Abstract
Extralymphatic filariasis is an uncommon phenomenon that can be caused by several lymphatic filarial species, including zoonotic filaria of animal origins. In this study, we report a case of a 64-year-old Thai woman who presented with a lump in her left breast that was diagnosed with invasive ductal carcinoma. At the same time, a small nodule was found in her right breast, via imaging study, without any abnormal symptoms. A core needle biopsy of the right breast nodule revealed a filarial-like nematode compatible with the adult stage of Brugia sp. A molecular identification of the nematode partial mt 12rRNA gene and ITS1 suggested the causative species as closely related to Brugia pahangi, a zoonotic lymphatic filaria of animals such as cats and dogs. The sequence of the partial mt 12rRNA and ITS1 gene in this patient was 94% and 99% identical to the previously reported sequence of mt 12rRNA and ITS1 genes of B. pahangi. The sequence of ITS1 gene is 99% similar to B. pahangi microfilaria from infected dogs in Bangkok, which was highly suspected of having a zoonotic origin. As far as we know, this is the first case report of B. pahangi filariasis presented with a breast mass concomitantly found in a patient with invasive ductal carcinoma. This raised serious concern regarding the zoonotic transmission of filariasis from natural animal reservoirs.
Keywords: Filariasis, Extranodal filariasis, Extralymphatic filariasis, Breast filariasis, Brugia pahangi, Brugia spp.
1. Introduction
Lymphatic filariasis, commonly known as elephantiasis, occurs when filarial parasites are transmitted to humans through mosquito bites. Wuchereria bancrofti and Brugia malayi are two common species responsible for more than 90% of the cases [1]. Adult worms of these species dwell in the lymphatic system which trigger chronic inflammation and blockage of lymphatic drainage, resulting in lymphedema of peripheral extremities and hydrocele [2]. Extralymphatic presentation of microfilariae or adult worms is uncommon. Presentation of breast filariasis is rare and may be misdiagnosed as a breast tumor or cyst [3]. The majority of the reported cases have been from India, which is an endemic area of lymphatic filariasis [[4], [5], [6]]. Extralymphatic presentations of filariasis have also been caused by zoonotic filaria [3]. In this study, we report an unusual case of filariasis of the breast, caused by Brugia pahangi, a lymphatic filaria of cats and dogs. B. pahangi has been reported to cause a small number of zoonotic filariasis in people in Malaysia [7]. This is the first case report of zoonotic filariasis presenting with a breast mass caused by B. pahangi.
2. Case report
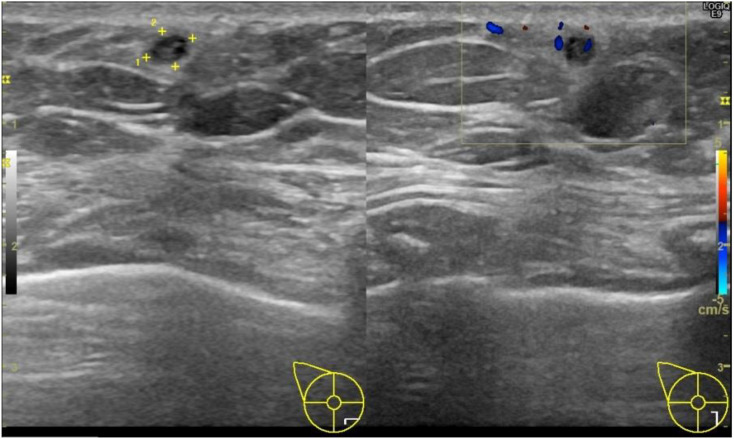
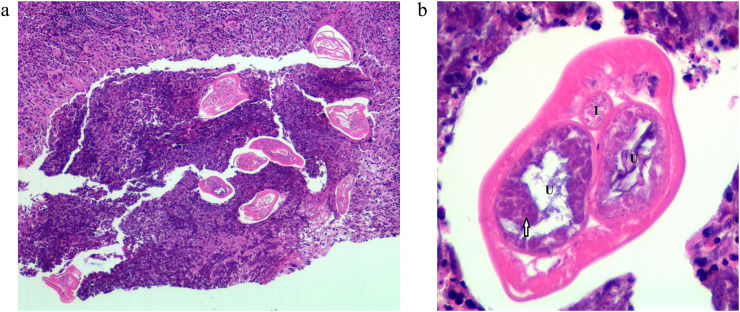
A 64-year-old woman came to the breast clinic at the Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand with a palpable left breast mass which she had had for three months. She noticed an occasionally bloody left nipple discharge for one month. She denied any history of mastalgia, skin changes, or any systemic abnormalities. There was no palpable mass detected on her right breast. The patient was previously healthy, without any reported family history of breast carcinoma. She lives in Nonthaburi province in the central region of Thailand, adjacent to Bangkok. She had no recent travel history and no pet cats or dogs. A physical examination revealed a non-movable, palpable mass, and approximately 2 cm in the upper inner quadrant (UIQ) of her left breast, with multiple axillary lymphadenopathies. A physical examination of her right breast was unremarkable. The patient was given a mammography and ultrasound. There were multiple irregular masses, with a microlobulated margin in the UIQ and the inner part of her left breast, with associated fine pleomorphic calcifications and duct extension in the inner central region, with a slightly thickened cortex of the left axillary lymph node. The largest mass was 18 × 14 mm which was highly suggestive of malignancy (BI-RADS Category 5). A concomitant abnormal finding in her right breast was also made. A small heterogeneous nodule with surrounding hyperechogenicity of suspicious abnormality was also revealed in the lower inner quadrant (LIQ) of her right breast, with no axillary lymphadenopathy as shown in Fig. 1 . An ultrasound-guided core needle biopsy was performed at the UIQ and LIQ masses of the left and right breasts, respectively. A histological examination of breast tissue from the left mass showed invasive ductal carcinoma with solid papillary features. The biopsy of the right mass revealed a degenerated nematode worm with the presence of pseudocelom. The worm was surrounded by chronic eosinophilic, granulomatous inflammation, and stromal fibrosis, without any evidence of malignancy. It had a thin, smooth cuticle with a few well-developed, low, broad muscle cells per quadrant. The transverse section revealed two uterine tubes with microfilariae inside, which was compatible with the adult stage of Brugia sp. (Fig. 2 a-b). Additional tests were performed, including a Giemsa-stained thick-blood film for microfilaria and a serum IgG4 for filaria. Both tests turned out negative. A complete blood count did not show eosinophilia. To further identify the causative filarial species, PCR-targeted mt 12S rRNA and ITS1 gene were performed using the protocol as previously described [[8], [9], [10]]. The PCR products were cloned into the T-Vector pMD20 and sequenced. The mt 12S rRNA sequence was blasted with an NCBI reference-sequence database which was 94% identical to B. pahangi (Genbank accession number AP017680.1 and submitted into the NCBI database under GenBank accession number MT887286). The ITS1 sequence was 99% identical to the sequence of B. pahangi microfilaria (Genbank accession number MK250800.1), previously reported from domestic dogs in Bangkok metropolitan. This emphasizes the possibility of zoonotic infection in this patient. The ITS1 sequence was submitted to Genbank under accession number MT732324. The patient was subjected to a left modified radical mastectomy for treatment of the invasive ductal carcinoma. She received pretreatment of anastrozole for two months before surgery. (The mastectomy operation was delayed due to the COVID-19 situation). In addition, the nodule on the right breast containing nematode was planned to be surgically removed at the same time; however, the mass subsided and could not be seen on ultrasound two months after the core needle biopsy. No additional medicine was given for breast filariasis due to the negative microfilaria and filarial serum IgG4. The lesion was resolved on its own without any treatment.
Fig. 1.
Ultrasonography of right breast revealed a small heterogeneous nodule (+), with indistinct margin surrounding hyperechogenicity in the lower inner quadrant (LIQ). No calcification was observed within the mass.
Fig. 2.
(a) A section from core needle aspiration of right breast mass revealed a nematode like worm with the presence of pseudocoelom and smooth thin cuticle surrounded with chronic eosinophilic and granulomatous inflammation. (b) Transverse section of nematode revealed a nematode with 2 uterine tubes (U) containing microfilariae (arrow) inside and one intestine (I). It has a few, fully-developed broad muscle per quadrant.
3. Discussion
B. pahangi is a lymphatic filarial species which is known to have mammals in feline and canine families as definitive hosts [11]. An infection in cats and dogs usually does not cause any clinical manifestation or lymphadenopathy [11]. In prior studies involving the lymphatic system, not the breast, humans have been considered an accidental host for B. pahangi [12]. A zoonotic infection of B. pahangi is uncommon, and happens to be localized in Southeast Asian countries such as Malaysia and Thailand [7,12]. In Thailand, B. pahangi have been recovered in cats and dogs in Surat Thani, Rayong, and Narathiwat provinces, as well as in Bangkok [13]. Patients with breast filariasis can present with a variety of clinical manifestations, ranging from asymptomatic to a breast lump which mimics inflammatory breast cancer [14]. A few case reports have shown that breast filariasis is mostly caused by Wuchereria bancrofti [[4], [5], [6],14]. The most common site of breast filariasis is the upper outer quadrant of the breast, and the lesion seldom invades the ductal part [5]. A mammogram study of the lesion may reveal calcified filarial worms in coiled or serpiginous form, or a mixture of both, but more commonly as a solitary nodule [14]. The filarial dance sign, which is a vigorous twirling movement of multiple curvilinear echoes, may be observed during a breast ultrasonography if the worm is still viable [15]. Extralymphatic filariasis caused by B. pahangi as observed in this patient was rare. Although humans are an accidental host of B. pahangi, the worm could undergo full development in this host [11]. Normally, the worm will die in human tissue within a certain period of time, and may or may not cause any symptoms, depending on the host's immune response and the location of the worm [11]. The majority of zoonotic filaria found in human tissue have been sexually mature female worms with the presence of a developing egg in uterus [3]. In our patient, a gravid adult female with evidence of microfilaria in utero was found in her breast tissue. The presence of blood microfilaria in a patient infected with B. pahangi is extremely rare. Only one previous study reported the presence of microfilaria in the blood sample of an 18-month-old boy who presented with lymphadenopathy in his groin area [12]. In addition, microfilaria has been found in the blood of volunteers who were experimentally inoculated with B. pahangi, which suggests that the female worm may be able to reach a sexually mature and gravid stage in humans [16]. Blood microfilaria and serum filarial IgG4 were negative in this patient. This may due to the fact that the level of IgG4 in this patient was not S under detectable threshold.
Zoonotic transmission of B. pahangi from animal reservoirs such as domestic cats and dogs was suspected in this patient. Such pets have been reported to be reservoirs of B. pahangi in several parts of Thailand, including the Bangkok metropolis, which is adjacent to the patient's hometown in Nonthaburi province [13]. Mosquitoes of the species Mansonia spp. and Armigeres subalbatus have been reported to be natural vectors of B. pahangi [17]. These species have previously been identified in several regions of Thailand [18]. The main treatment strategy for zoonotic filariasis in humans are surgical removal of the lesion and medication. The lesion resulting from zoonotic filariasis is mostly a solitary nodule; thus, surgical removal of the lesion may be adequate [19]. Drug treatments such as ivermectin and selamectin have been shown to effectively eliminate the worm in cats and dogs [20,21]. However, the necessity of giving those medications to people with zoonotic filariasis is still questionable. In our patient, only a single lesion was observed, and the worm did not seem to be viable. So, medication was not given to the patient, and her lesion resolved spontaneously. Identification of filarial species causing extralymphatic filariasis is essential for diagnosis, treatment, prevention, and disease control because the treatment strategies for zoonotic and human filariasis are different. Regarding zoonotic filariasis, eradication of microfilariae in natural reservoirs, including cats and dogs, is crucial in the control strategy [21]. To the best of our knowledge, this is the first case report of a Brugia pahangi infection in a human who presented with a breast mass concomitantly found with invasive ductal carcinoma. The presence of a host immunity which made the patient susceptible to B. pahangi infection is still questionable. Further study is required to reveal the predisposing factors of this infection and the nature of the disease's transmission.
.
Acknowledgements
The authors would like to thank Prof. Dr.Praphathip Eamsobhana and Prof. Dr.Sirichit Wongkamchai, Department of Parasitology and Assoc. Prof. Panitta sitthinamsuwan, Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University for their suggestions and assistances with this work. This work was funded by the Internal Departmental Funding, Department of Parasitology, Facutly of Medicine Siriraj Hospital, Mahidol University.
References
- 1.World Health Organization Lymphatic Filariasis [Fact Sheet] 2020. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis
- 2.Whitworth J.A., Hewitt K. Filariasis. Medicine. 2005;33:61–64. doi: 10.1383/medc.2005.33.8.61. [DOI] [Google Scholar]
- 3.Orihel T.C., Eberhard M.L. Zoonotic Filariasis. Clin. Microbiol. Rev. 1998;11:366–381. doi: 10.1128/cmr.11.2.366. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC106837/ accessed December 4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda D.K., Mohapatra D.P., Mohapatra M.M. Case Report: Filarial breast lump. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barwad A., Singh S.K., Phulware R. Breast filariasis. IDCases. 2018;14:e00453. doi: 10.1016/j.idcr.2018.e00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik A., Singh V., Dahiya S.K., Dutta V. Coexistence of filariasis with carcinoma breast – an incidental cytological finding. Med. J. Armed Forces India. 2015;71:S76. doi: 10.1016/j.mjafi.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan L.H., Fong M.Y., Mahmud R., Muslim A., Lau Y.L., Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol. Int. 2011;60:111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Wongkamchai S., Nochote H., Foongladda S., Dekumyoy P., Thammapalo S., Boitano J.J., Choochote W. A high resolution melting real time PCR for mapping of filaria infection in domestic cats living in brugian filariasis-endemic areas. Vet. Parasitol. 2014;201:120–127. doi: 10.1016/j.vetpar.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Nuchprayoon S., Junpee A., Nithiuthai S., Chungpivat S., Suvannadabba S., Poovorawan Y. Detection of filarial parasites in domestic cats by PCR-RFLP of ITS1. Vet. Parasitol. 2006;140:366–372. doi: 10.1016/j.vetpar.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Wongkamchai S., Monkong N., Mahannol P., Taweethavonsawat P., Loymak S., Foongladda S. Rapid detection and identification of Brugia malayi, B. pahangi, and Dirofilaria immitis by high-resolution melting assay. Vector Borne Zoonotic Dis. 2012;13:31–36. doi: 10.1089/vbz.2012.0971. [DOI] [PubMed] [Google Scholar]
- 11.Bowman A. American Association of Veterinary Parasitologists; 2014. Brugia pahangi.https://www.aavp.org/wiki/nematodes/spirurida/filarioidea/brugia-pahangi/ (accessed February 17, 2020) [Google Scholar]
- 12.Iamsa-ard W., Waewwab P., Pukdeeprayoon S., Wiriya-alongkorn W., Songklin P., Thamcharoen T., Tadthong U., Khlamsida T., Phol-Or W., Rojchanapanus S., Wiwatsattaya B. Outbreak investigation of autochthonous lymphatic filariasis in Wangchan district, Rayong, Thailand. Wkly Epidemiol. Surveill Rep. December 2013 – July 2014;46(2015):385–392. [Google Scholar]
- 13.Kaikuntod M., Thongkorn K., Tiwananthagorn S., Boonyapakorn C. Filarial worms in dogs in southeast asia. Vet. Integr. Sci. 2018;16:1–17. https://he02.tci-thaijo.org/index.php/vis/article/view/141464 (accessed February 18, 2020) [Google Scholar]
- 14.Das S., Lal H., Dey M., Mohindra N., Report Case. Bilateral breast filariasis mimicking inflammatory breast carcinoma. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaral F., Dreyer G., Figueredo-Silva J., Noroes J., Cavalcanti A., Samico S., Santos A., Coutinho A. Live adult worms detected by ultrasonography in human bancroftian filariasis. Am. J. Trop. Med. Hyg. 1994;50 doi: 10.4269/ajtmh.1994.50.753. [DOI] [PubMed] [Google Scholar]
- 16.Edeson J.F.B., Wilson T., Wharton R.H., Laing A.B.G. Experimental transmission of Brugia Malayi and B. Pahangi to man. Trans. R. Soc. Trop. Med. Hyg. 1960;54:229–234. doi: 10.1016/0035-9203(60)90066-3. [DOI] [PubMed] [Google Scholar]
- 17.Muslim A., Fong M., Mahmud R., Sivanandam S. Vector and reservoir host of a case of human Brugia Pahangi infection in Selangor, peninsular Malaysia. Trop. Biomed. 2013;30 [PubMed] [Google Scholar]
- 18.Tiawsirisup S., Sripatranusorn S., Oraveerakul K., Nuchprayoon S. Distribution of mosquito (Diptera: Culicidae) species and Wolbachia (Rickettsiales: Rickettsiaceae) infections during the bird immigration season in Pathumthani province, Central Thailand. Parasitol. Res. 2008;102:731–735. doi: 10.1007/s00436-007-0825-z. [DOI] [PubMed] [Google Scholar]
- 19.Paniz-Mondolfi A.E., Gárate T., Stavropoulos C., Fan W., González L.M., Eberhard M.L., Kimmelstiel F., Sordillo E.M. Zoonotic filariasis caused by novel Brugia sp. nematode, United States, 2011. Emerg. Infect. Dis. 2014;20:1248–1250. doi: 10.3201/eid2007.131654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taweethavonsawat P., Chungpivat S. Successful treatment of Brugia pahangi in naturally infected cats with ivermectin. Korean J. Parasitol. 2013;51:759. doi: 10.3347/kjp.2013.51.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarasombath P., Thammapalo S., Loymek S., Angkanasinsiri A., Priyavoravong P., Wongkamchai S. First study of topical selamectin efficacy for treating cats naturally infected with Brugia Malayi and Brugia Pahangi under field conditions. Parasitol. Res. 2019;118 doi: 10.1007/s00436-019-06248-3. [DOI] [PubMed] [Google Scholar]