Abstract
Purpose:
To compare the average intraocular pressure (IOP) among smokers, past smokers, and never smokers using the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry.
Design:
Retrospective database study of the IRIS Registry data.
Participants:
Intelligent Research in Sight Registry patients who were seen by an eye care provider during 2017.
Methods:
Patients were divided into current smoker, past smoker, and never smoker categories. The IOP was based on an average measurement, and separate analyses were performed in patients with and without a glaucoma diagnosis based on International Classification of Diseases, Ninth Edition and Tenth Edition, codes. Stratified, descriptive statistics by glaucoma status were determined, and the relationship between smoking and IOP was assessed with a multivariate linear regression model.
Main Outcome Measures:
Mean IOP.
Results:
A total of 12 535 013 patients were included. Compared with never smokers, current and past smokers showed a statistically significantly higher IOP by 0.92 mmHg (95% confidence interval [CI], 0.88–0.95 mmHg) and 0.77 mmHg (95% CI, 0.75–0.79 mmHg), respectively, after adjusting for age, gender, glaucoma, age-related macular degeneration, diabetic retinopathy, cataract, glaucoma surgery, cataract surgery, and first-order interactions. In addition, the difference in IOP between current and never smokers was the highest in the fourth decade, regardless of the glaucoma status (glaucoma group, 1.14 mmHg [95% CI, 1.00–1.29 mmHg]; without glaucoma group, 0.68 mmHg [95% CI, 0.65–0.71 mmHg]).
Conclusions:
Current smokers and past smokers have higher IOP than patients who never smoked. This difference is higher in patients with an underlying glaucoma diagnosis.
Smoking is a major cause of global morbidity and mortality.1,2 Mortality among middle-aged current smokers is 3 times higher than that of people of similar age, education level, alcohol consumption, and body mass index who never smoked.3 With the current rate of tobacco consumption, 450 million smoking-attributable deaths are estimated to occur between 2000 and 2050, with roughly half of them being in a younger adult population.4 Thus, smoking is a serious threat to public health. Understanding the extent to which smoking affects our health is critical to be able to treat, modify, and prevent any associated morbidities and mortalities.
In ophthalmology, smoking has been shown to be associated with various conditions5 such as exudative and nonexudative age-related macular degeneration (AMD),6,7 cataract,8,9 inflammatory diseases,10,11 and thyroid eye diseases.12,13 However, conflicting findings exist regarding the effects of smoking on glaucoma or intraocular pressure (IOP). Several cross-sectional population studies have found positive, statistically significant associations between smoking and increased IOP,14–16 whereas others have found no relationship between them.17–19
The American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry is the nation’s first comprehensive eye disease clinical database, started in 2014. As of September 1, 2019, 252.95 million patient visits from 60.78 million unique patients exist in the database.20 Given the enormous clinical dataset from more than 15 000 eye care providers in the United States,20 the IRIS Registry may provide a unique opportunity to perform epidemiologic studies at a population scale, allowing us to identify novel, subtle biomarkers related to vision-threatening conditions. Several studies already have been published using the IRIS Registry and have reported the epidemiologic features of common and rare conditions.21–26 Tobacco use, in particular, may be documented more completely because providers are incentivized to screen and document tobacco use in electronic health records as part of the Health Information Technology for Economic and Clinical Health Act.27,28 Thus, we wanted to take advantage of this unprecedented, real-world database and sought to determine associations between smoking and IOP in patients with or without glaucoma in this study.
Methods
The methods of data collection and aggregation of the IRIS Registry database have been described previously.29 The American Academy of Ophthalmology provided research access to a limited version of the IRIS Registry specifically meant for academic research to 4 different academic institutions in the spring of 2019 (American Academy of Ophthalmology IRIS Registry Data Analytic Centers). The version of the IRIS Registry data for this study was frozen on July 26, 2019. This version used by the IRIS Registry Analytic Centers was code-named as Rome (accessed on August 1, 2019, for this study). This study was conducted in accordance with the tenets of the Declaration of Helsinki. Given the use of de-identified data, the review was exempted by the University of Washington Institutional Review Board.
All analyses were performed within the Amazon Web Services virtual private cloud environment. A secure virtual private cloud tunnel was created with a secure shell tunnel to the Amazon Web Services Redshift cluster housing the IRIS Registry database. An r5a.2xlarge elastic computing instance was instantiated with 8 virtual computer processing units and 64 gigabytes of random access memory. Ubuntu version 18.04.2 was used as the base operating system for the computing instance.
All patients with a documented smoking history and at least 1 IOP measurement between January 1, 2017, and December 31, 2017, from the IRIS Registry dataset were included in the study. We excluded any persons younger than 20 years and those with a uveitis (291 569 patients) or ocular trauma (59 022 patients) diagnosis in 2017. Smoking exposure was defined as the smoking status recorded for each eye visit across all years. If any discrepancy in smoking status existed over the visits of each patient, we used a hierarchical heuristic to prioritize past over never over current recorded status over visits. The heuristic is necessary because we did not have access to visit month or day nor the order of visits. The heuristic behaves in the following fashion for those with more than 1 smoking status denoted in 2017. If there is at least 1 past smoker recorded, the patient is assigned to the past smoker group. By doing so, we assume that patients transition from active to past and not from past to active. If there is at least 1 never smoker listed (and no past smoker), the patient is assigned to the never smoker group. We chose this system so that the active smoker group would be as conservative as possible, given our study objective to examine the effect of smoking on IOP.
The following demographic and clinical factors were extracted: age; gender; smoking history (never, past, current); IOP (in millimeters of mercury); several ophthalmic disease diagnoses based on International Classification of Diseases, Ninth Edition and Tenth Edition, codes, including glaucoma, AMD, diabetic retinopathy (DR), cataract, uveitis, and ocular trauma; and history of remote (defined as before 2017) or recent (defined as during 2017) glaucoma procedures and cataract surgery based on Current Procedural Terminology codes (Table S1, available at www.ophthalmologyglaucoma.org, for codes). Intraocular pressure and clinical diagnoses data were exclusively from 2017, and no previous year’s data were used, with the exception of the cataract and glaucoma procedures. Race and ethnicity data were not available. For each patient, 1 eye was chosen at random for primary analyses, and the mean IOP was calculated for the chosen eye if multiple measures were obtained during the year 2017.
We decided a priori to exclude any IOPs of more than 40 mmHg (less than 2% of all IOP data in the IRIS Registry) because of the high likelihood of this being a measurement recording error or an acute hypertensive event. We did not exclude any IOPs on the low end because IOP measurements are bounded by 0 mmHg. Within our dataset, an IOP of 1 mmHg was the lowest value recorded. Mean and standard deviations were calculated for continuous variables. t Tests were used for pairwise statistics. A multivariate general linear regression model was fit to the data using a forward and backward stepwise, Akaike information criterion-based selection of the above-mentioned demographic and clinical covariates and all possible first-order interaction terms with smoking status. A sensitivity analysis for including bilateral IOP data was performed using 1000 bootstrapped estimates of coefficients using a mixed linear model compared against a simplified general linear model with age, gender, glaucoma diagnosis, and smoking status as covariates. Confidence intervals (CIs) were calculated at the 95% level, and the threshold of statistical significance was set to 0.05. All statistical analyses were performed with Python software (https://www.python.org/) and R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 52 378 244 unique patients were encountered from the IRIS Registry database from 2013 through 2017. Across all years, smoking was recorded in 62% of all patients, which was comparable with the rate of visual acuity recording (65%). Other variables were recorded at a lower rate, including IOP (44%), insurance (45%), and marital status (33%). The smoking variable was relatively consistent over visits: 85.3% had 1 smoking status documented across all years, whereas only 12.8% had 2 smoking statuses documented and 1.9% had 3 smoking statuses documented.
More than 14 903 371 million patients (23.5%) were seen during the year 2017. Of these, 12 535 013 patients (84.1%) had IOP and smoking status recorded and did not meet the exclusion criteria (Fig 1). These patients had a mean frequency of 3.1 visits/year. Data from 39 277 679 million clinical visits were used for analyses. Baseline demographic factors are shown in Table 1. The mean age was 63.2 years (standard deviation, 15.2 years) and 40.8% (n = 5 114 733) were male. Past smokers were statistically significantly older than current or never smokers by 9.1 years and 6.2 years, respectively (P < 2.2 × 10−16). Mean IOP was the highest in the current smoker group (15.84 mmHg) followed by never smokers (15.47 mmHg) and past smokers (15.45 mmHg; P < 2.2 × 10−16, analysis of variance, all pairwise comparisons with Tukey correction). The proportion of patients with glaucoma was the highest in the past smoker group (24.9%) compared with the never (21.9%) or current (17.9%) smokers (P < 2.2 × 10−16, chi-square test).
Figure 1.
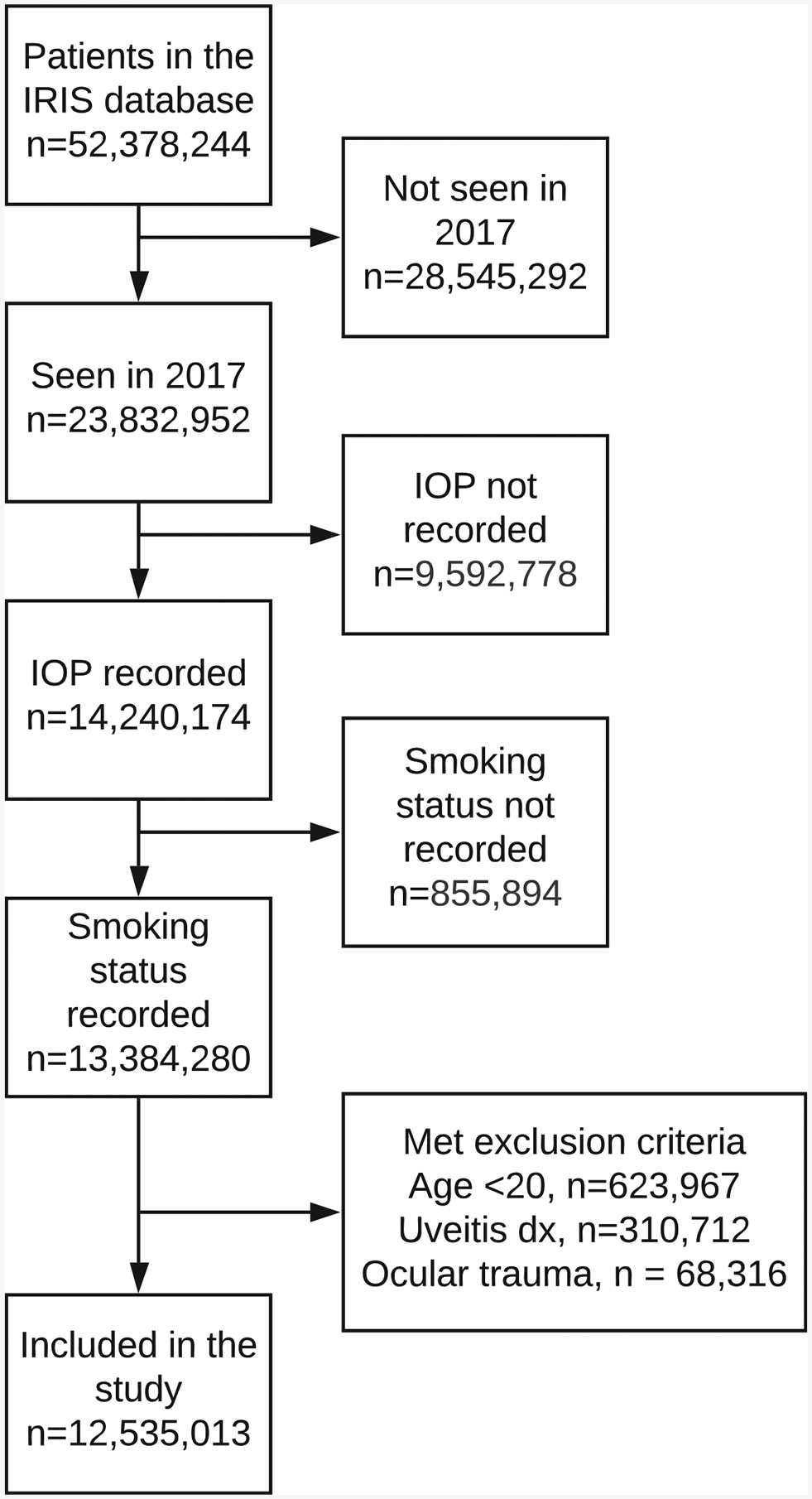
Flow diagram showing the Intelligent Research in Sight (IRIS) Registry data extraction. The number of patients excluded from the study at each step is shown. IOP = intraocular pressure.
Table 1.
Demographic and Baseline Clinical Characteristics of the Study Population
| Never | Past Smokers | Current Smokers | All | |
|---|---|---|---|---|
| Total no. of patients | 8 531 485 | 3 142 086 | 861 442 | 12 535 013 |
| Total no. of visits | 25 587 615 | 11 207 201 | 2 482 863 | 39 277 679 |
| Age (yrs) | ||||
| Mean (SD) | 61.8 (15.8) | 68.0 (12.4) | 58.9 (14.3) | 63.2 (15.2) |
| Range | 20–86 | 20–86 | 20–86 | 20–86 |
| Gender, no. (%) | ||||
| Male | 3 148 477 (36.9) | 1 546 178 (49.2) | 420 078 (48.8) | 5 114 733 (40.8) |
| Female | 5 367 402 (62.9) | 1 590 367 (50.6) | 420 078 (51.0) | 7 397 535 (59.0) |
| Unknown | 15 606 (0.2) | 5541 (0.2) | 1598 (0.2) | 22 745 (0.2) |
| Glaucoma, no. (%) | 1 798 707 (21.9) | 784 408 (24.9) | 153 784 (17.9) | 2 736 899 (21.8) |
| Diabetic retinopathy, no. (%) | 702 660 (8.2) | 299 771 (9.5) | 64 132 (7.4) | 1 066 563 (8.5) |
| Age-related macular degeneration, no. (%) | 1 028 665 (12.1) | 561 122 (17.9) | 94 181 (10.9) | 1 683 968 (13.4) |
| Cataract (%) | 4 750 134 (55.6) | 2 040 634 (64.9) | 483 442 (56.1) | 7 274 210 (58.0) |
| Cataract surgery, no. (%) | ||||
| In 2017 | 522 931 (6.1) | 250 688 (8.0) | 79 841 (9.3) | 853 460 (6.8) |
| Before 2017 | 860 661 (10.1) | 483 279 (15.4) | 75 179 (8.7) | 1 419 119 (11.3) |
| Glaucoma surgery, no. (%) | ||||
| In 2017 | 95 378 (1.1) | 39 735 (1.3) | 10 819 (1.3) | 145 932 (1.2) |
| Before 2017 | 202 785 (2.4) | 104 402 (3.3) | 15 467 (1.8) | 322 654 (2.6) |
| Right eye, no. (%) | 4 280 232 (50.2) | 1 570 641 (50.0) | 430 519 (50.0) | 6 253 621 (49.9) |
| IOP (mmHg), mean (SD) | 15.47 (3.26) | 15.45 (3.35) | 15.84 (3.48) | 15.49 (3.30) |
| IOP (mmHg), range | 1 −40 | 1–40 | 1 −40 | 1–40 |
IOP = intraocular pressure; SD = standard deviation.
The difference in IOP among the 3 smoking groups was seen at every year of life except for very early life (<30 years) and late life (60–79 years), regardless of glaucoma status (Fig 2). As would be expected, IOP was higher in patients with than without glaucoma for each smoking category, with a larger variability in IOP observed in the glaucoma group (Fig 2).
Figure 2.
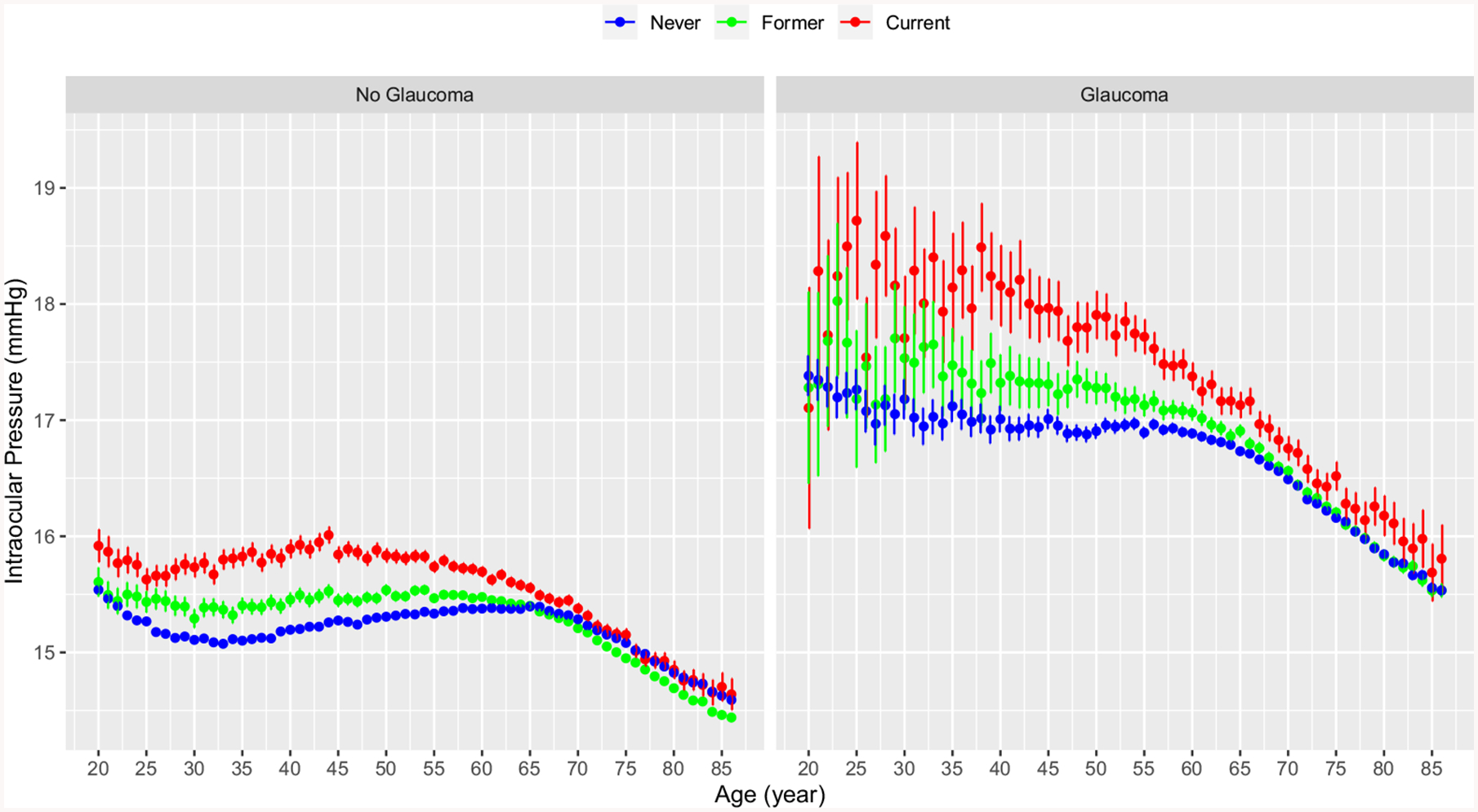
Graphs showing intraocular pressure by age and smoking status stratified by glaucoma status: (A) no glaucoma and (B) glaucoma. Patients who have never smoked are shown in blue, past smokers are shown in green, and current smokers are shown in red. The error bars represent the 95% confidence intervals.
The mean difference in IOP between current and never smokers was the highest in the fourth decade of life for both the glaucoma group (1.14 mmHg; 95% CI, 1.00–1.29 mmHg) and without glaucoma group (0.68 mmHg; 95% CI, 0.65–0.71 mmHg), followed by the third decade (1.01 mmHg; 95% CI, 0.80–1.23 mmHg) and the fifth decade (0.98 mmHg; 95% CI, 0.90–1.07 mmHg) for the glaucoma group and the fifth decade (0.64 mmHg; 95% CI, 0.62–0.66 mmHg) and the third decade (0.44 mmHg; 95% CI, 0.40–0.47 mmHg) for the without glaucoma group (Fig 3). The difference between current and never smokers was higher in patients with glaucoma than those without in all decades of life (Fig 3).
Figure 3.
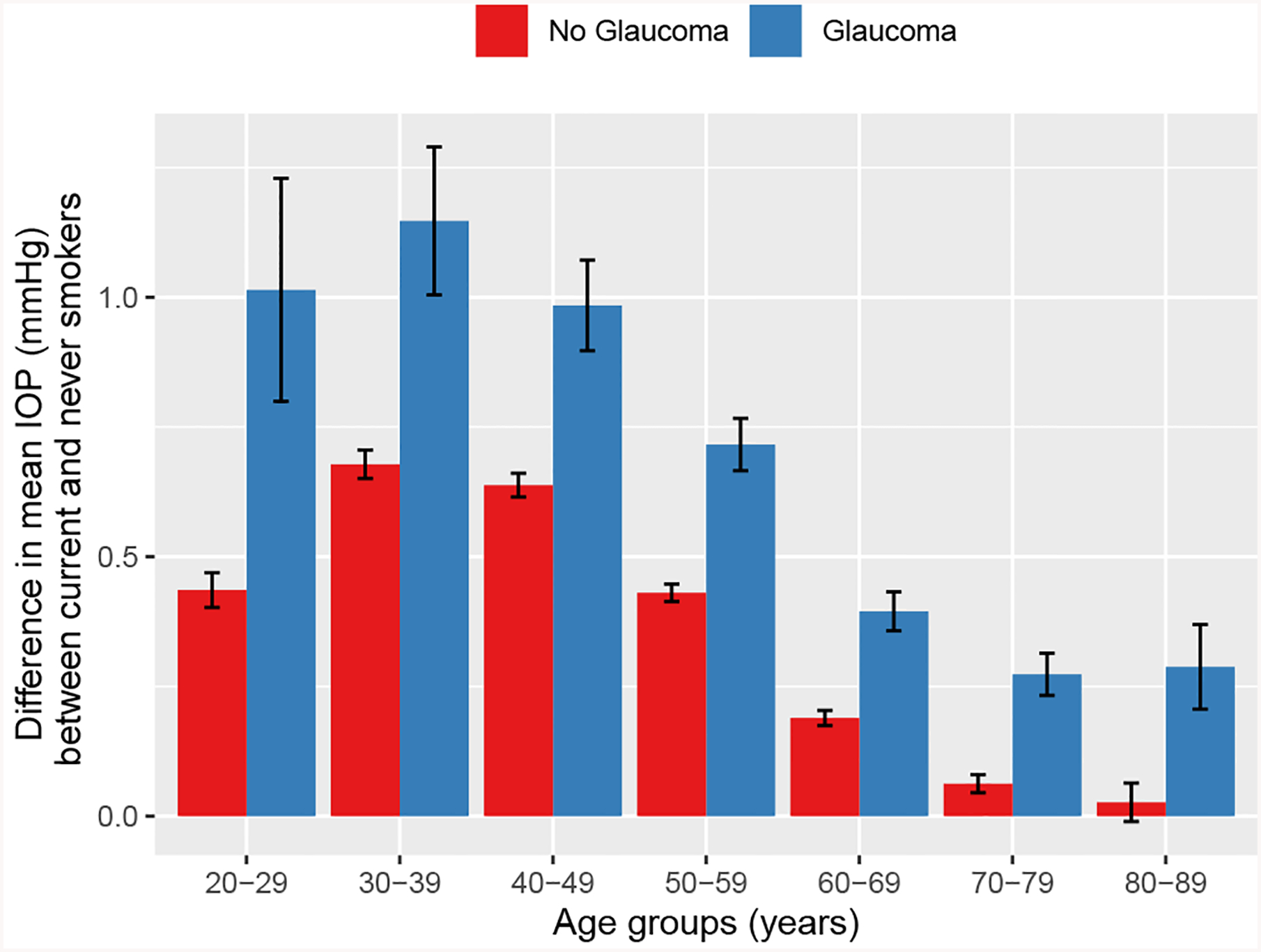
Box-and-whisker plot showing the mean difference in intraocular pressure (IOP) between current smokers and never smokers. Results are stratified by glaucoma (blue) compared with patients without glaucoma (red) and by decade of life (x-axis). The error bars represent the 95% confidence intervals.
The stepwise model selection yielded a model that contained all the demographic and clinical covariates as well as first-order interaction terms with smoking, with the exception of the interaction between smoking status and DR. Thus, in our multivariate regression model, we examined the effect of smoking status adjusted for age, gender, glaucoma, AMD, DR, cataract diagnosis, cataract surgery, glaucoma procedures, and all first-order interactions after stepwise selection (Table 2; Table S2, available at www.ophthalmologyglaucoma.org). Glaucoma was associated most strongly with IOP (β coefficient, 1.319; P < 2 10−16) followed by smoking status. Compared with never smokers, a 0.915-mmHg higher IOP was associated with current smokers and a 0.769-mmHg higher IOP was associated with past smokers, both of which were higher than any other covariates in the model, with the exception of glaucoma diagnosis. Females had higher IOPs than males by 0.095 mmHg, and a 0.0104-mmHg lower IOP was associated per each year of age (P <2 × 10−16). We performed a sensitivity analysis using data from both eyes and found little difference in the fitted coefficients (Table S3, available at www.ophthalmologyglaucoma.org).
Table 2.
Multivariate Linear Regression of Intraocular Pressure with Age, Gender, Smoking Status, Diagnoses, Surgeries, and Interaction Terms with Smoking Status
| β Coefficient | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Smoking | |||
| Never smoked | Reference | Reference | |
| Past smoker | 0.769 | 0.746–0.792 | <2 × 10−16 |
| Current smoker | 0.915 | 0.883–0.948 | <2 × 10−16 |
| Age (per year of life) | −0.0104 | −0.0106 to −0.0103 | <2 × 10−16 |
| Gender | |||
| Male | Reference | Reference | |
| Female | 0.095 | 0.091–0.020 | <2 × 10−16 |
| Unknown | −0.219 | −0.270 to −0.168 | <2 × 10−16 |
| Glaucoma | 1.319 | 1.313–1.324 | <2 × 10−16 |
| AMD | −0.200 | −0.207 to −0.193 | <2 × 10−16 |
| DR | 0.387 | 0.381–0.394 | <2 × 10−16 |
| Cataract | 0.327 | 0.318–0.328 | <2 × 10−16 |
| Glaucoma surgery | |||
| In 2017 | 0.596 | 0.575–0.617 | <2 × 10−16 |
| Before 2017 | −0.430 | −0.445 to −0.415 | <2 × 10−16 |
| Cataract surgery | |||
| In 2017 | 0.335 | 0.326–0.345 | <2 × 10−16 |
| Before 2017 | −0.735 | −0.743 to −0.728 | <2 × 10−16 |
AMD = age-related macular degeneration; DR = diabetic retinopathy.
Coefficients for interaction terms are included in Supplemental Table 2.
Discussion
In a review of the IRIS Registry of 12 535 013 unique patients who were seen during the year 2017 with IOP measurements and smoking status recorded, our study revealed that higher IOP is associated with smoking regardless of glaucoma status, after adjusting for age, gender, glaucoma, AMD, DR, cataract diagnosis, cataract surgery, glaucoma procedures, and all first-order interactions. Younger glaucoma patients who are current smokers showed the highest IOP overall. The difference in IOP between never smokers and current smokers was the greatest, followed by the difference between never smokers and past smokers.
Smoking and Intraocular Pressure
Although several studies have examined associations between cigarette smoking and IOP, the results have been conflicting. The Blue Mountains Eye Study of 3654 patients revealed mildly higher mean IOP (16.34 mmHg) in current smokers compared with past smokers (16.06 mmHg) and never smokers (16.03 mmHg). This difference in IOP between groups was still significant after controlling for age, gender, history of glaucoma, myopia, pseudoexfoliation, systolic blood pressure, family history of glaucoma, and diabetes (P = 0.017).14 This study included a much higher proportion of current smokers (17.3%) and past smokers (49.9%) compared with our study population (6.9% and 24.1%, respectively). Interestingly, the mean IOP difference between groups was strikingly similar to what we found in our study (0.31 mmHg in the Blue Mountains Eye Study vs. 0.37 mmHg in our study), even after controlling for multiple confounders that were not measured in our study. In contrast, an Australian population-based cross-sectional study of 4576 participants found no association between smoking and IOP in participants with or without glaucoma.17 However, their study excluded anyone with a history of glaucoma treatment, potentially biasing the glaucoma group to only those with mild or undiagnosed glaucoma. The Barbados Eye Study of 3752 black participants revealed significant positive associations between current smoking and IOP that are similar to our results; however, their study excluded those with a prior diagnosis of glaucoma.15
More recent studies reported equally conflicting results regarding smoking and IOP. A positive association between IOP and smoking history (multivariate regression coefficients, 0.143–0.149; P = 0.02–0.03) was found only in men and not in women in a Japanese cross-sectional study of 1320 residents 28 to 79 years of age.30 However, the number of men was approximately 3 times as high as the number of women, different from our predominantly female population. Similar to our study, a recent analysis of UK Biobank data from 110 573 participants with IOP measurements showed that smoking is associated with higher IOP by 0.19 mmHg in a multivariate model (P < 0.001) that controlled for many covariates such as age, gender, ethnicity, blood pressure, refractive error, and self-reported diabetes and glaucoma.31
Smoking and Glaucoma
Similar to IOP, associations between current smokers and glaucoma are unclear. Stronger evidence exists to suggest potential associations between current smokers and glaucoma than between past smokers and glaucoma. The Beaver Dam Eye Study reported no association between prevalence of glaucoma and cigarette smoking status after surveying 4926 participants.18 Their method of glaucoma diagnosis was more standardized than ours by obtaining stereoscopic funduscopic photographs of discs and visual fields from all participants. Nevertheless, no difference was found among current, past, and never smokers in this study. A prospective cohort of 32 570 black women in the Black Women’s Health Study found mostly no association between smoking and incidence of glaucoma except for younger age (<50 years) with higher consumption of tobacco (>15 cigarettes or >20 pack-years) compared with never smokers based on survey results.32 We found the highest IOP difference between current and never smokers in younger age (20–49 years), thus likely supporting our study results. The Nurses’ Health Study of more than 120 000 female registered nurses 30 to 55 years of age identified 450 incident cases of primary open-angle glaucoma during the follow-up of 1 035 227 person-years.33 Participants were grouped by gender and as never, past, and current smokers, and the smoking history was quantified as well. After controlling for age, hypertension, and black race, none of the groups showed significant associations between smoking and primary open-angle glaucoma.33 However, given that all participants were health professionals, their rate of smoking and regular eye examinations in which glaucoma could be detected may be different from the general population.
Other studies have shown positive associations between smoking and glaucoma. A large study of 16 797 Spanish participants with a mean age of 39 years (standard deviation, 12 years) detected 184 incident cases of glaucoma during 144 313 person-years (median follow-up, 8.5 years) based on questionnaires.34 In this study, current smokers were 88% more likely to develop glaucoma compared with never smokers (hazard ratio, 1.88; 95% CI, 1.26–2.81; P = 0.002) controlling for several covariates such as age, gender, body mass index, hypertension, and type 2 diabetes. No significant associations were found between past and never smokers (hazard ratio, 1.27; 95% CI, 0.88–1.82). The strengths of the study are its large sample size, longitudinal design, and validation of self-reported glaucoma diagnoses by ophthalmologists in a subset. The analysis of the National Health and Nutrition Examination Survey data from 3864 participants showed an increased odds of having glaucoma among current and past smokers per each increase in pack-per-day amount (odds ratio, 1.70; 95% CI, 1.08–2.67; P = 0.02).35 The dose-dependent relationship strengthens their findings. However, this was a cross-sectional study; thus, the temporal relationship between smoking and glaucoma was not ascertained. A meta-analysis of 7 studies (4 cross-sectional and 3 case-control studies) found a pooled odds ratio of 1.37 (95% CI, 1.00–1.87) between current smokers and glaucoma.36 A more recent systematic review of 17 studies (9 case-control, 5 cohort, and 3 cross-sectional studies) concluded that there is a potential association between primary open-angle glaucoma and heavy smoking, but this was based on primarily a few case-control studies.37 Many reasons for conflicting results across studies exist such as different study populations, designs, and methods of data collection and validation. Each study, including ours, provides their unique strengths and valuable information from which one can draw inferences.
Intraocular Pressure Associations
Lower IOP in the older age group was unexpected. Previous studies have found inconsistent relationships between IOP and age. The cross-sectional Singapore Malay Eye Study of 3280 participants reported an “inverted U-shape” relationship with increasing age.38 Although the decrease in IOP with older age was fairly mild (<0.5 mmHg), the pivotal age at which IOP rise stops and starts decreasing was during 60 to 69 years of age, consistent with what we found. Interestingly, the inverted U-shape relationship was observed in several other studies, such as the Beijing Eye Study and the EPIC-Norfolk Eye Study.39,40 In addition, the authors of the UK Biobank study claimed that there is “a hint of the same inverted U trend,” despite the overall positive linear associations found between IOP and age.31 Many of these studies controlled for several confounders, such as central corneal thickness, refractive error, blood pressure, and race, and found similar results as ours, which strengthens the validity of our dataset. Potential hypotheses for lower IOP in older patients include decreased production of aqueous humor, increased trabecular or uveoscleral outflow, or both, and change in the composition of the aqueous humor with age. In addition, cataract extraction may play a role, given that all IOPs showed a significantly lowering trend starting approximately late seventh decade of life. We found significantly lower IOP in patients who underwent cataract surgery before 2017, which supports this hypothesis.
Also interesting is the finding of higher IOP among current smokers compared with past smokers. We would have expected older past smokers to have a longer pack-year smoking history than current smokers. Higher adjusted IOP in current smokers suggests that a smoking effect may be conferred by current use, but declines or resolves with smoking cessationdafter the damage is already done. One study has shown higher IOP in long-term smokers compared with nonsmokers to be independent of chronic changes in corneal biomechanics such as corneal resistance factor and corneal hysteresis,41,42 and this may explain why current smoking status may have more impact than previous history of smoking. An increase in choroidal thickness has been shown after smoking, and chronic elevation in choroidal thickness may increase the episcleral venous pressure and IOP, which may impact both current and past smokers.43 In addition, lower IOP in past smokers also could reflect treated glaucoma; however, glaucoma patients in our sample showed higher IOP on average than nonglaucoma patients. Nevertheless, it is also possible that smoking may not increase true IOP, but rather may influence the corneal biomechanics, resulting in a higher measurement than true IOP. If so, smoking itself would not result in increased risk for glaucoma. The UK Biobank study supported this idea by showing that the Goldmann-correlated IOP was associated positively with smoking, whereas corneal-compensated IOP was associated negatively with smoking.31 These considerations warrant longitudinal analysis as part of future studies.
Using Big Data
When using big data such as the IRIS Registry, it is critical to pose questions and hypotheses within the confines of the dataset. Most of the IRIS Registry-related studies so far have relied on the combination of International Classification of Diseases and Current Procedural Terminology codes to minimize misclassifications or missing data.21–25 Smoking screening and cessation is one of the measures included in the Health Information Technology for Economic and Clinical Health Act, a program to incentivize providers to report quality measures to the Centers for Medicare and Medicaid Services.27,28 Thus, we hypothesized that smoking would be one of the most robustly recorded social history variables from the IRIS Registry by the eye care team. In addition, the IRIS Registry was designed to improve quality and to assist with reporting quality and outcome measures. Therefore, all the elements of quality documentation were critical components of the IRIS Registry.44
When we evaluated the recording rate and reliability of smoking exposure, smoking exposure was missing in only 7% of data from 2017. In addition, smoking was recorded as commonly as visual acuity (63% vs. 65%) throughout all years. Within the same individual, the value of recording (current vs. past vs. never smokers) changed between visits less than 15% of the time. The variation could suggest that providers were assessing the smoking status at each visit or a recording error. We do not have pack-per-year history or time since quitting data; thus, we are unable to evaluate a possible dose-response relationship of smoking and IOP, which is a limitation.
Glaucoma is a leading cause of irreversible blindness worldwide, but most of its known risk factors, such as old age, thin central corneal thickness, enlarged cup-to-disc ratio, and black race, are not modifiable.45 In our multivariate model that controlled for age, gender, and additional ophthalmic pathologic features, smoking was the most important predictor of IOP after the glaucoma diagnosis. The association between smoking and IOP was disproportional in the glaucoma group compared with the nonglaucoma group, suggesting an effect between cigarette smoking and glaucoma in IOP rise. Interestingly, the prevalence of glaucoma was the lowest in current smokers compared with never or past smokers. This may have been a result of age, because past smokers were the oldest, followed by never smokers and then current smokers. The stepwise reduction in IOP found between current smokers, past smokers, and never smokers also is noteworthy and should be considered while counseling patients. The global difference in IOP between current and never smokers (0.37 mmHg) may seem minimal, but when controlling for all the differences between groups, we found an even greater difference (0.915 mmHg). Importantly, in young patients with glaucoma (20–39 years), the IOP difference between current and never smokers was higher than 1 mmHg (range, 1.01–1.14 mmHg), which should be considered clinically significant. The Early Manifest Glaucoma Trial showed that every 1 mmHg of IOP reduction is associated with glaucoma risk reduction of 10% to 13%.46 Thus, counseling on smoking cessation should be considered in particular in young glaucoma patients.
The IRIS Registry is well positioned to report real-world epidemiologic features and outcomes of many ophthalmic conditions. The large number of data points in our study is an important strength, enabling us to observe the raw data without relying on complex statistical analyses. The availability of participants’ routine clinical data is another strength given that large studies often are based on surveys or claims data without relevant clinical data. However, whether the IRIS Registry data truly represents the real-world United States population is unknown. Given the disproportionate number of female patients compared with male patients in this study, a sampling bias may exist. With recent advances in artificial intelligence and natural language processing, study questions that require complex analyses and perhaps machine learning approaches using the IRIS Registry data likely will be possible in the future.47
However, several limitations exist. First, this was a retrospective review of a prospectively collected electronic medical record database. The quality of data depends largely on the reliable coding and extraction of the medical records. Any electronic medical record-based studies are at risk of potential misclassifications because of inaccurate coding, missing data, or incomplete data. For example, the vast majority of our cohort (>98%) did not specify the method used for IOP measurement, which is an important limitation. Our never smoker group could include some patients who do have a smoking history (and vice versa), making these groups in actuality more mixed than we acknowledge. However, we found the smoking recording to be consistent for more than 85% of all visits. In addition, the potential effect of the so-called noise of the data may be less likely to play a major role given the sheer number of more than 12.5 million IOP records included in our analyses. It is important to note that the difference in IOP that we found with the IRIS Registry database is exquisitely similar to that in the Blue Mountains Eye Study, a large population-based study with traditional methodologies. The subtle inverted U-shape relationship found in other population-based studies was replicated in our study, which may validate the quality of routinely collected data in the IRIS Registry.31,39,40 Second, we did not have access to all potential confounders of high IOP, such as black race; central corneal thickness; refraction status; IOP-lowering medication; and glaucoma severity, treatment status, or both. We also did not have access to month or day of visit, making it impossible to know the order of the IOP measurements, smoking statuses recorded, or timing of any procedures. These clinical data may be available for research in the near future. Furthermore, we adjusted for confounding variables that were available to us, but our results may be influenced by residual confounding or other unmeasured covariates. Despite the limitations in our dataset, we found similar results to other population-based studies. A more complete dataset is anticipated from the IRIS Registry, and better models may be explored in the future. Third, although the IRIS Registry captures a substantial amount of the United States population, it may overrepresent or underrepresent certain ethnicities or races that may have different risk factors for high IOP or glaucoma.48,49 Finally, we are able to evaluate only associations and not causality, similar to any other observational studies. Our results have led to interesting hypotheses, and prospective studies to test these hypotheses will be important.
In summary, smoking was associated with higher IOP when controlled for age, gender, and glaucoma status and other ophthalmic conditions known to raise IOP, which could have important public health implications. The largest difference in IOP was found between never and current smokers, in particular those who were diagnosed with glaucoma. Despite many limitations, big data such as the IRIS Registry provide an important resource for clinical epidemiologic studies in ophthalmology.
Supplementary Material
Acknowledgments.
The authors thank Leona Ding, MS, for aiding in the biostatistical approach.
Financial Disclosure(s):
The author(s) have made the following disclosure(s): S.P.: Financial support - Acumen, LLC; Equity owner - Verana Health.
J.W.M.: Consultant - Genentech/Roche, Sunovion, Alcon Research Institute, KalVista Pharmaceuticals, Ltd., ONL Therapeutics, LLC; Financial support - Bausch & Lomb, Lowy Medical Research Institute, Ltd.; Royalties - Massachusettes Eye and Ear/Valeant Pharmaceuticals; Equity owner - ONL Therapeutics, LLC; Patent - ONL Therapeutics, LLC, Valeant Pharmaceuticals.
J.A.H.: Consultant - Lowy Medical Research Institute, Merck, Alcon, Notal Vision; Board member - Celgene Corporation, Bristol-Myers Squibb; Financial support - Aura Bioscience, Lowy Medical Research Institute.
M.F.C.: Financial support - Genentech, Novartis; Equity owner - InTeleretina, LLC.
F.L.: Employee - American Academy of Ophthalmology.
Aaron Y. Lee: Financial support - Lowy Medical Research Institute, Verana Health, Topcon, Novartis, Carl Zeiss, Alcon.
Supported by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant nos.: K23 EY024921 and K23 EY029246); Lowy Medical Research Institute; Research to Prevent Blindness, Inc., New York, New York (unrestricted departmental funding); and the National Science Foundation (M.F.C.). The sponsors or funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- AMD
age-related macular degeneration
- CI
confidence interval
- DR
diabetic retinopathy
- IRIS
Intelligent Research in Sight
- IOP
intraocular pressure
Footnotes
HUMAN SUBJECTS: No human subjects were included in this study. Given the use of de-identified data, the review was exempted by the University of Washington Institutional Review Board. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Supplemental material available at www.ophthalmologyglaucoma.org.
References
- 1.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60–68. [DOI] [PubMed] [Google Scholar]
- 2.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014;127:807–812. [DOI] [PubMed] [Google Scholar]
- 3.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. [DOI] [PubMed] [Google Scholar]
- 4.Jha P Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–664. [DOI] [PubMed] [Google Scholar]
- 5.Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011;22:477–482. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. [DOI] [PubMed] [Google Scholar]
- 7.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276: 1141–1146. [PubMed] [Google Scholar]
- 8.Lindblad BE, Håkansson N, Wolk A. Smoking cessation and the risk of cataract: a prospective cohort study of cataract extraction among men. JAMA Ophthalmol. 2014;132: 253–257. [DOI] [PubMed] [Google Scholar]
- 9.Beltrán-Zambrano E, García-Lozada D, Ibáñez-Pinilla E. Risk of cataract in smokers: a meta-analysis of observational studies. Arch Soc Esp Oftalmol. 2019;94:60–74. [DOI] [PubMed] [Google Scholar]
- 10.Lin P, Loh AR, Margolis TP, Acharya NR. Cigarette smoking as a risk factor for uveitis. Ophthalmology. 2010;117: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne JE, Daniel E, Jabs DA, et al. Smoking as a risk factor for cystoid macular edema complicating intermediate uveitis. Am J Ophthalmol. 2008;145:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmunds MR, Boelaert K. Knowledge of thyroid eye disease in Graves’ disease patients with and without orbitopathy. Thyroid. 2019;29:557–562. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Cestari DM, Fortin E. Thyroid eye disease: what is new to know? Curr Opin Ophthalmol. 2018;29:528–534. [DOI] [PubMed] [Google Scholar]
- 14.Lee AJ, Rochtchina E, Wang JJ, et al. Does smoking affect intraocular pressure? Findings from the Blue Mountains Eye Study. J Glaucoma. 2003;12:209–212. [DOI] [PubMed] [Google Scholar]
- 15.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997;115: 1572–1576. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Ishikawa M, Karita K, et al. Association of blood pressure and body mass index with intraocular pressure in middle-aged and older Japanese residents: a cross-sectional and longitudinal study. Acta Med Okayama. 2014;68:27–34. [DOI] [PubMed] [Google Scholar]
- 17.Weih LM. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119:875. [DOI] [PubMed] [Google Scholar]
- 18.Klein BEK, Klein R, Ritter LL. Relationship of drinking alcohol and smoking to prevalence of open-angle glaucoma. Ophthalmology. 1993;100:1609–1613. [DOI] [PubMed] [Google Scholar]
- 19.Stewart WC, Crinkley CM, Murrell HP. Cigarette-smoking in normal subjects, ocular hypertensive, and chronic open-angle glaucoma patients. Am J Ophthalmol. 1994;117:267–268. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Ophthalmology. IRIS Registry data analysis: overview. https://www.aao.org/iris-registry/data-analysis/requirements; 2017. Accessed 12.08.19.
- 21.Parke DW 3rd, Lum F. Return to the operating room after macular surgery: IRIS Registry analysis. Ophthalmology. 2018;125:1273–1278. [DOI] [PubMed] [Google Scholar]
- 22.Willis J, Morse L, Vitale S, et al. Treatment patterns for myopic choroidal neovascularization in the United States: analysis of the IRIS Registry. Ophthalmology. 2017;124: 935–943. [DOI] [PubMed] [Google Scholar]
- 23.Repka MX, Lum F, Burugapalli B. Strabismus, strabismus surgery, and reoperation rate in the United States: analysis from the IRIS Registry. Ophthalmology. 2018;125: 1646–1653. [DOI] [PubMed] [Google Scholar]
- 24.Rao P, Lum F, Wood K, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS Registry. Ophthalmology. 2018;125:522–528. [DOI] [PubMed] [Google Scholar]
- 25.Atchison EA, Wood KM, Mattox CG, et al. The real-world effect of intravitreous anti-vascular endothelial growth factor drugs on intraocular pressure: an analysis using the IRIS Registry. Ophthalmology. 2018;125:676–682. [DOI] [PubMed] [Google Scholar]
- 26.Coleman AL. How big data informs us about cataract surgery: The LXXII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2015;160:1091–1103.e3. [DOI] [PubMed] [Google Scholar]
- 27.Rabius V, Karam-Hage M, Blalock JA, Cinciripini PM. “Meaningful use” provides a meaningful opportunity. Cancer. 2014;120:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan ASL, Young-Wolff KC, Carter-Harris L, et al. Disparities in the receipt of tobacco treatment counseling within the US context of the Affordable Care Act and meaningful use implementation. Nicotine Tob Res. 2018;20:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang MF, Sommer A, Rich WL, et al. The 2016 American Academy of Ophthalmology IRIS Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125:1143–1148. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida M, Take S, Ishikawa M, et al. Association of smoking with intraocular pressure in middle-aged and older Japanese residents. Environ Health Prev Med. 2014;19:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan MPY, Grossi CM, Khawaja AP, et al. Associations with intraocular pressure in a large cohort: results from the UK Biobank. Ophthalmology. 2016;123:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise LA, Rosenberg L, Radin RG, et al. A prospective study of diabetes, lifestyle factors, and glaucoma among African-American women. Ann Epidemiol. 2011;21:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang JH, Pasquale LR, Rosner BA, et al. Prospective study of cigarette smoking and the risk of primary open-angle glaucoma. Arch Ophthalmol. 2003;121:1762–1768. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-de-Arcelus M, Toledo E, Martínez-González MÁ, et al. Smoking and incidence of glaucoma: the SUN cohort. Medicine. 2017;96, e5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Law SM, Lu X, Yu F, et al. Cigarette smoking and glaucoma in the United States population. Eye. 2018;32:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonovas S, Filioussi K, Tsantes A, Peponis V. Epidemiological association between cigarette smoking and primary open-angle glaucoma: a meta-analysis. Public Health. 2004;118: 256–261. [DOI] [PubMed] [Google Scholar]
- 37.Jain V, Jain M, Abdull MM, Bastawrous A. The association between cigarette smoking and primary open-angle glaucoma: a systematic review. Int Ophthalmol. 2017;37:291–301. [DOI] [PubMed] [Google Scholar]
- 38.Wong TT, Wong TY, Foster PJ, et al. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an Asian population. Invest Ophthalmol Vis Sci. 2009;50:4097. [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Li J, Zheng Y, et al. Intraocular pressure in northern China in an urban and rural population: the Beijing Eye Study. Am J Ophthalmol. 2005;140:913–915. [DOI] [PubMed] [Google Scholar]
- 40.Foster PJ, Broadway DC, Garway-Heath DF, et al. Intraocular pressure and corneal biomechanics in an adult British population: the EPIC-Norfolk Eye Study. Invest Ophthalmol Vis Sci. 2011;52:8179–8185. [DOI] [PubMed] [Google Scholar]
- 41.Hafezi F. Smoking and corneal biomechanics. Ophthalmology. 2009;116:2259.e1. [DOI] [PubMed] [Google Scholar]
- 42.Mansouri K, Pajic B, Hafezi F. Effect of cigarette smoking on intraocular pressure. J Cataract Refract Surg. 2015;41: 682–683. [DOI] [PubMed] [Google Scholar]
- 43.Ulaş F, Çelik F, Doğan Ü, Çelebi S. Effect of smoking on choroidal thickness in healthy smokers. Curr Eye Res. 2014;39:504–511. [DOI] [PubMed] [Google Scholar]
- 44.Rich WL 3rd, Chiang MF, Lum F, et al. Performance rates measured in the American Academy of Ophthalmology IRIS Registry (Intelligent Research in Sight). Ophthalmology. 2018;125:782–784. [DOI] [PubMed] [Google Scholar]
- 45.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. ; discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 46.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment. Evidence-Based Eye Care. 2003;4:134–136. [Google Scholar]
- 47.Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badr M, Masis Solano M, Amoozgar B, et al. Central corneal thickness variances among different Asian ethnicities in glaucoma and nonglaucoma patients. J Glaucoma. 2019;28: 223–230. [DOI] [PubMed] [Google Scholar]
- 49.Nathan N, Joos KM. Glaucoma disparities in the Hispanic population. Semin Ophthalmol. 2016;31:394–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


