Highlights
-
•
There are few tools to facilitate shared decision-making after burn injury.
-
•
Mortality prognostication scores are thought to reflect long-term quality of life.
-
•
Higher rBaux and Ryan scores are negatively correlated with long-term physical health, but not mental health.
-
•
Burn size and mortality prognostication scores poorly explained the variance quality of life.
-
•
More accurate scores are needed to predict long-term quality of life
Keywords: Burn, Quality of life, Patient reported outcome measures
Abstract
Objective
Despite improved mortality rates after burn injury, many patients face significant long-term physical and psychosocial disabilities. We aimed to determine whether commonly used mortality prognostication scores predict long-term, health-related quality of life after burn injury. By doing so, we might add evidence to support goals of care discussions and facilitate shared decision-making efforts in the hours and days after a life-changing injury.
Methods
We used the multicenter National Institute of Disability, Independent Living and Rehabilitation Research Burn Model System database (1994–2019) to analyze SF-12 physical (PCS) and mental component (MCS) scores among survivors one year after major burn injury. Ninety percent of the observations were randomly assigned to a model development dataset. Multilevel, mixed-effects, linear regression models determined the relationship between revised Baux and Ryan Scores and SF-12 measures. Additionally, we tested a model with disaggregated independent and other covariates easily obtained around the time of index admission: age, sex, race, burn size, inhalation injury. Residuals from the remaining 10% of observations in the validation dataset were examined.
Results
The analysis included 1606 respondents (median age 42 years, IQR 28–53 years; 70% male). Median burn size was 16% TBSA (IQR 6–30) and 13% of respondents sustained inhalation injury. Higher revised Baux and Ryan Scores and age, burn size, and inhalation injury were significantly correlated with lower PCS, but were not correlated with MCS. Female sex, black race, burn size, and inhalation injury correlated with lower MCS. All models poorly explained the variance in SF-12 scores (adjusted r2 0.01–0.12).
Conclusion
Higher revised Baux and Ryan Scores negatively correlated with long-term physical health, but not mental health, after burn injury. Regardless, the models poorly explained the variance in SF-12 scores one year after injury. More accurate models are needed to predict long-term, health-related quality of life and support shared decision-making during acute burn care.
1. Introduction
Despite marked reductions in mortality after burn injury, burn survivors often face significant long-term physical and psychosocial disabilities that negatively affect their quality of life. [1] However, current prognostication scores emphasize in-hospital mortality (e.g., revised Baux and Ryan Scores) and have not been examined in relation to long-term, health-related quality of life [2,3]. As a result, the acute burn care, rehabilitation and palliative care communities lack tools that provide rapid estimates of long-term, health-related quality of life in the hours and days after burn injury to facilitate goals of care discussions and promote shared decision-making with patients and their families [[4], [5], [6]].
The revised Baux and Ryan scores use patient age, burn size and presence of inhalation injury to estimate in-hospital mortality. [7] These covariates are objectively measured, easy to obtain and immediately available in the hours and days after burn injury. Many reports have described the strong correlation between these scores and in-hospital mortality [3,[7], [8], [9]]. The simplicity of the revised Baux Score nomogram-based mortality estimation has made it the most widely used prognostication model for burn-related mortality in the world [3,5,7].
Although risk of in-hospital mortality is a critical point of discussion for patients with large injuries and/or numerous or advanced comorbidities, most patients and their families want to understand life might months to years after injury. Providing evidence-based expectations regarding long-term, health-related quality of life can be used to guide care plans, early goals of care discussions and shared decision-making that improve patient and family experiences. [10] Therefore, it is important that we develop support tools for these discussions and activities. Such tools have been used in the hours and days after other emergency conditions, such as non-burn injury, traumatic brain injury, respiratory failure, stroke and sepsis [[11], [12], [13], [14]]. Over the last two decades, reports have described long-term quality of life after burn injury derived from patient-reported outcome measures. [1,[15], [16], [17], [18], [19], [20], [21]] The findings from these reports are being used to help patients and their families understand how their burn injury will affect their health and function, satisfaction with life, community reintegration, and return to work or school [16]. However, to date no report has prognosticated long-term, health-related quality of life based on data available at the time of admission alone.
To address this gap, we aimed to determine whether common and immediately available mortality prognostication scores (i.e., revised Baux and Ryan Scores) predict long-term, health-related quality of life after burn injury. Additionally, we used disaggregated and other covariates known to affect quality of life after burn injury that would be readily available in the hours after injury to determine if these would improve model accuracy. By doing so, the findings might provide evidence for either expanded application of common mortality prognostication scores or a need to develop more accurate, but similarly practical, models.
2. Methods
2.1. Database
We used the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) Burn Injury Model System National Longitudinal Database (BMS) with records from 1994 – 2019. Funded BMS grantees collect information prospectively on survivors of burn injury, including demographics, clinical characteristics, and health outcomes using medical record review, interviews and self-report surveys at set intervals. The data are coordinated and managed by the BMS National Data and Statistical Center. The goals and ideology behind the BMS program have been previously described and an overview of the type of data available has been published. [16,18,22]
2.2. Sample
All records from patients ≥13 years collected by the BMS sites were included. Children younger than 13 years were excluded since they do not complete the quality of life measures used in the analysis (see Measures). Patients who were injured by self-immolation were not excluded. All participants met the BMS inclusion criteria based on at the time of their recruitment; the requirements and changes made to the recruitment criteria over time are detailed in Table 1 .
Table 1.
Summary of enrollment eligibility criteria for the Burn Model System (BMS) by year.
| 1994 – 2005 | 2006 – 2008 | 2009 – current |
|---|---|---|
|
2005 criteria with the below changes: Burn surgery for wound closure required TBSA requirements by age group were changed to >10% for those aged ≥65 years and ≥20% for those aged 18−64 years Participants were also required to receive primary treatment at a BMS center with reasonable expectation of follow-up treatment at a BMS center |
2006 criteria with the below changes: Exclusion of frostbite, toxic epidermal necrolysis, abrasions, necrotizing fasciitis, meningococcemia, and other non-burn skin diseases |
TBSA – total burn surface area, BMS - National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) Burn Injury Model System.
Baseline data were collected from consenting participants within 30 days of their discharge from their index hospitalization following their burn injury. Follow-up data were collected during specific follow-up windows: 6- (±2) months, 12- (±3) months, and 24- (±6) months after injury. Participants were considered lost to follow-up when phone calls, certified mailings, and clinic visits failed to result in data collection from the participant within the follow-up window.
2.3. Measures
Since BMS inception in 1994, the information and instruments used to collect data have evolved, resulting in some inconsistency in outcome measures collected at specific time points or sites. Demographic covariates utilized in this study included age in years, sex, and race/ethnicity. Ethnicity and race were combined to generate the categories of white non-Hispanic, Hispanic (white or nonwhite), Black non-Hispanic, and any other race (“other”). Clinical covariates used included burn size (percent total burn surface area burned, % TBSA) and presence of inhalation injury diagnosed with bronchoscopy or clinical syndrome per site-specific definitions (e.g., consistent history with or without facial or airway signs of injury, abnormal sputum production, hypoxemia). We did not include other covariates that have been associated with long-term outcomes since many of them require additional time, patient and family interviews, and diagnostic testing that may not be available during the initial 24−72 hours after admission. During early acute care for a major burn injury, use of time for interviews and non-clinically vital diagnostic tests are inappropriate.
The BMS National Database includes The Veterans RAND 12 Item Health Survey (VR-12) and 12-Item Short Form Health Survey (SF-12). We selected the SF-12 reported one year after injury and the nested physical (PCS) and mental component summary scores (MCS) as our outcome measures for several reasons:
-
i)
SF-12 is a widely validated, global measure of health-related quality of life;
-
ii)
SF-12 is the longest running patient reported outcome measure in the BMS database (i.e., largest number of participant responses);
-
iii)
VR-12 (collected 2013–2020) can be crosswalked to SF-12 for patients who responded to VR-12, but not SF-12 (collected 1998–2015); and
-
iv)
Many studies of long-term, health-related quality of life after injury or as a result of a medical condition have been reported, providing context to SF-12 scores.
The SF-12 is a health-related quality-of-life questionnaire that consists of twelve items that measure eight domains to assess physical and mental health. Physical health-related domains include general health, physical functioning, role, and pain. Mental health-related domains include vitality, social functioning, role, and mental health. The instrument has been validated across many condition. The VR-12 consists of items that measure eight principal domains including general health perceptions, physical functioning, role limitations due to physical and emotional problems, bodily pain, energy-fatigue, social functioning and mental health. VR-12 was developed from Veterans RAND 36 Item Health Survey (VR-36). VR-36 was developed from Medical Outcomes Study (MOS) RAND SF-36. The Veterans versions of the survey were designed to be comparable to the MOS version, but with improved survey characteristics. Therefore, the scores are readily crosswalked between one another.
2.4. Data analysis
Participant characteristics were described. Differences in proportions and medians between groups were examined with Chi squared and Mann-Whitney U tests, respectively. The BMS National Database was randomly divided into 90% of observations to develop the model and perform regression diagnostics and 10% of observations to test the model (i.e., plot observed and predicted physical and mental health component scores). The respondents were assigned random values between 0 and 1. Observations with a random value below 0.9 were assigned to comprise the development dataset. Observations with a random value greater than or equal to 0.9 were allocated to the validation dataset.
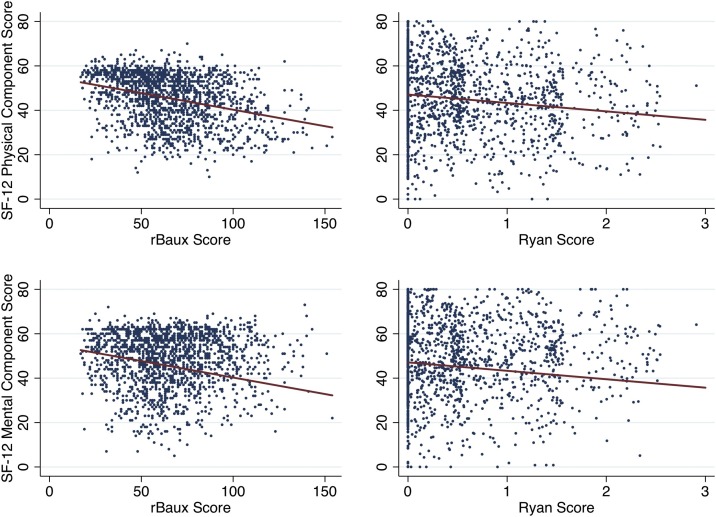
The analyses below were performed within the development dataset. We first examined scatter plots between independent covariates (i.e., model 1 – revised Baux Score, model 2 – Ryan Score, model 3 – age, sex, race, burn size, inhalation injury) and SF-12 outcomes (i.e., PCS and MCS, dependent covariates). These revealed loose linear relationships (Fig. 1 ). The common covariate model included the variables of age at time of burn injury, sex and race given that they are immediately available at index admission and have consistently been shown to impact recovery and long-term, health-related quality of life after burn injury. [1,23] We used multi-level (i.e., BMS site, participant), mixed-effects, generalized linear regression to determine the relationship between our independent and dependent covariates as outlined above and account for known and unknown differences that might exist between the BMS sites.
Fig. 1.
Linear regressions of revised Baux and Ryan Scores and SF-12 physical (PCS) and mental health (MCS) component scores at 1-year.
rBaux Score - revised Baux Score = age in years + % total burn surface area + 17, if inhalation injury; Ryan Score = sum of risk factors (age>60 years, >40% total burn surface area, inhalation injury); SF-12 – Short Form-12® Health Survey; these plots were generated the development dataset (i.e., random 90% of observations)
We examined each covariate to determine if the data met the assumptions required for linear regression (e.g., normality of distribution, influence, homoscedasticity, non-multicolinearity, normality of residuals, linearity). The covariates were examined with histograms, kernel density, symmetry, normal quantile and probability plots to assess normality. The covariates were normally distributed and did not require transformation. This was confirmed with Tukey’s ladder of powers, which suggested that each variable should be maintained in its current form. [24] There were a number of potential outliers on the plot that were confirmed by case-wise diagnostics; however, they represented true observations of prognostication scores, covariates and SF-12 outcomes. Therefore, we did not drop those participants from the models. Ultimately, these observations did not have significant influence on the regression coefficients as determined by DFBETA values for each covariate with limits above the absolute value of (number of covariates)/√(observations).
There were no residuals of any model ≥2 inter-quartile ranges above the third quartile or below the first quartile, providing evidence for normally distributed model residuals. There was weak evidence for heteroskedasticity among the variance of the residuals for the three models when White’s and Breusch-Pagan tests were applied. When the residual variances were plotted against the predicted values, the pattern widened only marginally at extremes of predicted values, indicating non-significant heterogeneity of variance. Regardless, robust standard errors were estimated. The tolerance for each independent variable (i.e., 1/variance inflation factor) was <0.1, which indicated low risk of multicolinearity. Augmented partial residual plots demonstrated relatively uniform patterns, with only minimal skewness at estimate extremes that was not improved with covariate transformations.
Lastly, we used the validation dataset (10% of observations) to predict PCS and MCS scores. The observed and predicted PCS and MCS scores were plotted. All analyses were performed with Stata v13.1 (Stata Corp., USA).
3. Results
3.1. Population and injury characteristics
Among the 4762 participants enrolled in the BMS database, 1606 responded to the SF-12 or VR-12 one year after injury (34% of possible respondents) (Table 2 ). The median age of respondents was 42 years [interquartile range (IQR) 25–53 years] and 1131 were male (70%). The majority of respondents identified as white race (1023, 64% of respondents), followed by Hispanic or Latino ethnicity (17%), black race (15%), and multiple or other races or ethnicities (4%). The median burn size of respondents was 16% total burn surface area (IQR 6–30%). Thirteen percent of respondents sustained inhalation injury. The median revised Baux Score of respondents was 63 (IQR 49–81). The percent of respondents who sustained each Ryan Score was: Ryan Score 0 - 63%, 1 - 30%, 2 - 8%, and 3 – 0.1%.
Table 2.
Characteristics of Burn Model System participants who either respondents or non-respondents to SF-12 physical (PCS) and mental (MCS) health component scores.
| Respondents |
Non-Respondents |
p-value | |||
|---|---|---|---|---|---|
| n = 1606 | % | n = 3066 | % | ||
| Age at injury (years) | |||||
| 13 - 18 | 125 | 7.8 | 453 | 14.8 | <0.01 |
| 19 - 39 | 610 | 37.9 | 1262 | 41.2 | |
| 40 - 64 | 720 | 44.8 | 1086 | 35.4 | |
| ≥65 | 151 | 9.5 | 265 | 8.6 | |
| Sex | |||||
| Male | 1131 | 70.4 | 2351 | 76.7 | <0.01 |
| Female | 475 | 29.6 | 715 | 23.3 | |
| Race | |||||
| White | 1023 | 64.2 | 1977 | 65.2 | 0.21 |
| Hispanic or Latino | 265 | 16.5 | 496 | 16.4 | |
| Black | 243 | 15.2 | 400 | 13.2 | |
| Multiple or other | 63 | 4.1 | 160 | 5.2 | |
| Injury | |||||
| % TBSA, median (IQR) | 16 | 6 - 30 | 14 | 6 - 28 | 0.02 |
| Inhalation injury | 213 | 13.4 | 373 | 12.3 | 0.28 |
| Injury severity | |||||
| Revised Baux score, median (IQR) | 63 | 49 - 81 | 58 | 42 - 75 | <0.01 |
| Ryan score | |||||
| 0 | 1004 | 62.5 | 2067 | 67.4 | 0.01 |
| 1 | 474 | 29.5 | 814 | 26.6 | |
| 2 | 127 | 7.9 | 179 | 5.8 | |
| 3 | 1 | 0.1 | 6 | 0.2 | |
Revised Baux Score = age in years + % total burn surface area + 17, if inhalation injury; Ryan Score = sum of risk factors (age>60 years, >40% total burn surface area, inhalation injury); SF-12 – Short Form-12® Health Survey; these plots were generated using the development dataset (i.e., random 90% of observations).
There were significant demographic and injury differences between participants enrolled in the BMS database who did not respond to the SF-12 one year after injury and those who did respond (Table 2). For example, non-respondents were slightly younger (median age 37 vs 42 years, p < 0.01), more often male (77% vs 70%, p < 0.01), had marginally smaller burn sizes (median % TBSA 14 vs 16, p = 0.02), and had lower revised Baux Scores (median score 58 vs 63, p < 0.01). The proportions of races and ethnicities (p = 0.21) and inhalation injury (0.28) were similar in both groups.
3.2. Regression models
Higher revised Baux Scores were significantly correlated with lower PCS scores one year after injury (coefficient -0.16, p < 0.01) (Fig. 1). However, the revised Baux Score accounted for little of the variance in PCS responses (r2 0.10) (Table 3 ). There was no evidence for a correlation between revised Baux Scores and MCS scores. Similarly, increasing Ryan Scores (1, 2, and 3) were strongly correlated with lower PCS scores at one year (-4.25, -8.91, and -13.58, respectively; p < 0.01). Only Ryan Score of 3 had a significant correlation with lower MCS scores (coefficient -2.84, p < 0.01). Lower Ryan Scores (i.e., 0–2) were not correlated with MCS scores. The Ryan Score model was even less able to explain the variance of PCS and MCS scores (r2 0.05 and 0.01, respectively).
Table 3.
Coefficients, robust standard errors and model characteristics from linear regression of prognostication scores and covariates with SF-12 physical (PCS) and mental (MCS) health component scores.
| Linear regression models |
|||||||
|---|---|---|---|---|---|---|---|
| Revised Baux Score |
Ryan Score |
Common covariates |
|||||
| Outcome | Independent covariates | Coefficient | rSE | Coefficient | rSE | Coefficient | rSE |
| SF-12 Physical Component Score one year after injury | Revised Baux Score | −0.16*** | 0.01 | ||||
| Ryan Score | |||||||
| 1 | −4.25*** | 0.93 | |||||
| 2 | −8.91*** | 1.19 | |||||
| 3 | −13.58*** | 0.35 | |||||
| Age at injury (years) | −0.16*** | 0.01 | |||||
| Sex | |||||||
| Female sex | −0.45 | 0.47 | |||||
| Race | |||||||
| Hispanic or Latino | −0.09 | 0.73 | |||||
| Black | −2.10 | 1.50 | |||||
| Mixed or other | −0.13 | 1.20 | |||||
| % TBSA | −0.14*** | 0.03 | |||||
| Inhalation injury | −3.88*** | 0.46 | |||||
| Constant | 56.55*** | 1.33 | 48.63*** | 1.53 | 53.26*** | 1.26 | |
| Random effects constant | 3.76*** | 0.93 | 3.29** | 1.25 | 2.38** | 0.98 | |
| Adjusted R2 | 0.10 | 0.05 | 0.12 | ||||
| Wald chi2 | 168.41*** | 47,409.15*** | 314.80*** | ||||
| SF-12 Mental Component Score one year after injury | Revised Baux Score | −0.01 | 0.01 | ||||
| Ryan Score | |||||||
| 1 | 0.74 | 0.45 | |||||
| 2 | −0.17 | 0.47 | |||||
| 3 | −2.84*** | 0.30 | |||||
| Age at injury (years) | 0.02 | 0.02 | |||||
| Sex | |||||||
| Female sex | −4.46*** | 0.45 | |||||
| Race | |||||||
| Hispanic or Latino | 2.01** | 0.88 | |||||
| Black | −3.13*** | 0.48 | |||||
| Mixed or other | −1.15 | 1.68 | |||||
| % TBSA | −0.03*** | 0.01 | |||||
| Inhalation injury | −2.17*** | 0.84 | |||||
| Constant | 48.85*** | 0.37 | 47.97*** | 0.38 | 47.56*** | 0.95 | |
| Random effects constant | 0.26** | 0.17 | 0.27** | 0.17 | 0.17** | 0.03 | |
| Adjusted R2 | 0.02 | 0.01 | 0.05 | ||||
| Wald chi2 | 3.52* | 1,000.05*** | 314.80*** | ||||
Revised Baux Score = age in years + % total burn surface area + 17, if inhalation injury; Ryan Score - sum of risk factors (age>60 years, >40% total burn surface area, inhalation injury); sex - male is referent; race - white is referent; TBSA - total burn surface area; site - regional burn center site contributing to the National Institute of Disability, Independent Living, and Rehabilitation Research (NIDILRR) Burn Model System (BMS) database; rSE - robust standard errors; *** p < 0.01, ** p < 0.05, * p < 0.1; estimates were generated with multi-level, mixed-effects, linear regression models using the development dataset (i.e., random 90% of observations).
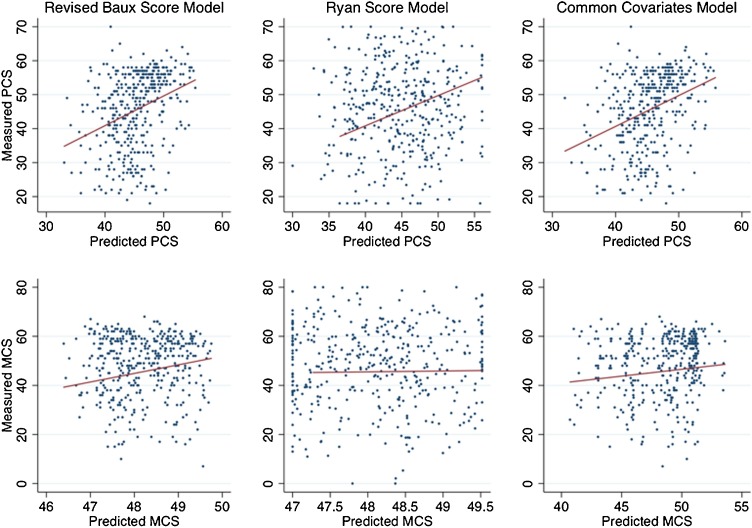
In the common covariates model (i.e., age, sex, race, burn size, inhalation injury), only covariates also contained within the revised Baux Score were significantly correlated with lower PCS scores (coefficients -0.16, -0.14, and -3.88, respectively; p < 0.01) (Table 3). Gender and race were not correlated with PCS scores. This model also poorly explained the variance in PCS scores (r2 0.12). Age was not correlated with MCS scores; however, female gender (coefficient -4.46, p < 0.01), Hispanic or Latino ethnicity (coefficient -2.01, p < 0.01), black race (coefficient -3.13, p < 0.01), larger burn size (coefficient -0.03, p < 0.01) and inhalation injury (coefficient -2.17, p < 0.01) were all significantly correlated lower MCS scores. Consistent with the other models, the covariates model poorly explained the variance in MCS scores. Fig. 2 depicts the predicted and observed SF-12 measures for each model and highlights the large residuals.
Fig. 2.
Observed and predicted SF-12 physical (PCS) and mental health (MCS) component scores at 1-year using revised Baux Score, Ryan Score and common covariates models.
Revised Baux Score = age in years + % total burn surface area + 17, if inhalation injury; Ryan Score = sum of risk factors (age >60 years, >40% total burn surface area, inhalation injury); common covariates model included: age in years, sex, race, % total burn surface area, and inhalation injury; SF-12 – Short Form-12® Health Survey; estimates were generated using the validation dataset (i.e., random 10% of observations).
4. Discussion
This study was performed to determine whether commonly used burn-related mortality prognostication scores and other immediately or easily available covariates can accurately predict quality of life after burn injury. The findings suggest that higher revised Baux and Ryan Scores are correlated with lower physical health, but not with mental health one year after injury. Additionally, the findings highlight the importance of including gender and race, and potentially other sociodemographic and injury-related covariates, into quality of life prediction models to more accurately estimate long-term psychosocial health. Lastly, currently used covariates immediately available at index admission are insufficient for accurately predicting long-term, health-related quality of life after burn injury. In order to support early goals of care discussions and shared decision making after burn injury, additional work must be done to identify easily obtainable covariates that predict quality of life more accurately (e.g., pre-injury health and function, socioeconomic status, number and severity of comorbidities, depth and distribution of burn injury).
We found that the mortality prognostication scores were correlated with physical health, but not mental health. It has been documented by several reports that burn size is a poor predictor of long-term outcomes, particularly those not directly related to physical complaints. [1,25,26] A report of 42 burn survivors with a median follow-up of 14 years described predictors of quality of life after injury [27]. The authors determined that non-injury and non-acute care factors were predictive of long-term psychosocial health, including body image, sexual function, strength of social support, and co-existing mental health disorders. There were no attempts to use covariates that would have been available at the time of index admission. Xie et al. reported that age at injury and facial burns were negatively correlated with MCS two years after injury among 20 burn survivors in China [28]. The authors also reported that current body image and temperature sensitivity were predictors of health-related quality of life. A systematic review of predictors of quality of life after injury reported injury severity, avoidance coping, lack of emotional and social support, higher levels of neuroticism, and unemployment after injury were all associated with low physical and mental health scores. [4] We observed that female sex and black race correlated with worse mental health, but not physical health. Similar findings have been demonstrated in polytrauma patients and patients with spinal cord injuries, suggesting that this is not a burn injury-specific phenomenon [27,29]. Given the lack of correlation between burn size and many long-term outcomes, professional burn care and survivorship societies suggest de-emphasizing the role of burn size during shared-decision making conversations. As example of these findings in current practice and during the COVID-19 pandemic response, the collaboration between the Organization for Delivery of Burn Care and Phoenix Society for Burn Survivors emphasize that burn size, although an important determinant of mortality, should not be a consideration in triage related to quality of life determinations, even during crisis standards of care [30]. Therefore, it is important for future models that aim to estimate long-term, health-related quality of life immediately after injury to consider predictors of physical and mental health in addition to burn size. However, caution should be exercised when using other non-modifiable or difficult-to-modify pre-injury factors that are associated with poor long-term, health-related quality of life (e.g., gender, race, ethnicity, socioeconomic status). Further, some pre-injury factors may be associated with structural, institutional and individual biases (e.g., comorbidities, substance use) that should prompt us to double-down on care and recovery efforts and not devalue patients’ potentials to experience good quality of life.
Our models purposefully included only covariates within widely used mortality prognostication models, as well as other immediately available covariates known to be correlated with long-term quality of life (e.g., sex, race and ethnicity). [1,18,31,32] However, all three models poorly explained the variance in PCS and MCS one year after injury. A number of other models have used additional covariates obtained from cross-sectional studies to predict long-term, health-related quality of life measures, including SF-12, to improve model accuracy (e.g., social support, predominant coping mechanism, concurrent psychiatric disorder, pain interference, work status, biomarkers) [4,18,33]. Although many of the covariates significantly influence quality of life, they may not be immediately available or often feasible to obtain during early acute care period, which limits their utility in models that aim to predict quality of life immediately after injury to support goals of care discussions and shared decision making. [4] However, a comprehensive model that may more accurately estimate recovery trajectory and outcomes to support expectation and goal setting at hospital discharge is also needed.
More must be done to identify covariates that: i) are easily obtainable from patients, their families, or the medical record early in acute burn care; ii) are highly correlated with long-term, health-related quality of life measures; and iii) thoroughly explain the variance of specific measures. Clear contenders for covariates that meet these criteria include pre-injury socioeconomic status, comorbidities or comorbidity index (e.g., Charlson or NCI Comorbidities Index), and functional and/or frailty status (e.g., Functional Independence Measure, Clinical Frailty Score, Short Musculoskeletal Function Assessment), as well as depth and distribution of burn (in addition to burn size). [4,[34], [35], [36]] Given the complexity and potentially complicated relationship between each of these covariates, newer analytic techniques may prove useful (e.g., computer vision, deep learning) [37,38]. A recent report of the use of machine learning in burn care and research returned 15 retrospective observation studies related to: burn diagnosis and healing (n = 5), antibacterial response in the burn wound (n = 4), hospital length of stay (n = 2), and mortality (n = 4). [39] Although algorithm performance was assessed differently in each report, all demonstrated improved performance of machine learning over traditional biostatistics methodologies. These techniques may be particularly useful for determining the impacts of depth and distribution of burn injury on long-term health and function. Subsequent work to develop models that predict long-term quality of life may consider using machine learning techniques to improve model accuracy.
Several limitations should be considered before interpreting these findings. First, a large proportion of participants recorded within the BMS database did not respond to SF-12 one year after injury. BMS uses a redundant and iterative process for communicating with participants to minimize losses to follow-up. Although most of the covariates used in the three models were statistically different between respondents and non-respondents, the differences may be clinically irrelevant (e.g., 70 vs 77% male, median TBSA 16 vs 14%, median revised Baux Score 63 vs 58). Regardless, some of the losses to follow-up are inevitable despite additional retention efforts, particularly among young people, transient and marginalized populations, people with substance abuse disorders and the uninsured. [40] Second, several recent reports have demonstrated that conventional burn-related mortality prognostication scores, including the revised Baux Score, may no longer be the most accurate predictors of mortality given advances in burn care systems and increasing prevalence of significant comorbidities [8,9]. However, these scores remain the most commonly used worldwide and remain easy to capture at the time of admission [3,7]. In light of these reports, we aimed to determine their relationship to long-term quality of life (i.e., not mortality) and provide additional evidence for either their expanded use or the need to identify more accurate, but similarly simple, models. Lastly, we did not prospectively collect data at the time of index admission regarding pre-injury function or comorbidities. These covariates significantly contribute to models that predict long-term, health-related quality of life [1,25,26,[41], [42], [43], [44]]. Future models certainly must consider these covariates if feasibly collected within hours or days of burn injury. Despite these limitations, the findings can be used to generate discussion on collection of specific covariates and developing models to predict long-term, health-related quality of life that rely on data available early during acute burn care to support goals of care discussions and efforts toward shared decision-making.
5. Conclusion
Burn care providers often extrapolate in-patient mortality prognostication scores to long-term quality of life after a major burn injury to facilitate goals of care discussions and shared decision-making with patients and their families. However, these data show that current mortality prognostication scores should not be used to estimate long-term health-related quality of life.
Therefore, we recommend de-emphasizing burn size and considering additional covariates feasibly collected at the time of index admission (e.g., pre-injury socioeconomic status, comorbidities or comorbidity index, functional and/or frailty status, depth and distribution of burn) when developing models of recovery and communicating with patients and families.
Funding statement
The contents of this report were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant #90DPBU0004, #90DPBU0001 and #90DPGE0004). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (DHHS). The contents of this report do not necessarily represent the policy of NIDILRR, ACL, DHHS, and do not assume endorsement by the Federal Government.
Declaration of interests
The authors have no real or potential conflicts of interests to disclose.
References
- 1.Chin T.L., Carrougher G.J., Amtmann D., McMullen K., Herndon D.N., Holavanahalli R. Trends 10 years after burn injury: a burn model system national database study. Burns. 2018;44:1882–1886. doi: 10.1016/j.burns.2018.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan C.M., Schoenfeld D.A., Thorpe W.P., Sheridan R.L., Cassem E.H., Tompkins R.G. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338:362–366. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 3.Osler T., Glance L.G., Hosmer D.W. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. J Trauma. 2010;68:690–697. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 4.Spronk I., Legemate C.M., Dokter J., van Loey N.E.E., van Baar M.E., Polinder S. Predictors of health-related quality of life after burn injuries: a systematic review. Crit Care. 2018;22:160. doi: 10.1186/s13054-018-2071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheckter C.C., Hung K.S., Rochlin D., Maan Z., Karanas Y., Curtin C. Trends and inpatient outcomes for palliative care services in major burn patients: a 10-year analysis of the nationwide inpatient sample. Burns. 2018;44:1903–1909. doi: 10.1016/j.burns.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell K., Maier R. Palliative care in the trauma ICU. Curr Opin Crit Care. 2016;22:584–590. doi: 10.1097/MCC.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 7.Halgas B., Bay C., Foster K. A comparison of injury scoring systems in predicting burn mortality. Ann Burns Fire Disasters. 2018;31:89–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Pompermaier L., Steinvall I., Elmasry M., Thorfinn J., Sjoberg F. Burned patients who die from causes other than the burn affect the model used to predict mortality: a national exploratory study. Burns. 2018;44:280–287. doi: 10.1016/j.burns.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Roberts G., Lloyd M., Parker M., Martin R., Philp B., Shelley O. The Baux score is dead. Long live the Baux score: a 27-year retrospective cohort study of mortality at a regional burns service. J Trauma Acute Care Surg. 2012;72:251–256. doi: 10.1097/TA.0b013e31824052bb. [DOI] [PubMed] [Google Scholar]
- 10.Asadi-Lari M., Tamburini M., Gray D. Patients’ needs, satisfaction, and health related quality of life: towards a comprehensive model. Health Qual Life Outcomes. 2004;2:32. doi: 10.1186/1477-7525-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grudzen C.R., Stone S.C., Morrison R.S. The palliative care model for emergency department patients with advanced illness. J Palliat Med. 2011;14:945–950. doi: 10.1089/jpm.2011.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steigleder T., Kollmar R., Ostgathe C. Palliative care for stroke patients and their families: barriers for implementation. Front Neurol. 2019;10:164. doi: 10.3389/fneur.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkman-Stoppelenburg A., Witkamp F.E., van Zuylen L., van der Rijt C.C.D., van der Heide A. Palliative care team consultation and quality of death and dying in a university hospital: a secondary analysis of a prospective study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turgeon A.F., Lauzier F., Zarychanski R., Fergusson D.A., Leger C., McIntyre L.A. Prognostication in critically ill patients with severe traumatic brain injury: the TBI-Prognosis multicentre feasibility study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amtmann D., Bocell F.D., Bamer A., Heinemann A.W., Hoffman J.M., Juengst S.B. Psychometric properties of the satisfaction with life scale in people with traumatic brain, spinal cord, or burn injury: a national institute on disability, independent living, and rehabilitation research model system study. Assessment. 2019;26:695–705. doi: 10.1177/1073191117693921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amtmann D., McMullen K., Bamer A., Fauerbach J.A., Gibran N.S., Herndon D. National institute on disability, independent living, and rehabilitation research burn model system: review of program and database. Arch Phys Med Rehabil. 2017 doi: 10.1016/j.apmr.2017.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeter L., Seaton M., Carrougher G.J., McMullen K., Mandell S.P., Amtmann D. Hospital-acquired complications alter quality of life in adult burn survivors: report from a burn model system. Burns. 2018 doi: 10.1016/j.burns.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Goverman J., Mathews K., Holavanahalli R.K., Vardanian A., Herndon D.N., Meyer W.J. The national institute on disability, independent living, and rehabilitation research burn model system: twenty years of contributions to clinical service and research. J Burn Care Res. 2017;38 doi: 10.1097/BCR.0000000000000361. e240-e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holavanahalli R.K., Schneider J.C., Miller A.C. Introduction to the NIDILRR burn model system (BMS) program: selected findings II. Arch Phys Med Rehabil. 2019 doi: 10.1016/j.apmr.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Klein M.B., Lezotte D.L., Fauerbach J.A., Herndon D.N., Kowalske K.J., Carrougher G.J. The National Institute on Disability and Rehabilitation Research burn model system database: a tool for the multicenter study of the outcome of burn injury. J Burn Care Res. 2007;28:84–96. doi: 10.1097/BCR.0b013E31802C888E. [DOI] [PubMed] [Google Scholar]
- 21.Osborne C.L., Petersson C., Graham J.E., Meyer W.J., 3rd, Simeonsson R.J., Suman O.E. The Burn Model Systems outcome measures: a content analysis using the International Classification of Functioning, disability, and health. Disabil Rehabil. 2017;39:2584–2593. doi: 10.1080/09638288.2016.1239767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amtmann D., Project Directors of the National Institute on Disability IL, Rehabilitation Research Burn Model System Centers P, Gibran N., Herndon D., Kowalske K. BMS letter to the editor #1: introduction to the burn model system centers program. Burns. 2016;42:944–946. doi: 10.1016/j.burns.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Goverman J., Mathews K., Goldstein R., Holavanahalli R., Kowalske K., Esselman P. Adult contractures in burn injury: a burn model system national database study. J Burn Care Res. 2017;38 doi: 10.1097/BCR.0000000000000380. e328-e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shachar N., Mitelpunkt A., Kozlovski T., Galili T., Frostig T., Brill B. The importance of nonlinear transformations use in medical data analysis. JMIR Med Inform. 2018;6:e27. doi: 10.2196/medinform.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider J.C., Trinh N.H., Selleck E., Fregni F., Salles S.S., Ryan C.M. The long-term impact of physical and emotional trauma: the station nightclub fire. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan C.M., Schneider J.C., Kazis L.E., Lee A., Li N.C., Hinson M. Benchmarks for multidimensional recovery after burn injury in young adults: the development, validation, and testing of the American Burn Association/Shriners Hospitals for Children young adult burn outcome questionnaire. J Burn Care Res. 2013;34:e121–42. doi: 10.1097/BCR.0b013e31827e7ecf. [DOI] [PubMed] [Google Scholar]
- 27.Gojowy D., Kauke M., Ohmann T., Homann H.H., Mannil L. Early and late-recorded predictors of health-related quality of life of burn patients on long-term follow-up. Burns. 2019;45:1300–1310. doi: 10.1016/j.burns.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Xie B., Xiao S.C., Zhu S.H., Xia Z.F. Evaluation of long term health-related quality of life in extensive burns: a 12-year experience in a burn center. Burns. 2012;38:348–355. doi: 10.1016/j.burns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Tee J.W., Chan C.H., Gruen R.L., Fitzgerald M.C., Liew S.M., Cameron P.A. Early predictors of health-related quality of life outcomes in polytrauma patients with spine injuries: a level 1 trauma center study. Global Spine J. 2014;4:21–32. doi: 10.1055/s-0033-1358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan C.M., Stoddard F.J., Schneider J.C. COVID-19 pandemic and the burn survivor community: a call for action. Burns. 2020 doi: 10.1016/j.burns.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goverman J., Mathews K., Nadler D., Henderson E., McMullen K., Herndon D. Satisfaction with life after burn: a burn model system national database study. Burns. 2016;42:1067–1073. doi: 10.1016/j.burns.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAleavey A.A., Wyka K., Peskin M., Difede J. Physical, functional, and psychosocial recovery from burn injury are related and their relationship changes over time: a Burn Model System study. Burns. 2018;44:793–799. doi: 10.1016/j.burns.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Kvannli L., Finlay V., Edgar D.W., Wu A., Wood F.M. Using the Burn Specific Health Scale-brief as a measure of quality of life after a burn-what score should clinicians expect? Burns. 2011;37:54–60. doi: 10.1016/j.burns.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Holmes M., Garver M., Albrecht L., Arbabi S., Pham T.N. Comparison of two comorbidity scoring systems for older adults with traumatic injuries. J Am Coll Surg. 2014 doi: 10.1016/j.jamcollsurg.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Lundgren R.S., Kramer C.B., Rivara F.P., Wang J., Heimbach D.M., Gibran N.S. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30:307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 37.Stylianou N., Akbarov A., Kontopantelis E., Buchan I., Dunn K.W. Mortality risk prediction in burn injury: comparison of logistic regression with machine learning approaches. Burns. 2015;41:925–934. doi: 10.1016/j.burns.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Liu N.T., Salinas J. Machine learning for predicting outcomes in trauma. Shock. 2017;48:504–510. doi: 10.1097/SHK.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 39.Liu N.T., Salinas J. Machine learning in burn care and research: a systematic review of the literature. Burns. 2015;41:1636–1641. doi: 10.1016/j.burns.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Bamer A., McMullen K., Gibran N., Holavanahalli R., Schneider J.C., Carrougher G.J. Factors associated with attrition of adult participants in a longitudinal database: A National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model System Study. J Burn Care Res. 2019 doi: 10.1093/jbcr/irz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan C.M., Lee A., Kazis L.E., Schneider J.C., Shapiro G.D., Sheridan R.L. Recovery trajectories after burn injury in young adults: does burn size matter? J Burn Care Res. 2015;36:118–129. doi: 10.1097/BCR.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 42.Schneider J.C., Bassi S., Ryan C.M. Barriers impacting employment after burn injury. J Burn Care Res. 2009;30:294–300. doi: 10.1097/BCR.0b013e318198a2c2. [DOI] [PubMed] [Google Scholar]
- 43.Schneider J.C., Gerrard P., Goldstein R., DiVita M.A., Niewczyk P., Ryan C.M. The impact of comorbidities and complications on burn injury inpatient rehabilitation outcomes. PM R. 2013;5:114–121. doi: 10.1016/j.pmrj.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Schneider J.C., Shie V.L., Espinoza L.F., Shapiro G.D., Lee A., Acton A. Impact of work-related burn injury on social reintegration outcomes: a life impact burn recovery evaluation (LIBRE) study. Arch Phys Med Rehabil. 2017 doi: 10.1016/j.apmr.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]