was noted in Nch (288 μg mg−1 fresh wt), PS2 + Nch (295.37 μg mg−1 fresh wt) and PS2 + Nz (290.27 μg mg−1 fresh wt) treatments over control.
Table 4.
Effect of nanocompounds and PGPR on sugar and protein content in Fenugreek plants
| Treatments | Total sugar (μg mg−1 dry wt) |
Reducing sugar (μg mg−1 dry wt) |
Nonreducing sugar (μg mg−1 dry wt) |
Protein (μg mg−1 fresh wt) |
|---|---|---|---|---|
| Control | 5.43 ± 0.26ab | 0.98 ± 0.43a | 4.22 ± 0.21a | 267.38 ± 3.57c |
| Nch | 5.30 ± 0.08ab | 1.03 ± 0.02ab | 4.05 ± 0.03a | 288.58 ± 4.55de |
| Nz | 5.29 ± 0.25a | 0.95 ± 0.10a | 4.11 ± 0.14a | 222.78 ± 7.22a |
| PS10 | 5.65 ± 0.01ab | 1.03 ± 0.87ab | 4.38 ± 0.09a | 241.61 ± 2.30ab |
| PS10 + Nch | 6.13 ± 0.06ab | 1.16 ± 0.07bc | 4.71 ± 0.02a | 277.06 ± 2.34cde |
| PS10 + Nz | 6.14 ± 0.66b | 1.16 ± 0.15bc | 4.73 ± 1.48a | 245.85 ± 3.35b |
| PS2 | 5.50 ± 0.31ab | 1.09 ± 0.23ab | 4.19 ± 0.28a | 273.26 ± 1.89cd |
| PS2 + Nch | 5.66 ± 0.99ab | 1.26 ± 0.12c | 4.29 ± 0.11a | 295.37 ± 1.17e |
| PS2 + Nz | 5.61 ± 0.66ab | 1.10 ± 0.05ab | 4.17 ± 0.82a | 290.27 ± 6.88de |
Values within a column followed by single letters (a, b, c) show significant varietal difference by Duncan’s test. Mean ± SD (standard deviation)
Catalase activity
Nanocompounds treated plants showed higher catalase activity than the control. Fenugreek plants treated with PS2 + Nch and PS2 showed maximum (23.84 U g−1 tissue) and minimum (4.07 U g−1 tissue) catalase activity, respectively. The rest of the treatments showed a remarkable variation in catalase activity (Fig. 2).
Fig. 2.
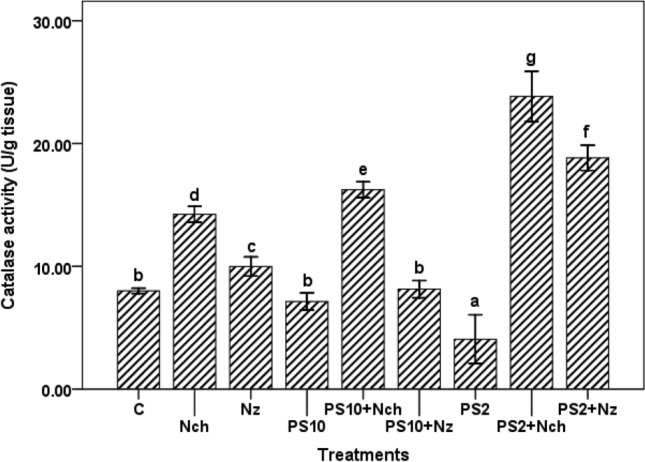
Catalase activity in Fenugreek plants under various treatments. Mean values with standard deviation bars
GC–MS analysis of plant metabolites under the treatments of nanocompounds and/or bacterial cultures
Occurrence of various metabolites in Fenugreek plants under different treatments was observed using GC–MS analysis. Peak areas of alcohols, ketones, phenols and acids of the treated plant samples were evaluated using Wiley library 9 and their abundance is plotted in Fig. 3, SM1-2. Level of phenols was maximum in combined treatment of nanocompounds and PGPR. Level of alcohol was high in Fenugreek leaf extract treated with Nch, Nz and PS2 + Nz treatments. Variation in ketone level was observed in all the treatments as compared to control.
Fig. 3.
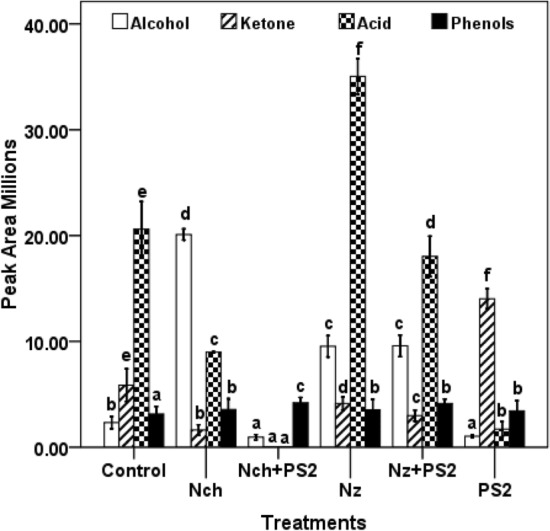
Peak area of alcohols, ketones, phenols and acids in leaf extracts of Fenugreek plants under different treatments. Mean values with standard deviation bars
Soil analysis
The results of soil analysis are presented in Supplementary material (SM3). None of the treatments affected soil pH after 45 days of the experiment. However, level of dissolved organic carbon was high in all the treatments but level of available phosphate was medium in comparison to control. Soil treated with only nanocompounds had medium to high level of available phosphate (56–73 kg ha−1). Increased level of available phosphate in treated soil could be related to phosphate solublizing property of the bacterial cultures used in the experiment. Available potassium was low in the control (> 112 kg ha−1) and the soil treated with only nanocompounds showed a slight increase in the available potassium (280–392 kg ha−1). Higher level of ammonical and nitrate nitrogen was observed in all the treatments than control. As per the observations, it can be concluded that no negative effects were detected in analysed soil properties.
Soil enzyme activities
Significant increase in FDA activity was observed in all the treated soil compared to control. FDA activity was maximum in PS10 + Nz (3.50 μg g−1 soil) followed by PS10 + Nch (2.72 μg g−1 soil), PS2 + Nch (2.63 μg g−1 soil), PS2 + Nz (2.46 μg g−1 soil), Nch (2.38 μg g−1 soil), Nz and PS10 (2.13 μg g−1 soil), PS2 (2.10 μg g−1 soil) and 1.36 μg g−1 in control after 45 days of the experiment. Maximum activity of dehydrogenase enzyme was observed in PS10 (3.65 μg g−1) followed by PS2 (3.26 μg g−1), Nch (2.75 μg g−1), PS10 + Nz (2.10 μg g−1), PS2 + Nz (2.05 μg g−1), Nz (2.00 μg g−1), PS10 + Nch (1.85 μg g−1), PS2 + Nch (1.50 μg g−1) treated soil and least activity was observed in control (1.46 μg g−1). Alkaline phosphatase was maximum in PS10 + Nz (41.35 μg g−1) followed by PS2 (40.24 μg g−1), PS10 (39.19 μg g−1), PS2 + Nch (39.02), PS10 + Nch and Nz (38.68 μg g−1), Nch (38.08 μg g−1), PS2 + Nz (37.65 μg g−1), and control had 37.13 μg g−1 activity (Fig. 4).
Fig. 4.
Effect of nanocompounds and PGPR on FDA hydrolysis, dehydrogenase and alkaline phosphatase enzyme activity. Mean values with standard deviation bars
Discussion
Significant improvement in plant health was observed under the combined application of bacterial cultures and nanocompound(s) in comparison to rest of the treatments. The same treatment was also found beneficial for the maintenance of soil health. Seed germination of Fenugreek was improved in treated seeds over control. Positive effect of nanocompounds on seed germination may be related due to increased availability of minerals and retention of water. Zeolites can store large amount of water due to high porosity which is made available to the seeds at the early stages of seed germination (Phillips and Edwards 2006). Our results are in accordance to an earlier study conducted by Khati et al. (2017, 2018) on maize plants. They reported that the application of nanochitosan and nanozeolite along with Bacillus sp. improved maize growth and soil health. Enhancement of percent seed germination in the presence of nanochitosan in wheat was also reported by Ma et al. (2014). Khodakovskaya et al. (2012) reported enhanced germination rate in tomato as penetration of Carbon nanotubes supports availability of water in seed coat. ZnONPs at a concentration of 20 µg ml−1 also promoted seed germination in onion, but at higher concentration ZnONPs showed reduction in seed germination (Raskar and Laware 2014). Application of TiO2 NPs gives better seed germination and boosts plumule and radicle growth of canola seedlings (Mahmoodzadeh et al. 2013). Application of nano-SiO2 also enhanced seed germination in tomato (Lycopersicum esculentum) (Siddiqui and Whaibi 2014).
Increase in agronomical parameters, such as plant height, fresh weight was observed in all the treated plants as compared to control. Increase in plant height might be due to an increased level of gibberallic acid which is found responsible for shoot elongation (Stepanova et al. 2007). Application of SNPs @ 50 ppm supported plant height, fresh weight, root length of wheat seedlings significantly (Najafi and Jamei 2014). Sharma et al. (2012) reported enhanced fresh weight, root length and vigor index of Brassica juncea seedlings on application of SNPs. Durairaj et al. (2015) observed a gradual increase in plant height, chlorophyll content, germination rate and protein content of Trigonella foenum-graecum (Fenugreek), when treated with 50 μg of nanoTiO2. Olanrewaju and Babalolo (2019) reported that application of Pseudomonas sp. and Bacillus subtilis enhanced the plant height, number of leaves, root length and yield of maize crop. Appanna (2007) reported that auxins produced by PGPR support plant growth and root elongation which improves uptake of nutrients for plants. Elsheery et al. (2020a) reported that zinc oxide nanoparticles improved mango growth and productivity.
Total chlorophyll, chlorophyll a, chlorophyll b and carotenoid content were the highest in Fenugreek plants under PS2 + Nz treatment. This may be due to the positive and protected mechanism of combined treatment of nanocompounds and PGPR. Our results on increased chlorophyll and carotenoids content are in accordance to the results reported by Jhanzab et al. (2015) on wheat when treated with SNPs. Enhanced level of chlorophyll content may lead to higher photosynthesis rate and ultimately a higher yield. Wu et al. (2010) also reported increase in chlorophyll content, nitrogen use efficiency and grain yield in rice, treated with nano-carbon. Spinacia oleracia treated with nano-anatase TiO2 showed 2.67 times more activity of rubisco carboxylase enzyme over control (Gao et al. 2006). They concluded that nano-anatase enhanced photosynthesis by molecular mechanisms of carbon reaction. Choudhary et al. (2017) reported that application of Cu-chitosan NPs @ 250 mg kg−1 showed increased plant height, stem diameter, root length, root number, and chlorophyll content in Zea mays over control. Zinc oxide nanoparticles @ 400 mg kg−1 was also reported to enhanced the chlorophyll content in Coriandrum sativum (Pullagurala et al. 2018). Application of silver nanoparticles (SNPs) improved sugar and chlorophyll content in Brassica juncea and Gloriosa superba and leads to a higher yield (Arora et al. 2012; Gopinath et al. 2014). Similar results were observed in a study where pear saplings, sprayed with Fe3O4 nanoparticles showed increase in total carbohydrate, total amino acids, nitrogen, and iron content (Raliya and Tarafdar 2013). According to Rastogi et al. (2019), Si-NPs directly interact with plants and improve growth and yield due to their size and physicochemical properties.
Increase in protein and sugar content in different treatments can be correlated to the findings by Raliya and Tarafdar (2013), who found that application of ZnO NPs @ 10 ppm concentration improve protein synthesis in Cyamopsis tetragonoloba. Similarly, silver nanoparticles showed a positive effect on carbohydrate, chlorophyll and protein contents of common bean, Brassica juncea and corn (Salama 2012). Siva (2016) reported an increased in protein content in rhizome of garlic with an average value of 1.699 μg ml−1 when treated with iron oxide nanoparticles Khati et al. (2017, 2018) also reported two fold increase in protein content of maize plants, when nanochitosan and nanozeolite (50 mg L−1) were applied with PGPR.
Higher level of antioxidant enzymes in treated samples shows improved stress tolerance in plants. Helaly et al. (2014) found that application of Nano-ZnO (100 mg L−1) enhanced the activity of superoxide dismutase, catalase and peroxidase enzymes in banana thereby improved tolerance to the stress condition. Similarly, application of nanochitosan @ 250 mg kg−1 protected pearl millet plants by upregulation of activities of CAT, POD and SOD by enhancing 2.59, 3.29 and 3.09-fold activity, respectively, as compared to control (Chandra et al. 2018). Application of silicon nanoparticles mitigates the stress by stimulating the antioxidant enzymes (Imtiaz et al. 2016).
The phenols and ketones play major role in growth, pigment biosynthesis and protection of plants from biotic/abiotic stress. Level of organic compounds, like aldehydes, ketones, phenols, etc. was high in maize leaves, treated with nanochitosan and nanozeolite in combination with PGPR (Khati et al. 2017, 2018). Phenolics, synthesized in plant as secondary metabolites act as signaling molecules to regulate physical functions of the plants (Xu et al. 2011). Goto et al. (2003) reported positive impact of silicon on phenolic contents indicating positive response towards defence mechanism in rice plants. Exposure of maize with nanosilica resulted in higher expression of phenolic compounds which might be effective against the infestation of phytopathogens (Suriyaprabha et al. 2013).
Combined treatment of bacterial cultures with nanocompound(s) was observed to enhance nutrient status of the experimental soil which proves that nanocompounds support bacterial population in soil. Enhanced bacterial population may provide essential micro and macronutrients to the plants through their biochemical activities and improve plants health (Timmusk et al. 2018). Slow release of essential nutrients by nanocompounds for long period in soil was reported by Mukhopadhyay (2014). Similarly, Deluca and DeLuca (1997) also reported the slow release pattern of nutrients by zeolites. The addition of zeolite, as a fertilizer has a significant effect on nitrogen content in the soil in assimilative forms (Polat et al. 2004).
Soil enzymes help in recycling of essential nutrients that enhances plant growth. The high sensitivity of soil enzymes to any change makes them a good marker for the presence of viable microbial cells responsible for secretion of these extracellular enzymes (Khalifa et al. 2017). In this study, FDA, dehydrogenase and alkaline phosphatase activity were higher in treated soil over control indicating no negative impact on soil health. Presence of higher activity in treated soil could be related to availability of more substrate and microbial population. Positive correlation between the activities of FDA, dehydrogenase, phosphatase, ATPase etc. and soil health has been found by many authors (Bandick and Dick 1999). According to Caldwell (2005), activities of exoenzymes are linked with the dynamic properties of soil ecosystems, including microbial communities and nutrient status. Cullen et al. (2011) and Fang et al. (2012) found positive/negligible impact of zero-valent iron and α-Fe2O3 and Fe3O4 NPs on FDA hydrolase, urease, acid phosphatase, dehydrogenase, amylase and catalase activity in soil. Kwak et al. (2017) observed similar results and found increased dehydrogenase activity in cultivated soils treated with 500 mg ZnONPs per kg dry soil for 56 days. Raliya and Tarafdar (2013) observed improvement in phytase, alkaline and acid phosphatase activity in cluster bean rhizosphere under ZnONPs treatment. Ju et al. (2020) reported that inoculation of Sinorhizobium meliloti enhanced the urease and β-glucosidase activity and increase soil fertility. Parul (2019) reported that application of Pseudomonas taiwanensis and Pantoeae agglomerans increased FDA, dehydrogenase, and amylase activities under maize cultivation. In contrast to our study, Shin et al. (2012) observed negative effect of Ag NPs (100 µg g−1) on FDA, acid phosphatase, urease, arylsulfatase, dehydrogenase and β-glucosidase. He et al. (2015) also witnessed negative effect of single-walled and multiwalled carbon nanotubes on alkaline phosphatase and invertase activity in treated soil. Inhibition of dehydrogenase activity was observed when soil was treated with silver nanoparticles (McGee et al. 2017). The toxicity concern of some nanocompounds necessitates the monitoring of soil health so as to utilize the nanocompounds to their full potential. In our findings we can suggest the controlled application of nanocompounds is beneficial for the plant health of Fenugreek while maintaining the soil health.
Conclusion
Our results showed that application of nanozeolite and nanochitosan along with PGPR significantly improved plant health parameters, such as biomass, photosynthetic pigments, sugar, protein, phenolic content and enhanced the activity of catalase enzymes which provide protection to plants. Nanocompounds used in this study along with PGPR had positive effect on the rhizospheric microorganisms and significantly influenced the microbial activities. These nanocompounds along with PGPR have been proven as a promising agent for plant protection and growth promotion. Further greenhouse and field studies using PGPR and nanoparticles are needed to validate our findings on different soil type and crops.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge facilities provided by Department of Microbiology of G.B.Pant University of Agriculture and Technology, Pantnagar.
Abbreviations
- ZnONPs
Zinc oxide nanoparticles
- CEC
Cation exchange capacity
- FDA
Fluorescein diacetate
- TOC
Total organic carbon
- Nch
Nanochitosan
- Nz
Nanozeolite
- SNPs
Silver nanoparticles
- Fe3O4
Iron oxide
- SWCNTs
Single-walled carbon nanotubes
Author contributions
SK: research work, participated in all the experiments. AS: provided the laboratory facilities and critical checking of the manuscript. PC: wrote the manuscript and helped in data analysis. PK: isolated and characterized the bacterial culture used in the experiment.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interests.
Contributor Information
Swati Kumari, Email: swtkmr16@gmail.com.
Anita Sharma, Email: anitasharma14@yahoo.co.in.
Parul Chaudhary, Email: parulchaudhary1423@gmail.com.
Priyanka Khati, Email: priyankakhati712@gmail.com.
References
- Aminiyan MM, Hosseini H, Heydariyan A. Microbial communities and their characteristics in a soil amended by nanozeolite and some plant residues: short time in-situ incubation. Eurasian J Soil Sci. 2018;7:9–19. [Google Scholar]
- Appanna V. Efficacy of phosphate solubilizing bacteria isolated from vertisols on growth and yield parameters of sorghum. Res J Microbiol. 2007;2:550559. [Google Scholar]
- Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Reg. 2012;66:303–310. [Google Scholar]
- Bandick AK, Dick RP. Field management effects on soil enzyme activities. Soil Biol Biochem. 1999;31(11):1471–1479. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke DJ, Pietrasiak N, Samia ACS. Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int J Mol Sci. 2015;10:23630–23650. doi: 10.3390/ijms161023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BA. Enzyme activities as a component of soil biodiversity: a review. Pedobiologia. 2005;49(6):637–644. [Google Scholar]
- Casida LE, Jr, Klein DA, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98(6):371–376. [Google Scholar]
- Chai H, Yao J, Sun J, Zhang C, Liu W, Zhu M, Ceccanti B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull Environ Contam Toxicol. 2015;94(4):490–495. doi: 10.1007/s00128-015-1485-9. [DOI] [PubMed] [Google Scholar]
- Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, Krishnendu AK. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep. 2015;5:15195. doi: 10.1038/srep15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra NS, Keelara VHP, Niranjan RS, Venkataramana M, Vijai KG, Naveen KK, Xiao FD, Jie YC, Andrei M, Bhim PS, Rakesh KS. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci Rep. 2018;8:2485. doi: 10.1038/s41598-017-19016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P, Sharma A. Response of nanogypsum on the performance of plant growth promotory bacteria recovered from nanocompound infested agriculture field. Environ Ecol. 2019;37(1B):363–372. [Google Scholar]
- Choudhary CR, Kumaraswamy VR, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.) Sci Rep. 2017;7:9754. doi: 10.1038/s41598-017-08571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen LG, Tilston EL, Mitchell GR, Collins CD, Shaw LJ. Assessing the impact of nano-and micro-scale zerovalent iron particles on soil microbial activities: particle reactivity interferes with assay conditions and interpretation of genuine microbial effects. Chemosphere. 2011;82(11):1675–1682. doi: 10.1016/j.chemosphere.2010.11.009. [DOI] [PubMed] [Google Scholar]
- DeLuca TH, DeLuca DK. Composting for feedlot manure management and soil quality. J Prod Agri. 1997;10(2):235–241. [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. [Google Scholar]
- Durairaj B, Santhoshkumar M, Xavier T. Effect of Fungal generated n-TiO2 on Fenugreek (Trigonella foenumgraecum) seed germination and seedling growth. J Global Biosci. 2015;4(5):2319–2325. [Google Scholar]
- Elliott ET, Cambardella CA, Cole CV. Modification of ecosystem processes by management and the mediation of soil organic matter dynamics. Plant Soil. 1993;22:129–138. [Google Scholar]
- Elsheery NB, Helaly MN, El-Hoseiny HM, Alam-Eldein SM. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10:558. [Google Scholar]
- Elsheery NI, Sunoj VSJ, Wen Y, Zhu JJ, Muralidharan G, Cao KF. Foliar application of nanoparticles mitigates the chilling effect onphotosynthesis and photoprotection in sugarcane. Plant Physiol Biochem. 2020;149:50–60. doi: 10.1016/j.plaphy.2020.01.035. [DOI] [PubMed] [Google Scholar]
- Fang G, Si Y, Tian C. Degradation of 2, 4-D in soils by Fe3O4 nanoparticles combined with stimulating indigenous microbes. Env Sci Pollut Res. 2012;19(3):784–793. doi: 10.1007/s11356-011-0597-y. [DOI] [PubMed] [Google Scholar]
- Gao FQ, Hong FH, Liu C, Zheng L, Su MY, Wu X, Yang F, Wu C, Yang P. Mechanism of nanoanatase TiO2 on promoting photosynthetic carbon reaction of spinach-inducing complex of rubisco-rubisco activase. Biol Trace Elem Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. London: Hindawi Publishing Corporation, Scientifica; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Gowri S, Karthika V, Arumugam A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity Gloriosa superba. J Nanostr Chem. 2014;4:1–11. [Google Scholar]
- Goto M, Ehara H, Karita S, Takabe K, Ogawa N, Yamada Y, Ogawa S, Yahaya MS, Morita O. Protective effect of silicon on phenolic biosynthesis and ultraviolet spectral stress in rice crop. Plant Sci. 2003;164:349–356. [Google Scholar]
- He F, Wang H, Chen Q, Yang B, Gao Y, Wang L. Short-term response of soil enzyme activity and soil respiration to repeated carbon nanotubes exposure. Soil Sediment Contam. 2015;24(3):250–261. [Google Scholar]
- Helaly MN, El-Metwally MA, El-Hoseiny H, Omar SA, El-Sheery NI. Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust J Crop Sci. 2014;8:612–624. [Google Scholar]
- Helaly MN, El-Hoseinyb H, El-Sheeryc NI, Rastogi A, Kalajif HM. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physio Biochem. 2017;118:31. doi: 10.1016/j.plaphy.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Hiscox JT, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57(12):1332–1334. [Google Scholar]
- Imtiaz M, Rizwan MS, Mushtaq MA, Ashraf M, Shahzad SM, Yousaf B, Saeed DA, Rizwan M, Nawaz MA, Mehmood S, Tu S. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: a review. J Environ Manage. 2016;183:521–529. doi: 10.1016/j.jenvman.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Jhanzab HM, Razzaq A, Jilani G, Rehman A, Hafeez A, Yasmeen F. Silver nano-particles enhance the growth, yield and nutrient use efficiency of wheat. Int J Agron Agri Res. 2015;7(1):15–22. [Google Scholar]
- Ju W, Liu L, Jin X, Duan C, Cui Y, Wang J, Ma D, Zhao W, Wang Y, Fang L. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere. 2020;254:126724. doi: 10.1016/j.chemosphere.2020.126724. [DOI] [PubMed] [Google Scholar]
- Khalifa MMA, Fetian NAH, Abdel Magid MS, El-Sheery NI. Effectiveness of potassium silicate in suppression white rot disease andenhancement physiological resistance of onion plants, and its role on the soil microbial community. Middle East J Agric Res. 2017;6:2. [Google Scholar]
- Khati P, Chaudhary P, Gangola S, Bhatt P, Sharma A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech. 2017;7:81. doi: 10.1007/s13205-017-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khati P, Chaudhary P, Bhatt P, Nisha KR, Sharma A. Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3 Biotech. 2018;8:141. doi: 10.1007/s13205-018-1142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khati P, Chaudhary P, Gangola S, Sharma A. Influence of nanozeolite on plant growth promotory bacterial isolates recovered from nanocompound infested agriculture field. Environ Ecol. 2019;37(2):521–527. [Google Scholar]
- Khati P, Sharma A, Chaudhary P, Singh AK, Gangola S, Kumar R. High-throughput sequencing approach to access the impact of nanozeolite treatment on species richness and evens of soil metagenome. Biocatal Agri Biotech. 2019;20:101249. [Google Scholar]
- Khodakovskaya MV, de Silva K, Biris AS, Dervishi E, Villagarcia H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. 2012;6(3):2128–2135. doi: 10.1021/nn204643g. [DOI] [PubMed] [Google Scholar]
- Kirk JOT, Allen RL. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Kukreti B, Sharma A, Chaudhary P, Agri U, Maithani D. Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil. 3 Biotech. 2020;10:345. doi: 10.1007/s13205-020-02329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J, Yoon S, An Y. Long-term effects of ZnO nanoparticles on exoenzyme activities in planted soils. Envi Eng Res. 2017;22(2):224–229. [Google Scholar]
- Loomis WE, Shull CA. Methods in plant physiology. 1. New York: McGraw-Hill Co.; 1937. [Google Scholar]
- Luck H. Catalase. In: Begmeyer HU, editor. Methods of enzymatic analysis. New York: Academic press; 1963. pp. 895–897. [Google Scholar]
- Lugtenberg BJ, Dekkers L, Bloemberg GV. Molecular determinants of rhizosphere colonization by Pseudomonas. Ann Rev Phytopath. 2001;39(1):461–490. doi: 10.1146/annurev.phyto.39.1.461. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Li YY, Yu CM, Wang Y, Li XM, Li N. Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. Int J Agric Bio. 2014;16:766–770. [Google Scholar]
- Mahmoodzadeh H, Nabavi M, Kashefi H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus) J Ornam Hortic Plants. 2013;3(1):25–32. [Google Scholar]
- McGee CF, Storey S, Clipson N, Doyle E. Soil microbial community responses to contamination with silver, aluminium oxide and silicon dioxide nanoparticles. Ecotoxicology. 2017;26:449–458. doi: 10.1007/s10646-017-1776-5. [DOI] [PubMed] [Google Scholar]
- Mintova S, Grand J, Valtchev V. Nanosized zeolites: Quo Vadis? C R Chim. 2016;19(1):183–191. [Google Scholar]
- Mukhopadhyay SS. Nanotechnology in agriculture prospects and constraints. Nanotechnol Sci Appl. 2014;7:63–71. doi: 10.2147/NSA.S39409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi S, Jamei R. Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata) J Stress Physio Biochem. 2014;10(1):316–325. [Google Scholar]
- Naser HM, Hanan E, Elsheery NI. Effect of biofertilizers and putrescine amine on the physiological features and productivity of date palm (Phoenix dactylifera, L.) grown on reclaimed-salinized soil. Trees. 2016;30:1149–1161. [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153(2):375–380. [Google Scholar]
- Ok CH, Anderson SH, Ervin EH. Amendments and construction systems for improving the performance of sand-based putting greens. Agro J. 2003;95(6):1583–1590. [Google Scholar]
- Olanrewaju OS, Babalola OO. Bacterial consortium for improved maize (Zea mays) production. Microorganisms. 2019;7:519. doi: 10.3390/microorganisms7110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- India Country Overview (2008) World Bank Report.https://www.worldbank.org/en/country/india/overview
- Parul (2019)Application of conventional and metagenomic approaches to analyse the effect of nanocompounds and indigenous bioinoculants on the health of soil and Zea mays. Ph.D. Thesis G.B.P.U.A&T Pantnagar
- Phillips N, Edwards J (2006) Cereal seed quality after drought. State of New South Wales trough NSW, Department of Primary Industries
- Polat E, Karaca M, Demir H, Onus AN. Use of natural zeolite (clinoptilolite) in agriculture. J Fruit Ornam Plant Res. 2004;12(1):183–189. [Google Scholar]
- Pullagurala VLR, Adisa IO, Rawat S, Kalagara S, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey J. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum) Plant Physiol Biochem. 2018;132:120–127. doi: 10.1016/j.plaphy.2018.08.037. [DOI] [PubMed] [Google Scholar]
- Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.) Agri Res. 2013;2:48–57. [Google Scholar]
- Raskar SV, Laware SL. Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int J Curr Microbiol App Sci. 2014;3(2):467–473. [Google Scholar]
- Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živčak M, Ghorbanpour M, El-Sheery NI, Brestic M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019;9:90. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam S, Manikam A. Total carbohydrate by anthrone method biochemical methods for agricultural science. New Delhi: Wiley Eastern Limited; 1992. [Google Scholar]
- Salama HMH. Effects of silver nanoparticles in some crop plants, Common bean (Phaseolus vulgaris L.) and corn (Zea mays L.) Int Res J Biotech. 2012;3:190–197. [Google Scholar]
- Schnurer J, Rosswall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol. 1982;43(6):1256–1261. doi: 10.1128/aem.43.6.1256-1261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol. 2012;167(8):2225–2233. doi: 10.1007/s12010-012-9759-8. [DOI] [PubMed] [Google Scholar]
- Shin YJ, Kwak JI, An YJ. Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere. 2012;88(4):524–529. doi: 10.1016/j.chemosphere.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Siddaiah NC, Keelara VHP, Niranjan RS, Venkataramana M, Vijai KG, Naveen KK, Xiao FD, Jie YC, Andrei M, Bhim PS, Rakesh KS. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci Rep. 2018;8:2485. doi: 10.1038/s41598-017-19016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MH, Al-Whaibi MH. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J Biological Sci. 2014;21(1):13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva GV. Iron oxide nanoparticles promotes agronomic traits of ginger (Zingiber officinale Rosc.) Int J Adv Res Biol Sci. 2016;3(3):230–237. [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyaprabha R, Karunakaran G, Kavitha K, Yuvakkumar R, Rajendran V, Kannan N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotech. 2013;8:133–137. doi: 10.1049/iet-nbt.2013.0004. [DOI] [PubMed] [Google Scholar]
- Szostak R. Handbook of molecular sieves. New York: Van Nostrand Reinhold; 1992. p. 524. [Google Scholar]
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatise activity. Soil Biol Biochem. 1969;1(4):301–307. [Google Scholar]
- Timmusk S, Seisenbaeva G, Behers L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci Rep. 2018;8:617. doi: 10.1038/s41598-017-18939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Ruo-chao H, Xiao-hai T, Xiao-ling W, Guo-hui M, Hai-tao T. Effect of adding Nano-carbon in slow release fertilizer on grain yield and nitrogen use efficiency of super hybrid rice. Hybrid Rice. 2010;4:1–5. [Google Scholar]
- Xu C, Zhang Y, Zhu L, Huang Y, Lu J. Influence of growing season on phenolic compounds and antioxidant properties of grape berries from vines grown in subtropical climate. J Agric Food Chem. 2011;59:1078–1086. doi: 10.1021/jf104157z. [DOI] [PubMed] [Google Scholar]
- Yang K, Xu NS, Su WW. Co-immobilized enzymes in magnetic chitosan beads for improved hydrolysis of macromolecular substrates under a time-varying magnetic field. J Biotechnol. 2010;148(2):119–127. doi: 10.1016/j.jbiotec.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Yien L, Zin NM, Sarwar A, Katas H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Inter J Biomater. 2012;9:632698. doi: 10.1155/2012/632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cock JH. Laboratory manual for physiological studies of rice. 2. Los Baños: International Rice Research Institute; 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



