Abstract
BACE1 (beta-site amyloid precursor protein cleaving enzyme 1) was initially cloned and characterized in 1999. It is required for the generation of all monomeric forms of amyloid-β (Aβ), including Aβ42, which aggregates into bioactive conformational species and likely initiates toxicity in Alzheimer’s disease (AD). BACE1 concentrations and rates of activity are increased in AD brains and body fluids, thereby supporting the hypothesis that BACE1 plays a critical role in AD pathophysiology. Therefore, BACE1 is a prime drug target for slowing down Aβ production in early AD. Besides the amyloidogenic pathway, BACE1 has other substrates that may be important for synaptic plasticity and synaptic homeostasis. Indeed, germline and adult conditional BACE1 knockout mice display complex neurological phenotypes. Despite BACE1 inhibitor clinical trials conducted so far being discontinued for futility or safety reasons, BACE1 remains a well-validated therapeutic target for AD. A safe and efficacious compound with high substrate selectivity as well as a more accurate dose regimen, patient population, and disease stage may yet be found. Further research should focus on the role of Aβ and BACE1 in physiological processes and key pathophysiological mechanisms of AD. The functions of BACE1 and the homologue BACE2, as well as the biology of Aβ in neurons and glia, deserve further investigation. Cellular and molecular studies of BACE1 and BACE2 knockout mice coupled with biomarker-based human research will help elucidate the biological functions of these important enzymes and identify their substrates and downstream effects. Such studies will have critical implications for BACE1 inhibition as a therapeutic approach for AD.
Keywords: Alzheimer’s disease, BACE1 inhibitors, Biomarkers, Clinical trials, Soluble amyloid, Synaptic
BACE1 (beta-site amyloid precursor protein [APP] cleaving enzyme 1) is an aspartyl protease of the pepsin family and was discovered in 1999. BACE1 is a type I transmembrane protein, which makes it distinct from other peptidases of the same family, such as cathepsin D and E, which do not harbor a transmembrane domain (1–4). BACE1 is widely expressed in the brain, particularly in neurons, oligodendrocytes, and astrocytes, with particular abundance in various neuronal cell types (1–4). At the subcellular level, BACE1 localizes on the plasma membrane and in the endosomal compartments and was detected in healthy synaptic terminals and dystrophic neurites surrounding amyloid-β (Aβ) plaques (2,5,6). BACE1 is homologous with another membrane-bound secretase of the pepsin family, BACE2. The two proteases share 59% of their amino acid sequence and are composed of identical structural domain (1–4).
The active site of both secretases consists of 2 aspartic acid residues in their extracellular domains; they have 21-residue helical transmembrane domains and short cytoplasmic C-terminal domains (1–4). Three disulfide bonds help stabilize their tertiary structure, and the secretases are known to be glycosylated on several asparagine residues. Posttranslational modifications are known on BACE1 secretase and are important for lipid raft localization, phosphorylation for cellular trafficking, and ubiquitination for degradation (see below).
BACE1 and BACE2 are expressed in the same cell types in the brain, only with BACE2 being much less abundant (7,8). However, BACE2 is thought to be more active in peripheral tissues, with particular functions in melanocytes (9) and pancreatic β cells (10).
BACE1: GENETIC AND EPIGENETIC
Genetic Mutations Affect BACE1 Cleavages
Mutations in the BACE1 gene have not yet been linked to Alzheimer’s disease (AD) pathogenesis. However, mutations in APP near β-secretase sites are assumed to be either protective or causing early onset. The most prominent one is the Swedish mutation (K670M671 to N670L671 mutation at the cleavage P2-P1 subsite), which increases processing of APP at the β site by 10- to 50-fold and causes early onset of AD (11).
Whole-genome sequencing studies identified a genetic variant of APP, significantly more frequent in Icelandic and Scandinavian populations, that provides resilience against age-related brain Aβ deposition and AD (12,13).
Intriguingly, an Ala residue at the cleavage P3, subsite mutated to Thr (A673T) in APP confers an intrinsic biochemical resistance to cleavage by BACE1, resulting in less Aβ production overall and also in generation of peptides that are less prone to aggregation (12,13).
In particular, the A673T mutation is associated with a different BACE1 recognition motif at the position P2 (A673T), resulting in 20% to 30% lower soluble APPβ (sAPPβ) levels compared with control subjects (12,13). The A673T mutation appears to protect against AD and age-related cognitive decline as a result because of suppressed cleavage of APP at the β site, and carriers of the A673T variant in humans have about 28% lower levels of Aβ40 and Aβ42 in plasma compared with control subjects (12,13).
In addition, the E682K mutation in APP, located at the P1, β site, favors Aβ production by suppressing the cleavage at the β’ site to favor cleavage at the β site (12,13).
DNA Methylation
Epigenome-wide association studies on neuronal and glial cells sorted from postmortem brains of patients with AD and healthy donor subjects have revealed genes specific to neuronal cells, including APP, that undergo Braak stage–associated methylation changes (14). Interestingly, DNA methylation regulates BACE1 expression, likely owing to increased SP1 transcription factor binding to CpG sites on the BACE1 promoter region (15,16). More recently, a large cluster of significantly hypomethylated enhancers in the CpH sites were identified in prefrontal cortex neurons of individuals with severe AD pathology, and hypomethylation of these enhancers in the DSCAML1 gene likely upregulate BACE1 transcripts in AD (17). Similar DNA hypomethylation in the promoter region of APP enhances the expression of AD-related genes, including APP and PSEN1, leading to increased Aβ production (17,18).
DNA Acetylation
Although BACE1 expression and/or its activity have been extensively studied at both transcriptional and posttranslational levels, evidence of altered expression of BACE1 due to epigenetic acetylation remains weak. BACE1 messenger RNA levels are significantly increased in 3×Tg mouse brains and in peripheral blood mononuclear cells from patients with AD but are much less elevated in mild cognitive impairment (MCI) compared with control subjects, and this increase is linked to H3 acetylation facilitating the accessibility of the BACE1 promoter (19). Decreasing acetylated H3 in the BACE1 promoter regions by galangin treatment in SH-SY5Y cells reduces the BACE1 messenger RNA level, likely related to upregulated endogenous HDAC1-mediated deacetylation (20).
Inhibition of histone acetyltransferase p300 by curcumin can also decrease acetylated H3 to reduce BACE1 transcripts (21). On the other hand, royal jelly peptides appear to regulate BACE1 expression through the control of HDAC1 (21). BACE1 messenger RNA levels are also regulated by diverse factors, including different transcription factors, that are summarized elsewhere (22,23).
Regulated BACE1 Expression by microRNA
Recently, the role of noncoding RNA, in particular microRNA, in regulating BACE1 has been gaining traction. Noncoding RNA, often referred to as microRNA, is 19 to 22 nucleotides long and often regulates gene and protein expression at the posttranscriptional level by binding to 3’ untranslated region RNA to form a silencing complex. Over the past 2 decades, additional noncoding RNA has been shown to negatively regulate BACE1 expression: miR-107, miR-29c, miR-339–5p, miR-186, miR-195, miR-135b, miR-135a, miR-124, and miR298/328 (see Table 1 for more details) (24–28).
Table 1.
Regulation of BACE1 Levels by miRNAs
| miRNA | Mechanism of Action | Relevance in AD Brain | Reference(s) |
|---|---|---|---|
| miR-107 | Downregulates BACE1 mRNA levels by binding to its 3’ UTR. Other targets downregulated by miR-107 include granulin, cofilin, CDK5R1, and ADAM10. | Decreased miR-107 levels correlated with increased BACE1 levels in temporal cortex | (135,136) |
| miR-29c | Targets the 3’ UTR of BACE1. Overexpression of miR-29c in cells reduced BACE1 protein expression and Aβ accumulation. | Decreased miR-29c expression levels correlated with increased BACE1 levels in sporadic AD | (137,138) |
| miR-186 | Suppresses BACE1 expression by targeting the 3’ UTR of BACE1 mRNA in primary neuronal cells. Inhibition of miR-186 increased BACE1 protein levels and Aβ levels in neuro-2a cells. | Gradual reduction in miR-186 levels in 13month-old mouse cortices during aging | (139) |
| miR-195 | Levels inversely correlated with the protein level of BACE1 in SAMP8 mice. miR-195 overexpression in N2a/WT cells decreased the BACE1 protein and Aβ levels. | Downregulated in human AD CSF samples | (140) |
| miR-124 | Targets BACE1 by binding to 3’ UTR. miR-124 mimetic dramatically downregulated BACE1 mRNA and protein, while inhibition of miR-124 significantly increased the expression in SH-SY5Y cells. | Expression significantly reduced in the hippocampus and anterior temporal cortex in AD brain | (141) |
| miR-298 and −328 | Recognize specific binding sites in the 3’ UTR of BACE1 mRNA and regulate BACE1 protein expression in N2a neuronal cells. | (142) |
Aβ, amyloid-β; AD, Alzheimer’s disease; BACE1, beta-site amyloid precursor protein cleaving enzyme 1; CSF, cerebrospinal fluid; miRNA, microRNA; mRNA, messenger RNA; SAMP8, senescence-accelerated prone mice; UTR, untranslated region; WT, wild-type.
The emerging field of AD transcriptomic signatures, including noncoding RNA that may be involved in negatively regulating BACE1 expression, can offer a promising platform for developing biomarkers. However, diagnostic and therapeutic applications of microRNAs remain challenging owing to multiple reasons, including restricted brain penetration and high-specificity concerns.
Post-translational Regulation of BACE1
BACE1 activity is regulated not only at the expression level but also by posttranslational modification. During the course of AD, BACE1 is subjected to numerous posttranslational modifications and plays a role in regulating signaling associated with Aβ production and AD. BACE1 is a typical aspartyl protease with 2 active aspartate motifs (D93TG and D289SG) located in each lobe. BACE1 is first synthesized in the endoplasmic reticulum as a 501-amino-acid immature precursor protein, proBACE1. During maturation, BACE1 is N-glycosylated at 4 Asn sites (Asn153, Asn172, Asn223, and Asn354) in the endoplasmic reticulum lumen (4), and its prodomain (residues 1–21) is removed by furin-like proprotein convertases in the endoplasmic reticulum/early Golgi compartment (3,4). Although the presence of prodomain is not sufficient to suppress BACE1 activity (16), unlike most aspartyl proteases, suppressing glycosylation by site-directed mutagenesis of these aspartic acid residues reduces the protease activity of BACE1 (29). BACE1 is also reported to undergo sugar modifications by bisecting N-acetylglucosamine, which is high in brains. In brains of patients with AD, higher N-acetylglucosamine activity may partially cause increased BACE1 activity and Aβ deposition (30).
The role of sumoylation in regulating BACE1 functionality/stability and activity is reported in both in vitro and in vivo AD mouse models; it was shown that sumoylation of residue K501 on BACE1 enhances its stability and Aβ-producing activity (31). By contrast, overexpression of nonsumoylated BACE1 mutant negated memory decline in wild-type mice and did not accelerate senile plaque formation (31).
Posttranslational modifications of BACE1 have been shown to alter its trafficking and/or localization (32). For example, palmitoylation of BACE1 at 4 cysteine residues (Cys474, Cys478, Cys482, and Cys485) in the transmembrane and C-terminal domains targets BACE1 to cholesterol-rich lipid rafts (32).
BACE1 undergoes phosphorylation at both Ser498 and Thr252, but phosphorylation at the Ser498 residue appears to regulate intracellular trafficking by shuttling/recycling BACE1 between endosome and plasma membrane (33). This modification at Ser498 has minimal or no effect on Aβ levels (34). Others noted that phosphorylation at Thr252 by p25/Cdk5 was associated with increased BACE1 activity and Aβ production (35). Similarly, acetylation of BACE1 has also been identified at 7 lysine resides (36).
While acetylation imparts BACE1 protein stability (36), ubiquitination of BACE1 promotes its degradation in the lysosome and is impaired in AD (37,38).
Overall, as an aspartyl protease, BACE1 requires low acidic environments to reach optimal proteolytic activity, ideally at ~pH 4.5 (39,40).
BACE1 PHYSIOLOGICAL FUNCTIONS AND PATHOPHYSIOLOGICAL IMPLICATIONS
Amyloidogenesis
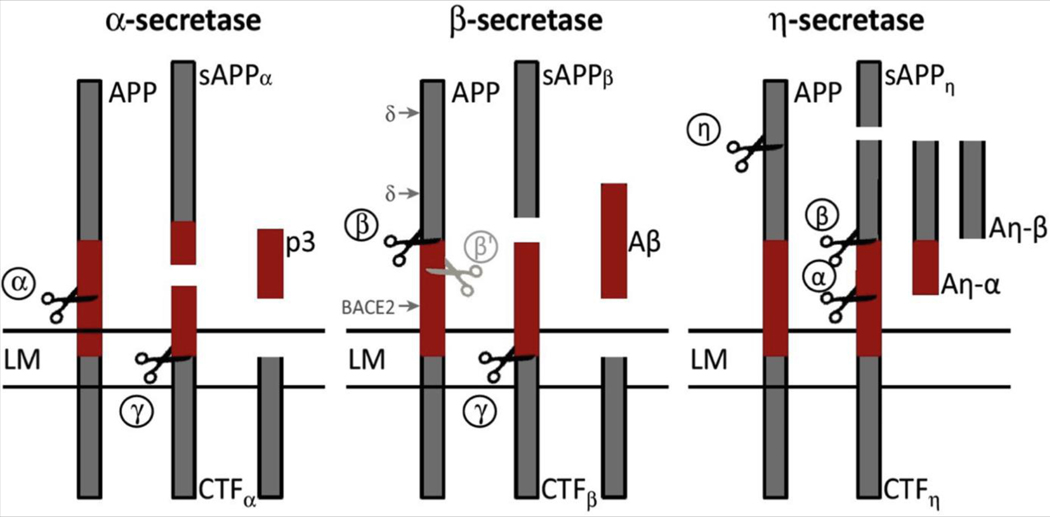
BACE1 is the β-secretase enzyme that cleaves the transmembrane APP and, together with γ-secretase, generates Aβ species that in AD form increasingly large and conformationally complex soluble regionally deposited brain aggregates (see Figure 1). BACE1 cleavage of APP represents the rate-limiting step for Aβ production.
Figure 1.
Schematic representation of amyloid precursor protein (APP) processing pathways. Aβ, amyloid-β; Aη-α, amyloid-η-α; Aη-β, amyloid-η-β; BACE2, beta-site amyloid precursor protein cleaving enzyme 2; CTF, C-terminal fragment; LM, lipid membrane; p3, p3 fragment of the amyloid precursor protein; sAPP, soluble amyloid precursor protein. [Adapted with permission from Barão et al. (143).]
For this reason, BACE1 has been extensively studied in the context of brain amyloidogenesis and proven to be directly involved in Aβ production based on data from several knockout (KO) mouse models (41–43). BACE1 has been pharmacologically targeted, with several inhibiting compounds entering clinical development and trials, effectively lowering Aβ concentrations in human individuals.
Cleavage of APP by β-secretase liberates the soluble N-terminus of APP, while the C-terminal fragment (CTF-β or C99) remains bound to the membrane. Two mutations at the β-secretase cleavage site (the Swedish mutation KM/NL and an Italian variant A673V) were reported to be linked to familial AD and consequently raise the sAPPβ level owing to strongly increased affinity of BACE1 for the changed recognition motif in APP (44).
The significant protective effect of the A673T variant against AD has provided a robust proof of principle for the pathophysiological and pharmacological model that reducing the β-cleavage of APP may offer a resilient mechanism against the disease (12). In addition, preliminary evidence suggests that a longtime preventive reduction of BACE1 activity by 20% to 30% may be sufficient to prevent AD. However, to fully exploit the clinical and pharmacological implications of the A673T mutation, it is essential to understand whether A673T mutation Aβ aggregates display different toxicity rates compared with wild-type carriers and which molecular pathways underlie the finding that the A673T allele also protects against non-AD cognitive decline (12).
High BACE1 enzymatic activity was found in human AD brain extracts, which is consistent with the finding that neurons produce the highest levels of Aβ (3,45). The highest BACE1 protein level was reported in postnatal brain in mouse. Notably, a relatively large accumulation of BACE1 was described in neuritic dystrophies in the vicinity of Aβ plaques both in AD amyloidogenic mouse models and in AD brains, most likely by a posttranslational mechanism (2,5,6). Inducing autophagy in mutant human neurons augments retention of BACE1 in distal axons by autophagy, leading to enhanced β-cleavage of APP (46). To produce Aβ, the fragment CTF-β is cleaved by β-secretase, which finally releases Aβ into the extracellular space and releases the APP intracellular domain into the cytoplasm (2,5,6).
In a parallel competing nonamyloidogenic pathway, APP is cleaved by either α-secretase or η-secretase to release 2 additional variants of the APP ectodomain, namely sAPP-α and sAPP-η (47). The η-secretase pathway is used as an alternative when BACE1 is inhibited, with the consequence of increased Aη-α activity with an effect on lowering neuronal activity by a so far unknown mechanism (47).
APP is a type I transmembrane protein and is highly expressed in neurons and abundant at the synapse (48–52). Its function remains elusive, although studies implicated it in maintenance of dendritic spines (53), neurotransmission (54), synaptic plasticity (55–58), and maintenance of excitation/inhibition balance (58). Soluble APP is a GABAB (gammaaminobutyric acid type B) ligand that modulates synaptic transmission (50). Rescue experiments in APP KO mice show that sAPPα is sufficient to restore defects in spine density (59), long-term potentiation, and spatial learning (60,61). Most of the ectodomain shedding of APP is performed by the α-secretase, which cleaves APP in the Aβ sequence and therefore is believed to protect against AD (47).
Although some evidence suggests that sAPPβ seems much less active in in vitro assays of neural activity and plasticity than sAPPα (62), both sAPPα and sAPPβ modulate basal synaptic transmission and short-term synaptic facilitation through binding to GABAB receptor subunit 1a at the synapse (50). The sushi domains of GABAB receptor subunit 1a are also able to bind full-length APP intracellularly (63). Interestingly, this interaction is crucial for axonal trafficking of the complex and affects presence of the receptor at the presynaptic terminals. Concomitantly, delivery of the complex to axonal cell surface diminishes the pool of APP available for BACE1 processing in endosomes and lowers Aβ production (63).
Synaptic Substrates
Initial evaluation of BACE1 KO mice focused on decreased production of Aβ shortly after BACE1 deficiency was revealed to be associated with subtle neurological deficits (43,64). Many of the BACE1 KO mouse phenotypes, such as the peripheral hypomyelination and synaptic deficits, are due to loss of function of substrates depending on the activation by BACE1. Lack of BACE1 was also reported to cause sensorimotor impairments, seizures, schizophrenia-like phenotypes, and retinal pathology (65–67). In 2012, the neuronal secretome of BACE1 was revealed by 2 independent studies in primary cultures (68,69), followed by a more complete repertoire of BACE1 substrates identified in mouse cerebrospinal fluid (CSF) (see Table 2). Among BACE1 substrates, NRG1 (neuregulin 1), SEZ6 (seizure-related protein 6), and CHL1 (close homologue of neural cell adhesion molecule L1) are known to have important neuronal functions and merit further discussion given the recent reports that BACE1 blockade in patients causes cognitive worsening.
Table 2.
BACE1 Substrates and Their Physiological Roles
| BACE1 Substrate | Physiological Role |
|---|---|
| APP | Regulates neurite outgrowth, synapse formation, and synaptic plasticity; also regulates metal homeostasis |
| APLP1 | Regulates neurotransmission and plasticity in CNS synapses |
| APLP2 | Regulates synaptic function and plasticity in CNS |
| Contactin 2 | Regulates axon guidance, cell adhesion, and neurite outgrowth |
| Jagged 1 | Balances astrogenesis and neurogenesis; notch signaling influences neural plasticity, long-term memory, and synapse remodeling transmitter release through astrocytes |
| CHL1 | Regulates axon guidance, cell adhesion, neuronal migration, and neurite outgrowth |
| Neurexin 1α and 3β | Regulates synapse assembly and maintenance |
| NRG1 | Regulates myelination, neuronal migration, and oligodendrocyte differentiation; also regulates synaptic transmission and plasticity via neurotransmitter receptors |
| SEZ6 | Regulates dendritic arborization and affects excitatory synapse development and maintenance and formation of neuronal circuits |
| SEZ6L | Regulates synapse maturation, tumor suppressor function, and free cholesterol levels |
| β (β1–4) Auxiliary Subunits of the VGSC Subtype Nav1 | Modulates cell surface expression of Nav1 sodium channels and thus controls excitability and propagation of action potentials in the neuronal membrane |
| VGSC Accessory Subunits KCNE1 and KCNE2 | Regulates cardiac and brain potassium channel subunit trafficking and maintenance of membrane excitability |
APLP1/2, amyloid-like protein 1/2; APP, amyloid precursor protein; CHL1, neural cell adhesion molecule L1; CNS, central nervous system; NRG1, neuregulin 1; SEZ6, seizure-related protein 6; SEZ6L, seizure-related protein 6 precursor protein; VEGFR1, vascular endothelial growth factor receptor 1; VGSC, voltage-gated sodium channels. [Adapted with permission from Das and Yan (144).]
NRG1 interacts with the epidermal growth factor receptor family of receptors to exert signaling cascades crucial for central nervous system development (70) and synaptic plasticity (70). BACE1 cleavage of NRG1 is essential for myelination in the central nervous system and peripheral nerves as well as for muscle spindle formation and maintenance (70). SEZ6 is important for dendritic branching, normal synaptic function, and motor coordination (1,71). In the mouse brain, soluble SEZ6 is almost exclusively produced by BACE1 (1,71). In its absence, achieved by genetic KO of SEZ6 or pharmacological inhibition of BACE1, synaptic plasticity is impaired. In particular, aberrant BACE1 processing of SEZ6 results in lower spine density and attenuated long-term potentiation in the hippocampus (1,71).
One of the most interesting substrates of BACE1 is CHL1. This cell adhesion molecule mediates axonal guidance in response to Sema3A (semaphorin 3A) (72,73). BACE1 cleavage yields an intracellular membrane-bound C-terminal fragment of CHL1 that is able to influence cytoskeleton dynamics, leading to growth cone collapse on presentation of the Sema3A cue. This particular substrate and/or its homologue L1CAM (L1 cell adhesion molecule), both cleaved by BACE1 (69,72–74), might be responsible for axonal organization defects in BACE1 KO mice (69,72–74). Intriguingly, axon guidance abnormalities in the hippocampus persist in the adult conditional KO of BACE1, confirming an important role of the secretase in adult circuitry architecture as well as its established developmental functions (69,72–74).
BACE2 Physiological Functions: A Brief Update
Much less is known about the brain-relevant substrates and functions of the sister secretase BACE2 that is more prominently expressed in the colon, kidney, and pancreas (7). In pancreatic β cells, the proproliferative plasma membrane protein Tmem27 and islet amyloid polypeptide are proposed BACE2 substrates (75,76). BACE2 also processes the PMEL (pigment cell–specific melanocyte protein) in pigment cells (9). Pharmacological inhibition of BACE2 results in depigmentation, the most consistent side effect seen in preclinical studies of BACE1/BACE2 inhibition (9). Thus, BACE1 and BACE2 shedding events seem to be tissue, cell type, and context dependent, revealing the intricacy of their functions (7). Inhibition of BACE2 brain substrates might contribute to some of the side effects seen with BACE1 inhibitors.
Human postmortem studies showed high expression levels of BACE2 and strong correlation with BACE1 expression in neurons and astrocytes of patients with AD but not of control subjects (77). Huentelman et al. reported that different single nucleotide polymorphism variations at the BACE2 locus are associated with AD risk and altered Aβ processing (78). This was the first genetic evidence of a role for BACE2 in AD pathophysiology. Larger genome-wide association studies are needed to confirm such important findings that may have significant pharmacological implications.
BACE1 BIOMARKERS: STATE OF THE ART ON THE VALIDATION AND QUALIFICATION FOR DRUG–BIOMARKER CODEVELOPMENT PIPELINES
During the past 15 years, a few human in vivo studies reported good diagnostic performance of CSF BACE1 concentration (supposed to reflect gene expression levels) and activity in discriminating among patients with AD dementia, patients with MCI, and cognitively healthy individuals (79–85). Some studies also showed an association between BACE1 biomarkers and other core CSF and neuroimaging biomarkers of AD as well as the presence of apolipoprotein E (APOE) ε4 allele (79–85). Significant predictive power regarding conversion from MCI to AD dementia has also been reported.
Regarding the blood matrix, BACE1 biomarkers have been investigated in both plasma (82,86,87) and platelets (88,89), displaying good correspondence with CSF and association with brain AD alterations.
A multicenter study reported good correspondence between CSF and plasma BACE1 concentrations (87). In particular, plasma BACE1 activity demonstrated good diagnostic performance in discriminating patients with AD dementia from patients with MCI and cognitively normal individuals (87). A recent study showed an association between plasma BACE1 concentrations and amyloid-positron emission tomography quantitative measures in a cohort of cognitively healthy individuals at risk for AD (86).
By contrast, some studies showed no acceptable diagnostic performance of BACE1 biomarkers (90–92) or no association between them and AD established biomarkers.
Moreover, lower levels of BACE1 were found in advanced dementia stages of AD, potentially owing to advanced neuronal and synaptic loss (93–95).
For more details about study populations and outcomes, see Supplemental Table S1.
Interstudy results variability may be partially explained by several differences in the study design, confounding factors (e.g., disease stage, sex, APOE genotype, comorbidities), and the methodology. Regarding the latter, preanalytical factors such as the sample collection, processing, and storage protocols, as well as analytical factors such as assays used, are likely the most relevant determinants. The conflicting data reported above call for harmonization and standardization of research protocols.
In summary, there are enough promising data to boost development of BACE1 biomarkers and investigate whether they may enrich the current AD biomarkers panel and potentially support different contexts of use in BACE1 clinical trials, including target engagement and proof of mechanism, dose finding, efficacy, and safety monitoring.
HUMAN CLINICAL TRIALS WITH BACE INHIBITORS: A SCHEMATIC OVERVIEW
All BACE inhibitors investigated in randomized clinical trials were discontinued for either futility or safety reasons.
A Phase 3 trial of verubecestat conducted in mild to moderate patients with AD (EPOCH) was terminated owing to futility (96).
A Phase 2/3 trial of atabecestat investigated in preclinical individuals with AD (EARLY) was discontinued owing to liver toxicity (97).
The Phase 3 trials of lanabecestat investigated in patients with prodromal AD and mild AD (AMARANTH and DAYBREAKALZ, respectively) were stopped owing to futility (98).
A Phase 2 trial of LY3202626 involving patients with mild AD (NAVIGATE-AD) was discontinued owing to interim futility analysis (99).
The phase 2/3 trials of umibecestat (CNP520) investigating asymptomatic individuals at risk for AD (i.e., APOE ε4 allele carriers) (GENERATION) were discontinued owing to cognitive worsening in the active treatment group (100).
The Phase 2 trial of elenbecestat (E2609), conducted in participants with MCI to moderate AD, was discontinued after recommendation by the Data Safety Monitoring Board owing to an unfavorable risk/benefit ratio (https://www.alzforum.org/therapeutics/elenbecestat).
See the Supplement for more details.
Potential Explanation Coming From Translational Data: Selectivity and Toxicity of BACE1 Inhibitors
To optimize next-generation BACE1 inhibitor clinical trials, it is essential to understand all major biological and pharmacological factors that might account for the high attrition rates of the previous trials. For this purpose, 3 points should be considered: 1) the biological rationale for BACE1 as a pharmacological target; 2) BACE 1/2 selectivity, druggability, and inhibition strength by dose adjustment as well as the overall benefit/risk ratio; and 3) timing of intervention with BACE1 inhibitors over the course of AD.
Regarding point 1, in consideration of all evidence reported in the sections above, we argue that the target has a good scientific rationale and was properly and extensively validated in experimental models of AD ahead of clinical studies. Regarding point 2, there are more than 40 known BACE1 substrates (see Table 2), and BACE inhibitors may block one or more of them, causing functional consequences. Current compounds tested in the clinic had variable amounts of selectivity for BACE1, but all exerted inhibition of BACE2 activity as well. Inhibiting BACE1 lowers Aβ production, but in combination with BACE2 inhibition the processing of a number of other substrates are blocked with potential negative impact, changing the benefit/risk ratio (see also Supplemental Table S2).
Provided these data have not been systematically disclosed during early development of these compounds, it is arguable that all compounds in clinical development have been tested in standard good laboratory practice/good clinical practice toxicity studies as requested and reviewed by regulatory authorities. Therefore, it is conceivable that these preclinical tests did not demonstrate systematic biological signatures indicating the observed effects. Dose levels selected for all prior and current ongoing studies may be too high, targeting more than 50% inhibition of BACE1, leading to unwanted side effects, while potentially lower levels of inhibition could have been therapeutically active.
In this regard, it is not possible to rule out that an excessive suppression of BACE1 activity has determined cognitive dysfunction in patients with AD by depleting Aβ monomers that have physiological functions and display poor toxicity. Aβ monomers can trigger or sustain intracellular signaling essential for synaptic plasticity and homeostasis (101–103).
Concerning point 3 above, timing of intervention with BACE1 inhibitors over the course of AD, robust evidence indicates that cerebral Aβ accumulation is one of the earliest mechanistic alterations of the whole pathophysiological dynamic of AD (104–107).
The stronger correlation found between tau biomarkers with neurodegeneration outcome measures and long-term cognitive scores than between Aβ markers and long-term cognitive scores has raised the question of whether Aβ pathophysiology triggers downstream pathways, including tau-mediated toxicity, and facilitates tau spreading (104–107). However, CSF and positron emission tomography longitudinal studies support the hypothetical pathophysiological model of AD for which amyloidosis proceeds, either promoting or being permissive to the spreading of tau pathology that is likely to drive disease clinical evolution (104–107). Such spatial and temporal dynamics of AD brain proteinopathies imply that Aβ-directed treatments should be initiated at the earliest preclinical stages of the disease and not in the dementia stages. If so, BACE1 inhibitors started prior to the spreading of tau pathology may represent the most suitable path to pursue (108).
Some detrimental effects were induced during the initial phase of treatment and were irrespective of disease stage. It is conceivable that these negative effects may be caused by acute synaptic impairment via BACE1 inhibition for some substrates other than APP and should be assessed for reversibility after off-treatment.
New Potential BACE Inhibition Strategies: Drug Repositioning Programs and Modulation of Posttranslational Modification
A pursuable path for BACE1 inhibition may be represented by drug repurposing (also called drug repositioning or reprofiling) pipelines that aim at identifying new therapeutic avenues for already approved or investigational drugs irrespective of their original medical indication. Two recent animal trials used chronic exposure to lithium chloride–a therapeutic agent approved for major psychiatric disorders–and showed slowdown of cognitive decline and histopathological alterations associated with reduced BACE1 activity (109,110).
In particular, Wilson et al. reported that an innovative experimental formulation of lithium microdose release is associated with the lowering of BACE1 gene expression and overall cerebral Aβ accumulation (110), thereby confirming previous translational studies pointing at a potential neuroprotective effect of lithium (111).
Modulation of posttranslational modification of BACE1 may represent a viable therapeutic avenue. For instance, it was shown that AD mouse models expressing S-palmitoylation-deficient BACE1 had a significant decrease in Aβ burden and improved memory function, indicating that posttranslational S-palmitoylation of BACE1 influences Aβ pathogenesis (32). In line with this, HEK293 cells treated with KMI-574 specifically caused dissociation of BACE1 from lipid raft to nonraft membranes, and BACE1 processing activity was reduced (112). Hence, blocking BACE1 activity in the raft membrane is another venue for reducing Aβ deposition.
OPEN ISSUES
Several genetic data studies (306 autosomal dominant mutations plus the APP gene duplication and trisomy 21) and multimodal biomarker studies indicate that an imbalance between Aβ production and clearance plays a critical and early role in AD pathogenesis (104–106,113,114). A large amount of evidence also supports the hypothesis that cerebral Aβ deposition begins decades before AD clinical onset and prior to cortical spreading of tau pathology. However, the full understanding of the molecular dynamics of Aβ species, from loss of proteostasis to synaptic toxicity (either tau mediated or not), has not yet been achieved. In this context, BACE1 is established to play a key role in Aβ homeostasis and may have an important function in synaptic plasticity.
Incomplete knowledge of the physiological functions of BACE and its downstream pathways may have contributed to the failures of BACE1 inhibitor clinical trials.
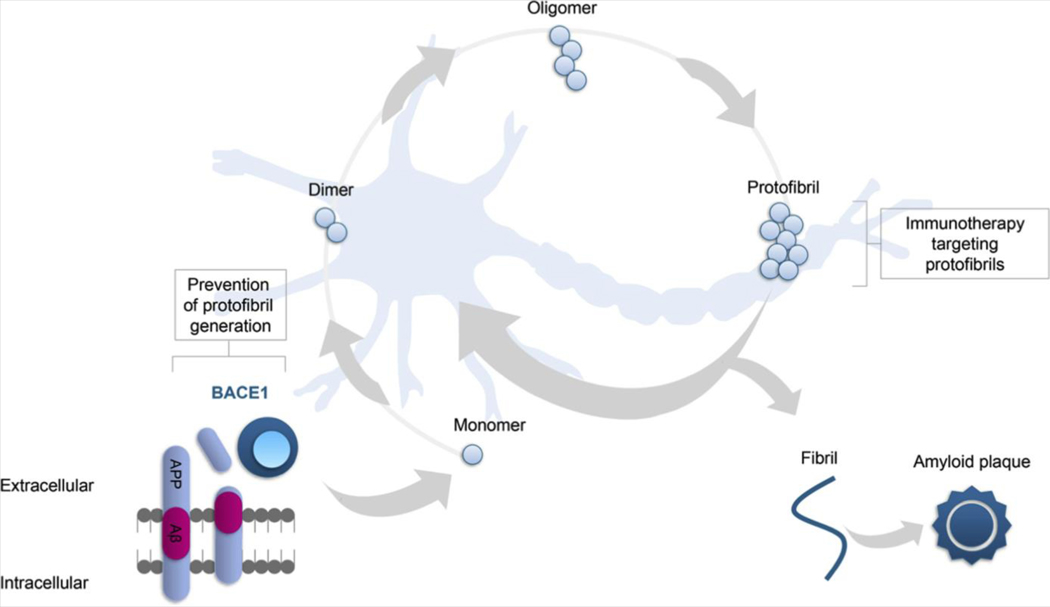
Soluble Aβ peptides, oligomers, protofibrils, fibrils, and plaques still remain attractive targets (see Figure 2).
Figure 2.
BACE1 (beta-site amyloid precursor protein cleaving enzyme 1) and the amyloid-β (Aβ) cycle. Despite the fact that several clinical trials investigating anti-Aβ compounds did not reach primary end points, Aβ peptides, oligomers, protofibrils, and plaques still remain attractive targets. Of note, the nature of the toxic Aβ species remains unclear. Evidence suggests that, besides fibrils, dimeric or oligomeric Aβ species, but not monomeric Aβ peptides, cause neuronal hyperactivity and downstream toxicity in the vicinity of Aβ plaques.
The recently discovered human APP Arctic (115) and E693Δ (Osaka) (116) mutations show a type of AD with low cerebral deposition of plaques, as indicated by modest Aβ-positron emission tomography radiotracer binding (117) and higher production of oligomers and protofibrils that are likely to be the initiators of Aβ toxicity (115,116,118,119). These findings indicated that other forms of Aβ, besides fibrils and plaques, may trigger brain toxicity and contribute to AD-synaptic failure (115,116,118,119). Such evidence has fostered the development of novel biomarkers for tracking all Aβ aggregation states that may be used for novel surrogate end points.
Time for Biomarkers of Synaptic Dysfunctions?
From a functional standpoint, synaptogenic mechanisms of AD cognitive decline–that is, network activation and deactivation deficits, abnormal oscillatory rhythmic activity, and network hypersynchrony–account for AD-related synaptic failure (120–122).
Clinical trials can benefit from resting-state and task-related functional magnetic resonance studies to detect aberrant patterns at the large-scale brain network level, including the default mode network (123–126). Brain networks’ functional shifts, as well as their association with molecular dynamics, have already been described in aging and AD (127,128). Very recently, it was shown that genetic risk factors with a pleiotropic biological effect, such as the APOE ε4 allele, affect the trajectories of the default mode network of cognitively healthy individuals at risk for AD (129,130).
Fluid biomarkers of synaptic dysfunction are currently under development (131–133). Neurogranin (a key regulator of the calcium-binding protein calmodulin), synaptogamin (a calcium sensor protein), and SNAP-25 (a component of the SNARE [soluble N-ethylmaleimide sensitive factor attachment protein receptor] complex) are the strongest candidates for in vivo tracking synaptic homeostasis (131–133).
CONCLUSIONS
Despite a number of robust discovery stage studies, a number of phase 3 small-molecule BACE1 inhibitor clinical trials did not reach primary end points, showed cognitive worsening, or were discontinued owing to safety reasons.
The failure of several BACE1 inhibitor clinical trials appears to involve insufficient understanding of BACE1 biology and physiology, limited knowledge of the natural history of AD and the optimal stage of disease at which to treat, and lack of biomarker-based outcomes and end points. In this regard, BACE1 trial failures may benefit from the previous pitfalls of γ-secretase pharmacological investigation (134).
The field needs to fully uncover the physiological functions of BACE1 substrates, including those involved in including synaptic homeostasis, and needs to better understand the physiological role(s) of BACE2.
During the past 20 years, several key genetic, epigenetic, and posttranslational factors have been established to influence BACE1 gene expression levels and enzymatic activity that may explain interindividual heterogeneity in BACE1-related pathophysiological processes and drug response. While the research community continues to debate the most plausible biological and pharmacological explanations for BACE1 clinical trial failures, there is emerging evidence encouraging a new generation of compounds with an ultra APP selectivity BACE inhibitory effect.
Robust evidence indicates that BACE1 concentrations and the rate of activity assessed in body fluids, including plasma, may serve as multiple contexts of use (including trial enrollment, proof of mechanism, response and toxicity dose estimation, and drug resistance prediction) in drug biomarker codevelopment programs (see Supplemental Figure S1).
Within this conceptual framework, BACE1-oriented therapies continue to represent a rational and central development area for time-sensitive and effective pathway (mechanism)– based preventive strategies for AD.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This research benefited from the support of the “Phoenix” program led by the Sorbonne University Foundation and sponsored by la Fondation pour la Recherche sur Alzheimer. During part of this work, HH was supported by the AXA Research Fund, the Fondation partenariale Sorbonne Université, and the Fondation pour la Recherche sur Alzheimer. The research leading to these results has received funding from the “Investissements d’avenir” program (Grant No. ANR-10-IAIHU-06) (Agence Nationale de la Recherche10-IA Agence Institut Hospitalo-Universitaire-6). RN is supported by Fondazione Turano.
HH is an employee of Eisai Inc. HH serves as senior associate editor for the journal Alzheimer’s & Dementia. He received lecture fees from Servier, Biogen, and Roche; research grants from Pfizer, Avid, and MSD Avenir (paid to the institution); travel funding from Functional Neuromodulation, Axovant, Eli Lilly, Takeda and Zinfandel, GE Healthcare, and Oryzon Genomics; and consultancy fees from Qynapse, Jung Diagnostics, Cytox, Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation. He participated on scientific advisory boards of Functional Neuromodulation, Axovant, Eisai, Eli Lilly, Cytox, GE Healthcare, Takeda and Zinfandel, Oryzon Genomics, and Roche Diagnostics. HH is coinventor of the following patents as a scientific expert and has received no royalties: “In vitro multiparameter determination method for the diagnosis and early diagnosis of neurodegenerative disorders” (Patent No. 8916388), “In vitro procedure for diagnosis and early diagnosis of neurodegenerative diseases” (Patent No. 8298784), “Neurodegenerative markers for psychiatric conditions” (Publication No. 20120196300), “In vitro multiparameter determination method for the diagnosis and early diagnosis of neurodegenerative disorders” (Publication No. 20100062463), “In vitro method for the diagnosis and early diagnosis of neurodegenerative disorders” (Publication No. 20100035286), “In vitro procedure for diagnosis and early diagnosis of neurodegenerative diseases” (Publication No. 20090263822), “In vitro method for the diagnosis of neurodegenerative diseases” (Patent No. 7547553), “CSF Diagnostic in vitro method for diagnosis of dementias and neuroinflammatory diseases” (Publication No. 20080206797), “In vitro method for the diagnosis of neurodegenerative diseases” (Publication No. 20080199966), and “Neurodegenerative markers for psychiatric conditions” (Publication No. 20080131921). SL received lecture honoraria from Servier and Roche. AV is an employee of Eisai Inc; he received lecture honoraria from Servier, Roche, and MagQu. MT holds shares in Johnson & Johnson. AATM, MI, BA, AK, LY, and LK are employees of Eisai Inc. NW and TK are employees of Eisai Company Ltd. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2020.02.001.
REFERENCES
- 1.Zhu K, Xiang X, Filser S, Marinkovic P, Dorostkar MM, Crux S, et al. (2018): Beta-site amyloid precursor protein cleaving enzyme 1 inhibition impairs synaptic plasticity via seizure protein 6. Biol Psychiatry 83:428–437. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, et al. (2007): Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: Implications for Alzheimer’s disease pathogenesis. J Neurosci 27:3639–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. (1999): Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741. [DOI] [PubMed] [Google Scholar]
- 4.Haniu M, Denis P, Young Y, Mendiaz EA, Fuller J, Hui JO, et al. (2000): Characterization of Alzheimer’s beta-secretase protein BACE: A pepsin family member with unusual properties. J Biol Chem 275:21099–21106. [DOI] [PubMed] [Google Scholar]
- 5.Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R (2013): The Alzheimer’s beta-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol 126:329–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadleir KR, Kandalepas PC, Buggia-Prevot V, Nicholson DA, Thinakaran G, Vassar R (2016): Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer’s disease. Acta Neuropathol 132:235–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voytyuk I, Mueller SA, Herber J, Snellinx A, Moechars D, van Loo G, et al. (2018): BACE2 distribution in major brain cell types and identification of novel substrates. Life Sci Alliance 1:e201800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassar R, Kuhn P-H, Haass C, Kennedy ME, Rajendran L, Wong PC, Lichtenthaler SF (2014): Function, therapeutic potential and cell biology of BACE proteases: Current status and future prospects. J Neurochem 130:4–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, et al. (2013): BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci U S A 110:10658–10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esterhazy D, Stutzer I, Wang H, Rechsteiner MP, Beauchamp J, Dobeli H, et al. (2011): Bace2 is a beta cell-enriched protease that regulates pancreatic beta cell function and mass. Cell Metab 14:365–377. [DOI] [PubMed] [Google Scholar]
- 11.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L (1992): A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1:345–347. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. (2012): A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488:96–99. [DOI] [PubMed] [Google Scholar]
- 13.Martiskainen H, Herukka S-K, Stancakova A, Paananen J, Soininen H, Kuusisto J, et al. (2017): Decreased plasma β-amyloid in the Alzheimer’s disease APP A673T variant carriers. Ann Neurol 82:128–132. [DOI] [PubMed] [Google Scholar]
- 14.Gasparoni G, Bultmann S, Lutsik P, Kraus TFJ, Sordon S, Vlcek J, et al. (2018): DNA methylation analysis on purified neurons and glia dissects age and Alzheimer’s disease-specific changes in the human cortex. Epigenetics Chromatin 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holler M, Westin G, Jiricny J, Schaffner W (1988): Sp1 transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev 2:1127–1135. [DOI] [PubMed] [Google Scholar]
- 16.Hussain I, Christie G, Schneider K, Moore S, Dingwall C (2001): Prodomain processing of Asp1 (BACE2) is autocatalytic. J Biol Chem 276:23322–23328. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Marshall L, Oh G, Jakubowski JL, Groot D, He Y, et al. (2019): Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun 10:2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West RL, Lee JM, Maroun LE (1995): Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 6:141–146. [DOI] [PubMed] [Google Scholar]
- 19.Marques SCF, Lemos R, Ferreiro E, Martins M, de Mendonca A, Santana I, et al. (2012): Epigenetic regulation of BACE1 in Alzheimer’s disease patients and in transgenic mice. Neuroscience 220:256–266. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H, Huang P, Wang X, Wu J, Wu M, Huang J (2015): Galangin-induced down-regulation of BACE1 by epigenetic mechanisms in SH-SY5Y cells. Neuroscience 294:172–181. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Deng Y, Yu D, Cao H, Wang L, Liu L, et al. (2014): Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer’s disease. PLoS One 9: e103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossner S, Sastre M, Bourne K, Lichtenthaler SF (2006): Transcriptional and translational regulation of BACE1 expression—Implications for Alzheimer’s disease. Prog Neurobiol 79:95–111. [DOI] [PubMed] [Google Scholar]
- 23.Tamagno E, Guglielmotto M, Monteleone D, Vercelli A, Tabaton M (2012): Transcriptional and post-transcriptional regulation of b-secretase. IUBMB Life 64:943–950. [DOI] [PubMed] [Google Scholar]
- 24.Alexandrov PN, Dua P, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ (2012): MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int J Biochem Mol Biol 3:365–373. [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. (2008): Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/ beta-secretase expression. Proc Natl Acad Sci U S A 105:6415–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian Q, Zhang J, He F-P, Bao W-X, Zheng T-T, Zhou D-M, et al. (2019): Down-regulated expression of microRNA-338–5p contributes to neuropathology in Alzheimer’s disease. FASEB J 33:4404–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W-X, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. (2008): The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci 28:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long JM, Ray B, Lahiri DK (2014): MicroRNA-339–5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem 289:5184–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlwood J, Dingwall C, Matico R, Hussain I, Johanson K, Moore S, et al. (2001): Characterization of the glycosylation profiles of Alzheimer’s beta-secretase protein Asp-2 expressed in a variety of cell lines. J Biol Chem 276:16739–16748. [DOI] [PubMed] [Google Scholar]
- 30.Kizuka Y, Kitazume S, Fujinawa R, Saito T, Iwata N, Saido TC, et al. (2015): An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer’s disease. EMBO Mol Med 7:175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao J, Qin M, Mahaman YAR, Zhang B, Huang F, Zeng K, et al. (2018): BACE1 SUMOylation increases its stability and escalates the protease activity in Alzheimer’s disease. Proc Natl Acad Sci U S A 115:3954–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vetrivel KS, Meckler X, Chen Y, Nguyen PD, Seidah NG, Vassar R, et al. (2009): Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem 284:3793–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, et al. (2001): Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem 276:14634–14641. [DOI] [PubMed] [Google Scholar]
- 34.Pastorino L, Ikin AF, Nairn AC, Pursnani A, Buxbaum JD (2002): The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total Ab. Mol Cell Neurosci 19:175–185. [DOI] [PubMed] [Google Scholar]
- 35.Song W-J, Son M-Y, Lee H-W, Seo H, Kim JH, Chung S-H (2015): Enhancement of BACE1 activity by p25/Cdk5-mediated phosphorylation in Alzheimer’s disease. PLoS One 10:e136950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costantini C, Ko MH, Jonas MC, Puglielli L (2007): A reversible form of lysine acetylation in the ER and Golgi lumen controls the molecular stabilization of BACE1. Biochem J 407:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang EL, Cameron AN, Piazza F, Walker KR, Tesco G (2010): Ubiquitin regulates GGA3-mediated degradation of BACE1. J Biol Chem 285:24108–24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang EL, Biscaro B, Piazza F, Tesco G (2012): BACE1 protein endocytosis and trafficking are differentially regulated by ubiquitination at lysine 501 and the Di-leucine motif in the carboxyl terminus. J Biol Chem 287:42867–42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasselli AG, Qahwash I, Emmons TL, Lu Y, Leone JW, Lull JM, et al. (2003): Employing a superior BACE1 cleavage sequence to probe cellular APP processing. J Neurochem 84:1006–1017. [DOI] [PubMed] [Google Scholar]
- 40.Turner RT 3rd, Loy JA, Nguyen C, Devasamudram T, Ghosh AK, Koelsch G, Tang J (2002): Specificity of memapsin 1 and its implications on the design of memapsin 2 (beta-secretase) inhibitor selectivity. Biochemistry 41:8742–8746. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. (2001): Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci 4:231–232. [DOI] [PubMed] [Google Scholar]
- 42.Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, et al. (2005): Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280:30797–30806. [DOI] [PubMed] [Google Scholar]
- 43.Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, et al. (2003): BACE1 (beta-secretase) transgenic and knockout mice: Identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci 24:646–655. [DOI] [PubMed] [Google Scholar]
- 44.Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, et al. (2009): A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science 323:1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, et al. (1992): Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359:322–325. [DOI] [PubMed] [Google Scholar]
- 46.Feng T, Tammineni P, Agrawal C, Jeong YY, Cai Q (2017): Autophagy-mediated regulation of BACE1 protein trafficking and degradation. J Biol Chem 292:1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, et al. (2015): η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TW, Wu K, Xu JL, McAuliffe G, Tanzi RE, Wasco W, Black IB (1995): Selective localization of amyloid precursor-like protein 1 in the cerebral cortex postsynaptic density. Brain Res Mol Brain Res 32:36–44. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues DI, Gutierres J, Pliassova A, Oliveira CR, Cunha RA, Agostinho P (2014): Synaptic and sub-synaptic localization of amyloid-β protein precursor in the rat hippocampus. J Alzheimers Dis 40:981–992. [DOI] [PubMed] [Google Scholar]
- 50.Rice HC, de Malmazet D, Schreurs A, Frere S, Van Molle I, Volkov AN, et al. (2019): Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science 363: eaao4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoe H-S, Fu Z, Makarova A, Lee J-Y, Lu C, Feng L, et al. (2009): The effects of amyloid precursor protein on postsynaptic composition and activity. J Biol Chem 284:8495–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schafer C, Rammner B, et al. (2014): Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344:1023–1028. [DOI] [PubMed] [Google Scholar]
- 53.Jung CKE, Herms J (2012): Role of APP for dendritic spine formation and stability. Exp Brain Res 217:463–470. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Wang Z, Sun L, Yang L, Li H, Cole AL, et al. (2014): The amyloid precursor protein controls adult hippocampal neurogenesis through GABAergic interneurons. J Neurosci 34:13314–13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, et al. (1999): Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 90:1–13. [DOI] [PubMed] [Google Scholar]
- 56.Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, et al. (1999): Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38:349–359. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, Wang Z, Wang B, Justice NJ, Zheng H (2009): Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J Neurosci 29:15660–15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Born HA, Kim J-Y, Savjani RR, Das P, Dabaghian YA, Guo Q, et al. (2014): Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzeimer’s disease. J Neurosci 34:3826–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weyer SW, Zagrebelsky M, Herrmann U, Hick M, Ganss L, Gobbert J, et al. (2014): Comparative analysis of single and combined APP/ APLP knockouts reveals reduced spine density in APP-KO mice that is prevented by APPsa expression. Acta Neuropathol Commun 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, et al. (2007): The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27:7817–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hick M, Herrmann U, Weyer SW, Mallm J-P, Tschape J-A, Borgers M, et al. (2015): Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol 129:21–37. [DOI] [PubMed] [Google Scholar]
- 62.Turner PR, O’Connor K, Tate WP, Abraham WC (2003): Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70:1–32. [DOI] [PubMed] [Google Scholar]
- 63.Dinamarca MC, Raveh A, Schneider A, Fritzius T, Fruh S, Rem PD, et al. (2019): Complex formation of APP with GABAB receptors links axonal trafficking to amyloidogenic processing. Nat Commun 10:1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, et al. (2001): BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet 10:1317–1324. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, et al. (2008): BACE1 gene deletion: Impact on behavioral function in a model of Alzheimer’s disease. Neurobiol Aging 29:861–873. [DOI] [PubMed] [Google Scholar]
- 66.Savonenko AV, Melnikova T, Laird FM, Stewart K-A, Price DL, Wong PC (2008): Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A 105:5585–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai J, Qi X, Kociok N, Skosyrski S, Emilio A, Ruan Q, et al. (2012): bSecretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med 4:980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhn P-H, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. (2012): Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J 31:3157–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou L, Barao S, Laga M, Bockstael K, Borgers M, Gijsen H, et al. (2012): The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem 287:25927–25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, et al. (2006): Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314:664–666. [DOI] [PubMed] [Google Scholar]
- 71.Pigoni M, Wanngren J, Kuhn P-H, Munro KM, Gunnersen JM, Takeshima H, et al. (2016): Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol Neurodegener 11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ou-Yang M-H, Kurz JE, Nomura T, Popovic J, Rajapaksha TW, Dong H, et al. (2018): Axonal organization defects in the hippocampus of adult conditional BACE1 knockout mice. Sci Transl Med 10: eaao5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright AG, Demyanenko GP, Powell A, Schachner M, Enriquez-Barreto L, Tran TS, et al. (2007): Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J Neurosci 27:13667–13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vassar R (2019): Editorial: Implications for BACE1 inhibitor clinical trials: Adult conditional BACE1 knockout mice exhibit axonal organization defects in the hippocampus. J Prev Alzheimers Dis 6:78–84. [DOI] [PubMed] [Google Scholar]
- 75.Rulifson IC, Cao P, Miao L, Kopecky D, Huang L, White RD, et al. (2016): Identification of human islet amyloid polypeptide as a BACE2 substrate. PLoS One 11:e147254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcarraz-Vizan G, Castano C, Visa M, Montane J, Servitja J-M, Novials A (2017): BACE2 suppression promotes β-cell survival and function in a model of type 2 diabetes induced by human islet amyloid polypeptide overexpression. Cell Mol Life Sci 74:2827–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holler CJ, Webb RL, Laux AL, Beckett TL, Niedowicz DM, Ahmed RR, et al. (2012): BACE2 expression increases in human neurodegenerative disease. Am J Pathol 180:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huentelman M, De Both M, Jepsen W, Piras IS, Talboom JS, Willeman M, et al. (2019): Common BACE2 polymorphisms are associated with altered risk for Alzheimer’s disease and CSF amyloid biomarkers in APOE ε4 non-carriers. Sci Rep 9:9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mulder SD, van der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, et al. (2010): BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis 20:253–260. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Z, Ewers M, Teipel S, Burger K, Wallin A, Blennow K, et al. (2007): Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry 64:718–726. [DOI] [PubMed] [Google Scholar]
- 81.Ewers M, Zhong Z, Burger K, Wallin A, Blennow K, Teipel SJ, et al. (2008): Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain 131:1252–1258. [DOI] [PubMed] [Google Scholar]
- 82.Wu G, Sankaranarayanan S, Wong J, Tugusheva K, Michener MS, Shi X, et al. (2012): Characterization of plasma b-secretase (BACE1) activity and soluble amyloid precursor proteins as potential biomarkers for Alzheimer’s disease. J Neurosci Res 90:2247–2258. [DOI] [PubMed] [Google Scholar]
- 83.Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. (2008): Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 65:1102–1107. [DOI] [PubMed] [Google Scholar]
- 84.Ewers M, Cheng X, Zhong Z, Nural HF, Walsh C, Meindl T, et al. (2011): Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer’s disease. J Alzheimers Dis 25:373–381. [DOI] [PubMed] [Google Scholar]
- 85.Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, et al. (2018): Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol 136:821–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vergallo A, Houot M, Cavedo E, Lemercier P, Vanmechelen E, De Vos A, et al. (2019): Brain Ab load association and sexual dimorphism of plasma BACE1 concentrations in cognitively normal individuals at risk for AD. Alzheimers Dement 15:1274–1285. [DOI] [PubMed] [Google Scholar]
- 87.Shen Y, Wang H, Sun Q, Yao H, Keegan AP, Mullan M, et al. (2018): Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biol Psychiatry 83:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGuinness B, Fuchs M, Barrett SL, Passmore AP, Johnston JA (2016): Platelet membrane β-secretase activity in mild cognitive impairment and conversion to dementia: A longitudinal study. J Alzheimers Dis 49:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bermejo-Bescos P, Martin-Aragon S, Jimenez-Aliaga K, Benedi J, Felici E, Gil P, et al. (2013): Processing of the platelet amyloid precursor protein in the mild cognitive impairment (MCI). Neurochem Res 38:1415–1423. [DOI] [PubMed] [Google Scholar]
- 90.Savage MJ, Holder DJ, Wu G, Kaplow J, Siuciak JA, Potter WZ (2015): Soluble BACE-1 activity and sAβPPβ concentrations in Alzheimer’s disease and age-matched healthy control cerebrospinal fluid from the Alzheimer’s Disease Neuroimaging Initiative-1 baseline cohort. J Alzheimers Dis 46:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perneczky R, Alexopoulos P (2014): Cerebrospinal fluid BACE1 activity and markers of amyloid precursor protein metabolism and axonal degeneration in Alzheimer’s disease. Alzheimers Dement 10:S425–S429.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu G, Sankaranarayanan S, Tugusheva K, Kahana J, Seabrook G, Sh X-P, et al. (2008): Decrease in age-adjusted cerebrospinal fluid beta-secretase activity in Alzheimer’s subjects. Clin Biochem 41:986–996. [DOI] [PubMed] [Google Scholar]
- 93.Alexopoulos P, Thierjung N, Grimmer T, Ortner M, Economou P, Assimakopoulos K, et al. (2018): Cerebrospinal fluid BACE1 activity and sAβPPβ as biomarker candidates of Alzheimer’s disease. Dement Geriatr Cogn Disord 45:152–161. [DOI] [PubMed] [Google Scholar]
- 94.Rosen C, Andreasson U, Mattsson N, Marcusson J, Minthon L, Andreasen N, et al. (2012): Cerebrospinal fluid profiles of amyloid β-related biomarkers in Alzheimer’s disease. Neuromolecular Med 14:65–73. [DOI] [PubMed] [Google Scholar]
- 95.Decourt B, Sabbagh MN (2011): BACE1 as a potential biomarker for Alzheimer’s disease. J Alzheimers Dis 24(suppl 2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, et al. (2018): Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G (2019): Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N Engl J Med 380:1483–1485. [DOI] [PubMed] [Google Scholar]
- 98.Strobel G (2019): BACE inhibitors: Postmortem on one, live updates on two. AlzForum (2019). https://www.alzforum.org/news/conferencecoverage/bace-inhibitors-postmortem-one-live-updates-two. [Google Scholar]
- 99.Albert C Lo, Duggan Evans C, Mancini M, Lin Q, Wang H, Liu P, et al. (2018): Results from the phase 2 NAVIGATE-AD clinical trial evaluating LY3202626 BACE inhibitor in patients with mild Alzheimer’s disease dementia. CTAD (Clinical Trials on Alzheimer’s Disease) 2018, Abstract LB1, S37. [Google Scholar]
- 100.NIA Office of Communications and Public Liaison (2019): Statement on discontinuation of BACE 1 inhibitor CNP520 in the Alzheimer’s Prevention Initiative Generation Study 1. National Institute on Aging, July 12. [Google Scholar]
- 101.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I (2009): Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12:1567–1576. [DOI] [PubMed] [Google Scholar]
- 102.Zimbone S, Monaco I, Giani F, Pandini G, Copani AG, Giuffrida ML, Rizzarelli E (2018): Amyloid beta monomers regulate cyclic adenosine monophosphate response element binding protein functions by activating type-1 insulin-like growth factor receptors in neuronal cells. Aging Cell 17:12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caruso G, Caraci F, Jolivet RB (2019): Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog Neurobiol 175:35–53. [DOI] [PubMed] [Google Scholar]
- 104.Jack CRJ, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. (2018): NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. (2019): Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study [published online ahead of print Jun 3]. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jack CR, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, et al. (2019): The bivariate distribution of amyloid-b and tau: Relationship with established neurocognitive clinical syndromes. Brain 142:3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jack CRJ, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. (2013): Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu K, Peters F, Filser S, Herms J (2018): Consequences of pharmacological BACE inhibition on synaptic structure and function. Biol Psychiatry 84:478–487. [DOI] [PubMed] [Google Scholar]
- 109.Nunes MA, Schowe NM, Monteiro-Silva KC, Baraldi-Tornisielo T, Souza SIG, Balthazar J, et al. (2015): Chronic nicrodose lithium treatment prevented memory loss and neurohistopathological changes in a transgenic mouse model of Alzheimer’s disease. PLoS One 10:e142267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson EN, Do Carmo S, Iulita MF, Hall H, Ducatenzeiler A, Marks AR, et al. (2017): BACE1 inhibition by microdose lithium formulation NP03 rescues memory loss and early stage amyloid neuropathology. Transl Psychiatry 7:e1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hampel H, Lista S, Mango D, Nistico R, Perry G, Avila J, et al. (2019): Lithium as a treatment for Alzheimer’s disease: The systems pharmacology perspective. J Alzheimers Dis 69:615–629. [DOI] [PubMed] [Google Scholar]
- 112.Ebina M, Futai E, Tanabe C, Sasagawa N, Kiso Y, Ishiura S (2009): Inhibition by KMI-574 leads to dislocalization of BACE1 from lipid rafts. J Neurosci Res 87:360–368. [DOI] [PubMed] [Google Scholar]
- 113.Karran E, Mercken M, De Strooper B (2011): The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov 10:698–712. [DOI] [PubMed] [Google Scholar]
- 114.Hardy JA, Higgins GA (1992): Alzheimer’s disease: The amyloid cascade hypothesis. Science 256:184–185. [DOI] [PubMed] [Google Scholar]
- 115.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, et al. (2001): The “Arctic” APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci 4:887–893. [DOI] [PubMed] [Google Scholar]
- 116.Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, et al. (2008): A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol 63:377–387. [DOI] [PubMed] [Google Scholar]
- 117.Scholl M, Wall A, Thordardottir S, Ferreira D, Bogdanovic N, Langstrom B, et al. (2012): Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology 79:229–236. [DOI] [PubMed] [Google Scholar]
- 118.O’Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE, et al. (2010): Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci 30:14411–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. (2008): Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Selkoe DJ (2002): Alzheimer’s disease is a synaptic failure. Science 298:789–791. [DOI] [PubMed] [Google Scholar]
- 121.Palop JJ, Mucke L (2016): Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 17:777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. (2007): Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seeley WW (2011): Divergent network connectivity changes in healthy APOE ε4 carriers: Disinhibition or compensation? Arch Neurol 68:1107–1108. [DOI] [PubMed] [Google Scholar]
- 124.Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT (2011): Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ε4 carriers: Relationships with memory performance. J Neurosci 31:7775–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koch W, Teipel S, Mueller S, Buerger K, Bokde ALW, Hampel H, et al. (2010): Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter? NeuroImage 51:280–287. [DOI] [PubMed] [Google Scholar]
- 126.Bassett DS, Sporns O (2017): Network neuroscience. Nat Neurosci 20:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hampel H, Lista S, Neri C, Vergallo A (2019): Time for the systemslevel integration of aging: Resilience enhancing strategies to prevent Alzheimer’s disease. Prog Neurobiol 181:101662. [DOI] [PubMed] [Google Scholar]
- 128.Franzmeier N, Duzel E, Jessen F, Buerger K, Levin J, Duering M, et al. (2018): Left frontal hub connectivity delays cognitive impairment in autosomal-dominant and sporadic Alzheimer’s disease. Brain 141:1186–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiesa PA, Cavedo E, Vergallo A, Lista S, Potier M-C, Habert M-O, et al. (2019): Differential default mode network trajectories in asymptomatic individuals at risk for Alzheimer’s disease. Alzheimers Dement 15:940–950. [DOI] [PubMed] [Google Scholar]
- 130.Chiesa PA, Houot M, Vergallo A, Cavedo E, Lista S, Potier MC, et al. (2019): Association of brain network dynamics with plasma biomarkers in subjective memory complainers [published online ahead of print Dec 23]. Neurobiol Aging. [DOI] [PubMed] [Google Scholar]
- 131.Ohrfelt A, Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, et al. (2016): The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. (2010): Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res 1362:13–22. [DOI] [PubMed] [Google Scholar]
- 133.Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, Fagan AM (2018): Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement 14:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Strooper B (2014): Lessons from a failed γ-secretase Alzheimer trial. Cell 159:721–726. [DOI] [PubMed] [Google Scholar]
- 135.Nelson PT, Wang W-X (2010): MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J Alzheimers Dis 21:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Augustin R, Endres K, Reinhardt S, Kuhn P-H, Lichtenthaler SF, Hansen J, et al. (2012): Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med Genet 13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lei X, Lei L, Zhang Z, Zhang Z, Cheng Y (2015): Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer’s disease. Int J Clin Exp Pathol 8:1565–1574. [PMC free article] [PubMed] [Google Scholar]
- 138.Zong Y, Wang H, Dong W, Quan X, Zhu H, Xu Y, et al. (2011): miR-29c regulates BACE1 protein expression. Brain Res 1395:108–115. [DOI] [PubMed] [Google Scholar]
- 139.Kim J, Yoon H, Chung D-E, Brown JL, Belmonte KC, Kim J (2016): miR-186 is decreased in aged brain and suppresses BACE1 expression. J Neurochem 137:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu H-C, Wang L-M, Wang M, Song B, Tan S, Teng J-F, Duan D-X (2012): MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res Bull 88:596–601. [DOI] [PubMed] [Google Scholar]
- 141.Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, et al. (2012): The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209:94–105. [DOI] [PubMed] [Google Scholar]
- 142.Boissonneault V, Plante I, Rivest S, Provost P (2009): MicroRNA298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J Biol Chem 284:1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barão S, Moechars D, Lichtenthaler SF, De Strooper B (2016): BACE1 physiological functions may limit its use as therapeutic target for Alzheimer’s disease. Trends Neurosci 39:158–169. [DOI] [PubMed] [Google Scholar]
- 144.Das B, Yan R (2019): A close look at BACE1 inhibitors for Alzheimer’s disease treatment. CNS Drugs 33:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.