Abstract
Contraception is widely used in the United States, and nurses in all settings may encounter patients who are using or want to use contraceptives. Nurses may be called on to anticipate how family planning intersects with other health care services and provide patients with information based on the most current evidence. This article describes key characteristics of nonpermanent contraceptive methods, including mechanism of action, correct use, failure rates with perfect and typical use, contraindications, benefits, side effects, discontinuation procedures, and innovations in the field. We also discuss how contraceptive care is related to nursing ethics and health inequities.
Keywords: birth control, contraception, family planning, reproductive health
Contraception is widely used in the United States, with an estimated 88.2% of all women ages 15 to 44 years using at least one form of contraception during their lifetime.1 Among women who could become pregnant but don’t wish to do so, 90% use some form of contraception.2 Thus, nurses in various settings are likely to encounter patients who are using contraception while presenting for a vast range of health care needs. Nurses will have many opportunities to support such patients by coordinating contraceptive use with other treatments, such as by identifying medications that interact with contraceptives or are teratogenic. Some patients, meeting with a nurse on an unrelated matter, may even seize the moment to ask questions about contraception.
Patients are best prepared to make informed decisions about contraceptive use when they have evidence-based information; nurses can better support patients’ reproductive goals by cultivating their own knowledge base. This article will prepare nurses at various practice levels and practice settings to meet the needs of patients who are current or potential contraceptive users. It describes the major categories of nonpermanent contraceptive methods and provides evidence-based updates. We also discuss inequities in contraceptive care that nurses can address using their current clinical knowledge and a reproductive justice approach.
Contraception in context.
In its position statement on reproductive health, the American Nurses Association (ANA) has asserted that clients have the right to make reproductive health decisions “based on full information and without coercion,” and that nursing professionals must be prepared to discuss “all relevant information about health choices that are legal.”3 Similarly, the American Academy of Nursing has issued policy recommendations that support “access to safe, quality sexual and reproductive health care and reproductive health care providers.”4 Aligning with these policies means that, across settings and in accordance with their scope of practice, nurses should be prepared to provide contraceptive counseling, services, and referrals.
Moreover, adopting a reproductive justice approach to care delivery can potentially improve the quality and equity of reproductive health care and outcomes significantly.5 Reproductive justice is a human rights framework that aligns with the ANA’s Code of Ethics for Nurses with Interpretive Statements,6, 7 and functions simultaneously as a theory, a practice, and a strategy. For more details, see Reproductive Justice.5, 7 Understanding contraception and contraceptive care in the context of both nursing ethics and reproductive justice will help nurses be best prepared for providing optimal care.
CONTRACEPTIVE METHODS: KEY CONSIDERATIONS
Three main considerations commonly arise in discussions of contraceptive methods: method safety and contraindications, failure rates, and return to fertility.
An important source for data about method safety comes from the Centers for Disease Control and Prevention (CDC): the U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC),8 which categorizes the safety of contraceptive methods in accordance with the specific health concerns of patients (see Table 18). In this article we’ll highlight the common contraindications and drug interactions categorized as U.S. MEC 4: “A condition that represents an unacceptable health risk if the contraceptive method is used.”8 We recommend that readers familiarize themselves with the U.S. MEC, which includes a comprehensive list of such conditions; it’s available free online (www.cdc.gov/mmwr/volumes/65/rr/pdfs/rr6503.pdf) and as an app.
Table 1.
U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC): Categorization of Safety for Specific Health Conditions8
| Category | Condition | Safety Recommendation |
|---|---|---|
| U.S. MEC 1 | A condition for which there is no restriction for the use of the contraceptive method. | Can use the method. |
| U.S. MEC 2 | A condition for which the advantages of the contraceptive method generally outweigh the theoretical or proven risks. | Can use the method. |
| U.S. MEC 3 | A condition for which the theoretical or proven risks of the contraceptive method generally outweigh its advantages. | Should not use the method unless no other method is appropriate and acceptable. |
| U.S.MEC4 | A condition for which the contraceptive method poses an unacceptable health risk. | Should not use the method. |
Failure rates represent a way to assess the efficacy of various contraceptive methods. For a given method, the failure rate is the percentage of users who have an unintended pregnancy during the first year of use; a lower failure rate indicates higher efficacy. For context, consider that up to 85% of women who have unprotected intercourse will experience an unintended pregnancy within a year.9 Failure rates for perfect and typical use of a given contraceptive method are also distinguished. Perfect use reflects method use when instructions are followed exactly and consistently; typical use reflects real-life use, when the method may not be used consistently or perfectly.
Many people have questions about the timing of return to fertility after stopping contraceptive use. The return to fertility is relatively rapid after cessation of almost all hormonal and nonhormonal methods, with the exception of depot medroxyprogesterone acetate (DMPA). For example, in one study among women who discontinued combined hormonal contraception, pregnancy rates were 57% at three months and 81% at 12 months after cessation.10 Conversely, ovulation may not resume for 15 to 49 weeks after one’s last DMPA injection, according to one systematic review.10
Method safety, efficacy, and return to fertility are not the only considerations that influence contraceptive choice. It’s important for nurses and other providers to understand that individuals will value different features of various contraceptive methods. Personal preferences (such as for a hormonal or nonhormonal method, ease and comfort with mode of use, partner acceptance, effects on the sexual experience, strength of desire to avoid pregnancy, and religious or spiritual beliefs and practices), medical considerations (such as whether the method protects against sexually transmitted infections [STIs], potential side effects), and structural factors (such as immediate and ongoing costs, ability to begin or stop use without needing access to health care)—all of these elements play a role.11–14 Seeing the whole picture will better equip nurses to help patients choose a method most aligned with their preferences and needs.
In this article, we describe the most common nonpermanent contraceptive methods; summarize their efficacy, mechanisms of action, uses, common adverse effects, and contraindications; and review the modes of administration of each type. Emergency contraception lies beyond the scope of this article and is not addressed.
HORMONAL CONTRACEPTIVES
Combined hormonal contraceptives
(CHCs) are among the most commonly prescribed and well-researched types of medication in use.1, 15 Synthetic estrogen and progestin revolutionized modern family planning when this combination first came on the market in pill form in 1960. Today CHCs can be delivered through a pill, patch, or vaginal ring with similar failure rates: less than 1% with perfect use and 7% to 9% with typical use.9, 16, 17
In CHCs, both progestins and estrogen inhibit the hypothalamic–pituitary–ovarian axis, which controls the reproductive cycle (see Figure 1).18 Progestins prevent pregnancy by inhibiting the luteinizing hormone (LH) surge, thus suppressing ovulation, thickening the cervical mucus, lowering fallopian tube motility, and causing the endometrium to become atrophic.18 Estrogens prevent pregnancy by suppressing follicle-stimulating hormone (FSH) production, which prevents the development of a dominant follicle.18 Progestin is responsible for the majority of both contraceptive action and side effects; the addition of estrogen helps prevent irregular or unscheduled bleeding.9
Figure 1.
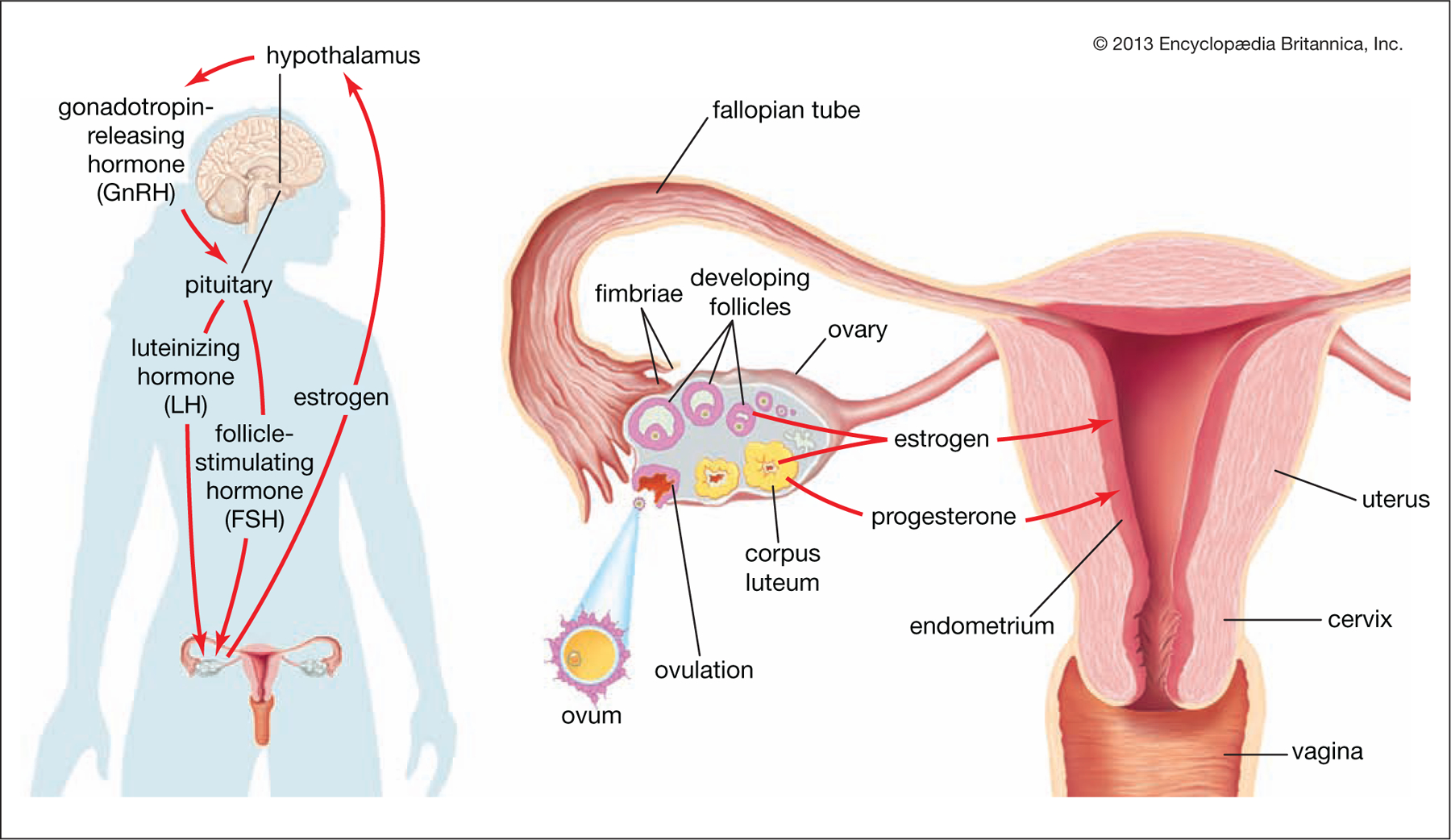
The Hormonal Regulation of Ovulation
At left: the hypothalamus secretes gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to secrete the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH stimulate the growth and maturation of the ovarian follicles. The mature follicle secretes estrogen, inhibiting the hypothalamus from further GnRH production (until the next reproductive cycle). At right: after ovulation, blood levels of LH and FSH fall, and the ruptured follicle, now a corpus luteum, secretes estrogen and progesterone to prepare the uterine lining for fertilization and implantation. Adapted with permission from Encyclopædia Britannica, © 2013 by Encyclopædia Britannica, Inc.
Traditionally, users take CHCs for three weeks, then placebo pills or nothing for one week. The hormone-free week prompts “withdrawal bleeding,” caused by withdrawal from active CHC ingredients, that mimics the menstrual cycle and may provide assurance that the user isn’t pregnant.18 Nurses can educate their patients that withdrawal bleeding is not actual menses and isn’t clinically necessary.18, 19
Common side effects of CHCs include lighter, shorter periods (40% to 50% reduction in menstrual flow); irregular bleeding (breakthrough bleeding or spotting); amenorrhea; nausea; breast tenderness; emotional lability; headaches; and reduced premenstrual syndrome symptoms (such as bloating, cramping, and acne).18 CHCs are also associated with reduced risk of ovarian, endometrial, and colon cancer, and are essential in treating polycystic ovarian syndrome.18 As with other methods, it’s difficult to predict which individuals will experience which side effects and how severe these will be. Certain side effects, particularly amenorrhea, may be considered beneficial by some people but unacceptable by others.20 These may be referred to as “noncontraceptive benefits” of these methods.
CHC contraindications (U.S. MEC 4–category conditions) include being age 35 years or older and smoking 15 or more cigarettes per day; being less than 21 days postpartum; having a systolic blood pressure of 160 mmHg or greater, or a diastolic blood pressure of 100 mmHg or greater; having had major surgery with prolonged immobilization; experiencing migraine with aura; and being at elevated risk for recurrent deep vein thrombosis or pulmonary embolism.8
CHCs are still effective when taken concurrently with many medications, including most commonly used antibiotics. But concurrent use of certain medications—including rifampin (Rifadin) or rifabutin (Mycobutin) therapy, the antiretroviral drug fosamprenavir (Lexiva), and certain anticonvulsants—can reduce CHC effectiveness.8 In such cases, use of a nonhormonal backup contraceptive method is recommended.
CHC pills.
Numerous CHC pills are currently available on the market. Typically, pills contain a ombination of 10 to 35 mcg ethinyl estradiol and one of the four generations of progestins. Different formulations have different side effect profiles, so patients may need to try another formulation if an undesirable side effect occurs.
Pills should be taken at about the same time every day to maintain ovulation suppression. This frequent dosing is one of the major drawbacks of pill use, and missing a pill is common, regardless of age.16 In general, nurses should counsel patients that a missed pill should be taken as soon as it is remembered. Ovulation suppression is not guaranteed if more than 48 hours have elapsed since the last pill was taken. Missing a single pill will have little effect on effectiveness, but if two pills are missed, the most recent pill should be taken as soon as possible, and a backup method (such as condoms) should be used for seven days.18
Pills can be initiated at any time. A “Sunday start” has been popular in the past because it typically ensures that the withdrawal bleed does not occur on weekend days. Recently, a “quick start,”starting the pill on the day of visit, has become more popular because, at least initially, it’s associated with better adherence, and there is no increase in the incidence of irregular bleeding.21
Extended and continuous use are increasingly popular dosing regimens. Extended use involves using the CHC for longer than the typical month-long cycle, thereby giving the user an extended time between withdrawal bleeds. This can be achieved by taking pills specifically designed for such regimens or by simply skipping the placebo pills in a 28-day pill pack (though users will run out of pills more quickly). Continuous use involves taking CHCs without interruption for an indefinite time. Extended and continuous use regimens have been associated with improved ovulation suppression, increased medication adherence, high user acceptability, decreases in scheduled bleeding, and less breakthrough bleeding over time.19, 22 Moreover, decreasing or eliminating periods can be preferable for patients who have period-related mood changes, headaches, painful cramping, heavy menses, or other estrogen-related changes. While extended and continuous use regimens have primarily been studied regarding CHC pills, there is evidence of similar efficacy among CHC patch and vaginal ring users.23
CHC transdermal patch.
The CHC transdermal patch (Xulane), a thin square about two inches across, contains 150 mcg norelgestromin and 35 mcg ethinyl estradiol (see Figure 2). It can be placed on the stomach, upper arm, buttock, or back, and must be completely attached to the skin to be effective. The patch is replaced every week for three weeks; during the fourth week no patch is worn and a withdrawal bleed occurs. Weekly application is appealing for those who don’t want the burden of daily pill taking. In 2014, the patch became available as a generic product.
Figure 2.
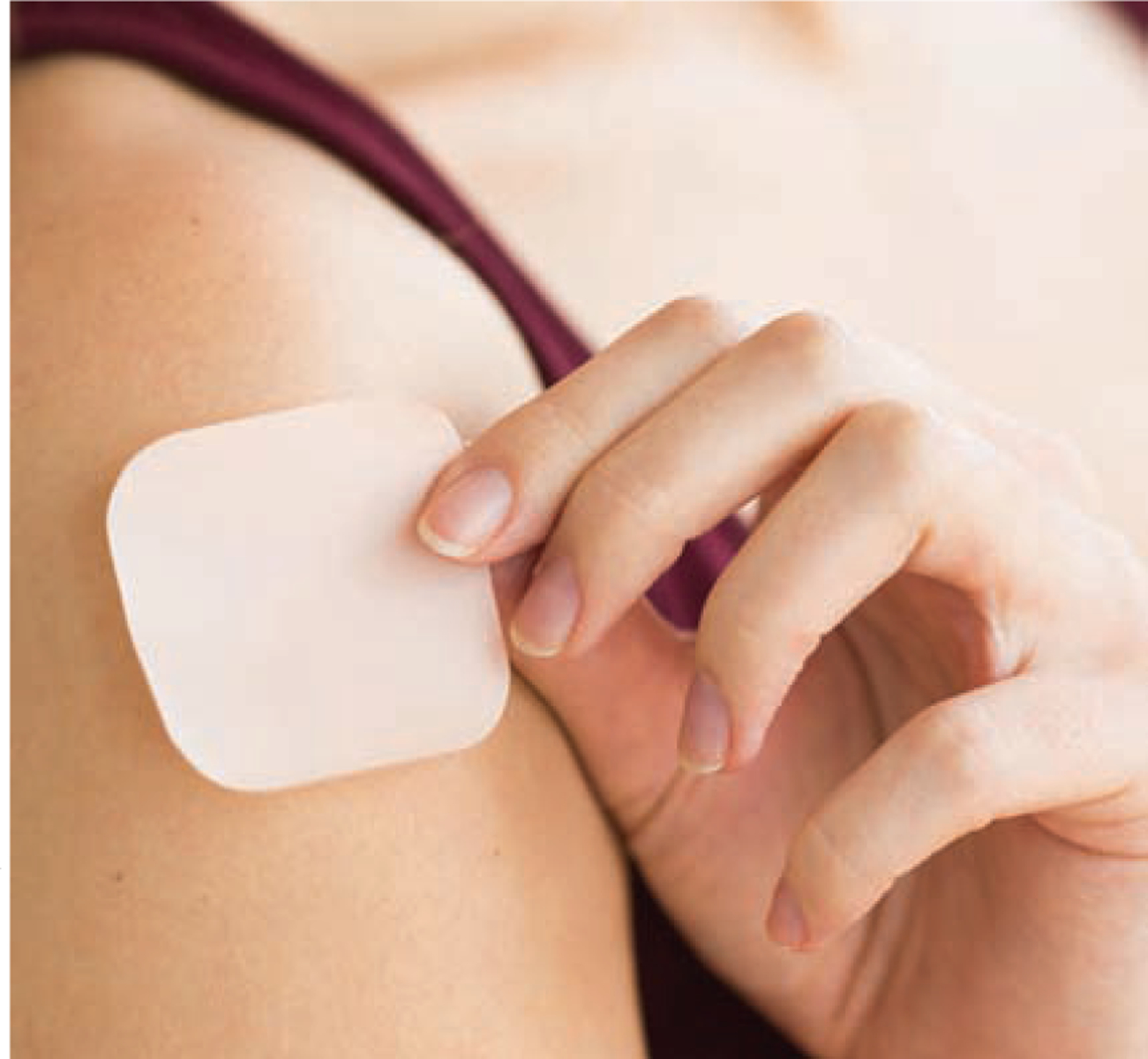
The Transdermal Patch
While contraindications for CHCs apply to all delivery methods, there are some additional concerns with the patch. Findings from early research suggested there was an increased risk of venous thromboembolism (VTE) with the patch compared to CHC pills, but later research has yielded conflicting results.24, 25 The U.S. Food and Drug Administration (FDA) recommends that the same guidelines regarding VTE be applied to both methods: CHC pills and the patch should be avoided in patients at high risk for clots, such as those who have a history of or current VTE or surgery requiring immobilization.24, 26 The patch also causes skin irritation in about 20% of users, though only about 3% discontinue the method for this reason.17
CHC vaginal ring.
The ring (NuvaRing) is a clear, flexible ring about two inches in diameter that is placed in the vagina for 21 days and removed for seven days to allow for withdrawal bleeding; it’s replaced monthly (see Figure 3). It releases 15 mcg/day of ethinyl estradiol and 120 mcg/day of etonogestrel. Users can simply place the ring in the vaginal canal themselves. As with the patch, the less frequent applications can be appealing and can lead to increased adherence.17 The ring’s internal placement ensures the steady delivery of hormones, which allows for lower serum concentrations than occur with either the patch or pills. As a result, the ring generally has milder side effects than are seen with other CHC delivery methods.17 Some users may experience increased vaginal irritation and discharge.17 There is also some evidence of reduced vaginal dryness, which may appeal to perimenopausal women and others who tend to experience such dryness.
Figure 3.
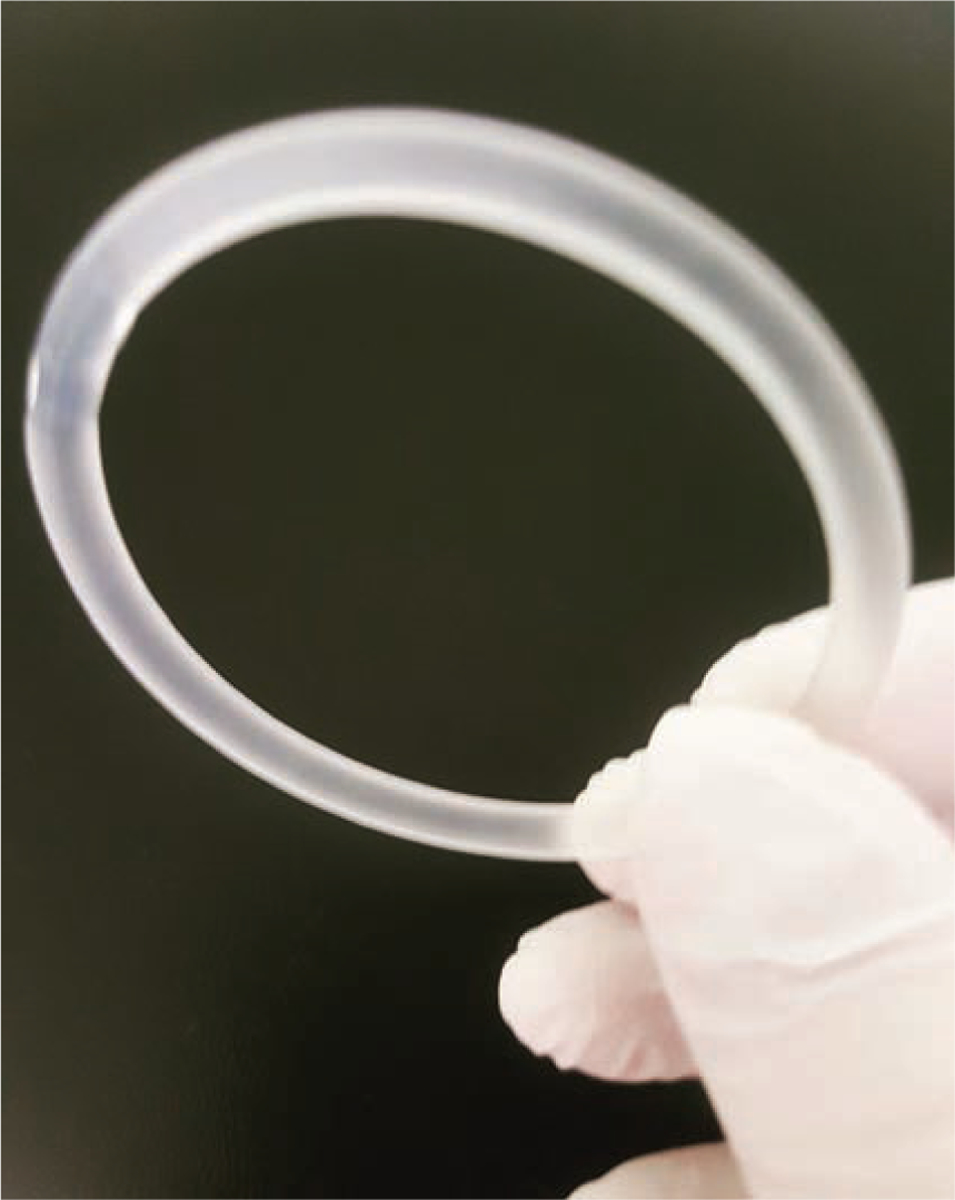
The Vaginal Ring
Ring users may have concerns about their risk for pregnancy if the ring is removed intentionally or accidentally. The ring can be removed for up to three hours without diminishing its contraceptive effect. This gives users the option of removing it during sex if they prefer. The manufacturer recommends rinsing the device in cool or lukewarm water prior to reinsertion.27 If the ring is out for more than three hours, users should take extra steps to protect against pregnancy. As with any device, users should consult the package insert for more specific instructions.
Progestin-only methods
include pills, injections, implants, and intrauterine devices (IUDs). Without concomitant estrogen, progestin-only methods pose less risk of VTE than CHCs.28 While the safety of the CHC pill, patch, and ring are addressed collectively in the U.S. MEC, the progestin-only methods are given separate safety profiles. Like CHCs, progestin-only methods require a prescription.
Progestin-only pills (POPs).
POPs are generally made with first-generation progestins, and dosage amounts are substantially lower than those found in any CHC. Like CHCs, POPs should be taken at the same time of day. They are used continuously, with no hormone-free interval. Despite their pharmacokinetic differences, failure rates are often reported together: Hatcher and colleagues report that for both types of pills, the failure rate is less than 1% with perfect use and 7% with typical use.9 That said, POPs have a higher failure rate when not taken at the same time every day, because effective drug levels are maintained in the bloodstream for only 22 hours.9 Nurses should caution patients that they must be vigilant about adhering to the dosing schedule. The most common side effects of POPs are unscheduled bleeding and spotting, likely due to the shorter daily window of efficacy and the absence of estrogen.18
POPs are considered safe in many clinical scenarios wherein CHCs are contraindicated (as noted above). As with CHCs, patients should use a nonhormonal backup method when taking certain medications, including rifampin or rifabutin therapy, the antiretroviral drug fosamprenavir, and certain anticonvulsants.8
DMPA injection.
DMPA (Depo-Provera) is available as a 150 mg/mL intramuscular injection or a 104 mg/mL subcutaneous injection given every 12 to 13 weeks.18, 29 Injections must be administered by a provider. The failure rate is less than 1% with perfect use and 4% with typical use.9 In addition to the aforementioned progestin mechanisms of action, DMPA also affects the hypothalamic–pituitary–ovarian axis at the hypothalamus, inhibiting ovulation through suppression of gonadotropin-releasing hormone.18
Irregular periods are a common side effect. One systematic review found that, after a year of regular use, only 12% of DMPA users had regular periods and 46% had amenorrhea.30 Although personal preferences vary, amenorrhea may be seen as beneficial by patients with anemia, endometriosis, fibroids, dysmenorrhea, or menorrhagia.9 Other potential side effects include weight gain, impaired glucose metabolism, bone mineral density loss, headache, and mood changes (specifically depression).18 Because DMPA is one of the more discrete methods available, it may appeal to people wishing to keep their contraception private.
DMPA has few contraindications and almost no drug interactions. Additional benefits include decreased risk of endometrial cancer and pelvic inflammatory disease, reduced incidence of epileptic seizures, and reduced frequency of sickle cell crises.9, 29
Implants.
Implants and IUDs containing progestin, as well as IUDs without hormones, are collectively referred to as long-acting reversible contraception (LARC). LARC insertions and removals are within the scope of practice of advanced practice clinicians, including NPs and certified nurse midwives. Once inserted, LARCs involve little user effort to maintain contraceptive efficacy.
The single-rod implant (Implanon, Nexplanon), which is about the size of a matchstick, is inserted in the upper arm and can remain in place for up to three years (see Figure 4). The implant contains 68 mg of etonogestrel that is released incrementally at slowly diminishing rates, from 60 to 70 mcg/day initially to 25 to 30 mcg/day by the end of the third year.31 Failure rates with both typical and perfect use are below 1%.9 The most commonly reported reasons for discontinuation include irregular bleeding (10%), emotional lability (2%), and weight gain (2%).32 The implant method can appeal to people who want a long-term, reversible, highly effective method but are uncomfortable with having devices in the vagina or uterus or with insertion procedures at those sites.18 The implant is safe for the vast majority of people, though there are contraindications for some specific conditions, such as active breast cancer.8
Figure 4.

The Single-Rod Implant
IUDs with progestin (also called intrauterine systems [IUSs]).
With both typical and perfect use, IUDs have failure rates below 1%.9 Those with progestin alter the cervical mucus such that sperm cannot pass through the cervix to access the upper reproductive tract.
Four levonorgestrel (LNG) IUDs are available on the U.S. market, with similar effectiveness but varying doses, duration, and side effects.33 The naming convention uses a number to indicate the average number of micrograms of LNG released per day. The LNG-IUS 20 (Mirena) and LNG-IUS 12 (Kyleena) can be used up to five years. The LNG-IUS 20 (Liletta, designed as a lower-cost version of Mirena) can be used up to four years, and the LNG-IUS 8 (Skyla) up to three years. The LNG-IUS 12 and LNG-IUS 8 are smaller in size, which makes insertion easier. Amenorrhea occurs in 20% of LNG-IUS 20 users after one year, in 12% of LNG-IUS 12 users after one year, and in 12% of LNG-IUS 8 users after three years.
Contraindications to IUD use include current purulent cervicitis, chlamydia infection, gonorrhea infection, or pelvic inflammatory disease at the time of insertion.21 If pelvic inflammatory disease develops after insertion, a course of antibiotics may be prescribed, and removal may be warranted.
Despite their safety and efficacy, IUD use in the United States is lower than in other parts of the industrialized world.34 IUDs have a fraught history, the legacy of which may affect patient and provider attitudes (see Are IUDs Safe?8, 9, 35–40). This is slowly starting to change, and recent substantial declines in unintended pregnancies are attributed, in part, to an increase in the use of LARCs.41
NONHORMONAL METHODS
Nonhormonal methods include the copper IUD, barrier methods with and without spermicides, and behavioral methods. Nonhormonal methods generally have fewer risks and side effects because, by definition, they don’t involve exposure to exogenous or synthetic hormones. As with hormonal methods, the effectiveness, safety, and ease of use of various nonhormonal methods are important user considerations and will strongly influence individual choices.
Copper IUD.
The most effective reversible nonhormonal method is the copper IUD (Paragard), which has a failure rate below 1% with both typical and perfect use; the device can be used for up to 10 years, and must be inserted by a skilled provider.9, 42 Copper ions are spermicidal. The copper IUD does not affect ovulation or timing of the menstrual cycle, but it is associated with heavier menstrual bleeding and cramping.43 In a three-year Australian study among 211 users, of the 59 women who discontinued use though still requiring contraception, 28 did so because of heavy bleeding.44 This side effect may be felt more acutely by users switching from a hormonal method that lessened their normal flow; anticipatory guidance from nurses can help prepare such users for this possibility.
The copper IUD may be an appealing option for those who are limited by contraindications to CHCs or progestin-only methods. In addition to the aforementioned contraindications for progestin-containing IUDs, copper IUDs are contraindicated for women with copper allergies, uterine infections, or uterine cancer.8
Barrier methods (with or without spermicides)
include condoms and diaphragms used at the time of intercourse. Efficacy is highly dependent on user behavior, and failure rates with typical and perfect use vary widely. For the male condom, failure rates with typical and perfect use are 13% and 2%, respectively; for the female condom, 21% and 5%, respectively; and for the diaphragm, 17% and 16%, respectively.9
Condoms are available over the counter. Those made from polyurethane or latex prevent the transmission of STIs, including HIV infection. Nonlatex condoms made of lambskin are available for individuals with latex sensitivity, but don’t protect against STIs.
Diaphragms are inserted into the vaginal canal such that they block the cervical os and can be placed up to an hour before intercourse. They require a prescription, and have traditionally come in multiple sizes, thus requiring fitting by a provider. Diaphragms are used with a spermicide to increase their effectiveness. In the United States, all commercially available spermicides contain nononoyl-9 (N-9) and are sold over the counter. N-9 may cause irritation or allergic reactions, and increases the risk of urinary tract infections.8 The irritation can cause genital lesions, which may increase the risk of HIV acquisition. For women with HIV, N-9 irritation is suspected of increasing viral shedding, which increases the likelihood of transmission to partners. Thus, spermicide use is contraindicated in people at high risk for contracting HIV and is not recommended for people who have HIV.8
Behavioral methods
include withdrawal, lactational amenorrhea (LAM), and fertility awareness-based methods (FABMs). Withdrawal (often called “pulling out”) involves removal of the penis from the vaginal canal during intercourse but before ejaculation. The failure rates are 20% with typical use and 4% with perfect use.9 Withdrawal requires good communication and mutual agreement, as well as adequate physical control by the ejaculating partner. Research indicates that only a very small proportion of individuals use withdrawal as their primary contraceptive method; but because it’s also commonly used in conjunction with other methods and might not be considered a “real” method, its use may be underreported.45 Withdrawal may be an option for people who don’t want to use other contraceptive methods for religious or cultural reasons.
LAM relies on the natural suppression of the LH surge that occurs during exclusive breastfeeding. It’s highly effective when infants are exclusively fed breast milk on demand, when infants are under six months of age, and when the woman has not yet resumed menses.18 If breastfeeding is nonexclusive or the infant is older than six months, efficacy drops.
FABMs involve avoiding unprotected intercourse during an estimated fertile window, which is determined through a variety of strategies of varying effectiveness. There are limited data about failure rates for each approach46; but collectively, the FABMs appear to have failure rates of 15% with typical use and from 0.4% to 5% with perfect use.9 These methods may involve tracking the menstrual cycle, basal body temperature, cervical mucus, or LH levels in order to calculate the likely fertile period. Midcycle, the LH surge preceding ovulation is followed by an increase in progesterone, causing a small but measurable increase in basal body temperature. The timing of ovulation varies, even among women with similar cycle lengths.47 Some FABM users might not fully comprehend how the method works,48 and nurses can help them reach a better understanding of their menstrual cycle.
Although FABMs have traditionally been a low-tech contraceptive method, several mobile apps that support FABMs are now available. An app user inputs the relevant data, and the app uses an algorithm to generate fertility window predictions. Apps algorithms vary, as does the accuracy of their predictions.49, 50 Nurses should explain to patients that most health apps aren’t regulated by the FDA, and very few have been evaluated in peer-reviewed scientific studies.51 In one study, nearly 20% of FABM apps contained erroneous medical information.50 Moreover, there is evidence that some app companies’ advertising overstates their product’s efficacy.52
For recent developments in contraception, see Innovations in Hormonal and Nonhormonal Methods.53–62
DISPARITIES IN ACCESS AND USE
Because of economic hardship and institutionalized racism, homophobia, and transphobia, many people have compromised access to the full spectrum of contraceptive options. Studies indicate that such socioeconomic factors play a role in the higher rates of unintended and unwanted pregnancies observed among Black and Latina women compared with white women in the United States, as well as influencing user preferences.14, 63 Black and Latina women tend to report lower rates of overall contraceptive use and prescription contraceptive use, but higher rates of condom use and tubal ligation or sterilization.64, 65
Disparate patterns of contraceptive use and options are also related to bias and discrimination within the health care system. Barriers to high-quality contraceptive care may emerge in the forms of limited knowledge about contraceptive options, limited access to health care generally, receiving biased care from providers, and reproductive coercion. For example, there is evidence to suggest that providers are more likely to recommend IUDs to Black and Latina women with low socioeconomic status than to white women with such status.66 Explanations for this pattern include that some providers subconsciously see certain women (that is, women of color or low socioeconomic status) as “not needing” more children, needing a lower-maintenance method, or needing more help to effectively prevent pregnancy.67 But pressuring certain patients into using LARCs undermines their reproductive autonomy and risks continuing historically coercive and racist U.S. contraception policies. As frontline providers, nurses can address these disparities by engaging in reflexive nursing practices and working to undo institutionalized racism.68
Members of sexual and gender minorities—including those who identify as lesbian, gay, bisexual, queer, transgender, or gender nonbinary—also require access to contraceptive services. But they often have limited access to safe, affirming health care of all types. Members of these minorities have pregnancy and childbearing histories, plans, and desires as diverse as those of any other population. Many nonheterosexual women have been pregnant and given birth, and many have a desire to do so.69 Others regularly have sex that could lead to pregnancy, and need and want reliable and consistent contraception.70, 71 Still others may rarely or never have penile–vaginal intercourse, and use contraception mainly for its noncontraceptive benefits, such as menstrual regulation, or acne or endometriosis treatment.72
Many transgender or nonbinary individuals who have a uterus and ovaries are capable of becoming pregnant through penile–vaginal intercourse.73 Testosterone therapy in transgender men is not a reliable contraceptive method, though this misconception is common.74 Access to effective contraception may be especially critical for transgender men or transmasculine people, since many desire menses suppression.75, 76 Clinical and anecdotal evidence also suggest that menstruation and pregnancy may trigger or heighten feelings of gender dysphoria or may put safety at risk by “outing” one as transgender or transmasculine.77, 78 Some members of these minorities may achieve amenorrhea and pregnancy prevention with sterilization. Others may want to stop menstruating but retain the possibility of becoming pregnant later in life. Nurses can let such patients know that this may be possible with progestin-only IUDs. Estrogen-containing contraceptives may cause amenorrhea but are contraindicated in people on masculinizing hormone therapy.
An essential component of patient-centered nursing practice is the delivery of individualized care; this includes avoiding assumptions about a patient’s reproductive health priorities and needs based on membership in a particular group. Individuals from any marginalized or stigmatized group who have experienced bias and discrimination in health care might have learned to expect the same from future encounters. It’s important for nurses in all clinical settings to understand how such history can affect patients’ current experiences and the nurse–patient relationship. By applying nursing skills such as taking thorough health histories, listening actively to patients’ reproductive health priorities, and referring patients to appropriate health care services, nurses may be able to improve these relationships and clinical outcomes.
CONCLUSION
It’s vital that nurses in all settings and specialties stay current on the latest evidence regarding contraception. First, this is essential to fulfilling the World Health Organization’s recommendation to provide comprehensive contraceptive patient education79 and the ANA’s ethical mandate to support the reproductive self-determination of all patients.6 Second, nurses can provide better patient-centered care if they can competently address patients’ family planning concerns and questions with current and evidence-based knowledge. We recognize that this is challenging, as new types of contraception, hormonal formulations, delivery systems, and indications for use are always being developed. For a list of resources that will help nurses stay up to date, see Resources for Nurses. Lastly, actively addressing the concerns of patients from stigmatized groups will ultimately contribute to efforts to resolve disparities in contraceptive care and work toward reproductive justice for all.▼
Reproductive Justice.
Reproductive justice is grounded in the following four principles, which posit that it’s a human right5, 7
to become pregnant and have children, and to determine how one wishes to give birth and create families.
to choose not to become pregnant or have children, and to have access to options for preventing or ending pregnancy.
to parent one’s children with dignity—including by having access to essential social supports, safe environments, and healthy communities—without fear of violence from individuals or the government.
to disassociate sex from reproduction, as healthy sexuality and pleasure are essential components of a full human life.
While the goal of reproductive justice is to address the systems and structures that create reproductive health inequities, making sure that people who need contraceptive services receive high-quality care is a crucial step toward that goal.
Are IUDs Safe?
Current intrauterine devices (IUDs) are among the most effective, safe, and convenient contraceptive methods available.8, 9 But there was a time when this was not the case. It’s important for nurses to understand why, as lingering fears and reservations about IUDs are incongruent with current recommendations.
In 1971, a new IUD called the Dalkon Shield was introduced and was on the market for three years. Its use was soon associated with increased risk of pelvic inflammatory disease, spontaneous abortion (often late in pregnancy), ectopic pregnancy, and infertility. But it took 10 years for the magnitude of the problem to fully emerge. Many factors caused these adverse events, some specific to the device and others specific to the state of the medical field. One of the biggest design flaws of the Dalkon Shield was its multifilament tail string. IUDs typically have monofilament tail strings that help providers to remove the device. But because removal of the Dalkon Shield required additional force, a cable-style, multifilament string was used. In contrast to monofilament strings, the multifilament string served as an easy vector for bacteria—such as those that cause chlamydia or gonorrhea—to move quickly from the vagina to the uterus. This led to a fivefold increase in pelvic inflammatory disease among women using the Dalkon Shield compared with those using other IUDs and a sevenfold increase in pelvic inflammatory disease among Dalkon Shield users compared with women using no contraception.35 Poor screening for and identification of sexually transmitted infections exacerbated the problem. Moreover, the manufacturer initially claimed it was safe to leave the Dalkon Shield in place when pregnancy did occur; this practice resulted in miscarriage, septic abortion, and several deaths.36
For a time, virtually all IUDs disappeared from the U.S. market, and fears about their use have persisted.37 Yet all current IUDs are approved for use in nulliparous women, adolescents and teenagers, and women at increased risk for pelvic inflammatory disease. Notably, the American Academy of Pediatrics recommends IUDs as a first-line contraceptive method for adolescents.38 The use of current IUDs is not associated with infertility, and fertility returns very rapidly upon removal.39, 40
Innovations in Hormonal and Nonhormonal Methods.
Hormonal contraceptives.
Combined hormonal contraceptives.
In 2018, the U.S. Food and Drug Administration (FDA) approved a new progestin–estrogen combined hormonal contraceptive, segesterone acetate plus ethinyl estradiol (Annovera). This is a vaginal ring that is placed for 21 days; removed, cleaned, and stored for seven days; and then reinserted for the start of a new cycle.53 The ring, which is slightly larger and thicker than the ethinyl estradiol–etonogestrel monthly ring (NuvaRing) and can be used for up to 13 cycles (one year), might be a good option for women who have difficulty picking up birth control at a pharmacy on a regular basis, are at risk for losing insurance coverage, or travel frequently. Unlike the NuvaRing, which requires refrigeration prior to dispensing, Annovera does not require refrigeration for long-term storage.
Progestin-only contraceptives.
The possibility of self-administration of depot medroxyprogesterone acetate (DMPA) by subcutaneous injection is being explored. There is evidence that self-administration improves method continuation.54 Interest has been documented among current DMPA users, who may encounter barriers obtaining or refilling their usual prescription.55
Nonhormonal contraceptives.
Single-size diaphragm.
In 2014, the FDA approved a single-size silicone diaphragm (Caya).56 This single-size option means that users no longer have to be fitted by a provider, although like other diaphragms it requires a prescription. In one study, 76% of users could correctly position this diaphragm with written instructions, and 94% could do so with coaching.57 The single-size diaphragm is described as fitting “most women,” though it will not fit those who previously used a diaphragm sized 50 to 60 mm or 85 to 90 mm.58 According to the manufacturers, contraindications include having a current vaginal infection, severe pelvic floor or uterine descent, small or absent retropubic recess, acute or frequent bladder infections, and being within the first six weeks postpartum.58 Users are instructed to insert the diaphragm before intercourse and to use it in combination with a water-based spermicidal gel. Several compatible gels are available. One study of a newer, lactic acid–based gel found its effectiveness comparable to that of gels containing nonoxynol-9.59
FDA-approved, fertility awareness–based method (FABM) mobile app.
Resources for Nurses.
U.S. Medical Eligibility Criteria for Contraceptive Use
www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html
A detailed document, a summary chart, a digital app, a slide set and more are available for reference regarding contraceptive safety for patients with specific health concerns.
U.S. Selected Practice Recommendations for Contraceptive Use
http://dx.doi.org/10.15585/mmwr.rr6504a1
These recommendations address common, often controversial or complex issues regarding initiation and use of specific contraceptive methods with an eye toward application in the clinical setting. The site includes helpful charts and algorithms.
Centers for Disease Control and Prevention: Reproductive Health: Contraception
www.cdc.gov/reproductivehealth/contraception/index.htm#Contraceptive-Effectiveness
The site includes a link to a chart showing the comparative effectiveness of contraceptive methods and abbreviated instructions for use.
Bedsider
Consumer-oriented, evidence-based decision aids about contraceptives are featured, including an interactive “method explorer” and numerous topic-specific articles and videos.
Footnotes
The authors and planners have disclosed no potential conflicts of interest, financial or otherwise.
A podcast with the authors is available at www.ajnonline.com.
For four additional continuing nursing education activities on the topic of contraception, go to www.nursingcenter.com/ce.
REFERENCES
- 1.Daniels K, Mosher WD. Contraceptive methods women have ever used: United States, 1982–2010. Natl Health Stat Report 2013(62):1–15. [PubMed] [Google Scholar]
- 2.Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 2018;97(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Nurses Association. ANA position statement onreproductive health. Silver Spring, MD; 2010. March https://www.nursingworld.org/practice-policy/nursing-excellence/official-position-statements/id/reproductive-health. [Google Scholar]
- 4.Olshansky E, et al. Sexual and reproductive health rights, access and justice: where nursing stands. Nurs Outlook 2018;66(4):416–22. [DOI] [PubMed] [Google Scholar]
- 5.Scott KA, et al. The ethics of perinatal care for black women: dismantling the structural racism in “mother blame” narratives. J Perinat Neonatal Nurs 2019;33(2):108–15. [DOI] [PubMed] [Google Scholar]
- 6.American Nurses Association. Code of ethics for nurses with interpretive statements. Silver Spring, MD; 2015. [Google Scholar]
- 7.Ross LJ, Solinger R. Reproductive justice: an introduction. Oakland, CA: University of California Press; 2017. [Google Scholar]
- 8.Curtis KM, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 9.Hatcher RA, et al. , editors. Contraceptive technology. 21st ed. Atlanta: Managing Contraception, LLC; 2018. [Google Scholar]
- 10.Barnhart K, et al. Return to fertility after cessation of a continuous oral contraceptive. Fertil Steril 2009;91(5):1654–6. [DOI] [PubMed] [Google Scholar]
- 11.Callegari L, et al. Racial and ethnic differences in contraceptive preferences—findings from the Examining Contraceptive Use and Unmet Need among women veterans (ECUUN) study [conference abstract]. Contraception 2016;94(4):410. [Google Scholar]
- 12.Gomez AM, Clark JB. The relationship between contraceptive features preferred by young women and interest in IUDs: an exploratory analysis. Perspect Sex Reprod Health 2014;46(3):157–63. [DOI] [PubMed] [Google Scholar]
- 13.He K, et al. Women’s contraceptive preference-use mismatch. J Womens Health (Larchmt) 2017;26(6):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AV, et al. Racial and ethnic differences in women’s preferences for features of contraceptive methods. Contraception 2016;93(5):406–11. [DOI] [PubMed] [Google Scholar]
- 15.Jones J, et al. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report 2012(60):1–25. [PubMed] [Google Scholar]
- 16.Chabbert-Buffet N, et al. Missed pills: frequency, reasons, consequences and solutions. Eur J Contracept Reprod Health Care 2017;22(3):165–9. [DOI] [PubMed] [Google Scholar]
- 17.Lopez LM, et al. Skin patch and vaginal ring versus combined oral contraceptives for contraception. Cochrane Database Syst Rev 2013;(4):CD003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy PA, et al. Contraception In: Schuiling KD, Likis FE, editors. Women’s gynecologic health. Sudbury, MA: Jones and Bartlett Learning; 2013. p. 209–60. [Google Scholar]
- 19.Jacobson JC, et al. Extended and continuous combined contraceptive regimens for menstrual suppression. J Midwifery Womens Health 2012;57(6):585–92. [DOI] [PubMed] [Google Scholar]
- 20.Polis CB, et al. There might be blood: a scoping review on women’s responses to contraceptive-induced menstrual bleeding changes. Reprod Health 2018;15(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis KM, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep 2016;65(4):1–66. [DOI] [PubMed] [Google Scholar]
- 22.Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015;42(4):669–81. [DOI] [PubMed] [Google Scholar]
- 23.Edelman A, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev 2014;(7):CD004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galzote RM, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health 2017;9:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepper NK, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception 2017;95(2):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Practice Committee of the American Society for Reproductive Medicine. Combined hormonal contraception and the risk of venous thromboembolism: a guideline. Fertil Steril 2017; 107(1):43–51. [DOI] [PubMed] [Google Scholar]
- 27.Merck and Company. NuvaRing (etonogestrel/ethinyl estradiol vaginal ring). Whitehouse Station, NJ; 2018. https://www.nuvaring.com/frequently-asked-questions. [Google Scholar]
- 28.World Health Organization. Medical eligibility criteria for contraceptive use. Geneva, Switzerland; 2015. https://apps.who.int/iris/bitstream/handle/10665/181468/9789241549158_eng.pdf?sequence=9. [PubMed] [Google Scholar]
- 29.Jacobstein R, Polis CB. Progestin-only contraception: injectables and implants. Best Pract Res Clin Obstet Gynaecol 2014;28(6):795–806. [DOI] [PubMed] [Google Scholar]
- 30.Hubacher D, et al. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: systematic review and evidence-based comparisons. Contraception 2009;80(2):113–8. [DOI] [PubMed] [Google Scholar]
- 31.Merck and Company. Prescribing information. Nexplanon (etonogestrel implant) Whitehouse Station, NJ; 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021529s011lbl.pdf. [Google Scholar]
- 32.Blumenthal PD, et al. Tolerability and clinical safety of Implanon. Eur J Contracept Reprod Health Care 2008;13 Suppl 1:29–36. [DOI] [PubMed] [Google Scholar]
- 33.Nelson AL. LNG-IUS 12: a 19.5 levonorgestrel-releasing intrauterine system for prevention of pregnancy for up to five years. Expert Opin Drug Deliv 2017;14(9):1131–40. [DOI] [PubMed] [Google Scholar]
- 34.Buhling KJ, et al. Worldwide use of intrauterine contraception: a review. Contraception 2014;89(3):162–73. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Elevated risk of pelvic inflammatory disease among women using the Dalkon Shield. MMWR Morb Mortal Wkly Rep 1983;32(17):221–2. [PubMed] [Google Scholar]
- 36.Henig RM. The Dalkon Shield disaster. Washington Post 1985. November 17 https://www.washingtonpost.com/archive/entertainment/books/1985/11/17/the-dalkon-shield-disaster/6c58f354-fa50-46e5-877a-10d96e1de610. [Google Scholar]
- 37.Sifferlin A. Why is the most effective form of birth control—the IUD—also the one no one is using? Time. 2014 Jun 30; https://time.com/the-best-form-of-birth-control-is-the-one-no-one-is-using. [Google Scholar]
- 38.Ott MA, et al. Contraception for adolescents. Pediatrics 2014;134(4):e1257–e1281. [DOI] [PubMed] [Google Scholar]
- 39.Hubacher D, et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 2001;345(8):561–7. [DOI] [PubMed] [Google Scholar]
- 40.Morgan KW. The intrauterine device: rethinking old paradigms. J Midwifery Womens Health 2006;51(6):464–70. [DOI] [PubMed] [Google Scholar]
- 41.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016;374(9):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copper Surgical. Prescribing information ParaGard T380A intrauterine copper contraceptive. Trumbull, CT; 2019. https://14wub23xi2gmhufxjmvfmt1d-wpengine.netdna-ssl.com/wp-content/uploads/2018/10/PARAGARD-PI.pdf. [Google Scholar]
- 43.Hall AM, Kutler BA. Intrauterine contraception in nulliparous women: a prospective survey. J Fam Plann Reprod Health Care 2016;42(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateson D, et al. User characteristics, experiences and continuation rates of copper intrauterine device use in a cohort of Australian women. Aust N Z J Obstet Gynaecol 2016;56(6):655–61. [DOI] [PubMed] [Google Scholar]
- 45.Jones RK, et al. Pull and pray or extra protection? Contraceptive strategies involving withdrawal among US adult women. Contraception 2014;90(4):416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peragallo Urrutia R, et al. Effectiveness of fertility awareness-based methods for pregnancy prevention: a systematic review. Obstet Gynecol 2018;132(3):591–604. [DOI] [PubMed] [Google Scholar]
- 47.Johnson S, et al. Can apps and calendar methods predict ovulation with accuracy? Curr Med Res Opin 2018;34(9):1587–94. [DOI] [PubMed] [Google Scholar]
- 48.Guzman L, et al. The use of fertility awareness methods (FAM) among young adult Latina and black women: what do they know and how well do they use it? Use of FAM among Latina and black women in the United States. Contraception 2013;88(2):232–8. [DOI] [PubMed] [Google Scholar]
- 49.Duane M, et al. The performance of fertility awareness-based method apps marketed to avoid pregnancy. J Am Board Fam Med 2016;29(4):508–11. [DOI] [PubMed] [Google Scholar]
- 50.Moglia ML, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol 2016;127(6):1153–60. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Food and Drug Administration. Device software functions including mobile medical applications. 2019. https://www.fda.gov/medical-devices/digital-health/device-softwarefunctions-including-mobile-medical-applications.
- 52.Polis CB. Published analysis of contraceptive effectiveness of Daysy and DaysyView app is fatally flawed. Reprod Health 2018;15(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.TherapeuticsMD. Prescribing information Annovera (segesterone acetate and ethinyl estradiol vaginal system). Boca Raton, FL; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209627s000lbl.pdf. [Google Scholar]
- 54.Kohn JE, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception 2018;97(3):198–204. [DOI] [PubMed] [Google Scholar]
- 55.Upadhyay UD, et al. Interest in self-administration of subcutaneous depot medroxyprogesterone acetate in the United States. Contraception 2016;94(4):303–13. [DOI] [PubMed] [Google Scholar]
- 56.U.S. Food and Drug Administration. Caya contoured diaphragm (K140305). Silver Spring, MD; 2018. 501(k) premarket notification (Diaphragm, contraceptive, and accessories); https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K140305. [Google Scholar]
- 57.Schwartz JL, et al. Contraceptive efficacy, safety, fit, and acceptability of a single-size diaphragm developed with enduser input. Obstet Gynecol 2015;125(4):895–903. [DOI] [PubMed] [Google Scholar]
- 58.Kessel medintim GmbH. Consulting guide Caya contoured diaphragm. Mörfelden-Walldorf, Germany; 2015. https://www.medintim.de/wp-content/uploads/2018/03/rz_flyer_caya_leitfaden_aerzte_en_180323_WEB.pdf. [Google Scholar]
- 59.Mauck CK, et al. A phase I randomized postcoital testing and safety study of the Caya diaphragm used with 3% Nonoxynol-9 gel, ContraGel or no gel. Contraception 2017;96(2):124–30. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Food and Drug Administration. FDA allows marketing of first direct-to-consumer app for contraceptive use to prevent pregnancy [press release]. Silver Spring, MD; 2018. August 10 https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-app-contraceptiveuse-prevent-pregnancy. [Google Scholar]
- 61.Sudjic O ‘I felt colossally naive’: the backlash against the birth control app. The Guardian 2018. July 21 https://www.theguardian.com/society/2018/jul/21/colossally-naive-backlashbirth-control-app. [Google Scholar]
- 62.Schimmoeller N, Creinin MD. More clarity needed for contraceptive mobile app Pearl Index calculations. Contraception 2018;97(5):456. [DOI] [PubMed] [Google Scholar]
- 63.Jackson AV, et al. Racial and ethnic differences in contraception use and obstetric outcomes: a review. Semin Perinatol 2017;41(5):273–7. [DOI] [PubMed] [Google Scholar]
- 64.Dehlendorf C, et al. Racial/ethnic disparities in contraceptive use: variation by age and women’s reproductive experiences. Am J Obstet Gynecol 2014;210(6):526.e1–526.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shreffler KM, et al. Surgical sterilization, regret, and race: contemporary patterns. Soc Sci Res 2015;50:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dehlendorf C, et al. Recommendations for intrauterine contraception: a randomized trial of the effects of patients’ race/ethnicity and socioeconomic status. Am J Obstet Gynecol 2010;203(4):319 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez AM, et al. Women or LARC first? Reproductive autonomy and the promotion of long-acting reversible contraceptive methods. Perspect Sex Reprod Health 2014; 46(3):171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timmins F Critical practice in nursing care: analysis, action and reflexivity. Nurs Stand 2006;20(39):49–54. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg AE, Gartrell NK. LGB-parent families: the current state of the research and directions for the future. Adv Child Dev Behav 2014;46:57–88. [DOI] [PubMed] [Google Scholar]
- 70.Everett BG, et al. Sexual orientation disparities in mistimed and unwanted pregnancy among adult women. Perspect Sex Reprod Health 2017;49(3):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Everett BG, et al. One in three: challenging heteronormative assumptions in family planning health centers. Contraception 2018;98(4):270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higgins JA, et al. Sexual minority women and contraceptive use: complex pathways between sexual orientation and health outcomes. Am J Public Health 2019;109(12):1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reisner SL, et al. A mixed methods study of the sexual health needs of New England transmen who have sex with nontransgender men. AIDS Patient Care STDS 2010;24(8):501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Light A, et al. Family planning and contraception use in transgender men. Contraception 2018;98(4):266–9. [DOI] [PubMed] [Google Scholar]
- 75.Chrisler JC, et al. Queer periods: attitudes toward and experiences with menstruation in the masculine of centre and transgender community. Cult Health Sex 2016;18(11):1238–50. [DOI] [PubMed] [Google Scholar]
- 76.Obedin-Maliver J, Makadon HJ. Transgender men and pregnancy. Obstet Med 2016;9(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carswell JM, Roberts SA. Induction and maintenance of amenorrhea in transmasculine and nonbinary adolescents. Transgend Health 2017;2(1):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pradhan S, Gomez-Lobo V. Hormonal contraceptives, intrauterine devices, gonadotropin-releasing hormone analogues and testosterone: menstrual suppression in special adolescent populations. J Pediatr Adolesc Gynecol 2019;32(5S):S23–S29. [DOI] [PubMed] [Google Scholar]
- 79.World Health Organization, Western Pacific Region. Integrating poverty and gender into health programmes: a sourcebook for health professionals (module on sexual and reproductive health). Manila, Philippines; 2008. http://www.wpro.who.int/publications/docs/22_October_2008_Module_on_SRH_web.pdf?ua=1. [Google Scholar]


