Abstract
At the population level, there is a parallel escalation in the healthcare burden of both, atrial fibrillation (AF) as well its risk factors. Compounding this relationship, AF is associated with escalating burden at an individual level, due its self-perpetuating and progressive nature. The mechanisms by which these risk factors interact to produce atrial remodelling and subsequent AF are unclear. This intersection is critical to the development of strategies to combat this disease at both the individual and population-level. It is well known that AF can manifest from disturbances in autonomic activity. At the population level, there is growing data to suggest a role of the autonomic nervous system in the future incidence of AF. Here, we provide an overview of the association of cardiac autonomic dysfunction with the incidence of AF, review the role of the autonomic nervous system (ANS) as an intermediary between risk factors and the development of AF and finally, we discuss the bidirectional relationship between AF and cardiac autonomic nervous system dysfunction; to determine whether this is implicated in the progression of AF.
Keywords: Atrial fibrillation, Autonomic nervous system, Autonomic dysfunction, Risk factors, Obesity
Introduction
The prevalence of atrial fibrillation (AF) has surged over the last two decades, such that it has become a significant burden to healthcare.1 Numerous risk factors that contribute to the development of the arrhythmia are also on the rise.2,3 Strategies to combat these risk factors in patients with AF undergoing catheter ablation; have become the cornerstone in the management of AF.4,5 It is clear that the atrial remodelling driven by these risk factors is reversible.2,5 However; the mechanisms by which these risk factors interact to produce atrial remodelling and subsequent AF are not entirely clear. It is well known that AF can manifest from autonomic perturbations;6-8 which several risk factors, themselves could also trigger.9-17 Indeed, there may be several interacting mechanisms and the role of the autonomic nervous system (ANS) as an intermediary between risk factors and the development of AF warrants review.
Studies that have used the cardiac ANS as a target in the management of AF have also provided some important insights. First, modulating the ANS is effective in treating AF.18,19 Second, it can also produce changes that result in longer term reduction in AF; therefore, implying that dysfunction of the cardiac ANS may contribute to atrial remodelling and may play a role in the burden of AF. Critically, the relationship between autonomic dysfunction and AF appears bi-directional; the presence of AF itself can result in a shift in autonomic tone20 cardiac autonomic remodelling 21-23 as well as abnormalities in reflexes involved in the regulation of cardiac volume.24 Last, cardiac autonomic dysfunction appears to heighten the incidence of AF in a large cohort study.25
Where other reviews have primarily addressed 26,27 the role of the ANS in AF-producing atrial electrophysiology and substrate, as well as modulating the ANS in AF; in this review we aim to provide the reader with an updated overview of the role that the ANS plays in the rising burden of AF both at an individual and population-level.
Cardiac autonomic dysfunction with incidence of atrial fibrillation
Although there have not been a great number of large-scale studies that have directly addressed this question, there is mounting indirect evidence through clinical sequelae of autonomic dysregulation to suggest that there is an association between the incidence of AF and cardiac autonomic dysfunction. The first indirect evidence from large population level prospective cohort studies came from those that assessed whether the presence of orthostatic hypotension (OH) was associated with an increased future risk of AF. The first of these studies, a prospective analysis of 33 346 community dwellers in the city of Malmö, Sweden28 demonstrated that the presence of OH; defined as a decrease in systolic blood pressure of ≥ 20 mmHg and/or a decrease in diastolic blood pressure of ≥ 10 mmHg within 3 minutes of standing was independently associated with the risk of AF during approximately 24 years follow up. A subgroup analysis indicated that hypertensive individuals with co-existing OH drove this effect.
In a prospective analysis of the 12 071 participants of the Atherosclerosis Risks in Communities (ARIC) study, there was a substantially increased risk of incident AF in patients with OH (18.4%) compared to those without (11.6%), over an 18-year follow up.29 When the authors adjusted for age, sex and the presence of concomitant risk factors (which perhaps also contribute to autonomic dysfunction), this relationship persisted and was similar in risk to either diabetes or hypertension in their multivariate model.
Psychological stress, in particular anger or hostility, is known to produce perturbations in autonomic activity.30 Here a surge in sympathetic activity and rise in catecholamines together with decreases in vagal activity have been observed in the laboratory setting in humans. In animals, correlated with an increase in catecholamines and reduced heart rate variability, protocols that reproduce social stress can trigger arrhythmia.31 In humans recent data by Lampert et al. 30 demonstrate that AF was more likely to occur during anger or stress and that this association was significantly attenuated in patients on ß-blockers (excluding sotalol). At a population level; the Framingham offspring cohort, which included 3873 participants in whom psychosocial questionnaires at a baseline visit were completed, the incidence of AF at up to 10 year follow up was higher in those with higher anger and hostility measures.32
Perhaps the only direct measure of autonomic function at a population level, albeit limited to spectral heart rate characteristics (from short 2-minute electrocardiograph recordings) also comes from an analysis participants of the Atherosclerosis Risks in Communities (ARIC) study by Agarwal et al.25 This large prospective cohort study (11 715 participants) also had a long follow up of approximately 20 years. Here, the major finding was that, despite adjustment for a variety of variables, lower resting heart rates and a lower overall variability of resting heart rate were highly suggestive of cardiac ANS dysfunction being associated with an increased risk of incident AF. Power spectral density – frequency domain characteristics of both heightened parasympathetic (High Frequency; HF) as well as sympathetic tone (ratio of Low frequency: High Frequency = LF:HF) were associated with incident AF. Given that the measurements are at rest and predict future incident AF; they are unlikely to implicate simple surges in the activity of both arms of the ANS as causative in in the development of AF. Rather, it is more likely that there is an underlying dysfunction of the ANS present even during sinus rhythm and even at rest. This independent association with future incident AF boosts the hypothesis that chronic disturbances of the cardiac ANS, rather than acute shifts, contributes to the development of AF.
The role of autonomic dysfunction as a trigger of atrial fibrillation
The question as to whether AF may be related to acute perturbations in the ANS arose from early observational data that assessed the time of onset of a paroxysm of AF during a 24 hour period and found that there was bimodal distribution strongly resembling the circadian rhythm.8 The cardiac ANS comprises of sympathetic and parasympathetic divisions that can be afferent/signal processing or efferent nerves together with a large network of interneurons that form a plexus often denoted “the little brain on the mammalian heart.”33,34 Extrinsic sympathetic innervation to the heart arise mostly from the extra vertebral sympathetic chain, among which, the stellate ganglion is chief.27 However, the vagus nerve (which contains mostly parasympathetic nerves) has also been found to augment sympathetic tone, with immunohistochemical assessment of the vagus showing adrenergic neuron staining.27 The vagus nerve contains a mix of both motor and sensory neurons that innervate structures in the heart (sino-atrial and atrio-ventricular nodes, both atrial and ventricular myocardium as well as intrinsic cardiac ganglia).27 Afferent (sensory) inputs into the vagus nerve can arise from the pulmonary vein- left atrial junctions, sites known to be critical for the pathogenesis of AF.35
The downstream effects of efferent atrial nerves on cellular electrophysiology to cause AF are well known and have been detailed previously.26,27 Adrenergic nerves release noradrenaline; which stimulates ß-adrenoreceptors through G-coupled proteins. The principal arrhythmogenic effect of adrenergic stimulation comes through its fundamental purpose enhancing myocardial calcium handling in order to produce cardiac contractility in the face of a “fight or flight” situation. 27 Adrenergic nerve- mediated activity of L-type calcium channels, increases calcium influx, resulting in action potential duration changes to enhance early after- depolarization. Owing to the increase in intra-cellular calcium, abnormalities in calcium-handling develop. First, there is calcium overload, resulting in the extrusion of calcium from the sarcoplasmic reticulum. Second, ryanodine type-2-receptor dysfunction occurs, exacerbating this process. The sodium/calcium exchanger ion channel (NCX), owing to its stoichiometry, disproportionately offloads 1 calcium ions from the cell in exchange for 3 sodium ions. This produces a net inward current responsible for triggering delayed after depolarization. Finally, adrenergic stimulation can also enhance automaticity. All these effects can occur at the level of the atrium and the pulmonary veins, triggering AF. 26,27 Cholinergic (parasympathetic) stimulation can result in shortening of the atrial effective refractory period by increasing the activity of IKACh (acetyl-choline receptor mediated inward rectifying potassium channel). This effect is heterogenous owing to the spatial differences in parasympathetic atrial innervation. 26 The combined contribution of both sympathetic and parasympathetic arms appears to be important in the development of AF with some caveats. In younger patients without structural heart disease, a spectrum of vagal triggers such as vaso-vagal syncope, after ingestion of a meal, at night, or during the recovery phase of exercise, can bring about a paroxysm of AF. 36
Can atrial fibrillation risk factors cause an autonomic dysfunction that promotes atrial fibrillation?
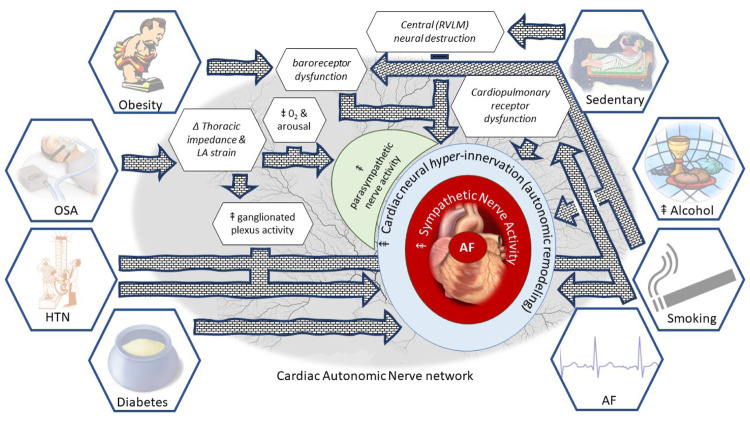
The parallel increases in the prevalence of both, the development of AF as well as its risk factors may be underpinned by a shared mechanism. Here, we review each risk factor for AF, with specific reference to its association with autonomic dysfunction. The features of autonomic dysfunction due to risk factors and the proposed avenues toward the development of AF are summarised in Figure 1.
Figure 1. “All roads lead to AF”: A “map” of the road to risk factors leading to autonomic dysfunction and then to AF.
Obesity
The role of obesity in the development of AF is well established. Firstly, through a strong epidemiological link, second through electrophysiological studies demonstrating atrial substrate development in obesity 2,37,38 and finally, through the finding that weight loss together with a comprehensive risk factor management strategy can result in improved freedom from AF after catheter ablation39 and may even reverse the progression of AF.40 It is also well established that obesity 9,41-45 including visceral obesity17 is associated with an increase in sympathetic tone, as assessed by direct muscle sympathetic nerve activity (MSNA). Importantly, 10% weight loss after gastric banding procedure in severely obese patients led to a reduction in MSNA concomitant with improved cardiac and sympathetic baroreflex function.46
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) has been identified as an independent risk factor for AF.5,10 The acute effect of obstruction results in thoracic impedance changes which increase cardiac venous return and left atrial stretch, with associated electrophysiological changes such as reduced atrial effective refractory period and consequent AF vulnerability.2,47 In parallel with these acute effects, there is also increased ganglionated plexus activity with reduced susceptibility to AF after ganglionated plexi ablation, atropine and vagotomy.2 These observations strongly implicate the ANS in the pathophysiology of AF due to OSA. Additionally, an acute obstructive event also initiates the diving reflex, hypoxia and arousals that induce surges in both parasympathetic and sympathetic activity, which could promote the onset of AF.26 Finally, repetitive airway obstruction can result in chronic effects that include the development of atrial fibrosis and electrical remodelling, both of which contribute to AF maintenance,2 OSA can also result in autonomic remodelling with both sympathetic and parasympathetic atrial hyperinnervation, baroreceptor dysfunction, and chronic sympathetic activation, all of which can produce AF.48 Interestingly, in addition to ganglionated plexus ablation, a variety of strategies to modulate the ANS, such as ß-blockers, renal denervation, low-level tragus stimulation and baroreceptor stimulation, have been shown to reduce AF vulnerability to OSA in pre-clinical studies.48 Further work is required to determine whether neuromodulation can be a useful adjunct in the treatment of OSA, particularly in those intolerant or non-adherent to continuous positive airway pressure treatment.
Hypertension
Epidemiological studies have consistently identified hypertension as an important risk factor for AF.49,50 Although, several mechanistic studies have identified a hypertension related atrial substrate, there is cumulative evidence that in this group, sympathetic overdrive may be a significant contributor to this risk.2,51-54 The ANS has achieved significant attention in the pathogenesis of hypertension.11 Techniques such as sympathetic nerve activity using MSNA, the spill-over of noradrenaline and heart rate variability have clearly shown a sympathetic “overdrive” that occurs at all severities of elevated blood pressure.11 Additionally, the increase in sympathetic activity parallels blood pressure increases; implicating a cause/effect link.55 Indeed, these findings have been further extended to show that sympathetic over-activity is associated with and may be contributory to hypertension related end-organ damage (vascular remodelling, left ventricular hypertrophy, and perhaps even diastolic dysfunction).11
In a small group of pre-hypertensive individuals, MSNA at baseline and at 8 year follow-up demonstrated a correlation between MSNA and BP increase.56 In a comparison of young black men, who have a raised risk of hypertension, to white men, there was an exaggerated vasomotor response to sympathetic activity: where MSNA, itself, was not different between the groups.57 This suggests factors other than gross sympathetic motor tone are important in mediating the role of increased sympathetic nerve activity and high blood pressure. Although arterial baroreceptors and chemoreceptor deficits in heart rate control have been well established; there is no impairment in baroreceptor control of vasomotor tone.11,55 Cardiopulmonary receptors; found typically in veno-atrial junctions of the heart,58 that respond to changes in blood volume and inhibit efferent sympathetic outflow24 have been shown to be reversibly impaired in patients with hypertension and left ventricular hypertrophy.59
Diabetes
The epidemiological association of AF with diabetes is well described and it is considered an independent risk factor for AF.49,60,61 Recent work has demonstrated that in patients undergoing catheter ablation for AF, glycaemic management results in significantly improved outcomes,62 highlighting the need for suitable targets to optimise (>10% reduction in HbA1c and a target of <6.5%).63 Although there are likely a number of responsible mechanisms;63 of which none have been clearly elucidated, it has been shown that diabetes (which is well known to cause autonomic neuropathy) can result in cardiac autonomic dysfunction; assessed using tests such as Valsalva manoeuvre, heart rate variability during deep breathing, heart rate and blood pressure responses to standing and the isometric handgrip test.64 It is proposed that cardiac autonomic dysfunction can cause tachycardia, reduced exercise capacity, orthostatic intolerance (together with peripheral blood vessel sympathetic denervation) and perhaps even silent myocardial ischemia.64
Table 1. Autonomic dysfunction and the incident risk of AF at a population level.
SD = Standard Deviation. CI = confidence Interval. HRV = Heart Rate Variability (SD normal-normal RR interval).
| Study | Measure | Population | Study size | Mean Age |
|---|---|---|---|---|
| Direct evidence of autonomic dysfunction | ||||
| Agarwal et al.25 | Heart Rate Variability | Atherosclerosis Risks in Communities (ARIC) | 11,715 | 54±6 |
| Indirect evidence of autonomic dysfunction | ||||
| Fedorowski et al.28 | Orthostatic hypotension | Malmö, Sweden | 33,346 | 46±7 |
| Agarwal et al.29 | Orthostatic hypotension | Atherosclerosis Risks in Communities (ARIC) | 12, 071 | 55±6 |
| Eaker et al.32 | Psychosocial: Anger and Hostility measures | Framingham offspring | 3873 | 49±10 |
In an animal model of diabetes, atrial refractoriness, atrial conduction velocity and AF inducibility were not different to control rats at baseline; however, diabetic rats showed a heightened susceptibility to AF from sympathetic stimulation, together with histologic evidence of sympathetic nerve remodelling.15 This link has also been demonstrated in humans, where the burden of AF in diabetics was strongly correlated with LF/HF ratio, a frequency domain spectral characteristic of heart rate variability thought to represent a marker of sympathetic activity.65 Overall (time-domain) heart rate variability was not reported in this study, which is a significant limitation. Direct measures of sympathetic nerve activity (MSNA and noradrenaline spill-over) show that diabetes is associated with both an increased central sympathetic drive as well as an impairment in the sympathetic responses to a carbohydrate load.16 Therefore, there is modest evidence that implicates autonomic dysfunction as a mediator of the elevated risk of AF associated with diabetes.
Alcohol excess
The role of alcohol as a dose-dependent risk factor for AF is well known.14 A recent meta-analysis has demonstrated that moderate levels of alcohol consumption are associated with increased risk of AF.66 A randomised intervention study has shown that in patients with AF, abstinence results in reduced recurrence at 6 months follow up.67 A variety of mechanisms have been proposed. However, the direct effect of alcohol as neurotoxin68 resulting in autonomic dysfunction is likely to be important. Acute ingestion of alcohol is associated with a decrease in heart rate variability69,70 and diminished vagal heart rate modulation in healthy adults.69 Sympathetic hyperactivity was observed in patients with coronary artery disease70 and with a history of AF, in whom alcohol ingestion was associated with increased sympathetic tone (assessed using heart rate variability) together with increases in ß-adrenergic density.71 A number of studies also report an increase in MSNA due to alcohol.72
Finally, acute alcohol ingestion, which is a cause of syncope and orthostatic intolerance, is associated with an impaired homeostatic reflex response to decreased venous return, elicited by a non-invasive technique (Lower Body Negative Pressure; LBNP).72,73 Whilst under normal circumstances, blood pressure is maintained due to vasoconstriction,74 alcohol ingestion results in significant decreases in blood pressure and absent vasoconstriction. Although this reflex deficit could potentially be confounded by the known vasodilatory effect of alcohol, Carter et al.72 showed a concomitant deficit in the MSNA response to LBNP, which provides strong evidence to the contrary. Moreover, separate studies report baroreceptor dysfunction due to alcohol,75 implicating cardiac afferent neurotoxicity. Therefore, not only can alcohol cause perturbations in autonomic tone and autonomic remodelling, it may also lead to autonomic deficits, thus, providing a pathway to the development of AF.
Smoking
There is an epidemiologic link between smoking and the long term risk of developing AF, with the highest risk in those with the largest intake, and somewhat decreased risk in those who quit.76 There is no direct evidence linking the ANS to smoking as a risk factor. The pathophysiologic link is presumed to be related to the development of other risk factors in smokers – such as inflammation, hypertension and vascular disease. Nevertheless, there is strong evidence that cigarette smoking causes an increase in sympathetic activity.12 Middlekauff et al.12 provide a comprehensive review of the role of nicotine as well as fine particulate matter from tobacco in the development of autonomic dysfunction. Both acute and chronic effects result in an increase in sympathetic nerve activity, which can at least partially account for the epidemiologic association of smoking with AF.
Smoking causes acute increases in heart rate, blood pressure and contractility due to the effect of nicotine to increase noradrenaline from peripheral sympathetic efferent nerve terminals77,78 as well as a rise in sympathetic nerve activity. Whilst several mechanisms may be responsible for chronic increases in SNA, a number of studies demonstrate a consistent attenuation of afferent nerves, particularly the baroreceptors found in the heart and blood vessels. While under normal circumstances, the baroreflex would counter the effect of nicotine on sympathoexcitation, smoking results in dysfunction, permitting unchecked sympathetic activity.12,79 This effect appears to be reversible; encouraging the role of smoking cessation as a component of risk factor management.
Physical inactivity
Physical activity is associated with a reduced risk of AF in a number of studies and it can offset AF risk in obese individuals.80,81 A sedentary lifestyle is not only strongly linked to both cardiovascular disease as well as mortality, it is associated with autonomic dysfunction.13 Similar to the other risk factors, as described above, physical inactivity is associated with sympathetic hyperactivity, and baroreceptor dysfunction owing to an enhanced baro-reflex initiated sympathetic outflow. More recently, destructive changes in the central nervous system centres that control sympathetic outflow (RVLM; Rostral Ventrolateral medulla) have been described that result in an increase in sympathetic nerve activity.13 Indeed, it is proposed that physical inactivity may contribute to the development of hypertension due to the increased sympathetic activity.13
Although the benefits of physical activity, especially in terms of AF risk are widely accepted; excessive physical activity, particularly endurance exercise, is also associated with an increased risk of AF.82 The mechanisms underlying this association are not well understood; although heightened parasympathetic activity that result in electrophysiological changes in the atrium (shortening refractory periods) due to acetylcholine dependent potassium channels may play a role.82,83 A rat model of endurance training has demonstrated a temporal association with vagal activation (both efferent atrial effects and baroreceptor augmentation) coupled with AF inducibility; that wanes with cessation of excessive physical activity.84
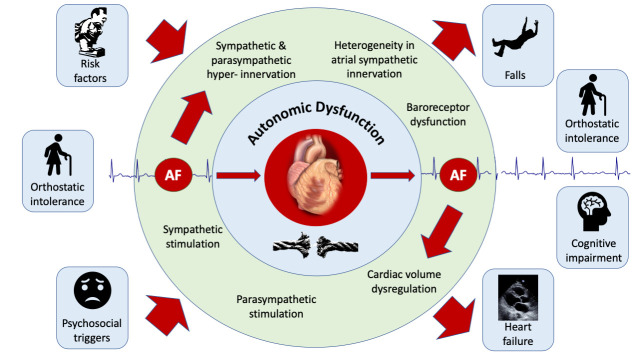
Bi-directional relationship between atrial fibrillation and cardiac autonomics: “AF begets AF”
The clinical progression of AF – and the dictum “AF begets AF” is well understood.85 There is overwhelming evidence that risk factors for AF may at least partially mediate their impact through autonomic dysfunction. Autonomic dysfunction may even potentiate risk factors such that cardiovascular disease, including AF may become much more likely in patients with more than one risk factor. Similarly, there is a strong link between autonomic dysfunction and the development of AF. Therefore, it is with substantial precedent that we hypothesise a bidirectional relationship between ANS dysfunction and the incidence of AF Figure 2
Figure 2. The bidirectional relationship of AF and the autonomic nervous system.
Traditionally, the role of the ANS in the development of AF has been moulded from the concept that acute perturbations in autonomic tone can cause functional (reversible) abnormalities in atrial electrophysiologic properties that result in a “bout” of AF.27 There is increasing evidence to support chronic changes in autonomic function (autonomic remodelling) at the level of the atria due to AF.21-23 Animal studies have shown that atrial phenol application to produce heterogenous sympathetic denervation results in electrophysiological substrate development as well as substantially increased AF inducibility.86 In another study in canines, 6 weeks of pacing induced AF produced hyperinnervation of efferent sympathetic nerves (assessed with both Positron Emission Tomography using C-11 hydroxyephedrine labelled sympathetic nerve terminals as well as tissue noradrenaline content.21 Heterogeneity of sympathetic innervation was also seen, which correlated with electrophysiological remodelling (atrial refractoriness). Finally, propranolol blunted these effects at 6 weeks, whereas others have shown that acutely, ß-blockade does not. These data suggest that AF itself promotes atrial autonomic remodelling.21 Further evidence of longer term autonomic remodelling (> 6 weeks of pacing induced AF in dogs) comes from immunohistochemical assessment of the cardiac intrinsic ganglia, which has shown an increase in both sympathetic22,23 and parasympathetic neurons.23
In patients with heart failure and chronic AF, assessment of single unit MSNA, which provides the advantage of detailed assessment of sympathetic activation per cardiac cycle and overcomes the limitations of multiunit MSNA recording during AF, has demonstrated an increase in sympathetic activity over heart failure patients in sinus rhythm.87 More importantly, variability in the R-R interval in AF was found to be associated with changes in sympathetic nerve activity. Specifically, lengthening of the R-R interval (with associated reduction in diastolic pressure) results in greater single unit MSNA.87 This strongly implicates that AF itself is responsible for the increase in sympathetic activity. Although the precise mechanisms by which this occurs has not been extensively evaluated, a longer R-R interval could unload arterial baroreceptors; augmenting sympathetic activity.26,87 Indeed, Gould et al.88 have shown impaired sympathetic responses to passive head-up tilt (which unloads baroreceptors) in patients with heart failure during AF in comparison to those in sinus rhythm. In patients with persistent AF referred for cardioversion, baroreflex abnormalities were corrected by restoring sinus rhythm.89
Finally, we have shown that patients who have symptomatic paroxysmal AF referred for catheter ablation and studied in sinus rhythm; have an impairment of the reflex response to Lower Body negative Pressure (LBNP) in comparison to age and sex matched healthy adults.24 The normal response to LBNP has already been discussed in the text. By decreasing cardiac venous return, LBNP deactivates receptors (known as cardiopulmonary, volume sensitive or stretch receptors) found mostly in veno-atrial junctions in the heart, and surrounding the pulmonary veins.35,58 This results in vasoconstriction in order to maintain blood pressure. Therefore, these receptors are important in the regulation of cardiac volume.74 Although the deficit could occur anywhere along the reflex arc, we demonstrated that successful AF ablation outcome (at a minimum of 3 months after the procedure and up to 2 years) resulted in improvement of this reflex. Moreover, these abnormalities were identified in sinus rhythm in patients with paroxysmal AF, as opposed to during AF only; highly suggestive that cardiac autonomic remodelling was responsible for these differences. Further, such abnormalities in autonomic function could also contribute to sequelae of AF; such as an independently increased risk of falls and orthostatic intolerance in older adults,90 diminished cerebral blood flow during AF,91 which may contribute to the risk of cognitive decline & dementia92 as well as heart failure93 owing to dysregulation of cardiac volume.
Clinical implications and perspective for future research
A thorough understanding of the important role that the ANS plays in the development of cardiovascular diseases, particularly AF, could present us more potentially effective methods to mitigate AF risk in susceptible individuals as well as halt its progression. Measurement of autonomic activity may provide a better marker of risk in individuals with one or more risk factors for AF. Strategies to manage risk factors could be refined to better modulate the ANS, such as individualised targets of weight loss using sympathetic activity as a guide, management of hypertension using agents that dampen down central sympathetic activity, advising particular kinds of physical exertion, or psychological techniques such as mindfulness or yoga to reduce autonomic perturbations that could trigger AF. In patients with established AF, neuromodulation techniques, which have already shown to be useful in reducing the burden of AF,18,26 may also delay its progression. Indeed, neuromodulation can also be considered in those who are unable to manage their risk factors by lifestyle adjustments or during the period in which they are making changes.
Future work should initially focus on a large-scale characterization of autonomic function across a wide spectrum of individuals with AF in order to determine whether autonomic dysfunction parallels AF severity with a focus on cardiovascular reflex testing in order to understand the role of afferent inputs that regulate central autonomic outflow.
Lastly, delineating the mechanisms responsible for afferent ANS dysfunction and its integration into higher centres to control overall autonomic activity warrants attention. This is an area that has been thus far neglected and may provide important insights into both the progression of AF; specifically, whether this represents an additional mechanism of atrial remodelling, as well as represent the missing mechanistic link between AF and its consequences such as heart failure,94 falls and orthostatic intolerance90 and perhaps even cognitive decline and dementia.92
Conclusions
Through a combination of epidemiological association as well as mechanistic studies, there is considerable evidence to suggest the concept that the ANS may form an integral link between lifestyle-based risk factors and the development of AF. Potentially this relationship may extend to be one of the drivers of disease progression. Therefore, we propose that the role of the ANS is quite literally an important section of the “road” that leads to AF.
References
- 1.Gallagher Celine, Hendriks Jeroen Ml, Giles Lynne, Karnon Jonathan, Pham Clarabelle, Elliott Adrian D, Middeldorp Melissa E, Mahajan Rajiv, Lau Dennis H, Sanders Prashanthan, Wong Christopher X. Increasing trends in hospitalisations due to atrial fibrillation in Australia from 1993 to 2013. Heart. 2019 Sep;105 (17):1358–1363. doi: 10.1136/heartjnl-2018-314471. [DOI] [PubMed] [Google Scholar]
- 2.Lau Dennis H, Nattel Stanley, Kalman Jonathan M, Sanders Prashanthan. Modifiable Risk Factors and Atrial Fibrillation. Circulation. 2017 Aug 08;136 (6):583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 3.Lavie Carl J, Pandey Ambarish, Lau Dennis H, Alpert Martin A, Sanders Prashanthan. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017 Oct 17;70 (16):2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 4.January Craig T, Wann L Samuel, Calkins Hugh, Chen Lin Y, Cigarroa Joaquin E, Cleveland Joseph C, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Furie Karen L, Heidenreich Paul A, Murray Katherine T, Shea Julie B, Tracy Cynthia M, Yancy Clyde W. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019 Jul 09;140 (2):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 5.Middeldorp Melissa E, Ariyaratnam Jonathan, Lau Dennis, Sanders Prashanthan. Lifestyle modifications for treatment of atrial fibrillation. Heart. 2020 Mar;106 (5):325–332. doi: 10.1136/heartjnl-2019-315327. [DOI] [PubMed] [Google Scholar]
- 6.Patterson Eugene, Po Sunny S, Scherlag Benjamin J, Lazzara Ralph. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 Jun;2 (6):624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Bettoni Marco, Zimmermann Marc. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002 Jun 11;105 (23):2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 8.Viskin S, Golovner M, Malov N, Fish R, Alroy I, Vila Y, Laniado S, Kaplinsky E, Roth A. Circadian variation of symptomatic paroxysmal atrial fibrillation. Data from almost 10 000 episodes. Eur. Heart J. 1999 Oct;20 (19):1429–34. doi: 10.1053/euhj.1999.1632. [DOI] [PubMed] [Google Scholar]
- 9.Lambert Elisabeth A, Esler Murray D, Schlaich Markus P, Dixon John, Eikelis Nina, Lambert Gavin W. Obesity-Associated Organ Damage and Sympathetic Nervous Activity. Hypertension. 2019 Jun;73 (6):1150–1159. doi: 10.1161/HYPERTENSIONAHA.118.11676. [DOI] [PubMed] [Google Scholar]
- 10.Gami Apoor S, Hodge Dave O, Herges Regina M, Olson Eric J, Nykodym Jiri, Kara Tomas, Somers Virend K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007 Feb 06;49 (5):565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Grassi Guido. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009 Oct;54 (4):690–7. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 12.Middlekauff Holly R, Park Jeanie, Moheimani Roya S. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J. Am. Coll. Cardiol. 2014 Oct 21;64 (16):1740–50. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 13.Mischel Nicholas A, Subramanian Madhan, Dombrowski Maryetta D, Llewellyn-Smith Ida J, Mueller Patrick J. (In)activity-related neuroplasticity in brainstem control of sympathetic outflow: unraveling underlying molecular, cellular, and anatomical mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2015 Jul 15;309 (2):H235–43. doi: 10.1152/ajpheart.00929.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voskoboinik Aleksandr, Prabhu Sandeep, Ling Liang-Han, Kalman Jonathan M, Kistler Peter M. Alcohol and Atrial Fibrillation: A Sobering Review. J. Am. Coll. Cardiol. 2016 Dec 13;68 (23):2567–2576. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Otake Hideki, Suzuki Hitoshi, Honda Takashi, Maruyama Yukio. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009 Sep;50 (5):627–41. doi: 10.1536/ihj.50.627. [DOI] [PubMed] [Google Scholar]
- 16.Straznicky Nora E, Grima Mariee T, Sari Carolina I, Eikelis Nina, Lambert Elisabeth A, Nestel Paul J, Esler Murray D, Dixon John B, Chopra Reena, Tilbrook Alan J, Schlaich Markus P, Lambert Gavin W. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes. 2012 Oct;61 (10):2506–16. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez Guy E, Beske Stacy D, Ballard Tasha P, Davy Kevin P. Sympathetic neural activation in visceral obesity. Circulation. 2002 Nov 12;106 (20):2533–6. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 18.Stavrakis Stavros, Stoner Julie A, Humphrey Mary Beth, Morris Lynsie, Filiberti Adrian, Reynolds Justin C, Elkholey Khaled, Javed Isma, Twidale Nicholas, Riha Pavel, Varahan Subha, Scherlag Benjamin J, Jackman Warren M, Dasari Tarun W, Po Sunny S. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol. 2020 Mar;6 (3):282–291. doi: 10.1016/j.jacep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katritsis Demosthenes G, Pokushalov Evgeny, Romanov Alexander, Giazitzoglou Eleftherios, Siontis George C M, Po Sunny S, Camm A John, Ioannidis John P A. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J. Am. Coll. Cardiol. 2013 Dec 17;62 (24):2318–25. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Linz Dominik, Ukena Christian, Mahfoud Felix, Neuberger Hans-Ruprecht, Böhm Michael. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J. Am. Coll. Cardiol. 2014 Jan 28;63 (3):215–24. doi: 10.1016/j.jacc.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Jayachandran J V, Sih H J, Winkle W, Zipes D P, Hutchins G D, Olgin J E. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000 Mar 14;101 (10):1185–91. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 22.Chang C M, Wu T J, Zhou S, Doshi R N, Lee M H, Ohara T, Fishbein M C, Karagueuzian H S, Chen P S, Chen L S. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001 Jan 02;103 (1):22–5. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Yu Yang, Wei Cao, Liu Li, Lian Ai Ling, Qu Xiu Fen, Yu Guang. Atrial fibrillation increases sympathetic and parasympathetic neurons in the intrinsic cardiac nervous system. Pacing Clin Electrophysiol. 2014 Nov;37 (11):1462–9. doi: 10.1111/pace.12450. [DOI] [PubMed] [Google Scholar]
- 24.Malik Varun, McKitrick Douglas J, Lau Dennis H, Sanders Prashanthan, Arnolda Leonard F. Clinical evidence of autonomic dysfunction due to atrial fibrillation: implications for rhythm control strategy. J Interv Card Electrophysiol. 2019 Apr;54 (3):299–307. doi: 10.1007/s10840-019-00508-z. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal Sunil K, Norby Faye L, Whitsel Eric A, Soliman Elsayed Z, Chen Lin Y, Loehr Laura R, Fuster Valentin, Heiss Gerardo, Coresh Josef, Alonso Alvaro. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. J. Am. Coll. Cardiol. 2017 Jan 24;69 (3):291–299. doi: 10.1016/j.jacc.2016.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linz Dominik, Elliott Adrian D, Hohl Mathias, Malik Varun, Schotten Ulrich, Dobrev Dobromir, Nattel Stanley, Böhm Michael, Floras John, Lau Dennis H, Sanders Prashanthan. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 2019 Jul 15;287 ():181–188. doi: 10.1016/j.ijcard.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 27.Chen Peng-Sheng, Chen Lan S, Fishbein Michael C, Lin Shien-Fong, Nattel Stanley. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 2014 Apr 25;114 (9):1500–15. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorowski A, Hedblad B, Engström G, Gustav Smith J, Melander O. Orthostatic hypotension and long-term incidence of atrial fibrillation: the Malmö Preventive Project. J. Intern. Med. 2010 Oct;268 (4):383–9. doi: 10.1111/j.1365-2796.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal Sunil K, Alonso Alvaro, Whelton Seamus P, Soliman Elsayed Z, Rose Kathryn M, Chamberlain Alanna M, Simpson Ross J, Coresh Josef, Heiss Gerardo. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PLoS ONE. 2013;8 (11) doi: 10.1371/journal.pone.0079030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampert Rachel, Burg Matthew M, Jamner Larry D, Dziura James, Brandt Cynthia, Li Fangyong, Donovan Theresa, Soufer Robert. Effect of β-blockers on triggering of symptomatic atrial fibrillation by anger or stress. Heart Rhythm. 2019 Aug;16 (8):1167–1173. doi: 10.1016/j.hrthm.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sgoifo A, de Boer S F, Westenbroek C, Maes F W, Beldhuis H, Suzuki T, Koolhaas J M. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am. J. Physiol. 1997 Oct;273 (4):H1754–60. doi: 10.1152/ajpheart.1997.273.4.H1754. [DOI] [PubMed] [Google Scholar]
- 32.Eaker Elaine D, Sullivan Lisa M, Kelly-Hayes Margaret, D'Agostino Ralph B, Benjamin Emelia J. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation. 2004 Mar 16;109 (10):1267–71. doi: 10.1161/01.CIR.0000118535.15205.8F. [DOI] [PubMed] [Google Scholar]
- 33.Armour J A. Potential clinical relevance of the 'little brain' on the mammalian heart. Exp. Physiol. 2008 Feb;93 (2):165–76. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 34.Ardell J L, Andresen M C, Armour J A, Billman G E, Chen P-S, Foreman R D, Herring N, O'Leary D S, Sabbah H N, Schultz H D, Sunagawa K, Zucker I H. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J. Physiol. (Lond.) 2016 Jul 15;594 (14):3877–909. doi: 10.1113/JP271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COLERIDGE J C, HEMINGWAY A, HOLMES R L, LINDEN R J. The location of atrial receptors in the dog: a physiological and histological study. J. Physiol. (Lond.) 1957 Apr 03;136 (1):174–97. doi: 10.1113/jphysiol.1957.sp005750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groh Christopher A, Faulkner Madelaine, Getabecha Shiffen, Taffe Victoria, Nah Gregory, Sigona Kathi, McCall Debbe, Hills Mellanie True, Sciarappa Kathleen, Pletcher Mark J, Olgin Jeffrey E, Marcus Gregory M. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. 2019 Jul;16 (7):996–1002. doi: 10.1016/j.hrthm.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Abed Hany S, Samuel Chrishan S, Lau Dennis H, Kelly Darren J, Royce Simon G, Alasady Muayad, Mahajan Rajiv, Kuklik Pawel, Zhang Yuan, Brooks Anthony G, Nelson Adam J, Worthley Stephen G, Abhayaratna Walter P, Kalman Jonathan M, Wittert Gary A, Sanders Prashanthan. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013 Jan;10 (1):90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Mahajan Rajiv, Lau Dennis H, Brooks Anthony G, Shipp Nicholas J, Manavis Jim, Wood John P M, Finnie John W, Samuel Chrishan S, Royce Simon G, Twomey Darragh J, Thanigaimani Shivshanker, Kalman Jonathan M, Sanders Prashanthan. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015 Jul 07;66 (1):1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 39.Pathak Rajeev K, Middeldorp Melissa E, Meredith Megan, Mehta Abhinav B, Mahajan Rajiv, Wong Christopher X, Twomey Darragh, Elliott Adrian D, Kalman Jonathan M, Abhayaratna Walter P, Lau Dennis H, Sanders Prashanthan. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015 May 26;65 (20):2159–69. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Middeldorp Melissa E, Pathak Rajeev K, Meredith Megan, Mehta Abhinav B, Elliott Adrian D, Mahajan Rajiv, Twomey Darragh, Gallagher Celine, Hendriks Jeroen M L, Linz Dominik, McEvoy R Doug, Abhayaratna Walter P, Kalman Jonathan M, Lau Dennis H, Sanders Prashanthan. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018 Dec 01;20 (12):1929–1935. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 41.Grassi G, Seravalle G, Cattaneo B M, Bolla G B, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995 Apr;25 (4 Pt 1):560–3. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 42.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo B M, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998 May 26;97 (20):2037–42. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Dell'Oro R, Turri C, Pasqualinotto L, Colombo M, Mancia G. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension. 2001 Dec 01;38 (6):1316–20. doi: 10.1161/hy1201.096117. [DOI] [PubMed] [Google Scholar]
- 44.Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994 Jun;89 (6):2634–40. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 45.Spraul M, Ravussin E, Fontvieille A M, Rising R, Larson D E, Anderson E A. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J. Clin. Invest. 1993 Oct;92 (4):1730–5. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert Elisabeth A, Rice Toni, Eikelis Nina, Straznicky Nora E, Lambert Gavin W, Head Geoffrey A, Hensman Chris, Schlaich Markus P, Dixon John B. Sympathetic activity and markers of cardiovascular risk in nondiabetic severely obese patients: the effect of the initial 10% weight loss. Am. J. Hypertens. 2014 Oct;27 (10):1308–15. doi: 10.1093/ajh/hpu050. [DOI] [PubMed] [Google Scholar]
- 47.Dimitri Hany, Ng Michelle, Brooks Anthony G, Kuklik Pawel, Stiles Martin K, Lau Dennis H, Antic Nicholas, Thornton Andrew, Saint David A, McEvoy Doug, Antic Ral, Kalman Jonathan M, Sanders Prashanthan. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012 Mar;9 (3):321–7. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Huang Bing, Liu Huafen, Scherlag Benjamin J, Sun Lihua, Xing Shifeng, Xu Jie, Luo Mei, Guo Yankai, Cao Guiqiu, Jiang Hong. Atrial fibrillation in obstructive sleep apnea: Neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 2020 Jan 22; () doi: 10.1016/j.tcm.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Benjamin E J, Levy D, Vaziri S M, D'Agostino R B, Belanger A J, Wolf P A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994 Mar 16;271 (11):840–4. [PubMed] [Google Scholar]
- 50.Huxley Rachel R, Lopez Faye L, Folsom Aaron R, Agarwal Sunil K, Loehr Laura R, Soliman Elsayed Z, Maclehose Rich, Konety Suma, Alonso Alvaro. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011 Apr 12;123 (14):1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau Dennis H, Mackenzie Lorraine, Kelly Darren J, Psaltis Peter J, Worthington Michael, Rajendram Arumuga, Kelly Douglas R, Nelson Adam J, Zhang Yuan, Kuklik Pawel, Brooks Anthony G, Worthley Stephen G, Faull Randall J, Rao Mohan, Edwards James, Saint David A, Sanders Prashanthan. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Heart Rhythm. 2010 Mar;7 (3):396–404. doi: 10.1016/j.hrthm.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Lau Dennis H, Mackenzie Lorraine, Kelly Darren J, Psaltis Peter J, Brooks Anthony G, Worthington Michael, Rajendram Arumuga, Kelly Douglas R, Zhang Yuan, Kuklik Pawel, Nelson Adam J, Wong Christopher X, Worthley Stephen G, Rao Mohan, Faull Randall J, Edwards James, Saint David A, Sanders Prashanthan. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010 Sep;7 (9):1282–90. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Lau Dennis H, Shipp Nicholas J, Kelly Darren J, Thanigaimani Shivshankar, Neo Melissa, Kuklik Pawel, Lim Han S, Zhang Yuan, Drury Karen, Wong Christopher X, Chia Nicholas H, Brooks Anthony G, Dimitri Hany, Saint David A, Brown Lindsay, Sanders Prashanthan. Atrial arrhythmia in ageing spontaneously hypertensive rats: unraveling the substrate in hypertension and ageing. PLoS ONE. 2013;8 (8) doi: 10.1371/journal.pone.0072416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kistler Peter M, Sanders Prashanthan, Dodic Miodrag, Spence Steven J, Samuel Chrishan S, Zhao Chongxin, Charles Jennifer A, Edwards Glenn A, Kalman Jonathan M. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur. Heart J. 2006 Dec;27 (24):3045–56. doi: 10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 55.Grassi G, Cattaneo B M, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998 Jan;31 (1):68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 56.Hering Dagmara, Kara Tomas, Kucharska Wiesława, Somers Virend K, Narkiewicz Krzysztof. Longitudinal tracking of muscle sympathetic nerve activity and its relationship with blood pressure in subjects with prehypertension. Blood Press. 2016 Jun;25 (3):184–92. doi: 10.3109/08037051.2015.1121708. [DOI] [PubMed] [Google Scholar]
- 57.Vranish Jennifer R, Holwerda Seth W, Young Benjamin E, Credeur Daniel P, Patik Jordan C, Barbosa Thales C, Keller David M, Fadel Paul J. Exaggerated Vasoconstriction to Spontaneous Bursts of Muscle Sympathetic Nerve Activity in Healthy Young Black Men. Hypertension. 2018 Jan;71 (1):192–198. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucker I H, Earle A M, Gilmore J P. The mechanism of adaptation of left atrial stretch receptors in dogs with chronic congestive heart failure. J. Clin. Invest. 1977 Aug;60 (2):323–31. doi: 10.1172/JCI108780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancia G, Grassi G, Giannattasio C. Cardiopulmonary receptor reflex in hypertension. Am. J. Hypertens. 1988 Jul;1 (3 Pt 1):249–55. doi: 10.1093/ajh/1.3.249. [DOI] [PubMed] [Google Scholar]
- 60.Huxley Rachel R, Filion Kristian B, Konety Suma, Alonso Alvaro. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am. J. Cardiol. 2011 Jul 01;108 (1):56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong Zhaohan, Liu Tong, Tse Gary, Gong Mengqi, Gladding Patrick A, Smaill Bruce H, Stiles Martin K, Gillis Anne M, Zhao Jichao. A Machine Learning Aided Systematic Review and Meta-Analysis of the Relative Risk of Atrial Fibrillation in Patients With Diabetes Mellitus. Frontiers in physiology. 2018;9 () doi: 10.3389/fphys.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donnellan Eoin, Aagaard Philip, Kanj Mohamed, Jaber Wael, Elshazly Mohamed, Hoosien Michael, Baranowski Bryan, Hussein Ayman, Saliba Walid, Wazni Oussama. Association Between Pre-Ablation Glycemic Control and Outcomes Among Patients With Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin Electrophysiol. 2019 Aug;5 (8):897–903. doi: 10.1016/j.jacep.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Sanders Prashanthan, Malik Varun, Lau Dennis H. Glycemic Control in Atrial Fibrillation: A Sweet Spot of Risk Factor Modification. JACC Clin Electrophysiol. 2019 Aug;5 (8):904–906. doi: 10.1016/j.jacep.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 64.Vinik Aaron I, Maser Raelene E, Mitchell Braxton D, Freeman Roy. Diabetic autonomic neuropathy. Diabetes Care. 2003 May;26 (5):1553–79. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 65.Rizzo Maria Rosaria, Sasso Ferdinando Carlo, Marfella Raffaele, Siniscalchi Mario, Paolisso Pasquale, Carbonara Ornella, Capoluongo Maria Carmela, Lascar Nadia, Pace Caterina, Sardu Celestino, Passavanti Beatrice, Barbieri Michelangela, Mauro Ciro, Paolisso Giuseppe. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. J. Diabetes Complicat. 2014 Oct 1;29 (1):88–92. doi: 10.1016/j.jdiacomp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher Celine, Hendriks Jeroen M L, Elliott Adrian D, Wong Christopher X, Rangnekar Geetanjali, Middeldorp Melissa E, Mahajan Rajiv, Lau Dennis H, Sanders Prashanthan. Alcohol and incident atrial fibrillation - A systematic review and meta-analysis. Int. J. Cardiol. 2017 Nov 01;246 ():46–52. doi: 10.1016/j.ijcard.2017.05.133. [DOI] [PubMed] [Google Scholar]
- 67.Voskoboinik Aleksandr, Kalman Jonathan M, De Silva Anurika, Nicholls Thomas, Costello Benedict, Nanayakkara Shane, Prabhu Sandeep, Stub Dion, Azzopardi Sonia, Vizi Donna, Wong Geoffrey, Nalliah Chrishan, Sugumar Hariharan, Wong Michael, Kotschet Emily, Kaye David, Taylor Andrew J, Kistler Peter M. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N. Engl. J. Med. 2020 Jan 02;382 (1):20–28. doi: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- 68.Harper Clive. The neurotoxicity of alcohol. Hum Exp Toxicol. 2007 Mar;26 (3):251–7. doi: 10.1177/0960327107070499. [DOI] [PubMed] [Google Scholar]
- 69.Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clin. Sci. 1994 Aug;87 (2):225–30. doi: 10.1042/cs0870225. [DOI] [PubMed] [Google Scholar]
- 70.Rossinen J, Viitasalo M, Partanen J, Koskinen P, Kupari M, Nieminen M S. Effects of acute alcohol ingestion on heart rate variability in patients with documented coronary artery disease and stable angina pectoris. Am. J. Cardiol. 1997 Feb 15;79 (4):487–91. doi: 10.1016/s0002-9149(96)00790-4. [DOI] [PubMed] [Google Scholar]
- 71.Mäki T, Toivonen L, Koskinen P, Näveri H, Härkönen M, Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am. J. Cardiol. 1998 Aug 01;82 (3):317–22. doi: 10.1016/s0002-9149(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 72.Carter Jason R, Stream Sarah F, Durocher John J, Larson Robert A. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am. J. Physiol. Endocrinol. Metab. 2011 May;300 (5):E771–8. doi: 10.1152/ajpendo.00674.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narkiewicz K, Cooley R L, Somers V K. Alcohol potentiates orthostatic hypotension : implications for alcohol-related syncope. Circulation. 2000 Feb 01;101 (4):398–402. doi: 10.1161/01.cir.101.4.398. [DOI] [PubMed] [Google Scholar]
- 74.Goswami Nandu, Blaber Andrew Philip, Hinghofer-Szalkay Helmut, Convertino Victor A. Lower Body Negative Pressure: Physiological Effects, Applications, and Implementation. Physiol. Rev. 2019 Jan 01;99 (1):807–851. doi: 10.1152/physrev.00006.2018. [DOI] [PubMed] [Google Scholar]
- 75.Abdel-Rahman A R, Merrill R H, Wooles W R. Effect of acute ethanol administration on the baroreceptor reflex control of heart rate in normotensive human volunteers. Clin. Sci. 1987 Jan;72 (1):113–22. doi: 10.1042/cs0720113. [DOI] [PubMed] [Google Scholar]
- 76.Chamberlain Alanna M, Agarwal Sunil K, Folsom Aaron R, Duval Sue, Soliman Elsayed Z, Ambrose Marietta, Eberly Lynn E, Alonso Alvaro. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011 Aug;8 (8):1160–6. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cryer P E, Haymond M W, Santiago J V, Shah S D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N. Engl. J. Med. 1976 Sep 09;295 (11):573–7. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 78.Haass M, Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997 Jan;10 (6):657–65. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 79.Middlekauff Holly R, Park Jeanie, Agrawal Harsh, Gornbein Jeffrey A. Abnormal sympathetic nerve activity in women exposed to cigarette smoke: a potential mechanism to explain increased cardiac risk. Am. J. Physiol. Heart Circ. Physiol. 2013 Nov 15;305 (10):H1560–7. doi: 10.1152/ajpheart.00502.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elliott Adrian D, Maatman Benjamin, Emery Michael S, Sanders Prashanthan. The role of exercise in atrial fibrillation prevention and promotion: Finding optimal ranges for health. Heart Rhythm. 2017 Nov;14 (11):1713–1720. doi: 10.1016/j.hrthm.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Elliott Adrian D, Linz Dominik, Mishima Ricardo, Kadhim Kadhim, Gallagher Celine, Middeldorp Melissa E, Verdicchio Christian V, Hendriks Jeroen M L, Lau Dennis H, La Gerche Andre, Sanders Prashanthan. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur. Heart J. 2020 Apr 14;41 (15):1479–1486. doi: 10.1093/eurheartj/ehz897. [DOI] [PubMed] [Google Scholar]
- 82.Elliott Adrian D, Linz Dominik, Verdicchio Christian V, Sanders Prashanthan. Exercise and Atrial Fibrillation: Prevention or Causation? Heart Lung Circ. 2018 Sep;27 (9):1078–1085. doi: 10.1016/j.hlc.2018.04.296. [DOI] [PubMed] [Google Scholar]
- 83.Estes N A Mark, Madias Christopher. Atrial Fibrillation in Athletes: A Lesson in the Virtue of Moderation. JACC Clin Electrophysiol. 2017 Sep;3 (9):921–928. doi: 10.1016/j.jacep.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 84.Guasch Eduard, Benito Begoña, Qi Xiaoyan, Cifelli Carlo, Naud Patrice, Shi Yanfen, Mighiu Alexandra, Tardif Jean-Claude, Tadevosyan Artavazd, Chen Yu, Gillis Marc-Antoine, Iwasaki Yu-Ki, Dobrev Dobromir, Mont Lluis, Heximer Scott, Nattel Stanley. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J. Am. Coll. Cardiol. 2013 Jul 02;62 (1):68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 85.Wijffels M C, Kirchhof C J, Dorland R, Allessie M A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 01;92 (7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 86.Olgin J E, Sih H J, Hanish S, Jayachandran J V, Wu J, Zheng Q H, Winkle W, Mulholland G K, Zipes D P, Hutchins G. Heterogeneous atrial denervation creates substrate for sustained atrial fibrillation. Circulation. 1998 Dec 08;98 (23):2608–14. doi: 10.1161/01.cir.98.23.2608. [DOI] [PubMed] [Google Scholar]
- 87.Ikeda Tatsunori, Murai Hisayoshi, Kaneko Shuichi, Usui Soichiro, Kobayashi Daisuke, Nakano Manabu, Ikeda Keiko, Takashima Shin-Ichiro, Kato Takeshi, Okajima Masaki, Furusho Hiroshi, Takamura Masayuki. Augmented single-unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. J. Physiol. (Lond.) 2012 Feb 01;590 (3):509–18. doi: 10.1113/jphysiol.2011.223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gould Paul A, Yii Michael, Esler Murray D, Power John M, Kaye David M. Atrial fibrillation impairs cardiac sympathetic response to baroreceptor unloading in congestive heart failure. Eur. Heart J. 2005 Dec;26 (23):2562–7. doi: 10.1093/eurheartj/ehi468. [DOI] [PubMed] [Google Scholar]
- 89.Field Michael E, Wasmund Stephen L, Page Richard L, Hamdan Mohamed H. Restoring Sinus Rhythm Improves Baroreflex Function in Patients With Persistent Atrial Fibrillation. J Am Heart Assoc. 2016 Feb 23;5 (2) doi: 10.1161/JAHA.115.002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malik Varun, Gallagher Celine, Linz Dominik, Elliott Adrian D, Emami Mehrdad, Kadhim Kadhim, Mishima Ricardo, Hendriks Jeroen M L, Mahajan Rajiv, Arnolda Leonard, Sanders Prashanthan, Lau Dennis H. Atrial Fibrillation Is Associated With Syncope and Falls in Older Adults: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2020 Apr;95 (4):676–687. doi: 10.1016/j.mayocp.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 91.Gardarsdottir Marianna, Sigurdsson Sigurdur, Aspelund Thor, Gardarsdottir Valdis Anna, Forsberg Lars, Gudnason Vilmundur, Arnar David O. Improved brain perfusion after electrical cardioversion of atrial fibrillation. Europace. 2020 Apr 01;22 (4):530–537. doi: 10.1093/europace/euz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diener Hans-Christoph, Hart Robert G, Koudstaal Peter J, Lane Deirdre A, Lip Gregory Y H. Atrial Fibrillation and Cognitive Function: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019 Feb 12;73 (5):612–619. doi: 10.1016/j.jacc.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 93.Anter Elad, Jessup Mariell, Callans David J. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009 May 12;119 (18):2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 94.Marrouche Nassir F, Brachmann Johannes, Andresen Dietrich, Siebels Jürgen, Boersma Lucas, Jordaens Luc, Merkely Béla, Pokushalov Evgeny, Sanders Prashanthan, Proff Jochen, Schunkert Heribert, Christ Hildegard, Vogt Jürgen, Bänsch Dietmar. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018 Feb 01;378 (5):417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]